The Impact of the Soil Environment and Surface Mulching on N2O Emissions from Farmland
Abstract
1. Introduction
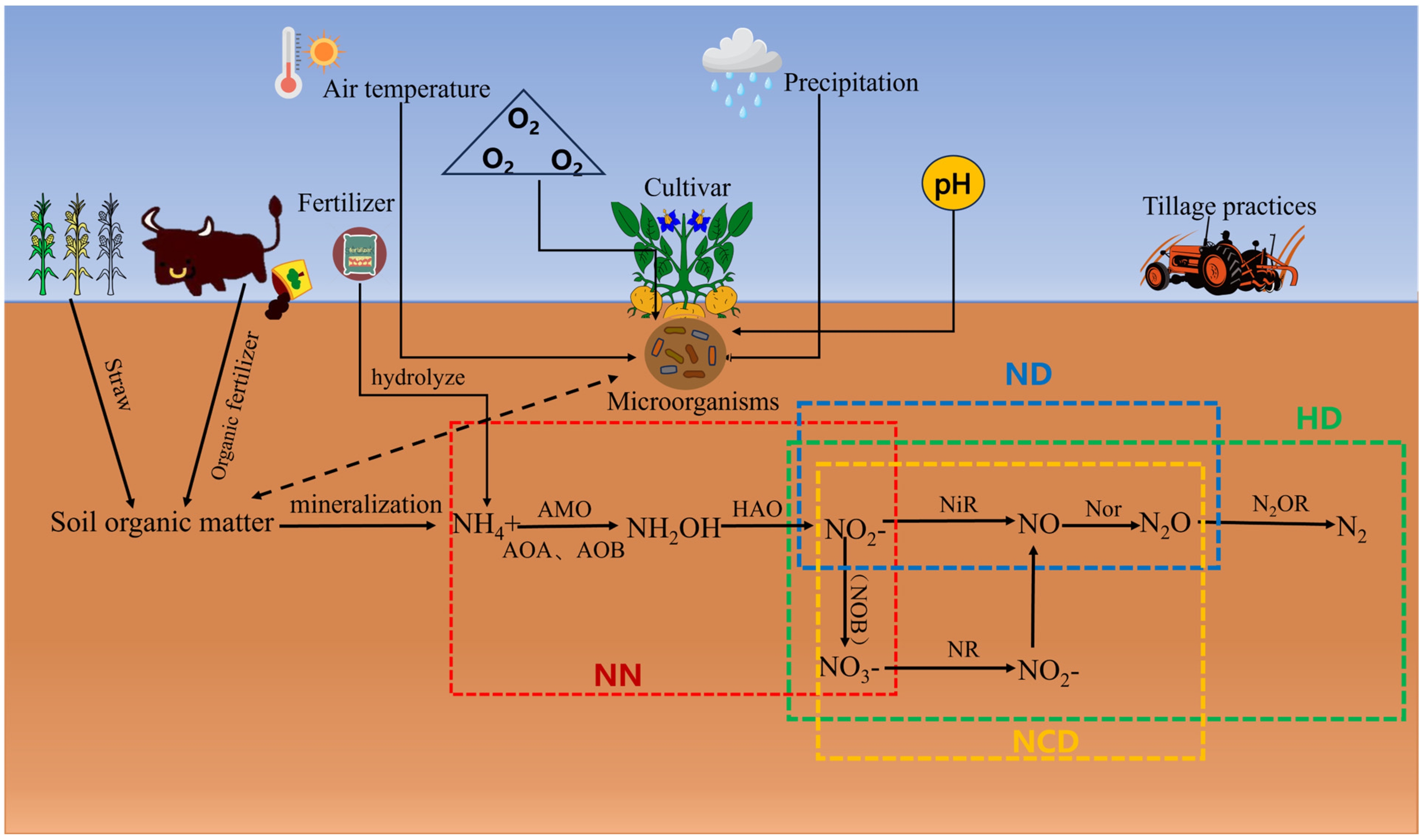
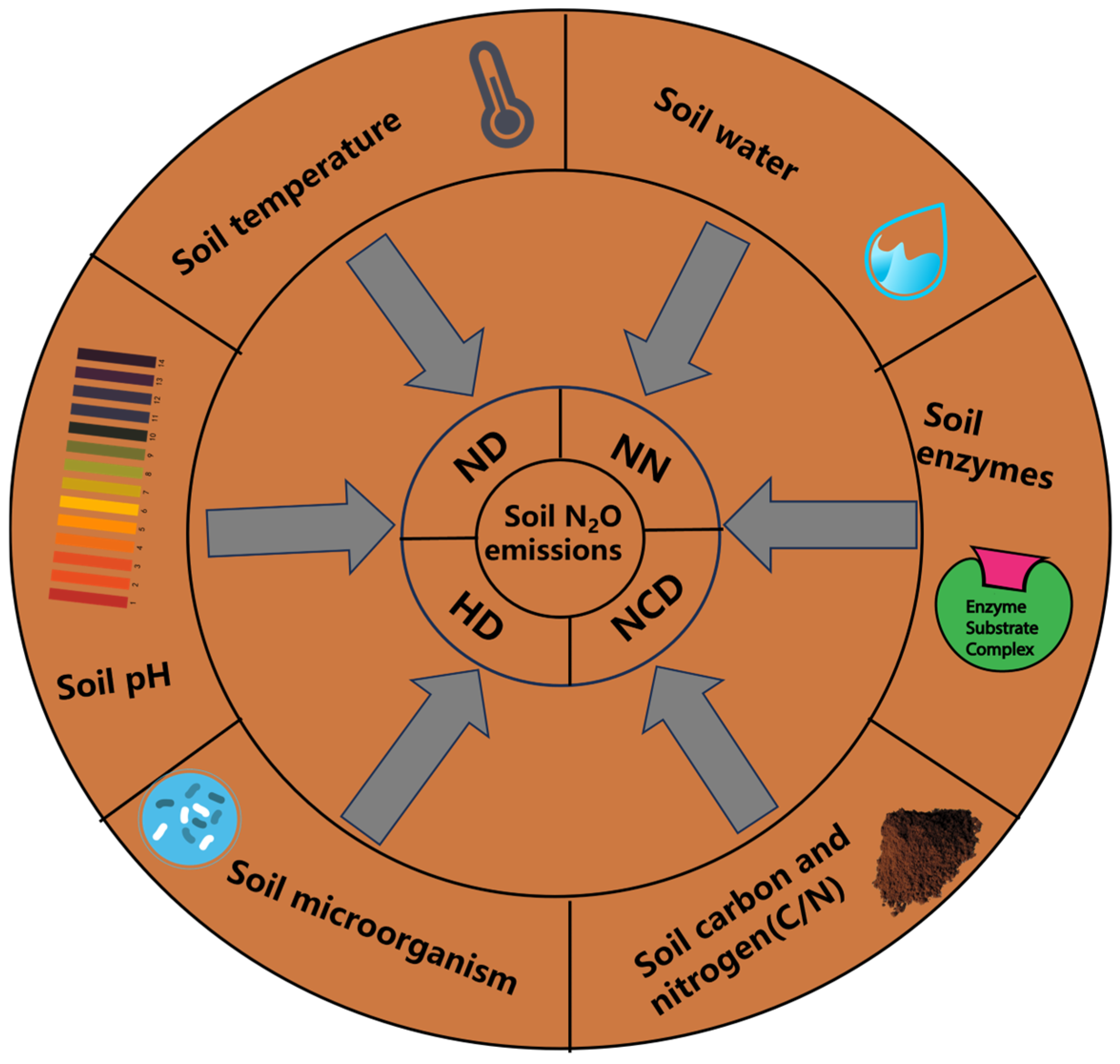
2. Environmental Factors Affecting Farmland N2O Emissions
2.1. Soil Temperature
2.2. Soil Water
2.3. Soil pH
2.4. Soil Enzymes
2.5. Soil Microorganism
2.6. Soil Carbon and Nitrogen
3. The Impact of Farmland Coverage on N2O Emissions
3.1. Plastic Film Mulching
3.2. Straw Mulching and Returning to Fields
4. Prospects and Outlook
- The complexity of the multi-pathway emissions mechanism. The primary mechanism for producing N2O in farmland is through the nitrification and denitrification processes facilitated by nitrifying bacteria. However, alternative pathways, such as fungal denitrification and chemical denitrification, also contribute to N2O production. Consequently, the mechanisms and pathways of N2O generation in agricultural settings remain incompletely understood. This complexity is particularly pronounced when multiple pathways operate simultaneously, making it challenging to isolate the contribution of a single pathway to overall N2O emissions.
- The optimization and innovation of coverage measures. The overarching goal of emission reduction efforts is to minimize greenhouse gas emissions without compromising agricultural productivity. Currently, research examining the effects of various mulching methods, mulching materials, straw application rates, and soil amendment amounts on soil N2O production and emissions is still relatively limited, highlighting an urgent need for further investigation in this area.
- Mechanisms of interaction between microorganisms and environmental factors. With the rapid advancement of microbial measurement technologies, the diversity of microorganisms involved in nitrification and denitrification continues to expand. Additionally, numerous environmental factors influence N2O production in farmland, and these factors interact in complex ways within the soil environment. Therefore, it is essential to employ networked causal analysis mathematical models to elucidate the correlation mechanisms between changes in soil environmental factors and N2O production, thereby providing a foundation for developing effective N2O emission reduction technologies in agricultural contexts.
- Integrated studies of soil–crop–atmosphere systems. Numerous studies have focused on the net emissions of N2O from farmland into the atmosphere; however, there is a notable paucity of research on the dynamic changes of N2O within the soil profile and its correlation with crop growth conditions. In this respect, there is an urgent need to enhance comprehensive research on N2O emissions in the context of the soil–crop–atmosphere continuum.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| AMO | Ammonia monooxygenase |
| HAD | Hydroxylamine dehydrogenase |
| NiR | Nitrite reductase |
| Nor | NO reductase |
| NR | Nitrate reductase |
| N2OR | N2O reductase |
| AOA | Ammonia-oxidizing archaebacteria |
| AOB | Ammonia-oxidizing bacteria |
| NOB | Nitrite-oxidizing bacteria |
| NN | Nitrification by nitrifying bacteria |
| ND | Denitrification by nitrifying bacteria |
| NCD | Denitrification coupled to nitrification reactions |
| HD | Heterotrophic denitrification |
| WFPS | Water filling pore space |
| OTUs | Operational taxonomic units |
References
- Melillo, J.M.; Steudler, P.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.; Wang, X.; Farooque, A.A.; Nawaz, R.A.; Pang, T.; Neokye, E.O. A review of greenhouse gas emissions from agricultural Soil. Sustainability 2024, 16, 4789. [Google Scholar] [CrossRef]
- Bouwman, A. Nitrogen oxides and tropical agriculture. Nature 1998, 392, 866–867. [Google Scholar] [CrossRef]
- Syakila, A.; Kroeze, C. The Global nitrous oxide budget revisited. Greenh. Gases Sci. Technol. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Luo, Z.; Lam, S.K.; Fu, H.; Hu, S.; Chen, D. Temporal and spatial evolution of nitrous oxide emissions in China: Assessment, strategy and recommendation. J. Clean. Prod. 2019, 223, 360–367. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Sophie Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Pereira, V.V.; Morales, M.M.; Pereira, D.H.; de Rezende, F.A.; de Souza Magalhães, C.A.; de Lima, L.B.; Marimon-Junior, B.H.; Petter, F.A. Activated biochar-based organomineral fertilizer delays nitrogen release and reduces N2O Emission. Sustainability 2022, 14, 12388. [Google Scholar] [CrossRef]
- Kool, D.M.; Dolfing, J.; Wrage, N.; Van Groenigen, J.W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- He, J.Z.; Shen, J.P.; Zhang, L.M.; Zhu, Y.G.; Zheng, Y.M.; Xu, M.g.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wrage, N.; van Groenigen, J.; Oenema, O.; Baggs, E.M. A novel dual-isotope labelling method for distinguishing between soil sources of N2O. Rapid Commun. Mass Spectrom. 2005, 19, 3298–3306. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Luo, Q.; Hu, A.; Wan, W.; Tian, D.; Ma, J.; Ma, T.; Luo, H.; Lu, S. Soil moisture–atmosphere feedback dominates land N2O nitrification emissions and denitrification reduction. Glob. Change Biol. 2022, 28, 6404–6418. [Google Scholar] [CrossRef]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Change Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef]
- Fan, W.; Sun, K.; Ma, M.; Mao, Y.; Xiang, W.; Xie, S. Mechanism and significance of Shewanella mediated N2O Emission under simulated typical Wetland conditions. Environ. Sci. Technol. 2020, 43, 1–7. [Google Scholar]
- Liu, Y.; Fang, Q.; Xu, S. Control factors of simultaneous nitrification/denitrification phosphorus removal and analysis of floa. Environ. Sci. Technol. 2019, 42, 27–33. [Google Scholar]
- Gong, Y.; Ren, L.; Peng, Y. The effect of pH on inhibitory kinetics of nitrification process and characteristic of N2O emission. Environ. Sci. Technol. 2019, 42, 10–15. [Google Scholar]
- Chen, X.; Zeng, X.C.; Kawa, Y.K.; Wu, W.; Zhu, X.; Ullah, Z.; Wang, Y. Microbial reactions and environmental factors affecting the dissolution and release of arsenic in the severely contaminated soils under anaerobic or aerobic conditions. Ecotoxicol. Environ. Saf. 2020, 189, 109946. [Google Scholar] [CrossRef]
- Gu, X.; Cai, H.; Fang, H.; Li, Y.; Chen, P. Effects of degradable film mulching on crop yield and water use efficiency in China: A Meta-Analysis. Soil Tillage Res. 2020, 202, 104676. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Jansen-Willems, A.B.; Lanigan, G.J.; Clough, T.J.; Andresen, L.C.; Müller, C. Long-term elevation of temperature affects organic N turnover and associated N2O emissions in a permanent grassland soil. Soil 2016, 2, 601–614. [Google Scholar] [CrossRef]
- Cai, Y.; Ding, W.; Luo, J. Nitrous oxide emissions from Chinese maize–wheat rotation systems: A 3-year field measurement. Atmos. Environ. 2013, 65, 112–122. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, Z.J.; Yuan, X.C.; Lin, W.S.; Xiong, D.C.; Yang, Y.S. Effects of precipitation exclusion and warming on soil soluble carbon and nitrogen in a young Cunninghamia lanceolata plantation. J. Appl. Econ. 2018, 29, 2217–2223. [Google Scholar]
- Alsajri, F.A.; Wijewardana, C.; Bheemanahalli, R.; Irby, J.T.; Krutz, J.; Golden, B.; Reddy, V.R.; Reddy, K.R. Morpho-Physiological, yield, and transgenerational seed germination responses of soybean to temperature. Front. Plant Sci. 2022, 13, 839270. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Z.; Wang, W.; Biederman, J.A.; Xu, X.; Ran, Q.; Qian, R.; Xu, C.; Zhang, B.; Wang, Y. Terrestrial N2O emissions and related functional genes under climate change: A global meta-analysis. Glob. Change Biol. 2020, 26, 931–943. [Google Scholar] [CrossRef]
- Ryden, J.C. Denitrification loss from a grassland soil in the field receiving different rates of nitrogen as ammonium nitrate. J. Soil Sci. 1983, 34, 355–365. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, S.; Xu, X.; Wang, Y.; Rui, Y.; Zhou, X.; Shen, Q.; Wang, J.; Jiang, L.; Luo, C. Fungi regulate the response of the N2O production process to warming and grazing in a Tibetan Grassland. Biogeosciences 2018, 15, 4447–4457. [Google Scholar] [CrossRef]
- Scarlett, K.; Denman, S.; Clark, D.R.; Forster, J.; Vanguelova, E.; Brown, N.; Whitby, C. Relationships between nitrogen cycling microbial community abundance and composition reveal the indirect effect of soil pH on oak decline. ISME J. 2021, 15, 623–635. [Google Scholar] [CrossRef]
- Liu, Y.; Cong, R.; Liao, S.; Guo, Q.; Li, X.; Ren, T.; Lu, Z.; Lu, J. Rapid soil rewetting promotes limited N2O emissions and suppresses NH3 volatilization under urea addition. Environ. Res. 2022, 212, 113402. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Huston, M.; Ingham, J.; Baker, A. Nitrous oxide laughing matter. Int. J. Oral Maxillofac. Surg. 2017, 46, 368–369. [Google Scholar] [CrossRef]
- Wu, D.; Dong, W.; Oenema, O.; Wang, Y.; Trebs, I.; Hu, C. N2O consumption by low-nitrogen soil and its regulation by water and oxygen. Soil Boil. Biochem. 2013, 60, 165–172. [Google Scholar] [CrossRef]
- Barton, L.; Murphy, D.V.; Butterbach-Bahl, K. Influence of crop rotation and liming on greenhouse gas emissions from a semi-arid soil. Agric. Ecosyst. Environ. 2013, 167, 23–32. [Google Scholar] [CrossRef]
- Chen, G.; Huang, G.; Huang, B.; Yu, K.; Wu, J.; Xu, H. Nitrous oxide and methane emissions from soil–plant systems. Nutr. Cycl. Agroecosyst. 1997, 49, 41–45. [Google Scholar] [CrossRef]
- Flechard, C.R.; Neftel, A.; Jocher, M.; Ammann, C.; Fuhrer, J. Bi-directional soil/atmosphere N2O exchange over two mown grassland systems with contrasting management practices. Glob. Change Biol. 2005, 11, 2114–2127. [Google Scholar] [CrossRef]
- Smith, K.; Thomson, P.; Clayton, H.; McTaggart, I.; Conen, F. Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos. Environ. 1998, 32, 3301–3309. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Ma, Y.; Sun, L.; Xiong, Z.; Huang, Q.; Sheng, Q. Methane and nitrous oxide emissions as affected by organic–inorganic mixed fertilizer from a rice paddy in southeast China. J. Soils Sediments 2013, 13, 1408–1417. [Google Scholar] [CrossRef]
- Oswald, R.; Behrendt, T.; Ermel, M.; Wu, D.; Su, H.; Cheng, Y.; Breuninger, C.; Moravek, A.; Mougin, E.; Trebs, I. HONO emissions from soil bacteria as a major source of atmospheric reactive nitrogen. Science 2013, 341, 1233–1235. [Google Scholar] [CrossRef]
- Cuhel, J.; Šimek, M.; Laughlin, R.J.; Bru, D.; Chèneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Cai, Z.; Mueller, C.; Zhang, J. Heterotrophic Nitrification is responsible for large rates of N2O emission from subtropical acid forest soil in China. Eur. J. Soil Sci. 2018, 69, 646–654. [Google Scholar] [CrossRef]
- Zhao, Y.; Ling, N.; Liu, X.; Li, C.; Jing, X.; Hu, J.; Rui, J. Altitudinal patterns of alpine soil Ammonia-Oxidizing community structure and potential nitrification rate. Appl. Environ. Microbiol. 2024, 90, e0007024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ju, X.; Wu, D. Transient nitrite accumulation explains the variation of N2O emissions to N fertilization in upland agricultural soils. Soil Boil. Biochem. 2023, 177, 108917. [Google Scholar] [CrossRef]
- Byrnes, B.H. Environmental effects of N fertilizer use—An overview. Fertil. Res. 1990, 26, 209–215. [Google Scholar] [CrossRef]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle–could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef]
- Kool, D.M.; Wrage, N.; Zechmeister-Boltenstern, S.; Pfeffer, M.; Brus, D.; Oenema, O.; Van Groenigen, J.W. Nitrifier denitrification can be a source of N2O from soil: A revised approach to the dual-isotope labelling method. Eur. J. Soil Sci. 2010, 61, 759–772. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Lee, B.H. Effect of Alcaligenes Faecalis on nitrous oxide emission and nitrogen removal in three hase fluidized bed process. J. Environ. Sci. Health A 2004, 39, 1791–1804. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Xia, L.; Yuan, W.; Zhou, Z.; Brüggmann, N. Role of chemical reactions in the nitrogenous trace gas emissions and nitrogen retention: A meta-analysis. Sci. Total Environ. 2022, 808, 152141. [Google Scholar] [CrossRef]
- Xu, M.P.; Ren, C.J.; Zhang, W.; Chen, Z.X.; Fu, S.Y.; Liu, W.C.; Han, X.H. Responses mechanism of C: N: P stoichiometry of soil microbial biomass and soil enzymes to climate change. J. Appl. Ecol. 2018, 29, 2445–2454. [Google Scholar]
- Woodward, E.E.; Edwards, T.M.; Givens, C.E.; Kolpin, D.W.; Hladik, M.L. Widespread use of the nitrification inhibitor nitrapyrin: Assessing benefits and costs to agriculture, ecosystems, and environmental Health. Environ. Sci. Technol. 2021, 55, 1345–1353. [Google Scholar] [CrossRef]
- Das, S.; Devi, T.; Goswami, M.; Yenuganti, M.; Bhardwaj, P.; Ghosh, S.; Kumar, P. Oxygen atom transfer promoted nitrate to nitric oxide transformation: A step-wise reduction of nitrate→ nitrite→ nitric oxide. Chem. Sci. 2021, 12, 10605–10612. [Google Scholar]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an Acid Cropped Soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, Y.; Liu, J.; Li, J.; Xu, G.; Luo, M.; Gao, M. Variation in N2O emission and N2O related microbial functional genes in straw-and biochar-amended and non-amended soils. Appl. Soil Ecol. 2019, 137, 57–68. [Google Scholar] [CrossRef]
- Bouwman, A.F. Direct emission of nitrous oxide from agricultural soils. Nutr. Cycl. Agroecosyst. 1996, 46, 53–70. [Google Scholar] [CrossRef]
- Bender, S.F.; Plantenga, F.; Neftel, A.; Jocher, M.; Oberholzer, H.-R.; Köhl, L.; Giles, M.; Daniell, T.J.; Van Der Heijden, M.G. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J. 2014, 8, 1336–1345. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, J.; Zhou, Z.; Wu, J.; Liu, Y.; Lin, J.-G.; Hong, Y.; Li, X.; Li, M.; Gu, J.D. Complex microbial nitrogen-cycling networks in three distinct anammox-inoculated wastewater treatment Systems. Water Res. 2020, 168, 115142. [Google Scholar] [CrossRef]
- Philippot, L.; Andert, J.; Jones, C.M.; Bru, D.; Hallin, S. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O Emissions from Soil. Glob. Change Biol. 2010, 17, 1497–1504. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Yoshihashi, T.; Ito, O.; Ono, H.; Ohnishi-Kameyama, M.; Yoshida, M.; Kawano, N.; Berry, W.L. Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant Soil 2008, 313, 89–99. [Google Scholar] [CrossRef]
- Giguere, A.T.; Taylor, A.E.; Myrold, D.D.; Mellbye, B.L.; Sayavedra-Soto, L.A.; Bottomley, P.J. Nitrite-oxidizing activity responds to nitrite accumulation in Soil. FEMS Microbiol. Ecol. 2018, 94, fiy008. [Google Scholar] [CrossRef]
- Knops, J.; Bradley, K.; Wedin, D. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol. Lett. 2002, 5, 454–466. [Google Scholar] [CrossRef]
- Trinsoutrot, I.; Recous, S.; Bentz, B.; Lineres, M.; Cheneby, D.; Nicolardot, B. Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci. Soc. Am. J. 2000, 64, 918–926. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhu, Y.G.; Xia, Y.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea: Important players in paddy rhizosphere soil? Environ. Microbiol. 2008, 10, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-C.; Guo, J.-H.; Song, H.; Liu, S.; Chen, J.-J.; Wang, J.-G. Effects of Ph and initial labile C/NO3− ratio on denitrification in a solar greenhouse vegetable soil. J. Plant Nutr. Fertil. 2017, 23, 1249–1257. [Google Scholar]
- Morley, N.; Baggs, E.M. Carbon and oxygen controls on N2O and N2 production during nitrate reduction. Soil Biol. Biochem. 2010, 42, 1864–1871. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Vidon, P.; Gurwick, N.P.; Allan, C.J.; Duval, T.P.; Lowrance, R. The role of riparian vegetation in protecting and improving chemical water quality in streams 1. J. Am. Water Resour. Assoc. 2010, 46, 261–277. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Bertrand, N.; Côté, D. Carbon dioxide and nitrous oxide emissions following fall and spring applications of pig slurry to an agricultural soil. Soil Sci. Soc. Am. J. 2004, 68, 1410–1420. [Google Scholar] [CrossRef]
- Mo, F.; Yu, K.L.; Crowther, T.W.; Wang, J.Y.; Zhao, H.; Xiong, Y.C.; Liao, Y.C. How plastic mulching affects net primary productivity, soil C fluxes and organic carbon balance in dry agroecosystems in China. J. Clean. Prod. 2020, 263, 121470. [Google Scholar] [CrossRef]
- Lamont, W.J. Plastics: Modifying the microclimate for the production of vegetable crops. HortTechnology 2005, 15, 477–481. [Google Scholar] [CrossRef]
- Cuello, J.P.; Hwang, H.Y.; Gutierrez, J.; Kim, S.Y.; Kim, P.J. Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl. Soil Ecol. 2015, 91, 48–57. [Google Scholar] [CrossRef]
- Nevins, C.J.; Lacey, C.; Armstrong, S. The synchrony of cover crop decomposition, enzyme activity, and nitrogen availability in a corn agroecosystem in the Midwest United States. Soil Tillage Res. 2020, 197, 104518. [Google Scholar] [CrossRef]
- Nan, W.-G.; Yue, S.-C.; Huang, H.-Z.; Li, S.-Q.; Shen, Y.F. Effects of plastic film mulching on soil greenhouse gases (CO2, CH4 and N2O) concentration within soil profiles in maize fields on the Loess Plateau, China. J. Integr. Agric. 2016, 15, 451–464. [Google Scholar] [CrossRef]
- García-Marco, S.; Ravella, S.R.; Chadwick, D.; Vallejo, A.; Gregory, A.S.; Cárdenas, L.M. Ranking factors affecting emissions of GHG from incubated agricultural soils. Eur. J. Soil Sci. 2014, 65, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, X.; Zhou, Y.; Peng, B.; Tang, L.; Luo, L.; Yao, B.; Deng, Y.; Tang, J.; Zeng, G. New insights into the activity of a biochar supported nanoscale zerovalent iron composite and nanoscale zero valent iron under znaerobic or zerobic conditions. RSC Adv. 2017, 7, 8755–8761. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Q.; Zhang, X.; Duan, P.; Yan, X.; Xiong, Z. Biochar-enriched soil mitigated N2O and NO emissions similarly as fresh biochar for wheat production. Sci. Total Environ. 2020, 701, 134943. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Luo, S.; Bu, L.; Chen, X.; Yue, S.; Li, S. Response of nitrous oxide emission to soil mulching and nitrogen fertilization in semi-arid farmland. Agric. Ecosyst. Environ. 2014, 188, 20–28. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, C.; Li, X.; He, X.; Hao, Q. Variations in nitrous oxide emissions as Manipulated by Plastic Film Mulching and Fertilization over Three Successive Years in a Hot Pepper-radish rotated vegetable production system. Agric. Ecosyst. Environ. 2020, 304, 107127. [Google Scholar] [CrossRef]
- Gao, N.; Yang, B.; Song, Q.; Li, X.; Chen, W.; Shen, Y.; Yue, S.; Li, S. Ammonia-oxidizing bacteria-driven autotrophic nitrification dominated nitrous oxide production in calcareous soil under long term plastic film mulching. Geoderma 2023, 435, 116523. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, P.; Liu, J.; Xiao, H.; Zhang, A.; Chen, S.; Chen, J.; Liu, H.; Zhu, X.; Hussain, Q.; et al. Microbial Effects of prolonged nitrogen fertilization and straw mulching on soil N2O emissions using metagenomic sequencing. Agric. Ecosyst. Environ. 2025, 382, 109476. [Google Scholar] [CrossRef]
- Kim, G.W.; Das, S.; Hwang, H.Y.; Kim, P.J. Nitrous oxide emissions from soils amended by cover-crops and under plastic film mulching: Fluxes, emission factors and yield-scaled emissions. Atmos. Environ. 2017, 152, 377–388. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, T.; Li, Z.; Tian, X.; Chen, J.; Zhang, J.; Li, S. Long-term effects of film mulching and fertilization regimes on gross N transformations in calcareous dryland soils. Appl. Soil Ecol. 2024, 204, 105747. [Google Scholar] [CrossRef]
- Fentabil, M.M.; Nichol, C.F.; Neilsen, G.H.; Hannam, K.D.; Neilsen, D.; Forge, T.A.; Jones, M.D. Effect of micro-irrigation type, N-source and mulching on nitrous oxide emissions in a semi-arid climate: An assessment across two years in a Merlot grape vineyard. Agric. Water Manag. 2016, 171, 49–62. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Gao, X.; Zheng, Z.; Xu, L.; Zhu, Z.; Jiang, J.; Miao, M. Effects of wheat straw mulching and wet treatment on soil improvement, greenhouse gas emission, nitrogen leaching, and vegetable yield. Hortic. Plant J. 2025, 5, 18. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, J.; Fan, J.; Zhang, F.; Huang, C. Grain yield and greenhouse gas emissions from maize and wheat fields under plastic film and straw mulching: A meta-analysis. Field Crops Res. 2021, 270, 108210. [Google Scholar] [CrossRef]
- Nawaz, A.; Lal, R.; Shrestha, R.K.; Farooq, M. Mulching affects soil properties and greenhouse gas emissions under long-term no-till and plough-till systems in Alfisol of central Ohio. Land Degrad. Dev. 2016, 28, 673–681. [Google Scholar] [CrossRef]
- Dix, B.A.; Hauschild, M.E.; Niether, W.; Wolf, B.; Gattinger, A. Regulating soil microclimate and greenhouse gas emissions with rye mulch in cabbage cultivation. Agric. Ecosyst. Environ. 2024, 367, 108951. [Google Scholar] [CrossRef]
- Yan, Z.; Tang, S.; He, Z.; Cheng, H.; Twagirayezu, G.; Zhao, J.; Xiang, R.; Hu, R.; Lin, S. Biochar addition under straw return reduces carbon dioxide and nitrous oxide emissions in acidic tea field soil. J. Environ. Manag. 2024, 370, 122498. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Zhu, K.; Ju, X.; Wu, D. The divergent role of straw return in soil O2 dynamics elucidates its confounding effect on soil N2O emission. Soil Biol. Biochem. 2024, 199, 109620. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Meng, S.; Zheng, X.; Zhou, Z.; Han, S.; Yang, Z. Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emissions from a wheat–maize rotation field in northern China. Agric. Ecosyst. Environ. 2011, 140, 226–233. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, D.; Schwenke, G.; Yang, B. The global warming potential of straw-return can be reduced by application of straw-decomposing microbial inoculants and biochar in rice-wheat production systems. Environ. Pollut. 2019, 252, 835–845. [Google Scholar] [CrossRef]
- Shan, J.; Yan, X. Effects of crop residue returning on nitrous oxide emissions in agricultural soils. Atmos. Environ. 2013, 71, 170–175. [Google Scholar] [CrossRef]
- Miller, M.N.; Zebarth, B.; Dandie, C.E.; Burton, D.L.; Goyer, C.; Trevors, J.T. Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol. Biochem. 2008, 40, 2553–2562. [Google Scholar] [CrossRef]
- Huang, T.; Yang, H.; Huang, C.; Ju, X. Effect of fertilizer N rates and straw management on yield-scaled nitrous oxide emissions in a maize-wheat double cropping system. Field Crops Res. 2017, 204, 1–11. [Google Scholar] [CrossRef]
- Baggs, E.M.; Stevenson, M.; Pihlatie, M.; Regar, A.; Cook, H.; Cadisch, G. Nitrous oxide emissions following application of residues and fertiliser under zero and conventional tillage. Plant Soil 2003, 254, 361–370. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Castellano, M.J.; Sawyer, J.E.; Pantoja, J. Cover crop effects on nitrous oxide emissions: Role of mineralizable carbon. Soil Sci. Soc. Am. J. 2013, 77, 1765–1773. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Enguang, N.; Khan, A.; Feng, Y.; Yang, G.; Wang, H. Straw mulching with inorganic nitrogen fertilizer reduces soil CO2 and N2O emissions and improves wheat yield. Sci. Total Environ. 2020, 741, 140488. [Google Scholar] [CrossRef]
- Li, J.; Ye, X.; Zhang, Y.; Chen, J.; Yu, N.; Zou, H. Maize straw deep-burying promotes soil bacteria community abundance and improves soil fertility. J. Soil Sci. Plant Nutr. 2021, 21, 1397–1407. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Liu, X.Y.; Qu, J.J.; Li, L.Q.; Zhang, A.F.; Jufeng, Z.; Zheng, J.W.; Pan, G.X. Can biochar amendment be an ecological engineering technology to depress N2O emission in rice paddies?—A cross site field experiment from South China. Ecol. Eng. 2012, 42, 168–173. [Google Scholar] [CrossRef]
- Case, S.D.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil–the role of soil aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Cui, F.; Yan, G.; Zhou, Z.; Zheng, X.; Deng, J. Annual emissions of nitrous oxide and nitric oxide from a wheat–maize cropping system on a silt loam calcareous soil in the North China Plain. Soil Biol. Biochem. 2012, 48, 10–19. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Liu, Y.; Liang, H.; Wang, T.; Zhao, Y.; Zhao, Q.; Peng, T. Differences in methane and nitrous oxide emissions and soil bacteria communities between straw return methods in central China. Environ. Sci. Pollut. Res. 2023, 30, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Hai, B.; Diallo, N.H.; Sall, S.; Haesler, F.; Schauss, K.; Bonzi, M.; Schloter, M. Quantification of key genes steering the microbial nitrogen cycle in the rhizosphere of sorghum cultivars in tropical agroecosystems. Appl. Environ. Microbiol. 2009, 75, 4993–5000. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; He, P.; Zhou, W. Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol. Biochem. 2013, 57, 30–42. [Google Scholar] [CrossRef]
- Liu, G.; Ma, J.; Yang, Y.T.; Yu, H.Y.; Zhang, G.; Xu, H. Effects of straw incorporation methods on nitrous oxide and methane emissions from a wheat-rice rotation system. Pedosphere 2019, 29, 204–215. [Google Scholar] [CrossRef]
| Coverage Methods | Effects on N2O Emissions | Reference |
|---|---|---|
| Plastic film | Increased N2O emissions from soil nitrogen and temperature increase in radish season and lower emissions from low soil water content in pepper season | (Zhao, M. et al., 2020) [76] |
| Plastic film | Lower N2O production in long-term mulched soils attributed to reduced nitrifying microbial activity | (Gao, N. et al., 2023) [77] |
| Straw mulch | Reduced N2O emissions without fertilizer compared to fertilizer under straw mulch | (Zhao, Y. et al., 2025) [78] |
| Plastic film | Increase in soil nitrogen content by cover crop residues compared to no cover contributes to cumulative N2O emissions | (Kim, G.W. et al., 2017) [79] |
| Plastic film | Long-term mulching negatively affects microorganisms by reducing N2O | (Gao, N. et al., 2024) [80] |
| Bark mulch | Bark mulch as a strategy for mitigating N2O emission in sandy loam soils of vineyard systems | (Fentabil, M.M. et al., 2016) [81] |
| Straw mulch | Under high moisture conditions, high straw volume mulching was more effective in reducing N2O emissions than medium straw volume mulching | (Zhang, Z. et al., 2025) [82] |
| Straw mulch | A meta-analysis revealed that both straw and mulch increased N2O emission | (Wang, H. et al., 2021) [83] |
| Plastic film | Mulching increases N2O emissions by influencing soil properties | (Nawaz, A. et al., 2021) [84] |
| Plastic film | Planting rye may reduce soil temperature to suppress N2O emissions | (Dix, B.A. et al., 2024) [85] |
| Straw mulch | Straw as a carbon source, prompting nitrogen fertilization to increase N2O emissions | (Yan, Z. et al., 2024) [86] |
| Straw mulch | Increase in cumulative N2O emissions under conventional N fertilization, no effect under optimized N inputs | (Wei, H. et al., 2024) [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Chang, L.; Khan, K.S.; Chai, S.; Chai, Y.; Han, F. The Impact of the Soil Environment and Surface Mulching on N2O Emissions from Farmland. Sustainability 2025, 17, 2502. https://doi.org/10.3390/su17062502
Chen Q, Chang L, Khan KS, Chai S, Chai Y, Han F. The Impact of the Soil Environment and Surface Mulching on N2O Emissions from Farmland. Sustainability. 2025; 17(6):2502. https://doi.org/10.3390/su17062502
Chicago/Turabian StyleChen, Qian, Lei Chang, Khuram Shehzad Khan, Shouxi Chai, Yuwei Chai, and Fanxiang Han. 2025. "The Impact of the Soil Environment and Surface Mulching on N2O Emissions from Farmland" Sustainability 17, no. 6: 2502. https://doi.org/10.3390/su17062502
APA StyleChen, Q., Chang, L., Khan, K. S., Chai, S., Chai, Y., & Han, F. (2025). The Impact of the Soil Environment and Surface Mulching on N2O Emissions from Farmland. Sustainability, 17(6), 2502. https://doi.org/10.3390/su17062502





