Abstract
Removing arsenic from industrial wastewater remains a crucial task. To protect public health and safety and address environmental pollution, there is an urgent need for a material that can efficiently remove arsenic from wastewater. In this study, a simple and highly efficient adsorbent, namely, a Co/Mn bimetallic-based organic framework (CoMn-MOF-74) adsorbent, was prepared by a hydrothermal synthesis method. Experimental results demonstrate that CoMn-MOF-74 exhibits excellent adsorption capacity for arsenic ions in wastewater. It was found that the optimal Co/Mn molar ratio of the adsorbent is 1:1. The CoMn-MOF-74 adsorbent compensates for the deficiencies in the adsorption performance of Co-MOF-74 and Mn-MOF-74, increasing the adsorption rate and the highest adsorption capacity. The maximum adsorption rate of CoMn-MOF-74 is 93.4%, and the highest adsorption capacity is 531 mg/g. Fitting CoMn-MOF-74 according to two categories of models, specifically, the adsorption isotherm and adsorption kinetics models, indicated that CoMn-MOF-74 adheres to the Langmuir model and pseudo-second-order kinetic model. The adsorption process is mainly chemical adsorption and monolayer adsorption. Analysis by XPS revealed that metal–oxygen groups and hydroxyl groups play important roles in the adsorption process. In conclusion, the CoMn-MOF-74 adsorbent shows excellent prospects in the field of arsenic adsorption from wastewater and is a promising arsenic-removing adsorbent.
1. Introduction
Environmental pollution has become a crucial issue in all countries. Combating human-induced environmental pollution can improve the ecological environment and protect public health. One of the major problems is the generation of large quantities of industrial wastewater from smelting processes [1]. Usually, industrial wastewater production brings about the generation of various heavy metals, such as copper, zinc, chromium, and arsenic. Among them, arsenic (As) is extremely toxic, and being in a highly arsenic-contaminated environment can have adverse effects on the human body and may cause a number of serious illnesses, such as skin cancers, lung cancers, organ failure [2], hypertension [3], peripheral vascular disease [4], and so on. Arsenic also represents a substantial danger to the ecological environment and organisms [5], mainly through water and air infiltration into surface water, groundwater, and soil.
Arsenic exists in a variety of forms in aqueous solution, mainly in the form of arsenite (As(III)) and arsenate (As(V)) ions, which are determined by environmental conditions such as the redox potential and pH of the arsenic-containing solution [6]. In groundwater with a pH of 6–8.5, As(III) under reducing conditions is dominated by H3AsO3; under oxidizing conditions, As(V) exists in a mixture of H2AsO3− and HAsO42−, respectively [7]. The toxicity and mobility of As(III) are greater than those of As(V) [8]. The main methods currently used to control arsenic in wastewater are adsorption [9], co-precipitation [10], ion exchange [11], and oxidation [12]. Among these methods for removing arsenic ions from wastewater, adsorption is considered to be one of the most effective and simplest methods, with the strengths of being affordable, being extremely efficient, and having a distinct competitive advantage [13]. The focal point of adsorption is the preparation of adsorbents [14]. The adsorbent is the most effective method for controlling arsenic in wastewater. However, conventional adsorbents, for instance, biochar [15], zeolite [16], resin [17], iron-based oxides [18], and other materials examined with the aim of removing anions from solution, have limited the widespread use of adsorbents owing to their inferior adsorptive capacity, poor surface activity, and lack of selectivity. Novel porous materials, such as covalent organic frameworks (COFs) [19,20], metal–organic frameworks (MOFs) [21,22], and porous organic polymers (POPs) [23,24,25], have richer surface functionality and pore topology than conventional adsorbents of the past.
Metal–organic frameworks (MOFs) are porous materials with highly efficient structures—such as MOF-74—which possess uniform pore sizes, permanent voids, structural diversity, excellent resistance to pyrolysis, and open metal sites. These advantages make it an excellent adsorbent material. Due to its high porosity, flexibility and excellent crystallinity, MOF shows good performance in wastewater adsorption and purification and is therefore widely used for the adsorption of heavy metals and other inorganic pollutants in wastewater [26,27,28,29,30]. However, these monometallic MOFs can no longer meet the demand as scientists continue to pursue higher MOF properties. Therefore, bimetallic MOFs have attracted great interest [31,32,33,34]. These are created by adding an extra metal element to a monometallic MOF, which allows the framework of the MOF to be changed while maintaining the properties of the original MOF and the physical and chemical properties of the second metal. The bimetallic MOF can increase the number of active sites and active organic groups, resulting in enhanced adsorption performance. It has been shown by studies that bimetallic and polymetallic MOFs possess greater benefits for arsenic adsorption in water bodies compared to other materials [35,36,37]. However, relevant studies are still scarce. Therefore, more studies are needed on the removal of arsenic from water by MOF or MOF composites. In an attempt to obtain an efficient and economical arsenic adsorbent in water bodies, a novel Co-Mn-based MOF-74 adsorbent was synthesized by a hydrothermal method and used for arsenic adsorption from water. The system was investigated with zeta potential, FTIR, and XPS. In addition, a set of experiments were conducted to analyze and explain the arsenic adsorption performance and the mechanism of the adsorbent.
2. Materials and Methods
2.1. Materials
The experimental reagents used in this experiment were all analytically pure reagents and were not further purified. The arsenic-containing wastewater solution used in the experiment was obtained from a copper smelter in southwest China. The arsenic-containing wastewater solution was diluted with deionized water in order to achieve the concentration required for the experiment. Manganese chloride tetrahydrate (MnCl2·4H2O, 99%), cobalt chloride hexahydrate (CoCl2·6H2O, AR), 2,5-dicarboxyterephthalic acid (C8H6O4, 98%), anhydrous ethanol (CH3CH2OH), and N, N-dimethylformamide(DMF, 99.5%) were purchased from Aladdin, and ultrapure water was obtained in the laboratory using an ultrapure water filtration unit.
2.2. Synthetic Composite Material
CoMn-MOF-74 was synthesized by a hydrothermal method according to the previous literature [36,37]. An amount of 1.5 mmol of CoCl2·6H2O was weighed and dissolved with 1.5 mmol of MnCl2·4H2O and 0.56 mmol of 2,5-dicarboxyterephthalic acid in 45 mL DMF, and 3 mL of C2H5OH and 3 mL of H2O were added. The mixed liquids were ultrasonicated, and then the fully fused solution was placed into a polytetrafluoroethylene reactor for hydrothermal treatment at 150 °C for 12 h. Following the cooling process to room temperature, the resulting precipitate was filtered by vacuum filtration to obtain the sample. The sample was washed three times with DMF and three times with anhydrous ethanol. The sample powder was then dried at 60 °C for 24 h. We synthesized other Co/Mn molar ratios of adsorbents with total molar amounts of the two metal ions added in Co/Mn molar ratios of 1:0, 3:1, 2:1, 1:2, and 1:3. The synthesis method and conditions were the same as in the previous section.
2.3. Experimental Procedures
The crystal properties of the resultant Co/Mn-MOF-74 were probed via X-ray diffraction (XRD) with a scan rate set at 5° per minute over a scan angle ranging from 0° to 90°. Scanning electron microscopy (SEM) was employed to assess the morphological architecture of the samples. Fourier transform infrared spectroscopy (FTIR) was utilized to elucidate the chemical bonding patterns and compositional details of the specimens. The porosity of the samples was evaluated using a Brunauer–Emmett–Teller (BET) analyzer. The concentration of trace metal and non-metal ions in the solution was quantified by means of inductively coupled plasma atomic emission spectroscopy (ICP-OES, model PQ9000, Analytik Jena AG, Jena, Germany).
2.4. Batch Adsorption Procedures
Batch experiments were carried out at ambient temperature using copper smelting industrial wastewater to study the adsorption and removal efficiency of different solution pH levels, adsorbent adsorption times, solution concentrations and adsorbent dosages on the interaction process of wastewater with CoMn-MOF-74. All the batch tests were carried out by placing 100 mL conical flasks into a thermostatic shaking chamber at 180 r/min at room temperature. An amount of 50 mL of the wastewater that contained arsenic and had a concentration of 30 mg/L was transferred into a conical flask, and the pH of the arsenic-containing wastewater was adjusted to 11.0 ± 0.2 with 0.1 M HCl/NaOH solution (we measured the pH of the arsenic-containing solution with a pH meter to ensure that it was stabilized correctly during the titration of the acid–base solution). Then 10 mg of CoMn-MOF-74 adsorbent was added, and the wastewater was placed into a shaking chamber and shaken at 25 °C for 24 h at a rotation rate of 180 revolutions per minute [38]. The solution was then extracted from the water, and the water was then mixed into the other water. After equilibration, it was filtered using a vacuum filter to separate the adsorbed CoMn-MOF-74 for later characterization. Inductively coupled plasma emission spectrometry was used to determine the arsenic residue in the filtrate. Kinetic adsorption tests were carried out on CoMn-MOF-74 at time intervals of 5~7200 s. The results are summarized as follows. The experimental data were analyzed using a pseudo-first-order kinetic model, pseudo-second-order kinetic model, and intra-particle diffusion model. The CoMn-MOF-74 was utilized to carry out isothermal adsorption tests in arsenic-laden wastewater having a concentration interval of 80–1000 mg/L. The experimental data were then examined and fitted with the Langmuir and Freundlich isothermal models [39]. The effect of pH on the adsorption of arsenic was investigated by changing the pH of arsenic-containing wastewater (pH = 2–12); the impact of varying the quantity of CoMn-MOF-74 (at levels of 0.2 g/L, 0.40 g/L, 0.60 g/L, 0.80 g/L, 1.00 g/L, 1.20 g/L) on the arsenic adsorption performance was explored [40]; and the effect of addition on the adsorption of arsenic was probed by changing the initial concentration of arsenic-containing wastewater (80–1000 mg/L) for the purpose of probing the role of concentration in the adsorption of arsenic. In order to prevent the effect of experimental errors, all experiments were performed three times. Equation (1) was utilized to calculate the arsenic-adsorbing efficiency and equation (2) was employed to determine the adsorption capacity.
where C0 is the initial arsenic concentration of the untreated wastewater and Ce is the final arsenic concentration (mg/L) obtained from the treated wastewater using Co/Mn-MOF-74. V is used to signify the volume (mL) of the arsenic-laden effluent, and M is employed to denote the quantity (mg) of the adsorbent material.
3. Results and Discussion
3.1. Characterization of CoMn-MOF-74
The morphological characteristics of CoMn-MOF-74 were examined by means of scanning electron microscopy (SEM). As illustrated in Figure 1a, the adsorbent exhibits prismatic columns aggregated into an irregular dumbbell-shaped structure. As shown in Figure 1c below, the elemental maps presented by SEM images and EDS images reveal that C, O, Co, and Mn elements are uniformly distributed on the surface of the CoMn-MOF-74 adsorbent. X-ray diffraction (XRD) was primarily applied to conduct the physical phase analysis of the specimens. Figure 2b displays the XRD image of the CoMn-MOF-74 material, which shows clear and sharp peaks indicating that the MOF has good crystallinity. Among them, CoMn-MOF-74 has obvious and prominent characteristic peaks at 6.9°, 11.9°, 21.6° 25.6°, 30°, and 31.5°;significantly, a high and sharply defined peak occurs at 6.9°, which shows that the synthesized adsorbent has a good degree of crystallinity, which is basically in agreement with the previously reported XRD patterns [41]. Overall, these results affirm the successful preparation of the adsorbent Co/Mn-MOF-74. The N2 adsorption–desorption curve as well as the pore size distribution are illustrated in Figure 2a. From the IUPAC classification, the material was classified as a type IV isothermal material with an H3 apparent hysteresis curve. The BET surface area of this synthesized material was 10.9384 m2/g, and the homogeneous pore size was 11.5962 nm.

Figure 1.
SEM images (a) and adsorbent element content (b) and elemental mapping of CoMn-MOF-74 (c).

Figure 2.
(a) N2 adsorption–desorption isotherms and BJH curves. (b) XRD correlation patterns.
3.2. The Effect of Co/Mn Molar Ratio on Arsenic Adsorption
According to previous reports [40,42], during the synthesis of bimetallic metal–organic frameworks (MOFs), adding metal elements in different molar ratios will lead to differences in the performance of the synthesized MOFs in adsorbing arsenic ions. An appropriate ratio of metal elements can enhance the adsorption performance of MOFs for arsenic ions. Therefore, it is necessary to determine the optimal molar ratio for the preparation of bimetallic MOFs to ensure the best effect when adsorbing arsenic ions.
In order to determine the optimal Co/Mn molar ratio, various preparation methods with different ratios were carried out during the preparation process for the adsorption of arsenic ions. As shown in the Figure 3, with the change in the Co/Mn molar ratio, the arsenic adsorption performance of the adsorbent also changes. When the Co/Mn molar ratio is 1:1, the arsenic adsorption rate of the adsorbent reaches 93.42%, achieving the best result. Under other molar ratios, the adsorption performance is lower than that at a molar ratio of 1:1. When observing this phenomenon, the reason why the adsorption performance is the best when the Co/Mn molar ratio is 1:1 may be due to the following aspects: (1) The quantity of active sites. The molar ratio during synthesis directly affects the number of active sites in the adsorbent. (2) The synergistic effect between metal ions. The synergistic effect between the two metal ions in bimetallic MOF-74 will change according to different molar ratios. There exists an optimal molar ratio in bimetallic MOF-74, which can maximize the synergistic effect between the two metal ions and enhance the adsorption effect of the adsorbent. Therefore, in this study, a Co/Mn molar ratio of 1:1 was selected to synthesize the adsorbent and conduct the adsorption experiment.

Figure 3.
Bimetallic MOF-74 with different Co/Mn molar ratios.
3.3. Effect of Initial pH on the Adsorption of As
The adsorption of arsenic in arsenic-containing wastewater was investigated under the influence of different pH values, the zeta potential of CoMn-MOF-74 adsorbent was tested at each pH value, and the adsorption mechanism of CoMn-MOF-74 was further analyzed based on the results of the test. The starting concentration of the arsenic-containing solution was 30 mg/L. An amount of 50 mL of the arsenic solution was put into a 100 mL conical flask, and the pH of it was modified to be in the scope spanning from 2 to 12 with the utilization of NaOH and HCl solutions. The concentration level of the solution was analyzed by the ICP-OES instrument, and the zeta potentials of the adsorbent were analyzed along with those at each pH value. To prevent experimental deviations, three sets of concurrent tests were conducted for each set of experiments.
In the experiment, the change in pH value of arsenic-containing solution will affect the adsorbent adsorption, the surface charge of the adsorbent will change at different pH values, and the type of arsenate in the arsenic-containing solution will also change with the change in pH value. Therefore, pH value is a key factor in the study of adsorbent adsorption [42]. As shown in the Figure 4a,b, for the smelting wastewater with the co-existence of As(III) and As(V), the arsenic-removal efficiency by CoMn-MOF-74 exhibited a gradual increase as the pH value shifted from acidic to alkaline conditions (pH range: 2–12), with the highest removal at PH = 11, and the adsorption performance decreased dramatically at PH = 12, which may be due to the structural decomposition of the adsorbent that started to take place in the presence of strong alkali. The type of arsenate is contingent upon the pH of the solution [13]. When the pH is less than 2.1, it exists as H3AsO4; when the pH is between 2.1 and 6.8, arsenate (H3AsO4) further dissociates to form hydrogen arsenate (H2AsO4−); and when the pH is greater than 6.7, it exists in the form of HAsO42− [43]. However, in the pH range of less than 8, As(III) exists mainly as neutral HAsO2, while in the pH range greater than 9, As(III) exists mainly as H2AsO3− [42,44]. From the figure, it can be seen that the surface of CoMn-MOF-74 is positively charged only at PH = 2–3 and negatively charged in the rest of the cases. Electrostatic adsorption is by the positive charge on the surface of the adsorbent attracted to the arsenate ion, which is negatively charged. However, it can be seen through the figure that a greater number of negative charges are generated on the surface of CoMn-MOF-74; this results in a robust electrostatic repulsion occurring between the negatively charged H2AsO4− and HAsO42− anions and the negatively charged surface of CoMn-MOF-74, which suggests that the electrostatic adsorption effect is not the main mechanism of arsenic adsorption by CoMn-MOF-74.

Figure 4.
Impact of pH on As adsorption (a) and zeta potential (b) of CoMn−MOF−74.
3.4. Effect of CoMn-MOF-74 Dosage
The dosage of CoMn-MOF-74 serves as a crucial factor in dictating the adsorptive capacity of the adsorbent and in examining the intensity of the adsorption interaction [45]. The effect of CoMn-MOF-74 adsorbent dosage on the removal of arsenic ions was investigated in the range of 0.20–1.0 g/L at pH = 11. The removal efficiency of arsenic ions increased with the increase in adsorbent dosage and resulted in the removal of arsenic ions from wastewater after adsorption.
As shown in Figure 5a, the removal rate of arsenic ions was 93.4% when the dosage of Co/Mn-MOF-74 was 0.6 g/L. It is noteworthy that with the continued addition of the adsorbent, the change in the removal efficiency was minimal, indicating that an adsorption equilibrium state had been reached. Therefore, although increasing the adsorbent dosage could provide more active sites and functional groups, the arsenic-removal efficiency did not increase significantly. Meanwhile, in the experiment, dosage tests for Co-MOF-74 and Co/Mn-MOF-74 adsorbents were also carried out, and the maximum arsenic-removal rates of these two adsorbents were obtained, which were 48.8% and 90.15%, respectively. When comparing the three adsorbents, Co/Mn-MOF-74 exhibits a more outstanding arsenic-removal rate with a lower adsorbent dosage, demonstrating greater economic and high-efficiency advantages in practical applications.
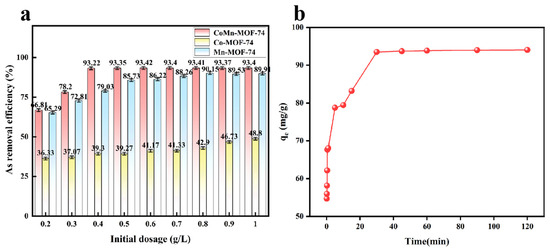
Figure 5.
Effect of CoMn-MOF-74 dosage and comparison of adsorption efficiency of three adsorbents (a). Influence of time on As ion adsorption with CoMn-MOF-74 (b).
3.5. Effect of Adsorption Time and Adsorption Dynamics Research
Exploring the adsorption time represents a crucial index when studying the adsorption of an adsorbent. The adsorption time of the adsorbent CoMn-MOF-74 in wastewater containing arsenic ions was investigated under the conditions of PH = 11; the initial concentration of the solution was 30 mg/L, and the dosage of the adsorbent was 10 mg. The time course of the adsorption of arsenic ions by CoMn-MOF-74 was divided into three phases, and the results over are shown in Figure 5b. In the range of 0–5 min, the process of rapid adsorption occurs, with a maximum adsorption amount of 78.75 mg/g at 5 min/ From 5 to 30 min, the adsorption speed of the adsorbent slows down; this is the slow adsorption stage, where the highest adsorption amount is 93.5 mg/g at 30 min. After 30 min, the adsorption amount produces a small change but does not show an obvious change, as the adsorbent reaches the adsorption equilibrium, and the maximum adsorption amount reaches 94.05 mg/g. This situation is because the adsorbent has many metal active sites at the beginning of the reaction, which can be rapidly combined with arsenic ions in solution; however, as the reaction proceeds and the reaction time grows, the adsorption active sites are slowly taken over, and the arsenic ions in the wastewater also decrease, which leads to a slowdown in the rate of adsorption, which finally reaches the adsorption equilibrium state. The adsorption mechanism of CoMn-MOF-74 was analyzed in more depth using three kinetic models: pseudo-first-order kinetic model (PFO) [46], pseudo-second-order kinetic model (PSO) [47], and intra-particle diffusion model [48]. the PFO, PSO, and intra-particle diffusion models are modeled as Equations (3)–(5), respectively:
where qe represents the amount adsorbed at equilibrium adsorption time in mg/g and qt represents the amount adsorbed at a specific adsorption time in mg/g. k1 (1/min) denotes the rate constants of the modeled PFO, k2 (mg/g·min) denotes the rate constants of the modeled PSO, and k3 (mg/g·min0.5) denotes the rate constants of the modeled endo-diffusion. t is the reaction time (unit is min), and C signifies a correlation index reflecting the boundary thickness. The reaction behavior of arsenic in smelting wastewater on CoMn-MOF-74 was analyzed by fitting kinetic curves using experimental data. The fits in Figure 6a–c show the pseudo-primary and pseudo-secondary adsorption kinetics and intra-particle diffusion modeling of arsenic by CoMn-MOF-74. From the analysis of the data in Table 1, the correlation coefficients R2 of the pseudo-first-order model and the pseudo-second-order model are 0.93818 and 0.99973, respectively, Among them, R2(PSO) = 0.99973, which is greater than that of the pseudo-first-order model. It can be inferred from this that the pseudo-second-order model shows a higher degree of agreement with the adsorption process of the adsorbent. This suggests that the adsorption of arsenic by CoMn-MOF-74 is mainly dominated by chemisorption.
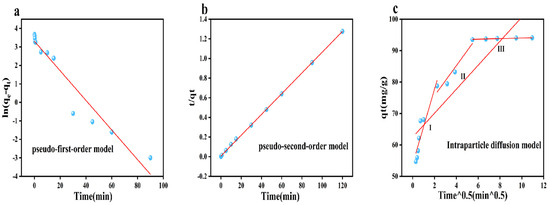
Figure 6.
Pseudo-first-order (a), pseudo-second-order (b), and intra-particle diffusion (c) models.

Table 1.
Kinetic model parameters.
3.6. Effect of Initial Concentration and Adsorption Isotherms
In order to study the adsorption effect of the adsorbent at different concentrations and to investigate the optimal adsorption capacity of CoMn-MOF-74 for arsenic ions, the adsorbent was put into solution with a concentration of 80–1000 mg/L under the condition of solution PH = 11. The adsorption capacity of the adsorbent was elevated with increasing concentration, and the adsorbent reached the saturation adsorption capacity of 531 mg/g after adsorption equilibrium when the initial solution concentration was 1000 mL/L. The adsorption capacity of the adsorbent was 531 mg/g after adsorption equilibrium. Meanwhile, Co-MOF-74 and Mn-MOF-74 were added to the experiment as control groups. The saturation adsorption capacity of Co-MOF-74 reached 282.7 mg/g, and that of Mn-MOF-74 reached 426.375 mg/g. The maximum adsorption capacity of CoMn-MOF-74 far exceeded that of these two single-metal adsorbents, demonstrating its superior adsorption performance.
The comparison of the saturation adsorption capacities of CoMn-MOF-74 and other adsorbents for arsenic ions is shown in Table 2. The results indicate that the adsorption capacity of CoMn-MOF-74 for As ions is much larger than that of most adsorbents. This demonstrates that CoMn-MOF-74 shows remarkable potential in adsorbing arsenic ions and exhibits outstanding adsorption performance towards them. This is because the adsorption process of arsenic ions by CoMn-MOF-74 occurs through chemisorption, which indicates that functional groups on adsorbent play important roles in the adsorption of arsenic. As a result, the maximum adsorption capacity of CoMn-MOF-74 for arsenic ions reaches 531 mg/g. This shows that CoMn-MOF-74 has a good adsorption potential and excellent adsorption performance for arsenic ions. Meanwhile, the processes of adsorption of arsenic ions by various adsorbents to reach the adsorption equilibrium state are compared in Table 2. The faster the adsorption rate, the richer the active sites of the adsorbent. It was found that the adsorption equilibrium time of CoMn-MOF-74 adsorbent was only 30 min, which was more than most of the adsorbents; there were two kinds of adsorbents whose adsorption equilibrium time was the same as that of CoMn-MOF-74 adsorbent, but the maximum adsorption amount qm was much smaller than that of CoMn-MOF-74 adsorbent. It can be seen that CoMn-MOF-74 has abundant active sites.
Adsorption isotherms are of great significance in elucidating the adsorption mechanism of heavy metallic ions interacting with the adsorbent material. They effectively mirror the adsorption quantity as well as the migration direction of heavy metal ions. To study the adsorption performance, the adsorption process presented in Figure 7b–d can be described and analyzed by using three isotherm models, namely, Langmuir [49], Freundlich [50], and Temkin [51]. The Langmuir adsorption isotherm model is based on the fact that the nature and affinity of the adsorbent is relatively homogeneous and the thickness of the adsorbent layer is equal to the mass of the adsorbent molecules, based on which it represents monolayer adsorption [52]. The Freundlich adsorption isotherm model is capable of describing the non-ideal adsorption course and also accounts for the inhomogeneous characteristics of the adsorbent, and it is fitting for multilamellar adsorption [53]. The Temkin adsorption isotherm model primarily depicts the quantifiable correlation present between the adsorbed amounts of two materials under isothermal and isobaric conditions [54]. The equations of the Langmuir, Freundlich, and Temkin models are as follows in Equations (6) to (8):
where qm in the equation represents the maximum adsorption amount in mg/g; Ce is the concentration of arsenic after adsorption equilibrium in mg/g; KL represents the Langmuir equilibrium constant in L/mg; KT represents the Temkin model equilibrium constant in L/mg; f represents the correlation constant in the equation of the Temkin model; KF represents the Freundlich model equilibrium constant in [(mg/g) (L/mg)1/n]; and n is the Freundlich model coefficient.
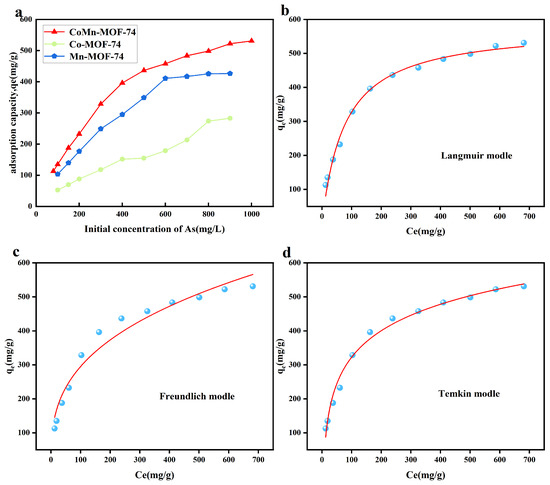
Figure 7.
The impact of the starting As ion concentration on the adsorption capacity of CoMn-MOF-74/Co-MOF-74/Mn-MOF-74 (a). The isotherm models used for As ion adsorption on CoMn-MOF-74: Langmuir (b), Freundlich (c), and Temkin (d).
From Table 3, it can be seen that the Freundlich model has an R2 of 0.96288 and the Temkin model has an R2 value of 0.98835, indicating that the reactive groups interact with As ions. And the Langmuir model’s R2 = 0.99119 also outperforms both the Freundlich and Temkin models. Therefore, the adsorption process of CoMn-MOF-74 is more inclined to monolayer adsorption.


Table 2.
Comparison of different adsorbents documented in the literature related to As ions.
Table 2.
Comparison of different adsorbents documented in the literature related to As ions.
| Adsorbent | Adsorption Equilibrium Time | pH | qm (mg/g) | Reference |
|---|---|---|---|---|
| Cu-ZIF-8 | 1 h | 7 | 238.11 | [38] |
| MnFe2O4-MIL-53 (Fe) | 4 h | 7 | 402 | [55] |
| NF/MIL-101 (Cr) | 5–20 h | 4 | 132.615 | [56] |
| Zn-MOF-74 | 2.5 h | 7 | 325 | [57] |
| FeCo-MOF-74 | 1 h | 7 | 292.29 | [42] |
| FeMn-MOF-74 | 0.5 h | 7 | 161.6 | [58] |
| Zn-MOF-74/rGO/PAM | 0.5 h | 10 | 282.4 | [59] |
| CoMn-MOF-74 | 0.5 h | 11 | 531 | This work |

Table 3.
The R2 values representing the correlation coefficients of the Langmuir, Freundlich, and Temkin.
3.7. Adsorption and Regeneration Cycle Experiment
The regeneration and readsorption tests of the adsorbent play a crucial role in its market application value. Four cycles of desorption experiments were carried out on the CoMn-MOF-74 adsorbent, using 50 mL of 0.1 M NaOH solution as the eluent for the adsorbed adsorbent [60]. As illustrated in Figure 8, after the first cycle, the adsorption rate of the adsorbent decreased, with the adsorption rate being 87.42% after the first cycle. After four cycles of experiments, the adsorption rate reached 68.71%. It can be clearly seen that the adsorption performance gradually decreased with the increase in the number of cycles. This may be owing to the reduction in metal active sites on the CoMn-MOF-74 after elution and the loss of a small amount of the adsorbent during the elution process. However, the adsorption rate remained at 68.74% after four cycles of experiments and remained above 80% after the second cycle, demonstrating that this is a potential and feasible adsorbent.
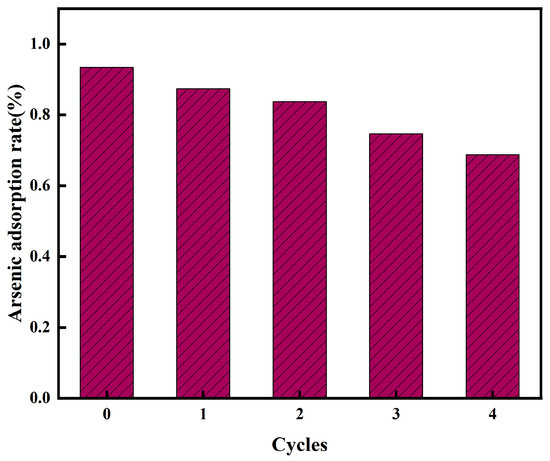
Figure 8.
Removal (%) of As on CoMn-MOF-74 under different regeneration cycles.
4. Reaction Mechanism of Arsenic Adsorption
The FTIR spectra of the prepared samples show that the bimetallic CoMn-MOF-74 displays almost identical absorption bands, as illustrated in Figure 9a. The typical absorption peak at about 1408 cm−1 is associated with (C-O) stretching vibrations, which may be related to the presence of 2, 5-dihydroxyterephthalic acid in the MOF [61]. In addition, the absorption band associated with the stretching vibration of the (C=O) group that appeared at 1633 cm−1 for CoMn-MOF-74 disappeared [62] or shifted to a lower value at 1592 cm−1 compared to the organic linker. This proved that all carboxyl groups of dihydroxyterephthalic acid were deprotonated, and there was no unreacted dihydroxyterephthalic acid on the surface or within the pores of the prepared MOF-74 [63]. The desired MOF was successfully synthesized. Another appearance at 1119 and 1207 cm−1 is associated with hydroxyl groups on metal ions (M-OH) [64]. In addition, moderate-intensity bands at 575 and 476 cm−1 are characteristic of Mn-Olinker (M=Co or Mn) vibrations. For CoMn-MOF-74, the absorption bands at 582 and 682 cm−1 are characteristic of M-O (M=Mn or Co) vibrations [65]. The IR peak at 588 cm−1 of the sample with adsorbed arsenic ions is due to the stretching vibration of the As-O bond as compared to the pristine CoMn-MOF-74 sample. In contrast, the movement of the M-O bond (582 cm−1 for M-O and 588 cm−1 for the adsorbed arsenic ion sample) is due to the formation of new ligand interactions, such as Co-O-As, replacing the original Co-O type. M-OH plays a vital part in the adsorption of arsenic by CoMn-MOF-74, and the decrease in the area and peak energy of the M-OH oscillation peaks indicate that hydroxyl groups are involved in the adsorption process [57]. These outcomes indicate that the metal nodes in CoMn-MOF-74 play an important role in the adsorption process.
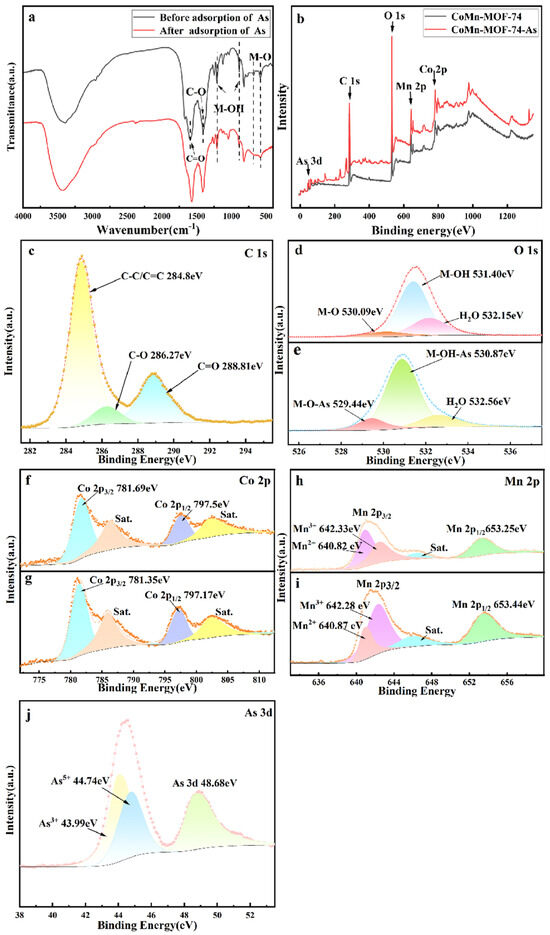
Figure 9.
FTIR spectra of CoMn-MOF-74 samples before and after use (a) and XPS survey scan (b). High-resolution C 1 s (c), high-resolution O 1s (d,e), high-resolution Co 2p (f,g), high-resolution Mn 2p (h,i), and high-resolution As 3d (j).
In addition, CoMn-MOF-74 before and after adsorption was investigated using XPS. The total investigation spectra clearly showed the presence of C 1s, O 1s, Co 2p, and Mn 2p as well as As 3d inside CoMn-MOF-74, and the appearance of As 3d indicated that arsenic in the wastewater was transferred by adsorption to the CoMn-MOF-74 adsorbent. According to the representation in Figure 9c, the C 1s spectral peaks are located at 284.8 eV, 286.27 eV, and 288.81 eV, and the functional groups that correspond to them are C-C and C=C bonds, C-O and C=O, respectively. The results obtained from the spectrum of C 1s indicate that the surface of C surrounds the oxygen-rich groups [55]. In the O 1s energy spectrum, M stands for Co and Mn, the peak at 530.09 eV denotes the bonding of O with metal ions to form the M-O bond, hydroxide and metal ions form an M-OH peak and H2O, and the peaks formed are 531.4 eV and 532.15 eV, respectively. After the adsorption of arsenic ions, the M-O bond’s spectral intensity enhancement area increased and the position shifted a little because the M-O bond participated in the reaction to form a new Co-O-As bond at 529.44 eV during the adsorption of arsenic, and the M-OH peak underwent a similar reaction and appeared as a Co-OH-As bond at 530.87 eV. This indicates the reaction between diverse forms of functionalized oxygen groups on the surface of CoMn-MOF-74 and arsenic. The Co 2p spectrum shows two main peaks at 797.5 eV and 781.69 eV, corresponding to Co 2p1/2 and Co 2p3/2, respectively. Two other broad peaks located around 786.26 and 802.03 eV were identified as satellite peaks (denoted as “Sat.”) [66], indicating the presence of cobalt mainly in the form of Co2+ cation [42]. The Mn 2p spectrum has two well-defined peaks corresponding to Mn 2p1/2 and Mn 2p3/2 in the Mn-O bond [67]. In addition, satellite peaks of Mn located at 646.33 eV (denoted as “Sat.”) and 2p3/2 at 640.82 eV and 642.33 eV also indicate the presence of Mn2+ and Mn3+ in the Mn-O bond [68], suggesting the presence of Mn in the form of Mn2+ and Mn3+. In addition, the As 3d spectrum showed two peaks at 44.8 eV and 45.6 eV for As3+ and As5+, respectively, indicating that arsenic was successfully adsorbed by the adsorbent (Figure 9j). After the adsorption of arsenic, a broad peak of As5+ at 48.68 eV indicated that arsenic ions formed a binding with other ions in CoMn-MOF-74.
To verify the elemental distribution of CoMn-MOF-74 after arsenic adsorption, SEM-EDS mapping was performed. As illustrated in Figure 10a, the high-magnification SEM image shows that the voids on the surface of CoMn-MOF-74 become very rough after the adsorption of arsenic is fulfilled. The distributions of elements containing Co, Mn, and As are shown in Figure 9b–d. The elements containing Co, Mn, and As were uniformly distributed in CoMn-MOF-74.
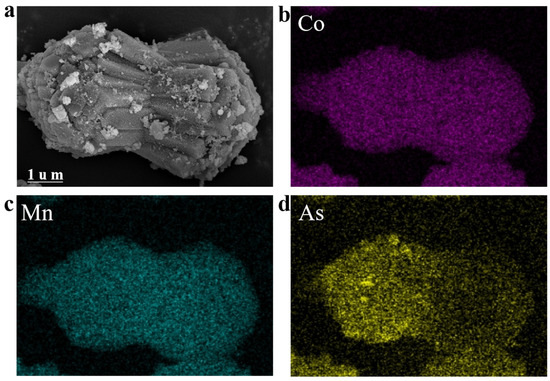
Figure 10.
(a) SEM images and (b–d) EDS mapping results of the reaction of CoMn-MOF-74 with As ion.
5. Conclusions
In this work, a bimetallic MOF was synthesized through an uncomplicated hydrothermal synthetic method. Its arsenic adsorption function was utilized to adsorption arsenic from arsenic-containing wastewater, and the removal effect of the experiment was good. The primary outcomes of this experiment are set forth as follows: The specific morphology of the adsorbent was observed using scanning electron microscopy. The results show that CoMn-MOF-74 exhibits prismatic aggregation into an irregular dumbbell-type structure. The results of the adsorption tests showed that the adsorption of CoMn-MOF-74 is very consistent with the pseudo-second-order kinetic model in terms of adsorption kinetics and agrees well with the Langmuir isotherm model, which indicates that the adsorption procedure of CoMn-MOF-74 was chemisorption, and the arsenic ions were attached to the surface of the adsorbent by adsorption. The time to reach the adsorption equilibrium was 30 min under the condition of arsenic-containing wastewater PH = 11 and temperature of 25 °C. The maximum adsorption amount was 531 mg/g, which was much higher than most of the reported adsorbents. In addition, CoMn-MOF-74 has good adsorption properties for As ions. SEM, XRD, FTIR, and XPS analyses show that the main adsorption behavior of CoMn-MOF-74 for adsorption of arsenic ions is monolayer adsorption when the adsorbent meets with the arsenic ions to form As-o-M complexes. The mechanism of arsenic adsorption from wastewater by CoMn-MOF-74 is mainly realized by releasing CO2+, Mn2+, and -OH. According to our experimental results in this paper as well as experimental characterization and testing, no negative effects were found from arsenic removal by the adsorbent. Also, the adsorbent can be recycled, which reduces the economic cost of the adsorbent. In summary, CoMn-MOF-74 is a promising adsorbent for arsenic adsorption with a simple synthesis process, strong adsorption capacity, and remediation of arsenic-contaminated environments.
Author Contributions
J.F.: conceptualization, methodology, formal analysis, writing—review and editing, visualization. G.Z.: conceptualization, methodology, formal analysis, visualization. X.Q.: conceptualization, methodology, writing—review and editing, supervision. M.G.: investigation, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported by the National Natural Science Foundation of China (Grant No. 52160011), the Yunnan Province Ten Thousand Talents Plan Young Talents Training Fund (No. KKRD201952029), and the University-Enterprise Cooperation Project of Kunming University of Science and Technology (No. KKZ4201552002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, B.; Dong, Q.; Zhang, M.; Wang, D.; Li, J.; Cheng, Y. Study on the Effect of Thermal Activation on Arsenic Removal from Industrial Wastewater. JOM 2024, 76, 1539–1547. [Google Scholar] [CrossRef]
- Abdollahi, N.; Moussavi, G.; Giannakis, S. A Review of Heavy Metals’ Removal from Aqueous Matrices by Metal-Organic Frameworks (MOFs): State-of-the Art and Recent Advances. J. Environ. Chem. Eng. 2022, 10, 107394. [Google Scholar] [CrossRef]
- Abhyankar, L.N.; Jones, M.R.; Guallar, E.; Navas-Acien, A. Arsenic Exposure and Hypertension: A Systematic Review. Environ. Health Perspect. 2011, 120, 494–500. [Google Scholar] [CrossRef]
- Yu, H.-S.; Lee, C.-H.; Chen, G.-S. Peripheral Vascular Diseases Resulting from Chronic Arsenical Poisoning. J. Dermatol. 2002, 29, 123–130. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.; Qi, X.; Shu, B.; Zhang, X.; Li, K.; Wei, Y.; Hao, F.; Wang, H. Efficient Removal of Arsenic from Copper Smelting Wastewater in Form of Scorodite Using Copper Slag. J. Clean. Prod. 2020, 270, 122428. [Google Scholar] [CrossRef]
- Zakhar, R.; Derco, J.; Čacho, F. An overview of main arsenic removal technologies. Acta Chim. Slovaca 2018, 11, 107–113. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (Modified) Natural Adsorbents for Arsenic Remediation: A Review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Huling, J.R.; Huling, S.G.; Ludwig, R. Enhanced Adsorption of Arsenic through the Oxidative Treatment of Reduced Aquifer Solids. Water Res. 2017, 123, 183–191. [Google Scholar] [CrossRef]
- Han, Y.-S.; Gallegos, T.J.; Demond, A.H.; Hayes, K.F. FeS-Coated Sand for Removal of Arsenic(III) under Anaerobic Conditions in Permeable Reactive Barriers. Water Res. 2011, 45, 593–604. [Google Scholar] [CrossRef]
- Chai, L.; Yue, M.; Yang, J.; Wang, Q.; Li, Q.; Liu, H. Formation of Tooeleite and the Role of Direct Removal of As(III) from High-Arsenic Acid Wastewater. J. Hazard. Mater. 2016, 320, 620–627. [Google Scholar] [CrossRef]
- An, B.; Liang, Q.; Zhao, D. Removal of Arsenic(V) from Spent Ion Exchange Brine Using a New Class of Starch-Bridged Magnetite Nanoparticles. Water Res. 2011, 45, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Wang, S.; Li, X.; Wang, X.; Jia, Y. Simultaneous Removal and Oxidation of Arsenic from Water by δ-MnO2 Modified Activated Carbon. J. Environ. Sci. 2020, 94, 147–160. [Google Scholar] [CrossRef]
- Cai, G.; Tian, Y.; Li, D.; Zhang, J.; Li, L.; Wang, Q.; Sun, H.; Zhang, H.; Wang, P. Self-Enhanced and Efficient Removal of As(III) from Water Using Fe–Cu–Mn Composite Oxide under Visible-Light Irradiation: Synergistic Oxidation and Mechanisms. J. Hazard. Mater. 2022, 422, 126908. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, L.; Arulmani, S.R.B.; Yan, J.; Li, Q.; Tang, J.; Wan, K.; Zhang, H.; Xiao, T.; Shao, M. Research Progress of Metal Organic Frameworks and Their Derivatives for Adsorption of Anions in Water: A Review. Environ. Res. 2022, 204, 112381. [Google Scholar] [CrossRef]
- Islam, A.; Teo, S.H.; Ahmed, M.T.; Khandaker, S.; Ibrahim, M.L.; Vo, D.-V.N.; Abdulkreem-Alsultan, G.; Khan, A.S. Novel Micro-Structured Carbon-Based Adsorbents for Notorious Arsenic Removal from Wastewater. Chemosphere 2021, 272, 129653. [Google Scholar] [CrossRef]
- Soni, R.; Shukla, D.P. Data on Arsenic(III) Removal Using Zeolite-Reduced Graphene Oxide Composite. Data Brief 2019, 22, 871–877. [Google Scholar] [CrossRef]
- Lu, Z.; Li, X.; Qi, X. Cobalt-Loaded Resin Can Effectively Remove Arsenic in Wastewater. Environ. Technol. Innov. 2021, 21, 101354. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Hu, B.; Zhao, X.; Guo, P. FeOOH and nZVI Combined with Superconducting High Gradient Magnetic Separation for the Remediation of High-Arsenic Metallurgical Wastewater. Sep. Purif. Technol. 2022, 285, 120372. [Google Scholar] [CrossRef]
- Gendy, E.A.; Ifthikar, J.; Ali, J.; Oyekunle, D.T.; Elkhlifia, Z.; Shahib, I.I.; Khodair, A.I.; Chen, Z. Removal of Heavy Metals by Covalent Organic Frameworks (COFs): A Review on Its Mechanism and Adsorption Properties. J. Environ. Chem. Eng. 2021, 9, 105687. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Lin, Y.; Wu, S.; Li, X.; Yang, C. Simultaneous Removal of Heavy Metals and Antibiotics from Anaerobically Digested Swine Wastewater via Functionalized Covalent Organic Frameworks. Environ. Res. 2025, 272, 121152. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Wen, J. Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities. Sustainability 2023, 15, 6686. [Google Scholar] [CrossRef]
- Amari, A.; Alawameleh, H.S.K.; Isam, M.; Maktoof, M.A.J.; Osman, H.; Panneerselvam, B.; Thomas, M. Thermodynamic Investigation and Study of Kinetics and Mass Transfer Mechanisms of Oily Wastewater Adsorption on UIO-66–MnFe2O4 as a Metal–Organic Framework (MOF). Sustainability 2023, 15, 2488. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, B.; Zhao, Y.; Wang, W.; Jia, Q. Adsorption of Heavy Metals with Hyper Crosslinked Polymers: Progress, Challenges and Perspectives. Chin. Chem. Lett. 2024, 110619. [Google Scholar] [CrossRef]
- Patra, K.; Pal, H. Recent Advances in Porous Organic Polymers (POPs): The Emerging Sorbent Materials with Promises towards Toxic and Radionuclides Metal Ions Separations. Mater. Today Sustain. 2024, 27, 100799. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, P.; Zhu, L.; Hua, M.; Huang, Y.; Chao, Y.; Wu, P.; Qiu, Z.; Zhu, W. Construction of Hydrophilic Hydroxyl-Rich Porous Organic Polymers for Efficient Removal of Heavy Metal Ions. Inorg. Chem. Commun. 2023, 153, 110821. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Jayaramulu, K.; Dubal, D.P.; Schneemann, A.; Ranc, V.; Perez-Reyes, C.; Stráská, J.; Kment, Š.; Otyepka, M.; Fischer, R.A.; Zbořil, R. Shape-Assisted 2D MOF/Graphene Derived Hybrids as Exceptional Lithium-Ion Battery Electrodes. Adv. Funct. Mater. 2019, 29, 1902539. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Rational Approach to Guest Confinement Inside MOF Cavities for Low-Temperature Catalysis|Nature Communications. Available online: https://www.nature.com/articles/s41467-019-08972-x (accessed on 21 October 2024).
- Wang, Y.; Jin, H.; Ma, Q.; Mo, K.; Mao, H.; Feldhoff, A.; Cao, X.; Li, Y.; Pan, F.; Jiang, Z. A MOF Glass Membrane for Gas Separation. Angew. Chem. Int. Ed. 2020, 59, 4365–4369. [Google Scholar] [CrossRef]
- MOF-Derived Porous Carbon-Supported Bimetallic Fischer–Tropsch Synthesis Catalysts|Industrial & Engineering Chemistry Research. Available online: https://pubs.acs.org/doi/10.1021/acs.iecr.1c03810 (accessed on 21 October 2024).
- Zhang, X.; Luo, J.; Wan, K.; Plessers, D.; Sels, B.; Song, J.; Chen, L.; Zhang, T.; Tang, P.; Morante, J.R.; et al. From Rational Design of a New Bimetallic MOF Family with Tunable Linkers to OER Catalysts. J. Mater. Chem. A 2019, 7, 1616–1628. [Google Scholar] [CrossRef]
- A Novel Ag/Zn Bimetallic MOF as a Superior Sensitive Biosensing Platform for HCV-RNA Electrochemical Detection—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0169433221012782?via%3Dihub (accessed on 21 October 2024).
- Chen, H.; Huo, Y.-q.; Cai, K.; Teng, Y. Controllable Preparation and Capacitance Performance of Bimetal Co/Ni-MOF. Synth. Met. 2021, 276, 116761. [Google Scholar] [CrossRef]
- Jian, M.; Wang, H.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Self-assembled one-dimensional MnO2@zeolitic imidazolate framework-8 nanostructures for highly efficient arsenite removal. Environ.Sci. Nano 2016, 3, 1186–1194. [Google Scholar] [CrossRef]
- Li, D.; Li, N.; Liu, W.; Xu, S.; Sun, Y.; Qiao, Z.; Zhong, C. Highly Water-Stable MOF-74 Synthesized by in-Situ Trace Polymer Modification. Polymer 2023, 281, 126112. [Google Scholar] [CrossRef]
- Jiang, H.; Niu, Y.; Wang, Q.; Chen, Y.; Zhang, M. Single-Phase SO2-Resistant to Poisoning Co/Mn-MOF-74 Catalysts for NH3-SCR. Catal. Commun. 2018, 113, 46–50. [Google Scholar] [CrossRef]
- Wang, H.; Qi, X.; Yan, G.; Shi, J. Copper-Doped ZIF-8 Nanomaterials as an Adsorbent for the Efficient Removal of As(V) from Wastewater. J. Phys. Chem. Solids 2023, 179, 111408. [Google Scholar] [CrossRef]
- Huang, P.; Qi, X.; Duan, X.; Jiang, W.; Yang, N.; Zhi, G.; Wang, J. Efficient Arsenate Capture Using Mixed-Metal La/Zr-MOF Internal Complexation. New J. Chem. 2024, 48, 5311–5325. [Google Scholar] [CrossRef]
- Jiang, N.; Du, B.; Gao, D.; Chai, Z.; Liu, C.; Zhu, X. Effective As(V) Removal Using in Situ Grown Ti-Based MOFs on ZnAl-LDHs. Mater. Sci. Eng. B 2024, 303, 117306. [Google Scholar] [CrossRef]
- Wen, Q.; Li, D.; Li, H.; Long, M.; Gao, C.; Wu, L.; Song, F.; Zhou, J. Synergetic Effect of Photocatalysis and Peroxymonosulfate Activated by Co/Mn-MOF-74@g-C3N4 Z-Scheme Photocatalyst for Removal of Tetracycline Hydrochloride. Sep. Purif. Technol. 2023, 313, 123518. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Zhang, A.; Liao, C. Preparation of Fe–Co Based MOF-74 and Its Effective Adsorption of Arsenic from Aqueous Solution. J. Environ. Sci. 2019, 80, 197–207. [Google Scholar] [CrossRef]
- Qu, G.; Jia, P.; Zhang, T.; Li, Z.; Chen, C.; Zhao, Y. UiO-66(Zr)-Derived t-Zirconia with Abundant Lattice Defect for Remarkably Enhanced Arsenic Removal. Chemosphere 2022, 288, 132594. [Google Scholar] [CrossRef]
- Li, Z.; Deng, S.; Yu, G.; Huang, J.; Lim, V.C. As(V) and As(III) Removal from Water by a Ce–Ti Oxide Adsorbent: Behavior and Mechanism. Chem. Eng. J. 2010, 161, 106–113. [Google Scholar] [CrossRef]
- Prola, L.D.T.; Machado, F.M.; Bergmann, C.P.; de Souza, F.E.; Gally, C.R.; Lima, E.C.; Adebayo, M.A.; Dias, S.L.P.; Calvete, T. Adsorption of Direct Blue 53 Dye from Aqueous Solutions by Multi-Walled Carbon Nanotubes and Activated Carbon. J. Environ. Manag. 2013, 130, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Prasad Panda, A.; Giri, M.; Jena, K.K.; Alhassan, S.M.; Kumar, S.A.; Jha, U.; Swain, S.K. Understanding the As(III) Oxidative Performance of MnO2 Polymorphs (α, β, and γ) and Synthesis of an Efficient Nanocomposite of Iron Ore Slime Derived 2-Line Ferrihydrite and γ-MnO2 for Sequestration of Total Arsenic from Aqueous Solution. Chem. Eng. J. 2022, 442, 136075. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, X.; Xiong, J.; Zhou, Q.; Zhu, Y.; Xie, Q.; Wang, S.; Yang, X.; Jiang, F. Aspartic Acid Derivative-Based MOFs: A Promising Green Material for Simultaneous Removal of Phosphorus and Arsenic(V) in Contaminated Spring Water. J. Water Process Eng. 2023, 52, 103547. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar]
- Pang, D.; Wang, C.-C.; Wang, P.; Liu, W.; Fu, H.; Zhao, C. Superior Removal of Inorganic and Organic Arsenic Pollutants from Water with MIL-88A(Fe) Decorated on Cotton Fibers. Chemosphere 2020, 254, 126829. [Google Scholar] [CrossRef]
- Gopi, S.; Ramu, A.G.; Yun, K. A Highly Stable Mesoporous Spinel Ferrite (CoxFe3−xO4) Derived from CoFe-MOF for Efficient Adsorption of Ultratrace As(III) Ions from Aqueous Solution. J. Environ. Chem. Eng. 2023, 11, 110106. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Zheng, L.-W.; Wei, X.-Y.; Xu, Y.; Shen, Y.-W.; Zhang, K.-G.; Yuan, C.-G. Facile Fabrication of Fe/Zr Binary MOFs for Arsenic Removal in Water: High Capacity, Fast Kinetics and Good Reusability. J. Environ. Sci. 2023, 128, 213–223. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Wang, S.; Zhang, L.; Sun, W. Bimetallic Coordination Polymer for Highly Selective Removal of Pb(II): Activation Energy, Isosteric Heat of Adsorption and Adsorption Mechanism. Chem. Eng. J. 2021, 425, 131474. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order Rate Equations from Langmuir and Freundlich Isotherms for Adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Ahmed, M.; Siddiqui, S.I.; Ahmed, S. Fe(III)–Sn(IV) Mixed Binary Oxide-Coated Sand Preparation and Its Use for the Removal of As(III) and As(V) from Water: Application of Isotherm, Kinetic and Thermodynamics. J. Mol. Liq. 2016, 224, 431–441. [Google Scholar] [CrossRef]
- Yan, G.; Qi, X.; Wang, H.; Shi, J. Magnetic MnFe2O4-MIL-53 (Fe) Composite as an Effective Adsorbent for As(V) Adsorption in Wastewater. Microporous Mesoporous Mater. 2022, 346, 112290. [Google Scholar] [CrossRef]
- Zhi, G.; Qi, X.; Li, Y.; Wang, J.; Wang, J. Efficient Treatment of Smelting Wastewater: 3D Nickel Foam @MOF Shatters the Previous Limitation, Enabling High-Throughput Selective Capture of Arsenic to Form Non-Homogeneous Nuclei. Sep. Purif. Technol. 2024, 328, 124927. [Google Scholar] [CrossRef]
- Yu, W.; Luo, M.; Yang, Y.; Wu, H.; Huang, W.; Zeng, K.; Luo, F. Metal-Organic Framework (MOF) Showing Both Ultrahigh As(V) and As(III) Removal from Aqueous Solution. J. Solid State Chem. 2019, 269, 264–270. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Zhang, W.; Yang, C.; Zhang, L.; Zhu, W.; Sun, J.; Li, G.; Li, T.; Wang, J. Amorphous Fe/Mn Bimetal–Organic Frameworks: Outer and Inner Structural Designs for Efficient Arsenic(III) Removal. J. Mater. Chem. A 2019, 7, 2845–2854. [Google Scholar] [CrossRef]
- Ploychompoo, S.; Liang, Q.; Zhou, X.; Wei, C.; Luo, H. Fabrication of Zn-MOF-74/Polyacrylamide Coated with Reduced Graphene Oxide (Zn-MOF-74/rGO/PAM) for As(III) Removal. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 125, 114377. [Google Scholar] [CrossRef]
- Wan, Z.; Xu, X.; Bi, Z.; Jiajia, D.; Li, Y.; Chen, M.; Huang, Z. Gadolinium Doping-Induced Electronic Structure Optimization of MIL-101-NH2: Efficient Adsorption of Arsenic (V) and Phosphorus and Electrochemical Regeneration. Sep. Purif. Technol. 2025, 357, 130133. [Google Scholar] [CrossRef]
- Salama, R.S.; Hassan, S.M.; Ahmed, A.I.; El-Yazeed, W.S.A.; Mannaa, M.A. The Role of PMA in Enhancing the Surface Acidity and Catalytic Activity of a Bimetallic Cr–Mg-MOF and Its Applications for Synthesis of Coumarin and Dihydropyrimidinone Derivatives. RSC Adv. 2020, 10, 21115–21128. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Z.-Z.; Xiang, S.; Chen, B. Perspective of Microporous Metal–Organic Frameworks for CO2 Capture and Separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Meng, F.; Fang, Z.; Li, Z.; Xu, W.; Wang, M.; Liu, Y.; Zhang, J.; Wang, W.; Zhao, D.; Guo, X. Porous Co3O4 Materials Prepared by Solid-State Thermolysis of a Novel Co-MOF Crystal and Their Superior Energy Storage Performances for Supercapacitors. J. Mater. Chem. A 2013, 1, 7235–7241. [Google Scholar] [CrossRef]
- El-Yazeed, W.S.A.; El-Reash, Y.G.A.; Elatwy, L.A.; Ahmed, A.I. Facile Fabrication of Bimetallic Fe–Mg MOF for the Synthesis of Xanthenes and Removal of Heavy Metal Ions. RSC Adv. 2020, 10, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Tuning 1-Hexene/n-Hexane Adsorption on MOF-74 via Constructing Co-Mg Bimetallic Frameworks—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1387181119302586?via%3Dihub (accessed on 8 November 2024).
- Wang, C.; Li, X.; Yang, W.; Xu, Y.; Pang, H. Solvent Regulation Strategy of Co-MOF-74 Microflower for Supercapacitors. Chin. Chem. Lett. 2021, 32, 2909–2913. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, W.B.; Zhao, L.; Xu, B.Q. MOF-Derived Binary Mixed Metal/Metal Oxide @carbon Nanoporous Materials and Their Novel Supercapacitive Performances. Phys. Chem. Chem. Phys. 2016, 18, 17941–17948. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, Y.J.; Kim, D.H.; Lee, Y.J. Bimetallic Metal–Organic Frameworks as Efficient Cathode Catalysts for Li–O2 Batteries. ACS Appl. Mater. Interfaces 2018, 10, 660–667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).