A Green Chemistry and Energy- and Cost-Effective Approach in Innovative Advanced Oxidation Processes Through Photoactive Microgels for Sustainable Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents, and Polymeric Precursors
2.2. Synthesis of the Photoactive Colloidal Microgels
- Step 1.
- Synthesis of NIPAM-co-AEMA polymeric microgel
- Step 2.
- Rose Bengal anchorage via carbodiimide coupling reaction: synthesis of NIPAM-co-AEMA-RB
2.3. Characterization of the Colloidal Microgels
2.4. Evaluation the Photo-Oxidation Kinetics of the Photoactive Colloidal Microgels
2.5. Studies of Diclofenac Pollutant Photo-Degradation
2.6. Photo-Oxidation of Furoic Acid and Green Synthesis of 5-Hydroxy-2(5H)-Furanone
3. Results and Discussion
3.1. Synthesis and Characterization of the Photoactive Colloidal Microgels
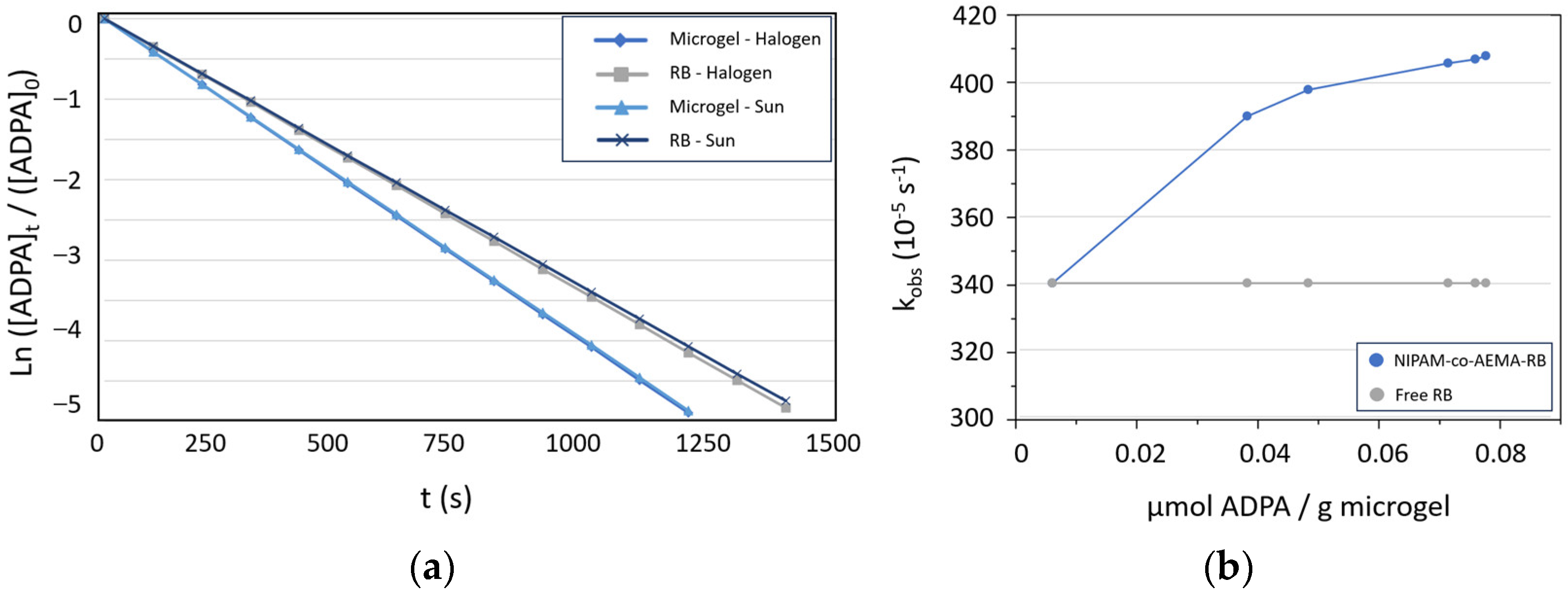
3.2. Evaluation the Photo-Oxidation Kinetics of the Photoactive Colloidal Microgels
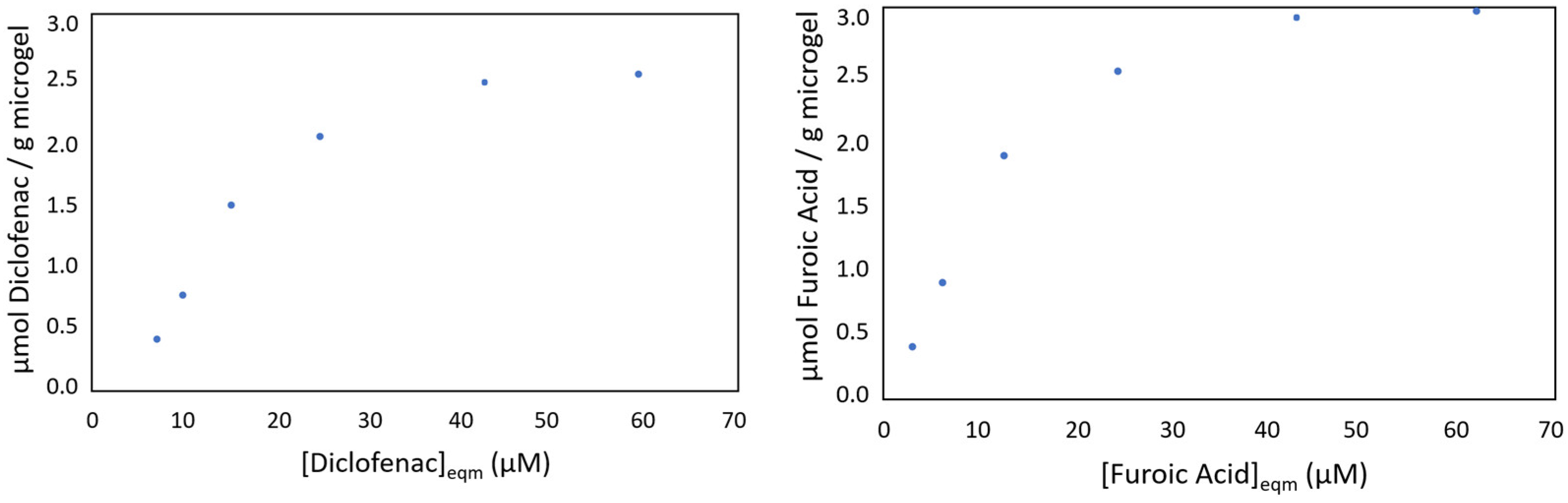
3.3. Studies of Diclofenac Pollutant Photo-Degradation
3.4. Studies of Photo-Oxidation of Furoic Acid and Green Synthesis of 5-Hydroxy-2(5H)-Furanone
3.5. Energy and Cost-Effective Calculations for Industrial Scale-Up
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CECs | Contaminants of Emerging Concern |
| WWTPs | Wastewater treatment plants |
| PNIPAM | Poly(N-isopropylacrylamide) |
| RB | Rose Bengal |
| EPs | Emerging pollutants |
| ADPA | 9,10-anthracenedipropionic acid |
| DLS | Dynamic light scattering |
| UV-Vis | Ultraviolet-visible spectroscopy |
| HPLC | High-Performance Liquid Chromatography |
| NIPAM | N-isopropylacrylamide |
| AEMA | Aminoethyl methacrylate |
| MBAM | N,N′-methylenebisacrylamide |
| EDC | 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide |
| MeOH | Methanol |
| t ½ | Half-reaction time |
| kobs | Observed rate constant or observed k |
| (O2(1Δg)) or 1O2 | Singlet oxygen |
| 3O2 | Molecular oxygen |
| hʋ | Light excitation or photon energy |
| TRL | Technology Readiness Level |
| CAPEX | Capital Expenditure |
| OPEX | Operational Expenditure |
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Andraos, J.; Matlack, A.S. Introduction to Green Chemistry; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Ncube, A.; Mtetwa, S.; Bukhari, M.; Fiorentino, G.; Passaro, R. Circular economy and green chemistry: The need for radical innovative approaches in the design for new products. Energies 2022, 16, 1752. [Google Scholar] [CrossRef]
- Zuin, V.G.; Eilks, I.; Elschami, M.; Kümmerer, K. Education in green chemistry and in sustainable chemistry: Perspectives towards sustainability. Green Chem. 2021, 23, 1594–1608. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Crini, G. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A review on emerging pollutants in the water environment: Existences, health effects and treatment processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Patel, N.A.V.E.E.N.; Khan, M.D.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging pollutants in aquatic environment: Source, effect, and challenges in biomonitoring and bioremediation-a review. Pollution 2020, 6, 99–113. [Google Scholar]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water–A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, B.; Fang, W. Sunlight-driven photocatalytic conversion of furfural and its derivatives. Green Chem. 2024, 26, 6261. [Google Scholar] [CrossRef]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F. Catalytic scissoring of lignin into aryl monomers. Adv. Mater. 2019, 31, 1901866. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Galindo, F. Singlet oxygen generation by photoactive polymeric microparticles with enhanced aqueous compatibility. Environ. Sci. Pollut. Res. 2014, 21, 11884–11892. [Google Scholar] [CrossRef]
- Huang, Y.M.; Lu, G.H.; Zong, M.H.; Cui, W.J.; Li, N. A plug-and-play chemobiocatalytic route for the one-pot controllable synthesis of biobased C4 chemicals from furfural. Green Chem. 2021, 23, 8604–8610. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: Theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Trombino, S.; Sole, R.; Di Gioia, M.L.; Procopio, D.; Curcio, F.; Cassano, R. Green chemistry principles for nano and micro-sized hydrogel synthesis. Molecules 2023, 28, 2107. [Google Scholar] [CrossRef]
- Rozhkova, Y.A.; Burin, D.A.; Galkin, S.V.; Yang, H. Review of microgels for enhanced oil recovery: Properties and cases of application. Gels 2022, 8, 112. [Google Scholar] [CrossRef]
- Das, A.; Babu, A.; Chakraborty, S.; Van Guyse, J.F.; Hoogenboom, R.; Maji, S. Poly (N-isopropylacrylamide) and Its Copolymers: A Review on Recent Advances in the Areas of Sensing and Biosensing. Adv. Funct. Mater. 2024, 34, 2402432. [Google Scholar] [CrossRef]
- Guerron, A.; Giasson, S. Multiresponsive microgels: Toward an independent tuning of swelling and surface properties. Langmuir 2021, 37, 11212–11221. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and emerging trends in advanced oxidation technologies and integration of advanced oxidation processes with biological processes for wastewater treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Green chemistry, biocatalysis, and the chemical industry of the future. ChemSusChem 2022, 15, e202102628. [Google Scholar] [CrossRef]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef]
- Ghogare, A.A.; Greer, A. Using singlet oxygen to synthesize natural products and drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef]
- Al-Nu’airat, J.; Oluwoye, I.; Zeinali, N.; Altarawneh, M.; Dlugogorski, B.Z. Review of chemical reactivity of singlet oxygen with organic fuels and contaminants. Chem. Rec. 2021, 21, 315–342. [Google Scholar] [CrossRef]
- Fabregat, V. Enhancing Emerging Pollutant Removal in Industrial Wastewater: Validation of a Photocatalysis Technology in Agri-Food Industry Effluents. Appl. Sci. 2024, 14, 6308. [Google Scholar] [CrossRef]
- Fabregat, V. Exploring the Role of pH and Solar Light-Driven Decontamination with Singlet Oxygen in Removing Emerging Pollutants from Agri-Food Effluents: The Case of Acetamiprid. Physchem 2025, 5, 9. [Google Scholar] [CrossRef]
- Koizumi, H.; Shiraishi, Y.; Tojo, S.; Fujitsuka, M.; Majima, T.; Hirai, T. Temperature-driven oxygenation rate control by polymeric photosensitizer. J. Am. Chem. Soc. 2006, 128, 8751–8753. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Suzuki, T.; Hirai, T. Temperature-and pH-responsive photosensitization activity of polymeric sensitizers based on poly-N-isopropylacrylamide. Polymer 2009, 50, 5758–5764. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kimata, Y.; Koizumi, H.; Hirai, T. Temperature-controlled photooxygenation with polymer nanocapsules encapsulating an organic photosensitizer. Langmuir 2008, 24, 9832–9836. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Kimata, Y.; Shiraishi, Y.; Hirai, T. Temperature-controlled changeable oxygenation selectivity by singlet oxygen with a polymeric photosensitizer. Chem. Commun. 2007, 18, 1846–1848. [Google Scholar] [CrossRef] [PubMed]
- Egil, A.C.; Carmignani, A.; Battaglini, M.; Sengul, B.S.; Acar, E.; Ciofani, G.; Ince, G.O. Dual stimuli-responsive nanocarriers via a facile batch emulsion method for controlled release of Rose Bengal. J. Drug Deliv. Sci. Technol. 2022, 74, 103547. [Google Scholar] [CrossRef]
- Don, T.M.; Lu, K.Y.; Lin, L.J.; Hsu, C.H.; Wu, J.Y.; Mi, F.L. Temperature/pH/Enzyme triple-responsive cationic protein/PAA-b-PNIPAAm nanogels for controlled anticancer drug and photosensitizer delivery against multidrug resistant breast cancer cells. Mol. Pharm. 2017, 14, 4648–4660. [Google Scholar] [CrossRef]
- Maki, Y.; Dobashi, T. Poly (N-isopropylacrylamide)-clay nanocomposite hydrogels with patterned thermo-responsive behavior. Trans. Mater. Res. Soc. Jpn. 2017, 42, 119–122. [Google Scholar] [CrossRef]
- Nowakowska, M.; Kȩpczyński, M.; Da̧browska, M. Polymeric Photosensitizers, 5. Synthesis and Photochemical Properties of Poly [(N-isopropylacrylamide)-co-(vinylbenzyl chloride)] Containing Covalently Bound Rose Bengal Chromophores. Macromol. Chem. Phys. 2001, 202, 1679–1688. [Google Scholar] [CrossRef]
- Nowakowska, M.; Kepczynski, M.; Szczubialka, K. New polymeric photosensitizers. Pure Appl. Chem. 2001, 73, 491–495. [Google Scholar] [CrossRef][Green Version]
- Nowakowska, M.; Szczubiałka, K. Photoactive polymeric and hybrid systems for photocatalytic degradation of water pollutants. Polym. Degrad. Stab. 2017, 145, 120–141. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Moya, P.; Marin, M.L.; Bosca, F. Synthesis of novel heterogeneous photocatalysts based on Rose Bengal for effective wastewater disinfection and decontamination. Catal. Today 2023, 413, 113948. [Google Scholar] [CrossRef]
- Flores, J.; Moya, P.; Bosca, F.; Marin, M.L. Photoreactivity of new rose bengal-SiO2 heterogeneous photocatalysts with and without a magnetite core for drug degradation and disinfection. Catal. Today 2023, 413, 113994. [Google Scholar] [CrossRef]
- Esser, P.; Pohlmann, B.; Scharf, H.D. The photochemical synthesis of fine chemicals with sunlight. Angew. Chem. Int. Ed. Engl. 1994, 33, 2009–2023. [Google Scholar] [CrossRef]
- Roy, J.S.; Messaddeq, Y. The Role of Solar Concentrators in Photocatalytic Wastewater Treatment. Energies 2024, 17, 4001. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Bharathi, V.D. Photocatalysis for removal of environmental pollutants and fuel production: A review. Environ. Chem. Lett. 2021, 19, 441–463. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Q.; Zhang, Y.; Zhang, K.; Hou, J.; Feng, M. Recent advances in solar-enhanced homogeneous water decontamination and disinfection: A review. Sep. Purif. Technol. 2023, 325, 124678. [Google Scholar] [CrossRef]
- Lee, B.C.Y.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging contaminants: An overview of recent trends for their treatment and management using light-driven processes. Water 2021, 13, 2340. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Luis, S.V.; Galindo, F. Improving photocatalytic oxygenation mediated by polymer supported photosensitizers using semiconductor quantum dots as ‘light antennas’. RSC Adv. 2017, 7, 35154–35158. [Google Scholar] [CrossRef]
- Burguete, M.I.; Fabregat, V.; Galindo, F.; Izquierdo, M.A.; Luis, S.V. Improved polyHEMA–DAQ films for the optical analysis of nitrite. Eur. Polym. J. 2009, 45, 1516–1523. [Google Scholar] [CrossRef]
- Fabregat, V.; Izquierdo, M.A.; Burguete, M.I.; Galindo, F.; Luis, S.V. Quantum dot–polymethacrylate composites for the analysis of NOx by fluorescence spectroscopy. Inorganica Chim. Acta 2012, 381, 212–217. [Google Scholar] [CrossRef]
- Fabregat, V.; Izquierdo, M.Á.; Burguete, M.I.; Galindo, F.; Luis, S.V. Nitric oxide sensitive fluorescent polymeric hydrogels showing negligible interference by dehydroascorbic acid. Eur. Polym. J. 2014, 55, 108–113. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Galindo, F.; Luis, S.V. Influence of polymer composition on the sensitivity towards nitrite and nitric oxide of colorimetric disposable test strips. Environ. Sci. Pollut. Res. 2017, 24, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Vincent, B. Poly (vinylpyridine) core/poly (N-isopropylacrylamide) shell microgel particles: Their characterization and the uptake and release of an anionic surfactant. Langmuir 2008, 24, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Vincent, B.; Burnett, G. Uptake and Release of Anionic Surfactant into and from Cationic Core− Shell Microgel Particles. Langmuir 2007, 23, 9237–9241. [Google Scholar] [CrossRef] [PubMed]
- Burakowska, E.; Zimmerman, S.C.; Haag, R. Photoresponsive crosslinked hyperbranched polyglycerols as smart nanocarriers for guest binding and controlled release. Small 2009, 5, 2199–2204. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Available online: https://www.aemet.es/documentos/es/serviciosclimaticos/datosclimatologicos/atlas_radiacion_solar/atlas_de_radiacion_24042012.pdf (accessed on 12 January 2025).
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA, 2003; Volume 66. [Google Scholar]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of techno-economic analysis and life cycle assessment for sustainable process design–A review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. Technoeconomic analysis of the recovery of phenols from olive mill wastewater through membrane filtration and resin adsorption/desorption. Sustainability 2021, 13, 2376. [Google Scholar] [CrossRef]
- Fabregat, V.; Pagán, J.M. Technical–Economic Feasibility of a New Method of Adsorbent Materials and Advanced Oxidation Techniques to Remove Emerging Pollutants in Treated Wastewater. Water 2024, 16, 814. [Google Scholar] [CrossRef]
- Dirección General del Agua, Ministerio para la Transición Ecológica y el Reto Demográfico. Mejora de la Eficiencia Energética e Integral de las Plantas de Tratamiento, Regeneración y Reutilización de Aguas Residuales—Informe Complementario; Dirección General del Agua, Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2020. [Google Scholar]
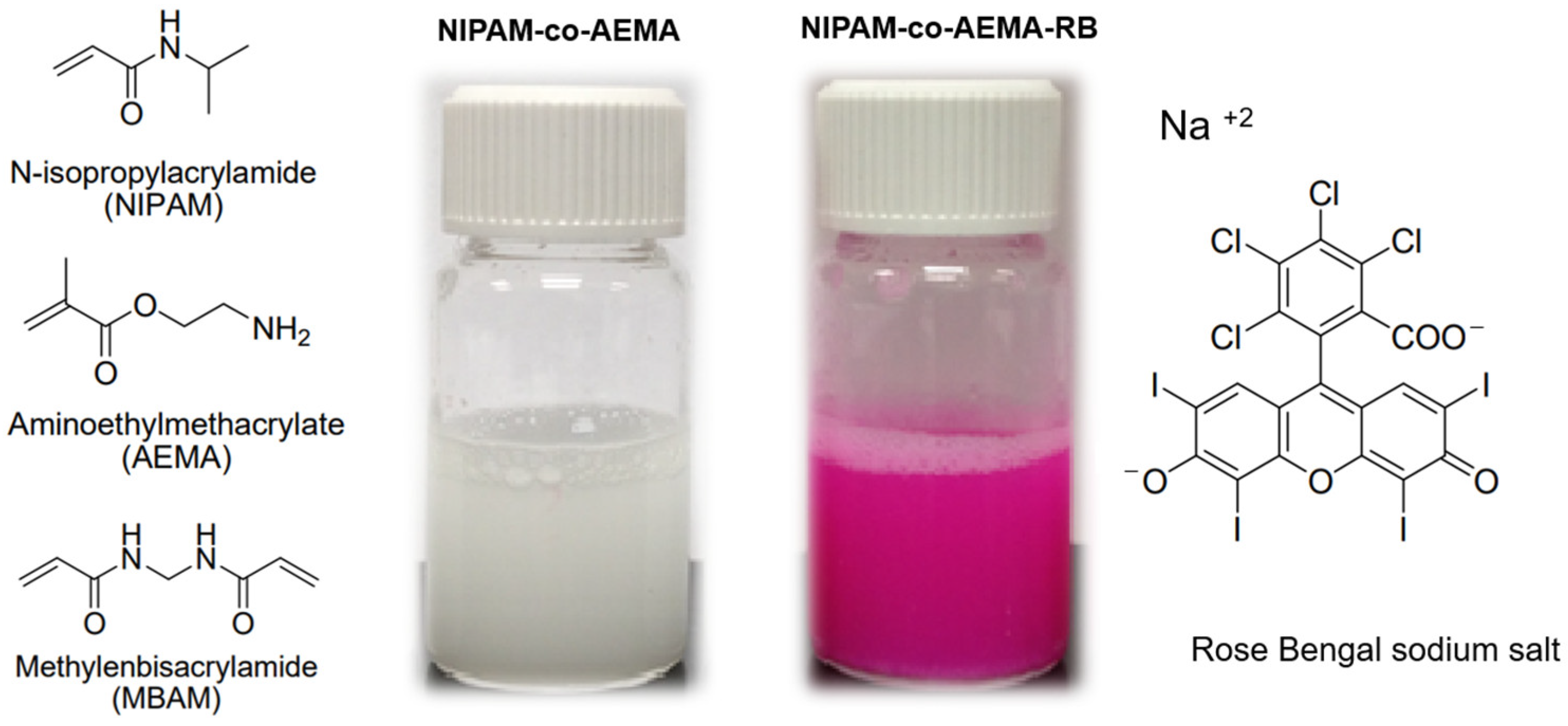
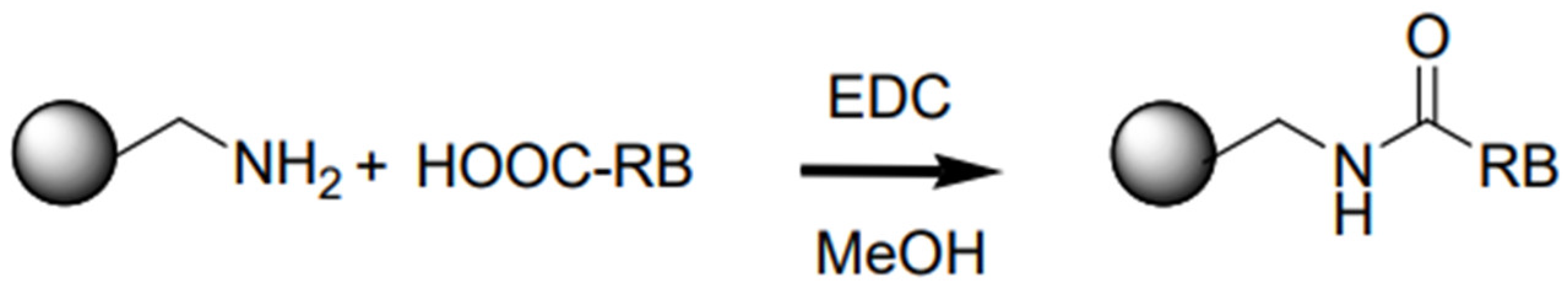
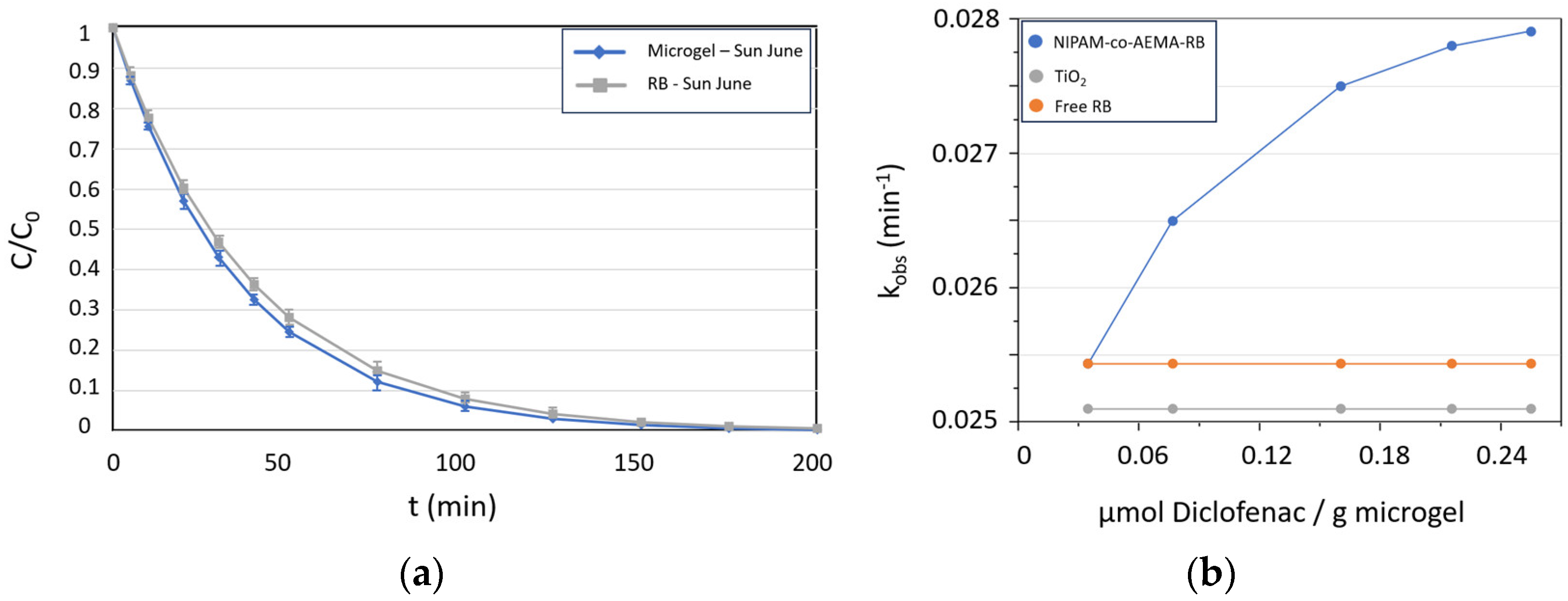
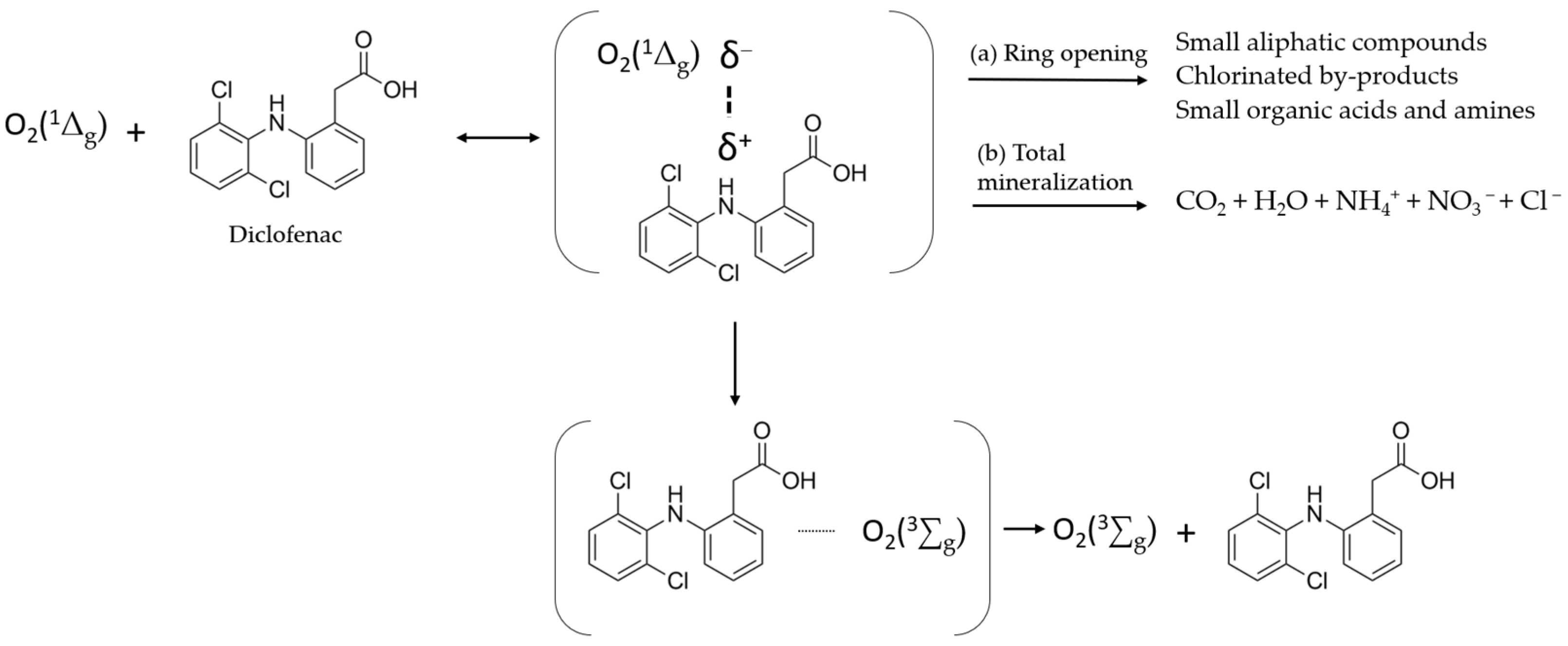
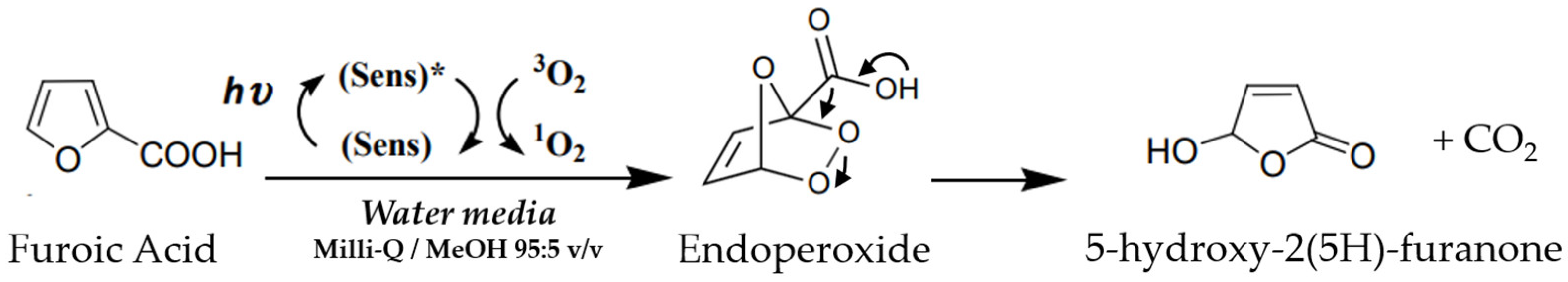
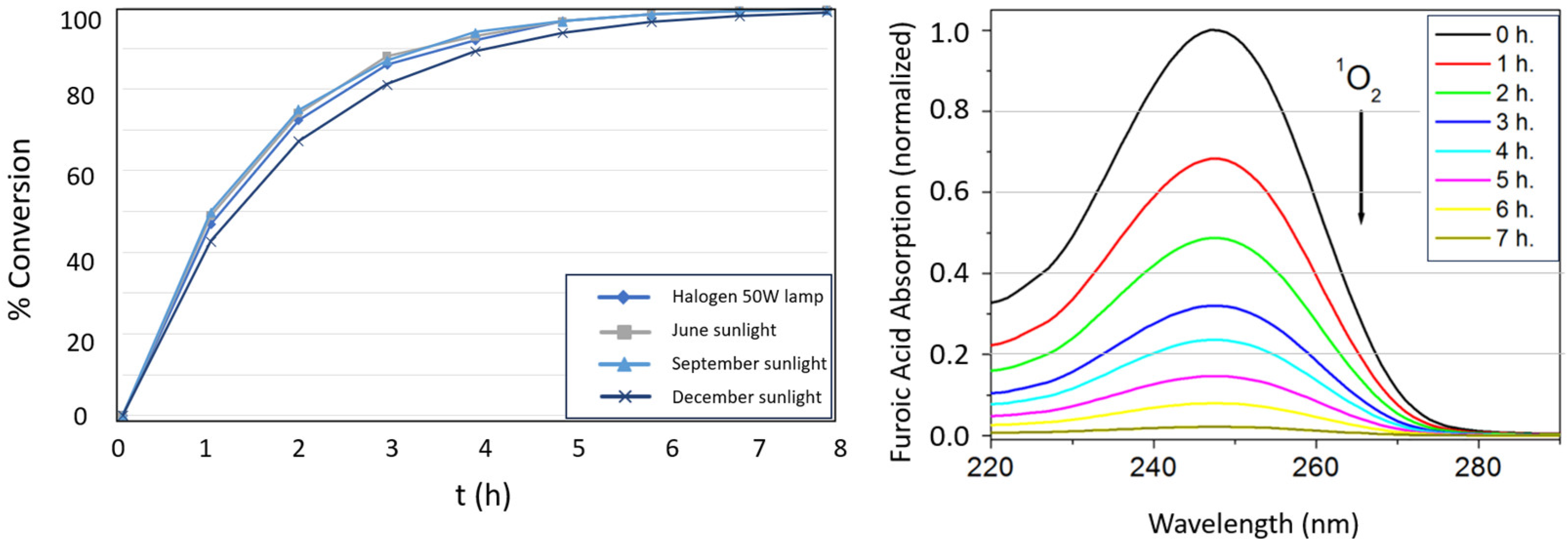
| Characterization Data | NIPAM-co-AEMA | NIPAM-co- AEMA-RB | |
|---|---|---|---|
| Effective diameter (nm) | pH = 3 | 732 | 724 |
| Natural pH of microgel | 655 | 669 | |
| pH = 7 | 631 | 634 | |
| pH = 11 | 488 | 498 | |
| Electrophoretic mobility (m2s−1V−1) | pH = 3 | 0.15 | 0.18 |
| Natural pH of microgel | 0.02 | 0.07 | |
| pH = 7 | 0.01 | 0.03 | |
| pH = 11 | −0.09 | −0.32 | |
| % AEMA (titration) | 0.92% | 0.92% | |
| RB loading (titration) | 0 | 33 µmol RB/g polym. | |
| RB loading (UV-Vis) | 0 | 33 µmol RB/g polym. | |
| Photosensitizer | t ½ (s) | t (>99%) (s) | kobs (10−5 s−1) | |
|---|---|---|---|---|
| Halogen lamp (sun-simulated) | Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | 170 | 1128 | 408 | |
| Free RB | 200 | 1332 | 346 | |
| Sun: natural irradiation (May 2024) | Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | 171 | 1135 | 406 | |
| Free RB | 204 | 1357 | 339 | |
| Photosensitizer | Solar Irradiation (kWh/m2) | t (>99%) (min) | kobs (min−1) | |
|---|---|---|---|---|
| Halogen lamp (sun-simulated) | Blank | 6.9 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 164 | 0.02808 | ||
| RB | 182 | 0.02530 | ||
| Sun: natural irradiation June | Blank | 7.2 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 165 | 0.02791 | ||
| RB | 181 | 0.02544 | ||
| Sun: natural irradiation September | Blank | 6.1 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 165 | 0.02788 | ||
| RB | 184 | 0.02532 | ||
| Sun: natural irradiation December | Blank | 3.3 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 169 | 0.02725 | ||
| RB | 188 | 0.02450 | ||
| Photosensitizer | Conversion of Furoic Acid at t = 420 min | t ½ (min) | kobs (min−1) | |
|---|---|---|---|---|
| Halogen lamp Irradiation = 6.9 kWh/m2 | Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | >99% | 61 | 0.01143 | |
| RB | 12% | 2277 | 0.00030 | |
| June sunlight Irradiation = 7.5 kWh/m2 | Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | >99% | 60 | 0.01140 | |
| RB | 13% | 2090 | 0.00033 | |
| September sunlight Irradiation = 6.1 kWh/m2 | Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | >99% | 61 | 0.01134 | |
| RB | 2498 | 0.00028 | ||
| December sunlight Irradiation = 2.9 kWh/m2 | Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | 98% | 74 | 0.00931 | |
| RB | 9% | 3087 | 0.00022 | |
| Process | Energy Consumption (kWh/m3) Reactor 500 mL | Reactor Scale-Up 1 m3. Energy Consp. (kWh/m3) | Reactor Scale-Up 100 m3. Energy Consp. (kWh/m3) | Reactor Scale-Up 1 Hm3. Energy Consp. (kWh/m3) | |||
|---|---|---|---|---|---|---|---|
| Aspen | Six-Tenths | Aspen | Six-Tenths | Aspen | Six-Tenths | ||
| Diclofenac (halogen lamp) | 537 | 24.38 | 25.69 | 4.12 | 4.07 | 0.27 | 0.26 |
| Diclofenac (sunlight) | 253 | 12.48 | 12.09 | 2.01 | 1.92 | 0.12 | 0.12 |
| Furoic Acid (halogen lamp) | 1050 | 48.36 | 50.21 | 7.85 | 7.96 | 0.48 | 0.50 |
| Furoic Acid (sunlight) | 375 | 18.05 | 17.95 | 2.92 | 2.85 | 0.17 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabregat, V.; Pagán, J.M. A Green Chemistry and Energy- and Cost-Effective Approach in Innovative Advanced Oxidation Processes Through Photoactive Microgels for Sustainable Applications. Sustainability 2025, 17, 2331. https://doi.org/10.3390/su17052331
Fabregat V, Pagán JM. A Green Chemistry and Energy- and Cost-Effective Approach in Innovative Advanced Oxidation Processes Through Photoactive Microgels for Sustainable Applications. Sustainability. 2025; 17(5):2331. https://doi.org/10.3390/su17052331
Chicago/Turabian StyleFabregat, Víctor, and Juana María Pagán. 2025. "A Green Chemistry and Energy- and Cost-Effective Approach in Innovative Advanced Oxidation Processes Through Photoactive Microgels for Sustainable Applications" Sustainability 17, no. 5: 2331. https://doi.org/10.3390/su17052331
APA StyleFabregat, V., & Pagán, J. M. (2025). A Green Chemistry and Energy- and Cost-Effective Approach in Innovative Advanced Oxidation Processes Through Photoactive Microgels for Sustainable Applications. Sustainability, 17(5), 2331. https://doi.org/10.3390/su17052331






