Development of a Concept for Closing the Water Cycle in the Surface Treatment of Ferrous and Non-Ferrous Metals
Abstract
1. Introduction
2. Materials and Methods
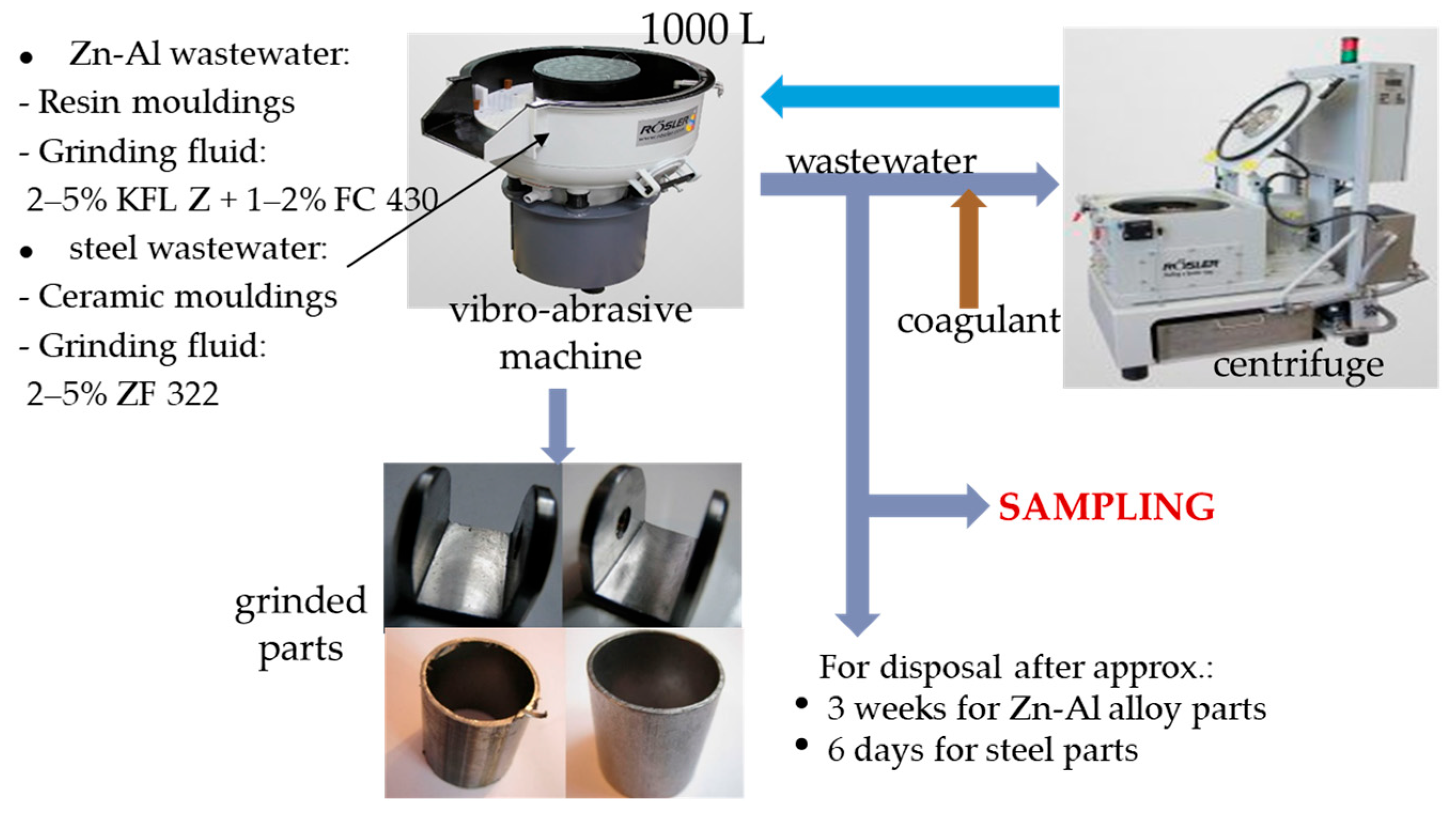
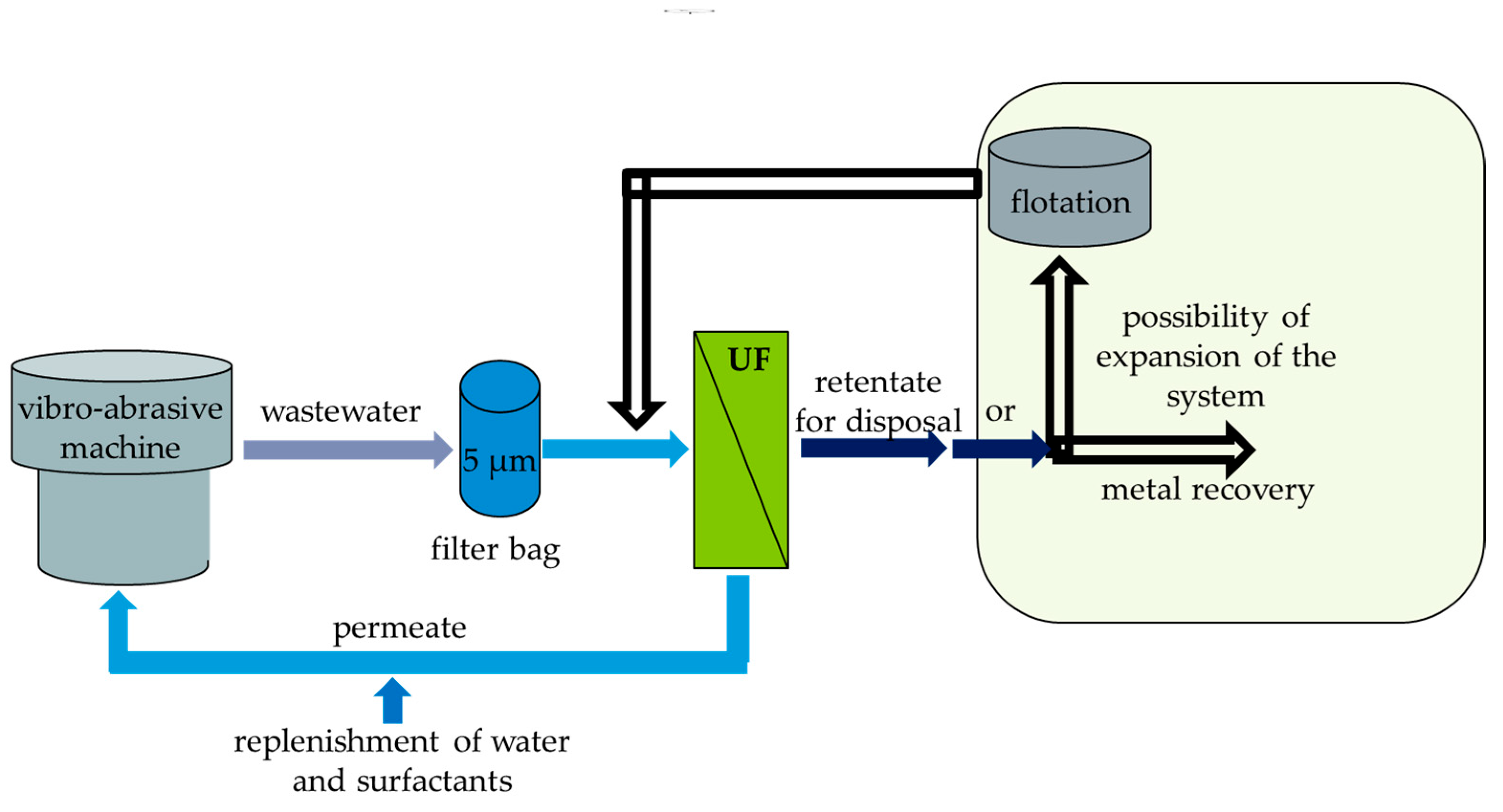
2.1. The Examined Wastewater
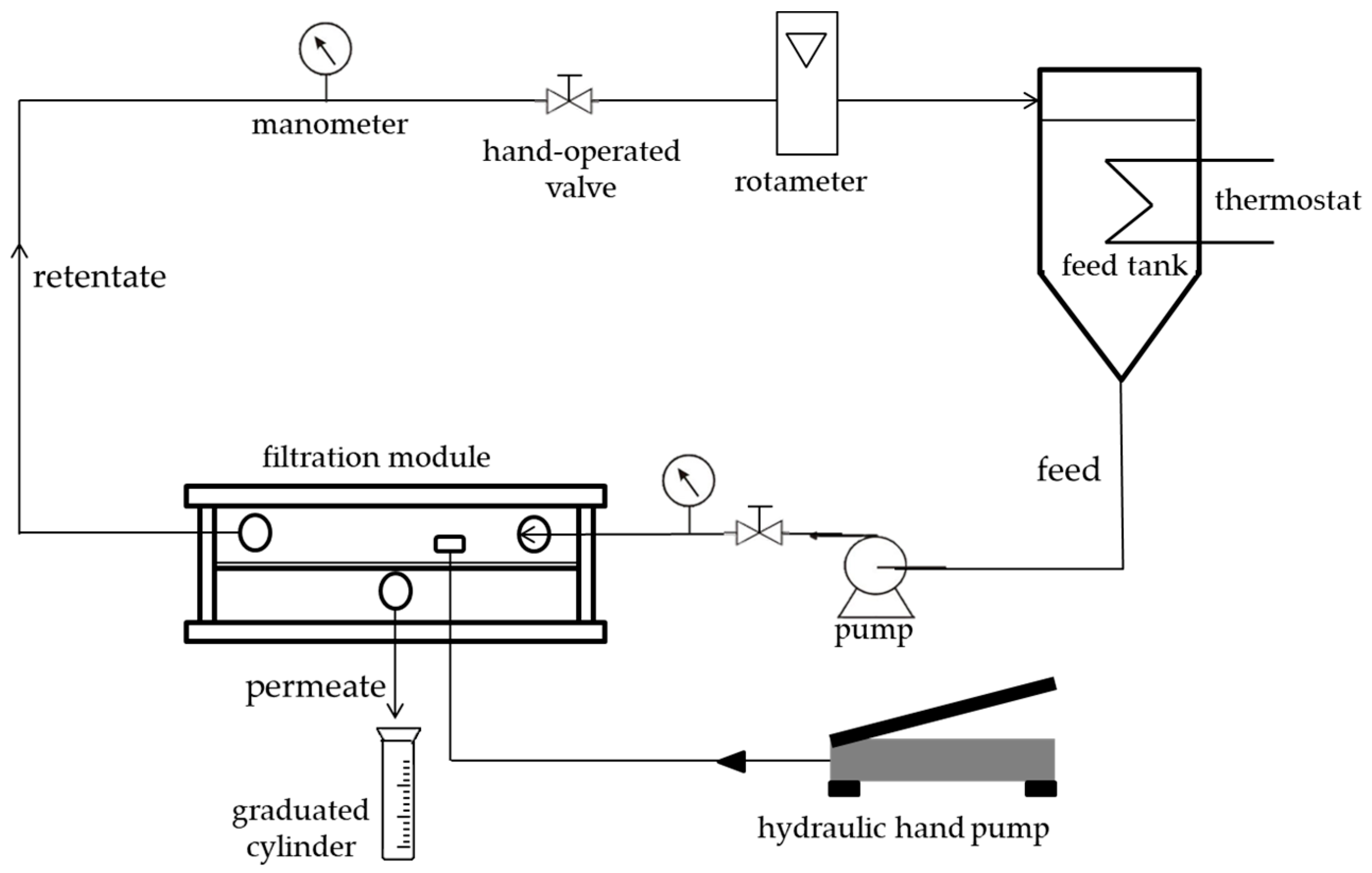
2.2. Membrane Filtration
2.3. Analytical Methods
- JA is the permeate flux (at constant temperature and pressure; mL (min × cm2)−1);
- V is the volume of filtrate (mL);
- A is the effective area of flat sheet membrane (cm2);
- t is the sampling time (min).
- R—the rejection coefficient (%);
- xp and xn—the values of the tested parameter in the permeate and the feed respectively (%, µS·cm−1, NTU or mg L−1, depending on the determined parameter).
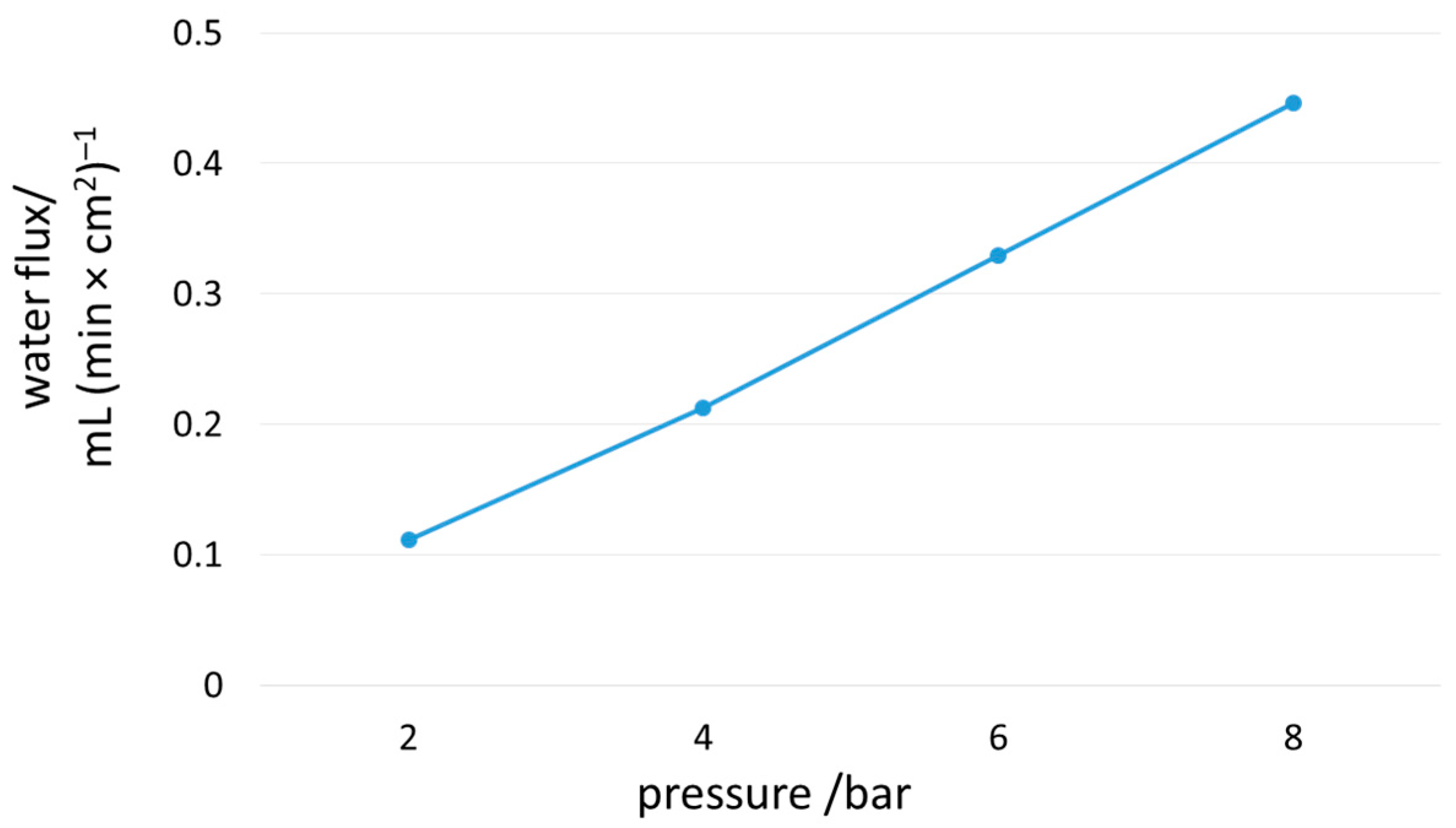
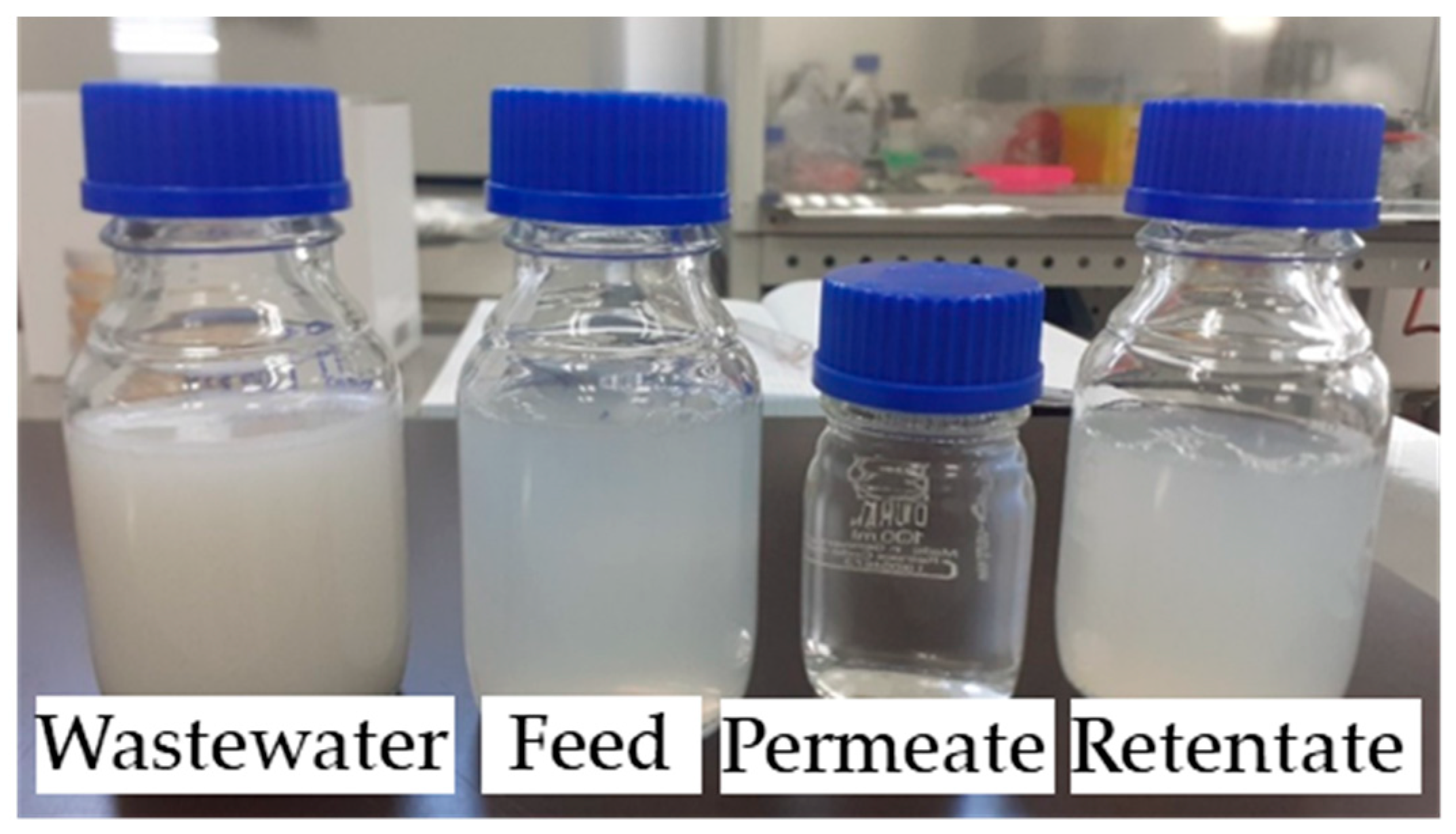
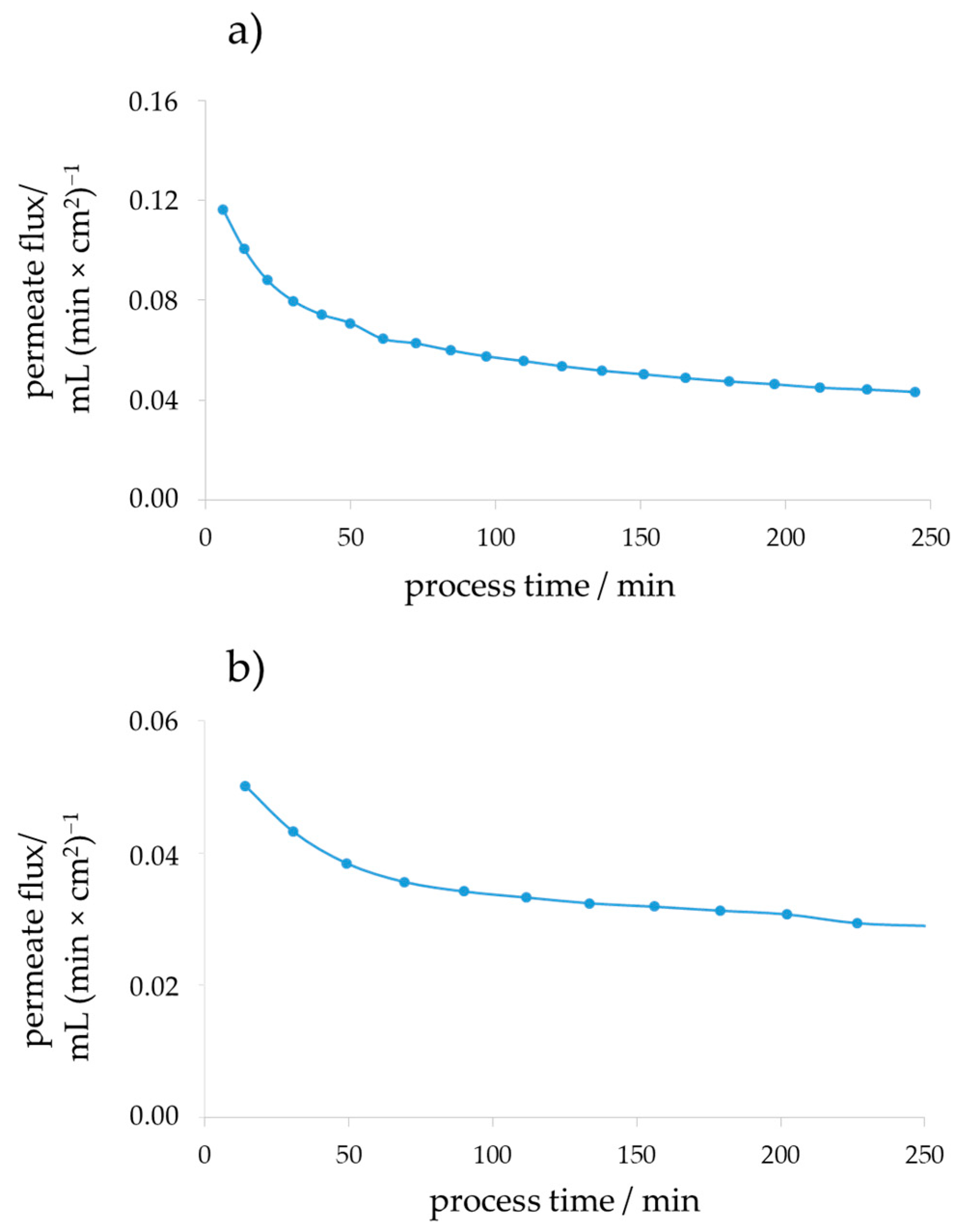
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0098 (accessed on 13 December 2024).
- OJ L 334/17; Directive 2010/75/EU on industrial emissions (IED). Council of the European Union: Avenue Marnix, Brussels, 2010.
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Meese, A.F.; Kim, D.J.; Wu, X.; Le, L.; Napier, C.; Hernandez, M.T.; Laroco, N.; Linden, K.G.; Cox, J.; Kurup, P.; et al. Opportunities and Challenges for Industrial Water Treatment and Reuse. ACS EST Eng. 2022, 2, 465–488. [Google Scholar] [CrossRef]
- Colla, V.; Matino, I.; Branca, T.A.; Fornai, B.; Romaniello, L.; Rosito, F. Efficient Use of Water Resources in the Steel Industry. Water 2017, 9, 874. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Singh, N.; Veeraraghavan, A.; Gupta, A.; Palani, S.G.; Mehdizadeh, M.; Omidi, A.; Al-Taey, D.K.A. Ascertaining and Optimizing the Water Footprint and Sludge Management Practice in Steel Industries. Water 2023, 15, 2177. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef]
- Chalaris, M.; Gkika, D.A.; Tolkou, A.K.; Kyzas, G.Z. Advancements and sustainable strategies for the treatment and management of wastewaters from metallurgical industries: An overview. Environ. Sci. Pollut. Res. 2023, 30, 119627–119653. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of electroplating industry wastewater: A review on the various techniques. Environ. Sci. Pollut. Res. 2022, 29, 72196–72246. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- Madan, J.; Singh, P.P. Sustainability in Foundry and Metal Casting Industry. In Sustainable Manufacturing Processes; Elsevier Inc.: Amsterdam, The Netherlands, 2023; pp. 29–52. [Google Scholar] [CrossRef]
- Petrinic, I.; Korenak, J.; Povodnik, D.; Hélix-Nielsen, C. A feasibility study of ultrafiltration/reverse osmosis (UF/RO)-based wastewater treatment and reuse in the metal finishing industry. J. Clean. Prod. 2015, 101, 292–300. [Google Scholar] [CrossRef]
- Yuzer, B.; Aydin, M.I.; Yildiz, H.; Hasançebi, B.; Selcuk, H.; Kadmi, Y. Optimal performance of electrodialysis process for the recovery of acid wastes in wastewater: Practicing circular economy in aluminium finishing industry. Chem. Eng. J. 2022, 434, 134755. [Google Scholar] [CrossRef]
- Dhokpande, S.R.; Deshmukh, S.M.; Khandekar, A.; Sankhe, A. A review outlook on methods for removal of heavy metal ions from wastewater. Sep. Purif. Technol. 2024, 350, 127868. [Google Scholar] [CrossRef]
- Sharma, P.; Dutta, D.; Udayan, A.; Kumar, S. Industrial wastewater purification through metal pollution reduction employing microbes and magnetic nanocomposites. J. Environ. Chem. Eng. 2021, 9, 106673. [Google Scholar] [CrossRef]
- Zheng, X.; Alam, O.; Zhou, Y.; Du, D.; Li, G.; Zhu, W. Heavy metals detection and removal from contaminated water: A critical review of adsorption methods. J. Environ. Chem. Eng. 2024, 12, 114366. [Google Scholar] [CrossRef]
- Karkou, E.; Teo, C.J.; Savvakis, N.; Poinapen, J.; Arampatzis, G. Industrial circular water use practices through the application of a conceptual water efficiency framework in the process industry. J. Environ. Manag. 2024, 370, 122596. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, J.; Li, E.; Xiong, F.; Zhang, B. Comprehensive benefit analysis of zero-liquid discharge system for cold-rolling wastewater in iron and steel industry. J. Water Process Eng. 2024, 63, 105537. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–50. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 503, 231. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions from Wastewater: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. [Google Scholar] [CrossRef]
- Wu, L.; Garg, S.; Waite, T.D. Progress and challenges in the use of electrochemical oxidation and reduction processes for heavy metals removal and recovery from wastewaters. J. Hazard. Mater. 2024, 479, 135581. [Google Scholar] [CrossRef]
- Barszcz, W.; Łożyńska, M.; Molenda, J. Impact of pyrolysis process conditions on the structure of biochar obtained from apple waste. Sci. Rep. 2024, 14, 10501. [Google Scholar] [CrossRef]
- Sharma, S.; Pathania, S.; Bhagta, S.; Kaushal, N.; Bhardwaj, S.; Bhatia, R.K.; Walia, A. Microbial remediation of polluted environment by using recombinant E. coli: A review. Biotechnol. Environ. 2024, 1, 8. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Nahiun, K.M.; Sarker, B.; Keya, K.N.; Mahir, F.I.; Shahida, S.; Khan, R.A. A Review on the Methods of Industrial Waste Water Treatment. Sci. Rev. 2021, 7, 20–31. [Google Scholar] [CrossRef]
- Garg, R.; Singh, S.K. Treatment technologies for sustainable management of wastewater from iron and steel industry—A review. Environ. Sci. Pollut. Res. 2022, 29, 75203–75222. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Aloulou, H.; Aloulou, W.; Duplay, J.; Baklouti, L.; Dammak, L.; Ben Amar, R. Development of Ultrafiltration Kaolin Membranes over Sand and Zeolite Supports for the Treatment of Electroplating Wastewater. Membranes 2022, 12, 1066. [Google Scholar] [CrossRef]
- Francis, L.; Al-Juboori, R.A.; Khatri, M.; Hilal, N. Nanostructured nanofiltration hollow fiber membranes for metal recovery from industrial wastewater. J. Water Process Eng. 2023, 56, 104281. [Google Scholar] [CrossRef]
- Engstler, R.; Reipert, J.; Karimi, S.; Vukušić, J.L.; Heinzler, F.; Davies, P.; Ulbricht, M.; Barbe, S. A Reverse Osmosis Process to Recover and Recycle Trivalent Chromium from Electroplating Wastewater. Membranes 2022, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Kaya, R.; Yuksekdag, A.; Korkut, S.; Turken, T.; Pasaoglu, M.E.; Ersahin, M.E.; Ozgun, H.; Koyuncu, I. Impact of membrane configuration on the performance and cost of a pilot-scale UF process treating surface water. Sep. Purif. Technol. 2023, 304, 122414. [Google Scholar] [CrossRef]
- Ayach, J.; Malti, W.; Duma, L.; Lalevée, J.; Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, J.; Marczak, M.; Maj, P.; Głowacki, D. The Influence of Vibro-Assisted Abrasive Processing on the Surface Roughness and Sub-Surface Microstructure of Inconel 939 Specimen Made by LPBF. Materials 2023, 16, 7429. [Google Scholar] [CrossRef]
- Bańkowski, D.; Spadło, S. Vibratory Machining Effect on the Properties of the Aluminum Alloys Surface. Arch. Foundry Eng. 2017, 4, 19–24. [Google Scholar] [CrossRef][Green Version]
- Mahata, S.; Shakya, P.; Babu, N.R.; Prakasam, P.K. In-process characterization of surface finish in cylindrical grinding process using vibration and power signals. Procedia CIRP 2020, 88, 335–340. [Google Scholar] [CrossRef]
- Van Tho, N.; Kolganova, E. Increasing the Efficiency of Vibro-Abrasive Treatment of Transport Machine Parts by Combining Processing Media. In Fundamental and Applied Scientific Research in the Development of Agriculture in the Far East (AFE-2022); AFE 2023; Lecture Notes in Networks and Systems; Zokirjon ugli, K.S., Muratov, A., Ignateva, S., Eds.; Springer: Cham, Switzerland, 2023; Volume 706. [Google Scholar] [CrossRef]
- Karakurt, I.; Ho, K.Y.; Ledford, C.; Gamzina, D.; Horn, T.; Luhmann, N.C.; Lin, L. Development of a magnetically driven abrasive polishing process for additively manufactured copper structures. Procedia Manuf. 2018, 26, 798–805. [Google Scholar] [CrossRef]
- Mohammadian, N.; Turenne, S.; Brailovski, V. Surface finish control of additively-manufactured Inconel 625 components using combined chemical-abrasive flow polishing. J. Mater. Process. Technol. 2018, 252, 728–738. [Google Scholar] [CrossRef]
- EN ISO 6878; Water quality—Determination of phosphorus—Ammonium molybdate spectrometric method. International Organization for Standardization: Geneva, Switzerland, 2004.
- EN ISO 11905-1; Water quality—Determination of nitrogen. Part 1: Method using oxidative digestion with peroxodisulphate. International Organization for Standardization: Geneva, Switzerland, 1997.
- ISO 6060; Water quality—Determination of chemical oxygen demand. Dichromate spectrometric method. International Organization for Standardization: Geneva, Switzerland, 1989.
- EN 1484; Water analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). European Committee for Standarization: Brussels, Belgium, 1997.
- ISO 7875-1; Water Quality—Determination of Surfactants. Part 1: Determination of anionic surfactants by measurement of the methylene blue index (MBAS). International Organization for Standardization: Geneva, Switzerland, 1996.
| Parameter/Analyte | Number of Cuvette Test | Applied Method | |
|---|---|---|---|
| Number of Norm | Name of Norm/Method | ||
| ∑P (as PO43−) | LCK 350 LCK 348 | EN ISO 6878 [43] | Determination of phosphorus. Ammonium molybdate spectrometric method |
| ∑N | LCK 338 | EN ISO 11905-1 [44] | Determination of nitrogen. Method using oxidative digestion with peroxodisulphate |
| COD | LCK 514 LCK 914 | ISO 6060 [45] | Determination of chemical oxygen demand. Dichromate spectrometric method. |
| TOC | LCK 387 | EN 1484 [46] | Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC) |
| Anionic Surfactants | LCK 432 | ISO 7875-1 [47] | Determination of anionic surfactants. Methylene Blue (MBA) method. |
| Cationic surfactants | LCK 331 | N/A | Determination of cationic surfactants. Bromophenol Blue method. |
| Nonionic Surfactants | LCK 333 | N/A | Determination of nonionic surfactants. TBPE (tetrabromophenolphthalein ethyl ester) method. |
| Organic Acids | LCK 365 | N/A | Determination of organic acids. Esterification spectrometric method |
| Parameter/Analyte | Unit | Determined Value/Concentration (±SD; n = 3) | |
|---|---|---|---|
| Zn-Al Wastewater | Steel Wastewater | ||
| Dry residue | % | 0.70 ± 0.02 | 3.50 ± 0.05 |
| Conductivity | mS·cm−1 | 2.610 ± 0.002 | 5.940 ± 0.002 |
| Turbidity | NTU | 2640 ± 88 | 5098 ± 2 |
| TSS | g·L−1 | 2.510 ± 0.001 | 4.950 ± 0.004 |
| COD | g·L−1 | 10.7 ± 0.1 | 43.0 ± 0.2 |
| TOC | g·L−1 | 13.6 ± 0.4 | 37.9 ± 0.3 |
| Organic acids | g·L−1 | 1.06 ± 0.01 | 2.53 ± 0.03 |
| ∑P (as PO43−) | mg·L−1 | 5.8 ± 0.1 | 535.0 ± 6.0 |
| ∑N | mg·L−1 | 91 ± 1 | 1160 ± 27 |
| Anionic surfactants | mg·L−1 | 314 ± 14 | 1085 ± 25 |
| Cationic surfactants | mg·L−1 | 0.51 ± 0.01 | 5.04 ± 0.14 |
| Non-ionic surfactants | mg·L−1 | 119 ± 2 | 377 ± 8 |
| Parameter/Analyte | Unit | Zn-Al Wastewater | Steel Wastewater | ||
|---|---|---|---|---|---|
| Determined Value in Permeate (±SD; n = 3) | Total Rejection Coefficient | Determined Value in Permeate (±SD; n = 3) | Total Rejection Coefficient | ||
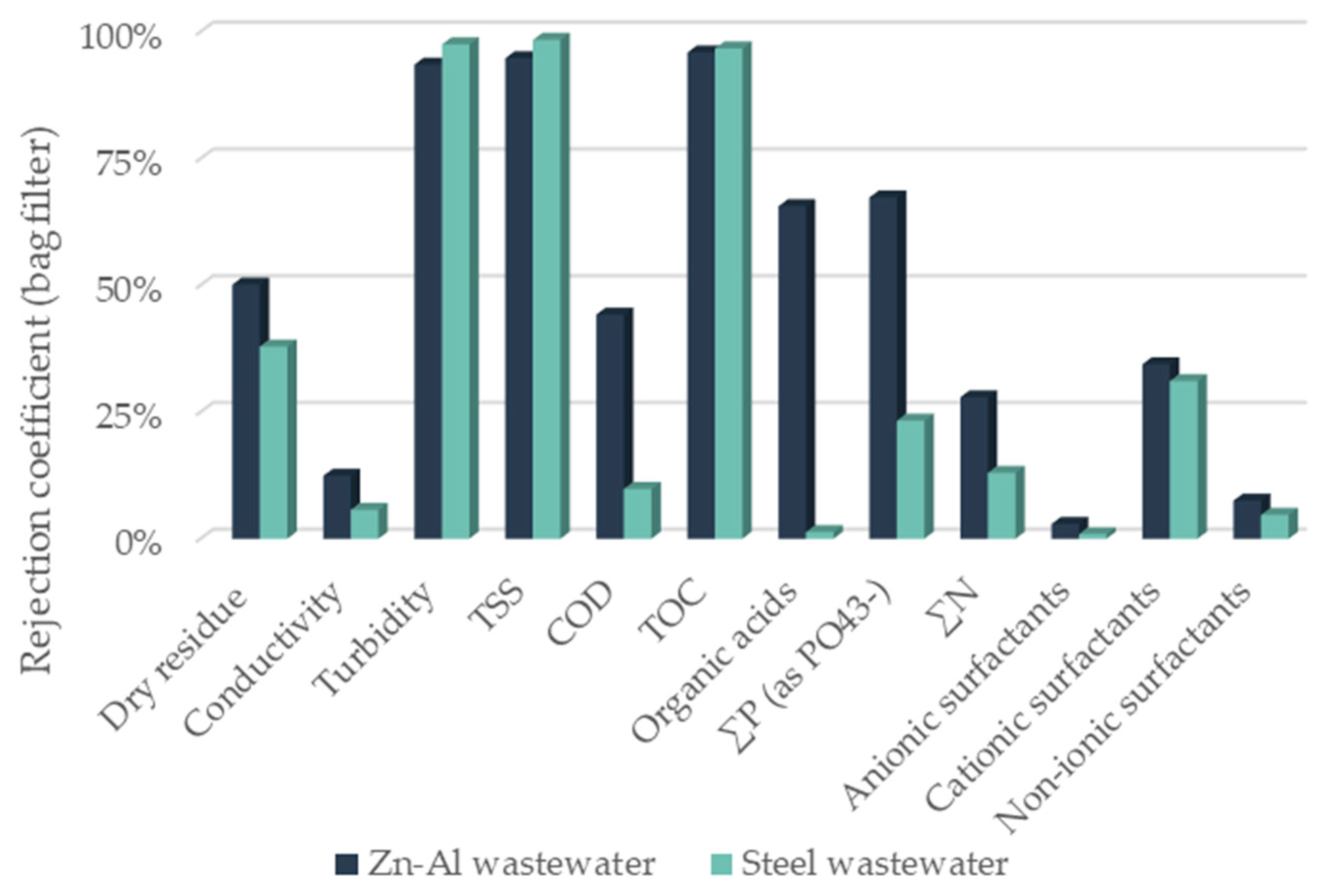
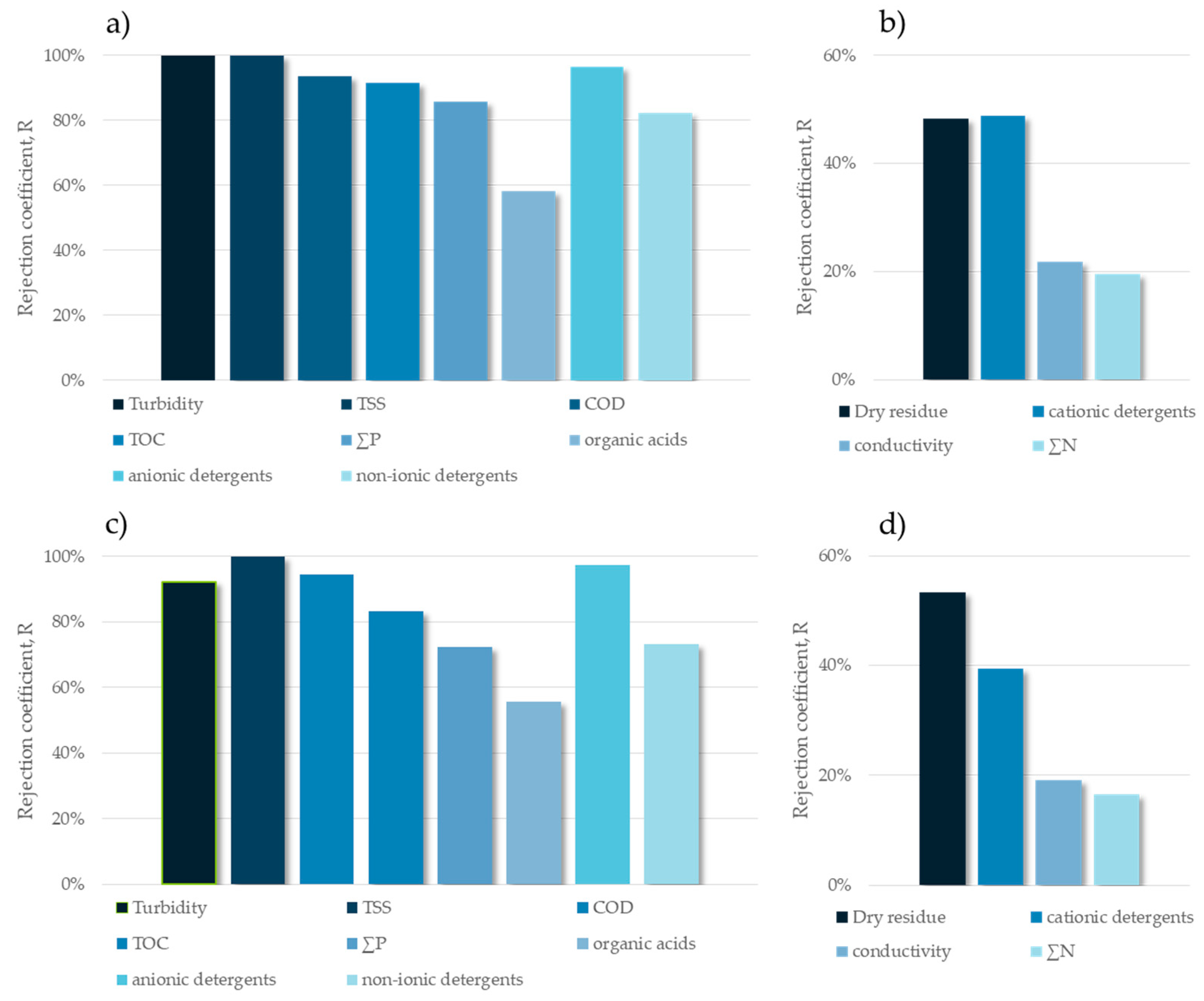
| Dry residue | % | 0.18 ± 0.01 | 73.8% | 0.98 ± 0.02 | 72.1% |
| Conductivity | µS·cm−1 | 1663 ± 1 | 36.2% | 4352 ± 2 | 26.8% |
| Turbidity | NTU | 0.25 ± 0.09 | 100% (99.99%) | 4.30 ± 0.16 | 99.9% |
| TSS | mg·L−1 | 0 ± 0 | 100% | 0 ± 0 | 100% |
| COD | mg·L−1 | 355 ± 9 | 96.7% | 2170 ± 52 | 95.0% |
| TOC | mg·L−1 | 26.7 ± 1.6 | 99.8% | 163.0 ± 4.6 | 99.6% |
| Organic acids | mg·L−1 | 143 ± 3 | 86.5% | 1017 ± 40 | 59.8% |
| ∑P (as PO43−) | mg·L−1 | 0.12 ± 0.01 | 98.0% | 105.5 ± 1.5 | 80.3% |
| ∑N | mg·L−1 | 49.8 ± 1.0 | 45.0% | 960.0 ± 9.2 | 17.3% |
| Anionic surfactants | mg·L−1 | 10.5 ± 0.9 | 96.6% | 22.2 ± 2.9 | 98.0% |
| Cationic surfactants | mg·L−1 | 0.16 ± 0.01 | 69.0% | 3.12 ± 0.10 | 38.0% |
| Non-ionic surfactants | mg·L−1 | 18.4 ± 1.1 | 84.6% | 88.3 ± 2.2 | 76.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska, J.; Rajewska, P. Development of a Concept for Closing the Water Cycle in the Surface Treatment of Ferrous and Non-Ferrous Metals. Sustainability 2025, 17, 2212. https://doi.org/10.3390/su17052212
Janiszewska J, Rajewska P. Development of a Concept for Closing the Water Cycle in the Surface Treatment of Ferrous and Non-Ferrous Metals. Sustainability. 2025; 17(5):2212. https://doi.org/10.3390/su17052212
Chicago/Turabian StyleJaniszewska, Jolanta, and Paulina Rajewska. 2025. "Development of a Concept for Closing the Water Cycle in the Surface Treatment of Ferrous and Non-Ferrous Metals" Sustainability 17, no. 5: 2212. https://doi.org/10.3390/su17052212
APA StyleJaniszewska, J., & Rajewska, P. (2025). Development of a Concept for Closing the Water Cycle in the Surface Treatment of Ferrous and Non-Ferrous Metals. Sustainability, 17(5), 2212. https://doi.org/10.3390/su17052212







