Carbon Content of Amazonian Commercial Tree Boles: Implications for Forest Management
Abstract
1. Introduction
2. Materials and Methods
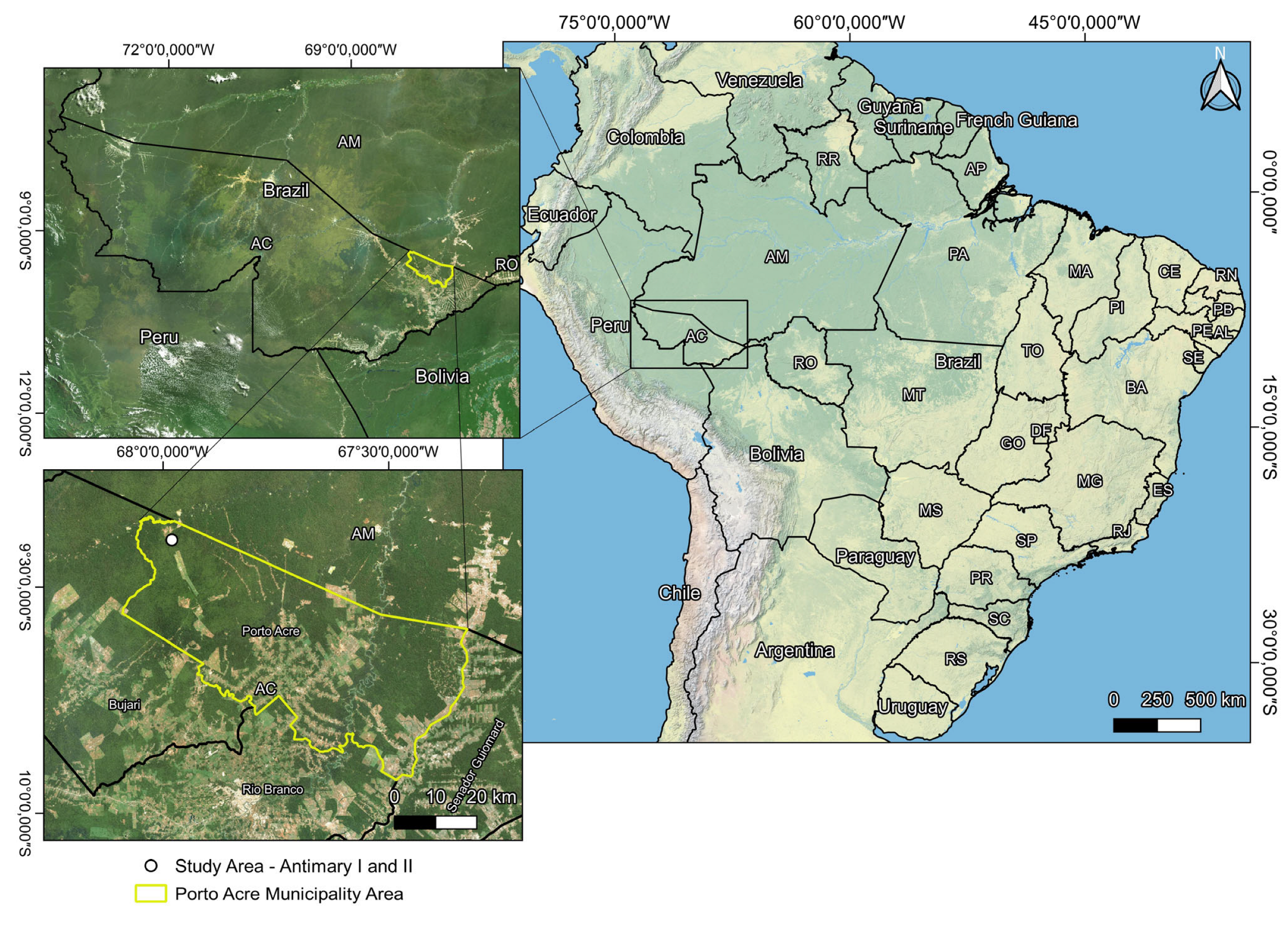
2.1. Study Area
2.2. Selection and Collection of Samples of Commercial Species
2.3. Statistical Analysis and Tree Classification
3. Results
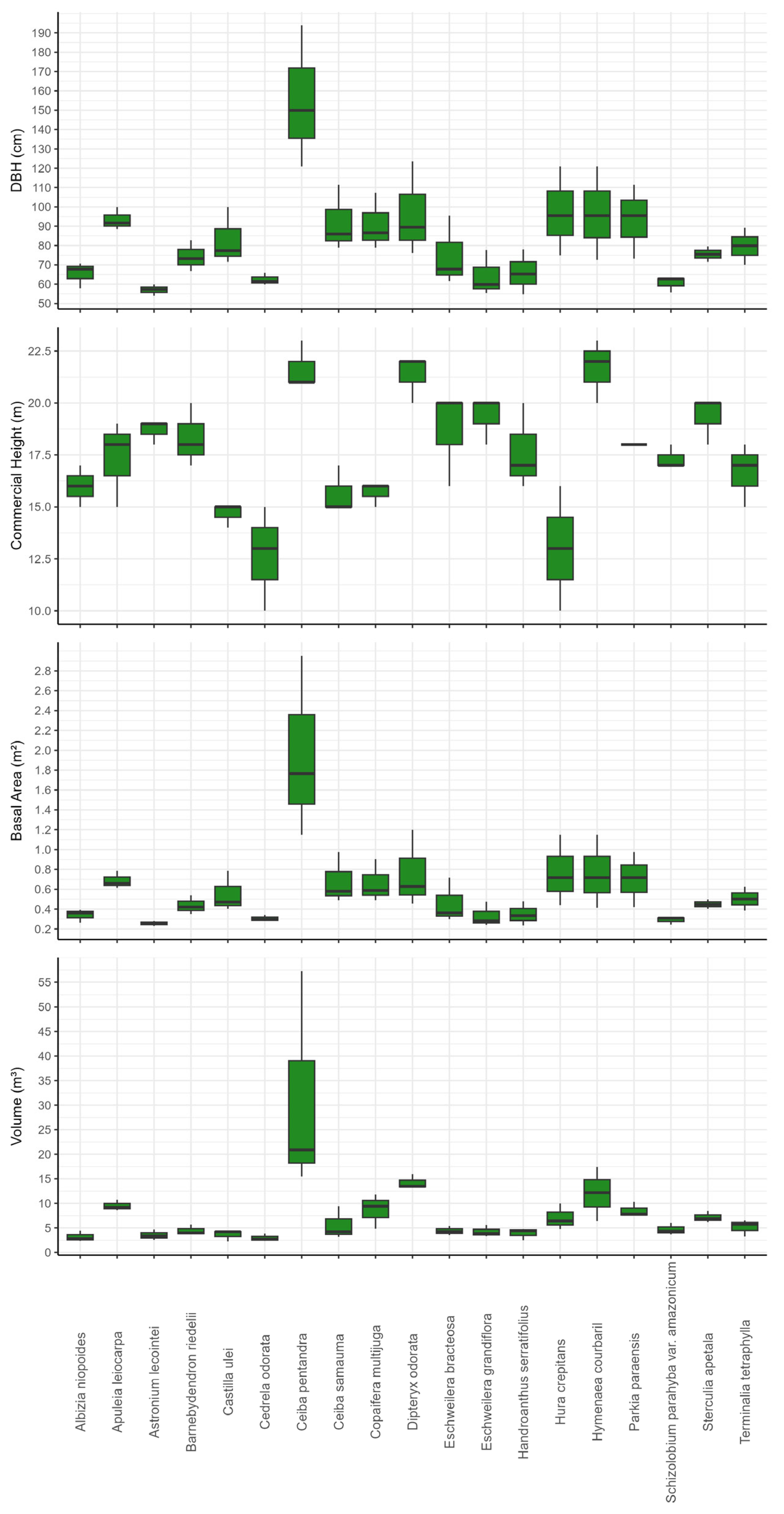
3.1. Variability of Diameter at Breast Height (DBH, Commercial Height, Basal Area and Volume in Commercial Boles
3.2. Variation in Carbon Content of the Commercial Bole Among Species
Comparison Between Species Using Tukey’s Post Hoc Test and Eta-Squared (η2)
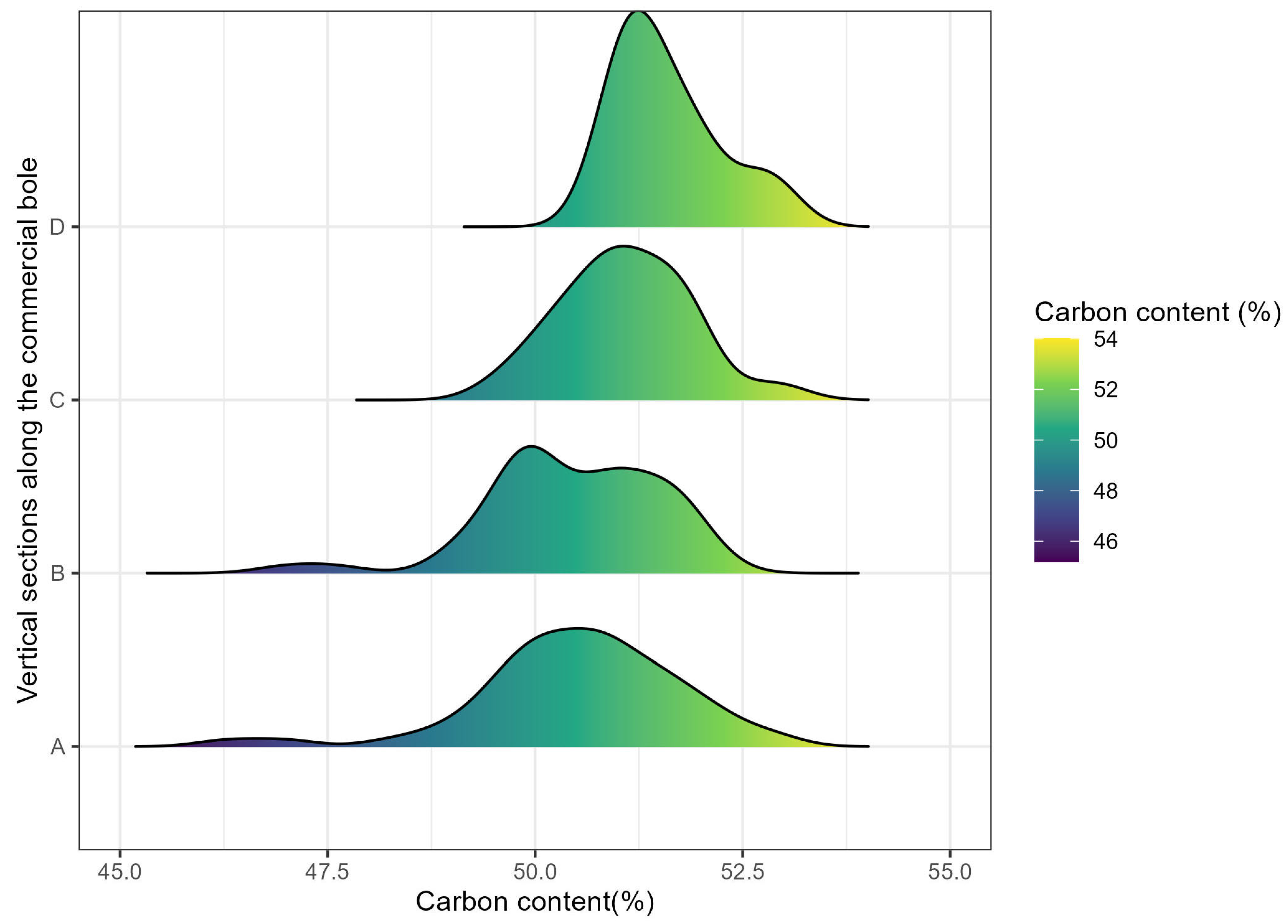
3.3. Variability of Carbon Content Along the Commercial Boles
4. Discussion
4.1. Variability in Biomass and Its Contribution to Timber Production
4.2. Variability of Carbon Content Along the Commercial Boles Among Species
4.3. Implications for Climate Change Policies and Forest Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fearnside, P.M. Effects of land use and forest management on the carbon cycle in the Brazilian Amazon. J. Sustain. For. 2001, 12, 79–97. [Google Scholar] [CrossRef]
- Flato, G.; Marotzke, J.; Abiodun, B.; Braconnot, P.; Chou, S.C.; Collins, W.; Cox, P.; Driouech, F.; Emori, S.; Eyring, V. Evaluation of climate models. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 741–866. [Google Scholar]
- Nieder, R.; Benbi, D.K. Carbon and Nitrogen in the Terrestrial Environment; Springer Science & Business Media: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Mooney, H. The carbon balance of plants. Annu. Rev. Ecol. Syst. 1972, 3, 315–346. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Romero, F.M.B.; Jacovine, L.A.G.; Torres, C.M.M.E.; Ribeiro, S.C.; da Rocha, S.J.S.S.; Novais, T.D.N.O.; de Oliveira Gaspar, R.; da Silva, L.F.; Vidal, E.; Leite, H.G.; et al. Aboveground biomass allometric models for large trees in southwestern Amazonia. Trees For. People 2022, 9, 100317. [Google Scholar] [CrossRef]
- de Lima, R.B.; Görgens, E.B.; da Silva, D.A.S.; de Oliveira, C.P.; Batista, A.P.B.; Caraciolo Ferreira, R.L.; Costa, F.R.C.; de Lima, R.A.F.; Aparício, P.d.S.; de Abreu, J.C.; et al. Giants of the Amazon: How does environmental variation drive the diversity patterns of large trees? Glob. Change Biol. 2023, 29, 4861–4879. [Google Scholar] [CrossRef]
- Lutz, J.A.; Furniss, T.J.; Johnson, D.J.; Davies, S.J.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Andrade, A.; Baltzer, J.; Becker, K.M.L.; et al. Global importance of large-diameter trees. Glob. Ecol. Biogeogr. 2018, 27, 849–864. [Google Scholar] [CrossRef]
- Brazil, CONAMA (Conselho Nacional do Meio Ambiente). Resolução No 406, de 02 de Fevereiro de 2009. Diário Oficial da União. No 26, 2 February 2009, Brasília, DF, Brazil. Available online: http://www.tjpa.jus.br/CMSPortal/VisualizarArquivo?idArquivo=8372 (accessed on 3 August 2020).
- Romero, F.M.B.; Jacovine, L.A.G.; Ribeiro, S.C.; Ferreira Neto, J.A.; Ferrante, L.; da Rocha, S.J.; Torres, C.M.; de Morais Junior, V.T.; Gaspar, R.D.; Velasquez, S.I.; et al. Stocks of carbon in logs and timber products from forest management in the southwestern Amazon. Forests 2020, 11, 1113. [Google Scholar] [CrossRef]
- Wyant, J.G.; Meganck, R.A.; Ham, S.H. A planning and decision-making framework for ecological restoration. Environ. Manag. 1995, 19, 789–796. [Google Scholar]
- Frelich, L.E.; Jõgiste, K.; Stanturf, J.A.; Parro, K.; Baders, E. Natural disturbances and forest management: Interacting patterns on the landscape. In Ecosystem Services from Forest Landscapes: Broadscale Considerations; Springer: Cham, Switzerland, 2018; pp. 221–248. [Google Scholar]
- Franklin, J.F.; Johnson, K.N.; Johnson, D.L. Ecological Forest Management; Waveland Press: Long Grove, IL, USA, 2018. [Google Scholar]
- Nunes, L.J.; Meireles, C.I.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M. Forest contribution to climate change mitigation: Management oriented to carbon capture and storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef]
- Roopsind, A.; Wortel, V.; Hanoeman, W.; Putz, F.E. Quantifying uncertainty about forest recovery 32-years after selective logging in Suriname. For. Ecol. Manag. 2017, 391, 246–255. [Google Scholar] [CrossRef]
- David, H.C.; Carvalho, J.O.; Pires, I.P.; Santos, L.S.; Barbosa, E.S.; Braga, N.S. A 20-year tree liberation experiment in the Amazon: Highlights for diameter growth rates and species-specific management. For. Ecol. Manag. 2019, 453, 117584. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Ussiri, D.A.; Lal, R. Carbon Sequestration for Climate Change Mitigation and Adaptation; Springer: Cham, Switzerland, 2017; pp. 287–325. [Google Scholar]
- Golden, D.; Smith, M.A.; Colombo, S. Forest carbon management and carbon trading: A review of Canadian forest options for climate change mitigation. For. Chron. 2011, 87, 625–635. [Google Scholar] [CrossRef]
- Thorburn, C. Seeing the forest for the carbon: Interrogating reduced emissions from deforestation and degradation (REDD). In Critical Reflections on Development; Palgrave Macmillan: London, UK, 2013; pp. 139–161. [Google Scholar]
- Miah, M.D. Reducing Emissions from Deforestation and Forest Degradation (REDD+). In Life on Land; Springer: Berlin/Heidelberg, Germany, 2020; pp. 797–807. [Google Scholar]
- Salma, A.; Fryda, L.; Djelal, H. Biochar: A key player in carbon credits and climate mitigation. Resources 2024, 13, 31. [Google Scholar] [CrossRef]
- Amowine, N. The impact of forestation and renewable energy utilisation on environmental efficiency in Africa. Econ. Res.-Ekon. Istraživanja 2023, 36, 2180412. [Google Scholar] [CrossRef]
- Vidal, E.; West, T.A.P.; Putz, F.E. Recovery of biomass and merchantable timber volumes twenty years after conventional and reduced-impact logging in Amazonian Brazil. For. Ecol. Manag. 2016, 376, 1–8. [Google Scholar] [CrossRef]
- Avila, A.L.; Schwartz, G.; Ruschel, A.R.; Lopes, J.C.; Silva, J.N.M.; Carvalho, J.O.P.; Dormann, C.F.; Mazzei, L.; Soares, M.H.M.; Bauhus, J. Recruitment, growth and recovery of commercial tree species over 30 years following logging and thinning in a tropical rain forest. For. Ecol. Manag. 2017, 385, 225–235. [Google Scholar] [CrossRef]
- Chambers, J.Q.; dos Santos, J.; Ribeiro, R.J.; Higuchi, N. Tree damage, allometric relationships, and above-ground net primary production in central Amazon forest. For. Ecol. Manag. 2001, 152, 73–84. [Google Scholar] [CrossRef]
- Nandal, A.; Yadav, S.S.; Rao, A.S.; Meena, R.S.; Lal, R. Advance methodological approaches for carbon stock estimation in forest ecosystems. Environ. Monit. Assess. 2023, 195, 315. [Google Scholar] [CrossRef]
- Goodman, R.C.; Phillips, O.L.; Baker, T.R. The importance of crown dimensions to improve tropical tree biomass estimates. Ecol. Appl. 2014, 24, 680–698. [Google Scholar] [CrossRef]
- Higuchi, N.; dos Santos, J.; Ribeiro, R.J.; Minette, L.; Biot, Y. Biomassa da parte aérea da vegetação da floresta tropical úmida de terra-firme da Amazônia brasileira. Acta Amaz. 1998, 28, 153. [Google Scholar] [CrossRef]
- Achard, F.H.; Stibig, F.; Eva, H.; Mayaux, P. Tropical forest cover monitoring in the humid tropics: TREES project. Trop. Ecol. 2002, 43, 9–20. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC22838 (accessed on 15 March 2025).
- d’Oliveira, M.V.N. Sustainable Forest Management for Small Farmers in Acre State in the Brazilian Amazon. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2000. [Google Scholar]
- Salimon, C.I.; Putz, F.E.; Menezes-Filho, L.; Anderson, A.; Silveira, M.; Brown, I.F.; Oliveira, L. Estimating state-wide biomass carbon stocks for a REDD plan in Acre, Brazil. For. Ecol. Manag. 2011, 262, 555–560. [Google Scholar] [CrossRef]
- Selivon, C.A. Plano de Operação Anual-POA, UPA-002. Fazenda Antimari I e II; Fox Laminados Ltd.: Rio Branco, Acre, Brazil, 2014. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- d’Oliveira, M.V.; Braz, E.M. Estudo da dinâmica da floresta manejada no projeto de manejo florestal comunitário do PC Pedro Peixoto na Amazônia Ocidental. Acta Amaz. 2006, 36, 177–182. [Google Scholar] [CrossRef]
- RADAMBRASIL. Folha SC. 19, Levantamento de Recursos Naturais (Geologia, Geomorfologia, Pedologia, Vegetação, Uso Potencial da Terra Rio Branco, v. 12. DNPM: Rio de Janeiro, RJ, Brazil. 1976. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv3591_cd.zip (accessed on 7 March 2025).
- IBGE (Instituto Brasileiro de Geografia e Estatística). Mapas Temáticos: Solos Estaduais/Acre. 2005. Available online: https://mapas.ibge.gov.br/tematicos/solos.html (accessed on 18 January 2024).
- Acre. SEMA (Secretaria do Meio Ambiente). Zoneamento Ecológico-Econômico do Estado do Acre, Fase II (Escala 1:250.000): Documento Síntese, 2nd ed.; Secretaria do Meio Ambiente (SEMA): Rio Branco, Acre, Brazil, 2010; 152p, Available online: https://drive.google.com/drive/folders/1K3MH1K6fenD7_e33EH1biwRS_ykU2RWa (accessed on 7 March 2025).
- Moro, M.F.; Martins, F.R. Métodos de levantamento do componente arbóreo-arbustivo. In Fitossociologia No Brasil, Métodos e Estudos de Caso; Felfili, J.M., Eisenlohr, P.V., d’Melo, M.M.d’.F., d’Andrade, L.A., Neto, J.A.A.M., Eds.; Universidade Federal de Viçosa: Viçosa, MG, Brazil, 2011; pp. 174–212. [Google Scholar]
- Silva, H.F.; Ribeiro, S.C.; Botelho, S.A.; Faria, R.A.V.B.; Teixeira, M.B.R.; Mello, J.M. Estimativa do estoque de carbono por métodos indiretos em área de restauração florestal em Minas Gerais. Sci. For. 2015, 43, 943–953. [Google Scholar] [CrossRef]
- Romero, F.M.B.; de Nazaré Oliveira Novais, T.; Jacovine, L.A.G.; Bezerra, E.B.; de Castro Lopes, R.B.; de Holanda, J.S.; Reyna, E.F.; Fearnside, P.M. Wood basic density in large trees: Impacts on biomass estimates in the southwestern Brazilian Amazon. Forests 2024, 15, 734. [Google Scholar] [CrossRef]
- Pulidori, E.; Gonzalez-Rivera, J.; Pelosi, C.; Ferrari, C.; Bernazzani, L.; Bramanti, E.; Tiné, M.R.; Duce, C. Thermochemical evaluation of different waste biomasses (citrus peels, aromatic herbs, and poultry feathers) towards their use for energy production. Thermo 2023, 3, 66–75. [Google Scholar] [CrossRef]
- Husch, B. Forest Mensuration and Statistics; Ronald Press: New York, NY, USA, 1963. [Google Scholar]
- Spurr, S.H. Forest Inventory; Ronald Press: New York, NY, USA, 1952. [Google Scholar]
- Peck, R.; Olsen, C.; Devore, J.L. Introduction to Statistics and Data Analysis; Cengage Learning: Independence, KY, USA, 2015. [Google Scholar]
- Parra-Frutos, I. Preliminary tests when comparing means. Comput. Stat. 2016, 31, 1607–1631. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practices of Statistics in Biological Research, 3rd ed.; W.H. Freeman: New York, NY, USA, 1995; 887p. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 2017; 735p. [Google Scholar]
- Mansourian, S.; Parrotta, J.A.; Sasaki, N.; Schmitt, C.B.; Thompson, I.D.; van Vliet, N. Impacts of forest and land management on biodiversity and carbon. In Understanding Relationships Between Biodiversity, Carbon, Forests and People: The Key to Achieving REDD+ Objectives; International Union of Forest Research Organizations: Vienna, Austria, 2012; pp. 53–82. [Google Scholar]
- Worbes, M.; Staschel, R.; Roloff, A.; Junk, W.J. Tree ring analysis reveals age structure, dynamics and wood production of a natural forest stand in Cameroon. For. Ecol. Manag. 2003, 173, 105–123. [Google Scholar] [CrossRef]
- Lu, D.; Ye, M.; Hill, M.C. Analysis of regression confidence intervals and Bayesian credible intervals for uncertainty quantification. Water Resour. Res. 2012, 48, W09550. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, H.; Liu, Y.; Liu, Z.; Xia, S.; Wu, Z.; Zhang, Y. Interdisciplinary perspectives on forest ecosystems and climate interplay: A review. Environ. Rev. 2024, 33, 1–21. [Google Scholar] [CrossRef]
- Wolfslehner, B.; Linser, S.; Pülzl, H.; Bastrup-Birk, A.; Camia, A.; Marchetti, M. Forest Bioeconomy—A New Scope for Sustainability Indicators; European Forest Institute: Joensuu, Finland, 2016; 31p. [Google Scholar]
- Palo, M.; Uusivuori, J.; Mery, G.; Korotkov, A.V.; Humphreys, D. World Forests, Markets and Policies: Towards a Balance; Springer: Dordrecht, The Netherlands, 2001; pp. 3–19. [Google Scholar] [CrossRef]
- Betts, M.G.; Phalan, B.T.; Wolf, C.; Baker, S.C.; Messier, C.; Puettmann, K.J.; Green, R.; Harris, S.H.; Edwards, D.P.; Lindenmayer, D.B.; et al. Producing wood at least cost to biodiversity: Integrating Triad and sharing–sparing approaches to inform forest landscape management. Biol. Rev. 2021, 96, 1301–1317. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, P.; DeLucia, E.H.; Schoettle, A.W.; Smolander, H. Photosynthetic light capture and processing from cell to canopy. In Resource Physiology of Conifers; Academic Press: Cambridge, MA, USA, 1995; pp. 3–38. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2021, 185, 34–48. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R. Relationships between biodiversity and carbon stocks in forest ecosystems: A systematic literature review. Trop. Ecol. 2017, 58, 1–14. Available online: https://www.researchgate.net/publication/281375930 (accessed on 15 March 2025).
- Reynolds, C.S. Ecological pattern and ecosystem theory. Ecol. Model. 2002, 158, 181–200. [Google Scholar] [CrossRef]
- Spake, R.; Doncaster, C.P. Use of meta-analysis in forest biodiversity research: Key challenges and considerations. For. Ecol. Manag. 2017, 400, 429–437. [Google Scholar] [CrossRef]
- da Silva, R.P. Alometria, Estoque e Dinâmica da Biomassa de Florestas Primárias e Secundárias na Região de Manaus (AM). Ph.D. Thesis, Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, AM, Brazil, 2007; 135p. Available online: https://repositorio.inpa.gov.br/bitstream/1/4966/1/Roseana_Silva.pdf (accessed on 15 March 2025).
- IPCC (Intergovernmental Panel on Climate Change). Forest lands. Intergovernmental Panel on Climate Change Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006; 83p, Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_04_Ch4_Forest_Land.pdf (accessed on 15 March 2025).
- REFLORA. Flora do Brasil 2020—Algas, Fungos e Plantas. 2019. Available online: http://floradobrasil.jbrj.gov.br/reflora/listaBrasil/PrincipalUC/PrincipalUC.do;jsessionid=21250D75FD7F1FEE1A402F5E30D08648#CondicaoTaxonCP (accessed on 7 March 2025).
- Romero, F.M.B.; Jacovine, L.A.G.; Torres, C.M.M.E.; Ribeiro, S.C.; de Morais Junior, V.T.M.; da Rocha, S.J.S.S.; Romero, R.A.B.; de Oliveira Gaspar, R.; Velasquez, S.I.S.; Staudhammer, C.L.; et al. Forest management with reduced-impact logging in Amazonia: Estimated aboveground volume and carbon in commercial tree species in managed forest in Brazil’s State of Acre. Forests 2021, 12, 481. [Google Scholar] [CrossRef]
- Fearnside, P.M. Biodiversity as an environmental service in Brazil’s Amazonian forests: Risks, value and conservation. Environ. Conserv. 1999, 26, 305–321. [Google Scholar] [CrossRef]
- Fearnside, P.M. The intrinsic value of Amazon biodiversity. Biodivers. Conserv. 2021, 30, 1199–1202. [Google Scholar] [CrossRef]
- Udali, A.; Chung, W.; Talbot, B.; Grigolato, S. Managing harvesting residues: A systematic review of management treatments around the world. For. Int. J. For. Res. 2024, 98, 117–135. [Google Scholar] [CrossRef]
- Nakagawa, M.; Hori, M.; Umemura, M.; Ishida, T. Relationships of wood density and wood chemical traits between stems and coarse roots across 53 Bornean tropical tree species. J. Trop. Ecol. 2016, 32, 175–178. [Google Scholar] [CrossRef]
- Brazil, MCTI (Ministério de Ciência, Tecnologia e Inovação). Fourth National Communication of Brazil to the United Nations Framework Convention on Climate Change; MCTI: Brasília, DF, Brazil, 2020; 531p, Available online: https://unfccc.int/documents/267657 (accessed on 15 March 2025).
- Brazil, MCTI (Ministério da Ciência, Tecnologia e Inovações). Quarta Comunicação Nacional do Brasil à Convenção Quadro das Nações Unidas Sobre Mudança do Clima, Sumário Executivo; MCTI: Brasília, DF, Brazil, 2021; 32p. Available online: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/sirene/publicacoes/comunicacoes-nacionais-do-brasil-a-unfccc/arquivos/4comunicacao/sumario_executivo_4cn_brasil_web.pdf (accessed on 15 March 2025).

| Family | Scientific Name | N | S.D. | Confidence Interval (95% CI) | ||
|---|---|---|---|---|---|---|
| L.L. | UL | |||||
| Euphorbiaceae | Hura crepitans | 3 | 49.706 | 1.83 | 48.16 | 51.36 |
| Fabaceae | Parkia paraensis | 3 | 50.53 | 0.29 | 50.32 | 50.74 |
| Malvaceae | Sterculia apetala | 3 | 50.39 | 0.56 | 49.98 | 50.80 |
| Lecythidaceae | Eschweilera bracteosa | 3 | 50.52 | 0.35 | 50.24 | 50.80 |
| Lecythidaceae | Eschweilera grandiflora | 3 | 50.54 | 0.58 | 50.16 | 50.92 |
| Moraceae | Castilla ulei | 3 | 49.98 | 0.40 | 49.63 | 50.33 |
| Meliaceae | Cedrela odorata | 3 | 51.60 | 0.18 | 51.46 | 51.74 |
| Fabaceae | Copaifera multijuga | 3 | 51.51 | 0.37 | 51.27 | 51.75 |
| Fabaceae | Dipteryx odorata | 3 | 51.60 | 0.31 | 51.42 | 51.78 |
| Fabaceae | Albizia niopoides | 3 | 49.50 | 0.43 | 49.16 | 49.84 |
| Fabaceae | Apuleia leiocarpa | 3 | 51.04 | 0.27 | 50.86 | 51.22 |
| Fabaceae | Barnebydendron riedelii | 3 | 49.57 | 0.49 | 49.18 | 49.96 |
| Bignoniaceae | Handroanthus serratifolius | 3 | 51.81 | 0.60 | 51.37 | 52.25 |
| Fabaceae | Hymenaea courbaril | 3 | 50.68 | 0.25 | 50.52 | 50.84 |
| Anacardiaceae | Astronium lecointei | 3 | 51.71 | 0.72 | 51.18 | 52.24 |
| Combretaceae | Terminalia tetraphylla | 3 | 49.83 | 0.27 | 49.61 | 50.05 |
| Fabaceae | Schizolobium parahyba var. amazonicum | 3 | 50.11 | 0.17 | 49.98 | 50.24 |
| Malvaceae | Ceiba pentandra | 3 | 49.08 | 3.36 | 47.18 | 50.98 |
| Malvaceae | Ceiba samauma | 3 | 49.45 | 0.21 | 49.28 | 49.62 |
| N, Mean, SD & CI | 19 | 50.48 | 0.42 | 50.07 | 50.90 | |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Families | 25.4 | 8 | 3.18 | 6.001 | <0.0001 |
| Residuals | 25.4 | 48 | 0.53 | ||
| Total | 50.8 | 56 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Species | 41.91 | 18 | 2.33 | 9.954 | <0.0001 |
| Residuals | 8.89 | 38 | 0.23 | ||
| Total | 50.80 | 56 |
| No. | Species Comparison * | Mean Difference | L.L. | U.L. | p-Value |
|---|---|---|---|---|---|
| 1 | Handroanthus serratifolius vs. Ceiba pentandra | 2.71 | 1.22 | 4.20 | <0.0001 |
| 2 | Dipteryx odorata vs. Ceiba pentandra | 2.52 | 1.03 | 4.01 | <0.0001 |
| 3 | Copaifera multijuga vs. Ceiba pentandra | 2.43 | 0.94 | 3.92 | <0.0001 |
| 4 | Handroanthus serratifolius vs. Ceiba samauma | 2.34 | 0.85 | 3.83 | <0.0001 |
| 5 | Astronium lecointei vs. Albizia niopoides | 2.33 | 0.84 | 3.82 | 0.0001 |
| 6 | Handroanthus serratifolius vs. Albizia niopoides | 2.29 | 0.80 | 3.78 | 0.0001 |
| 7 | Handroanthus serratifolius vs. Barnebydendron riedelii | 2.23 | 0.74 | 3.72 | 0.0002 |
| 8 | Dipteryx odorata vs. Ceiba samauma | 2.15 | 0.66 | 3.64 | 0.0004 |
| 9 | Cedrela odorata vs. Albizia niopoides | 2.10 | 0.61 | 3.59 | 0.0007 |
| 10 | Dipteryx odorata vs. Albizia niopoides | 2.10 | 0.61 | 3.59 | 0.0007 |
| 11 | Copaifera multijuga vs. Ceiba samauma | 2.06 | 0.57 | 3.55 | 0.0009 |
| 12 | Cedrela odorata vs. Barnebydendron riedelii | 2.03 | 0.54 | 3.52 | 0.0011 |
| 13 | Dipteryx odorata vs. Barnebydendron riedelii | 2.03 | 0.54 | 3.52 | 0.0011 |
| 14 | Copaifera multijuga vs. Albizia niopoides | 2.01 | 0.52 | 3.50 | 0.0013 |
| 15 | Copaifera multijuga vs. Barnebydendron riedelii | 1.94 | 0.45 | 3.43 | 0.0021 |
| 16 | Handroanthus serratifolius vs. Castilla ulei | 1.76 | 0.27 | 3.25 | 0.0081 |
| 17 | Hymenaea courbaril vs. Ceiba pentandra | 1.59 | 0.10 | 3.08 | 0.0260 |
| 18 | Cedrela odorata vs. Castilla ulei | 1.57 | 0.08 | 3.06 | 0.0304 |
| 19 | Dipteryx odorata vs. Castilla ulei | 1.57 | 0.08 | 3.06 | 0.0304 |
| 20 | Apuleia leiocarpa vs. Albizia niopoides | 1.55 | 0.06 | 3.04 | 0.0346 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, F.M.B.; Jacovine, L.A.G.; Carneiro, A.d.C.O.; Chaves, M.V.G.S.; Bezerra, E.B.; Lafuente, J.P.S.; da Rocha, S.J.S.S.; Lopes, M.T.G.; Fearnside, P.M. Carbon Content of Amazonian Commercial Tree Boles: Implications for Forest Management. Sustainability 2025, 17, 2960. https://doi.org/10.3390/su17072960
Romero FMB, Jacovine LAG, Carneiro AdCO, Chaves MVGS, Bezerra EB, Lafuente JPS, da Rocha SJSS, Lopes MTG, Fearnside PM. Carbon Content of Amazonian Commercial Tree Boles: Implications for Forest Management. Sustainability. 2025; 17(7):2960. https://doi.org/10.3390/su17072960
Chicago/Turabian StyleRomero, Flora Magdaline Benitez, Laércio Antônio Gonçalves Jacovine, Angélica de Cássia Oliveira Carneiro, Marcelo Vitor Gualberto Santos Chaves, Eronildo Braga Bezerra, Juan Pablo Sandoval Lafuente, Samuel José Silva Soares da Rocha, Maria Teresa Gomes Lopes, and Philip Martin Fearnside. 2025. "Carbon Content of Amazonian Commercial Tree Boles: Implications for Forest Management" Sustainability 17, no. 7: 2960. https://doi.org/10.3390/su17072960
APA StyleRomero, F. M. B., Jacovine, L. A. G., Carneiro, A. d. C. O., Chaves, M. V. G. S., Bezerra, E. B., Lafuente, J. P. S., da Rocha, S. J. S. S., Lopes, M. T. G., & Fearnside, P. M. (2025). Carbon Content of Amazonian Commercial Tree Boles: Implications for Forest Management. Sustainability, 17(7), 2960. https://doi.org/10.3390/su17072960








