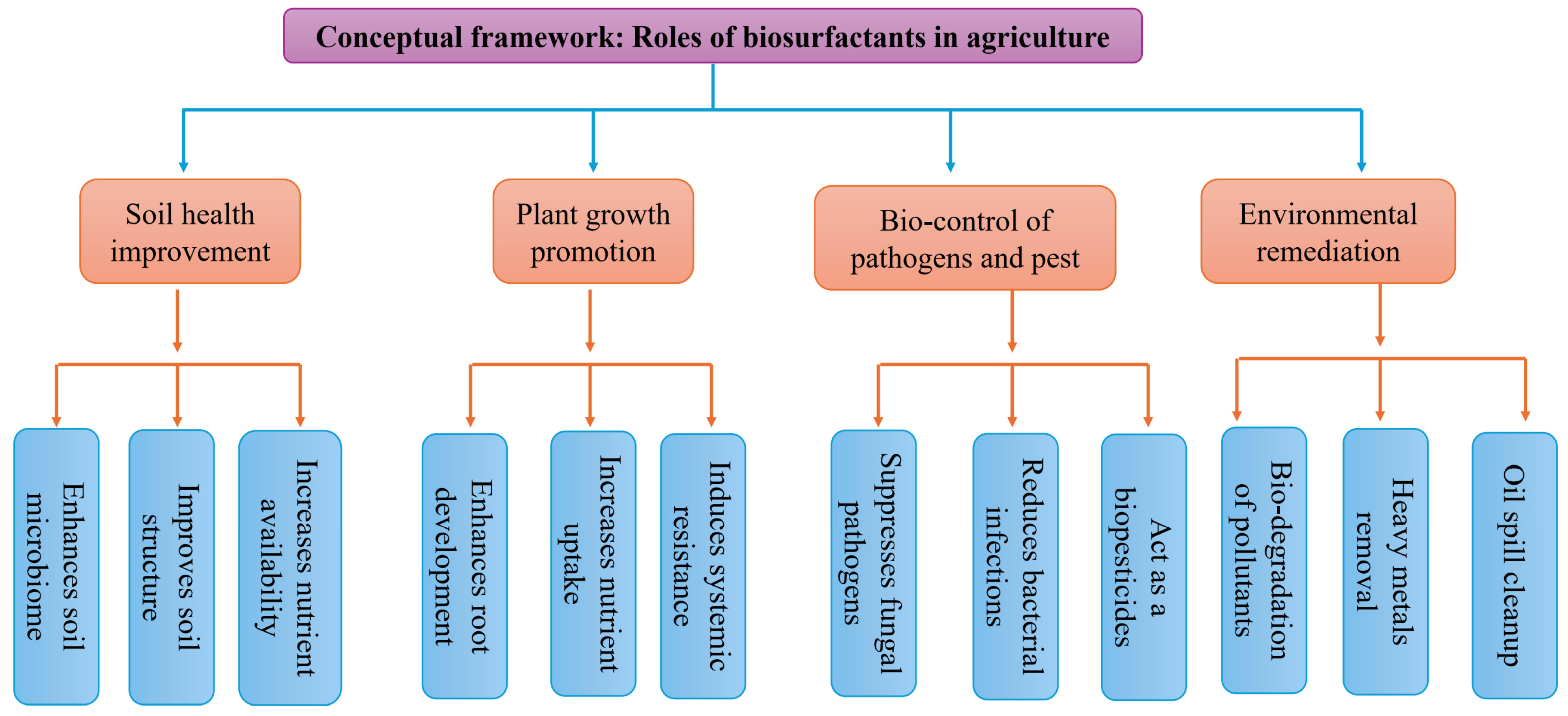
Unlocking the Potential of Biosurfactants in Agriculture: Novel Applications and Future Directions
Abstract
1. Introduction
2. Definition, Classification, and Structure of Microbial Surfactants
2.1. Glycolipids
2.2. Lipopeptides
2.3. Phospholipids
2.4. Fatty Acids
2.5. Polymeric Biosurfactants
3. Screening, Extraction, Purification, and Assessment of Biosurfactant Activity
3.1. Screening Methods for Biosurfactants
3.2. Extraction of Biosurfactants
3.3. Purification of Biosurfactants
3.4. Assessment of Biosurfactant Activity
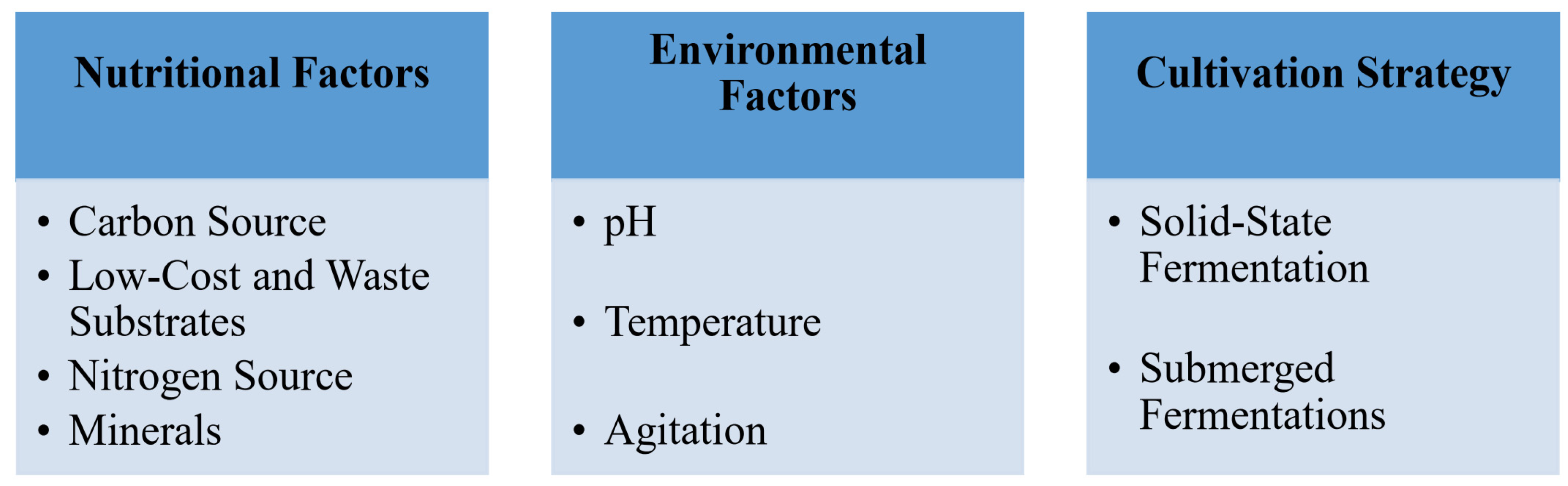
4. Factors Influencing Biosurfactant Production
4.1. Nutritional Factors
4.1.1. Carbon Source
4.1.2. Abundant and Cost-Effective Substrates
4.1.3. Nitrogen Source
4.1.4. Mineral Components
4.1.5. Environmental Factors
4.1.6. Cultivation Strategy
4.2. Molecular Characteristics of Biosurfactants
5. The Role of Biosurfactants in Bioremediation
5.1. Biosurfactants and Hydrocarbons
Petroleum Consuming Microorganisms
5.2. Biosurfactants and Heavy Metals
5.2.1. Microbial Remediation Techniques
Bio-Stimulation
Bio-Augmentation
Engineered Microbial Remediation
6. Biosurfactants in Biodegradation and Agricultural Waste Management
6.1. Role of Biosurfactants in Biodegradation Processes
6.2. Use of Biosurfactants in Bioconversion of Agricultural Waste
6.3. Case Studies of Successful Biosurfactant Applications in Biodegradation and Agricultural Waste Management
7. Biosurfactants in Soil Nutrient Availability and Soil Quality Improvement
7.1. Effects of Biosurfactants on Soil Nutrient Availability
7.2. Use of Biosurfactants to Improve Soil Quality
8. Biosurfactants as Pesticides and Soil Hydrophilization Agents
8.1. Use of Biosurfactants as Pesticides
8.2. Biosurfactants and Biological Control Agents Against Nematodes
8.3. Effects of Biosurfactants on Plant Pathogen Elimination
8.3.1. Biosurfactants and Biological Control Agents Against Fungi
8.3.2. Biosurfactants and Biological Control Agents Against Bacteria
8.4. Biosurfactant and Biological Control of Post-Harvest Diseases
8.5. Use of Biosurfactants for Soil Hydrophilization
8.6. Case Studies of Successful Biosurfactant Applications as Pesticides and Soil Hydrophilization Agents
9. Issues with Biosurfactant Application
9.1. Limitations in Discovery and Optimization
9.2. Toxicity and Environmental Safety Concerns
9.3. Economic and Industrial Challenges
10. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayeri, S.; Dehghanian, Z.; Lajayer, B.A.; Thomson, A.; Astatkie, T.; Price, G. CRISPR/Cas9-Mediated genetically edited ornamental and aromatic plants: A promising technology in phytoremediation of heavy metals. J. Clean. Prod. 2023, 428, 139512. [Google Scholar] [CrossRef]
- Devi, A.; Hansa, A.; Gupta, H.; Syam, K.; Upadhyay, M.; Kaur, M.; Lajayer, B.A.; Sharma, R. Microplastics as an emerging menace to environment: Insights into their uptake, prevalence, fate, and sustainable solutions. Environ. Res. 2023, 229, 115922. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Gautam, K.; Tyagi, V. Microbial surfactants: A review. J. Oleo Sci. 2006, 55, 155–166. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Fenibo, E.O.; Douglas, S.I.; Stanley, H.O. A review on microbial surfactants: Production, classifications, properties and characterization. J. Adv. Microbiol. 2019, 18, 1–22. [Google Scholar] [CrossRef]
- Huang, X.; Tang, X.; Liao, A.; Sun, W.; Lei, L.; Wu, J. Application of Cyclopropane with Triangular Stable Structure in Pesticides. J. Mol. Struct. 2025, 1326, 141171. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, S.; Sun, W.; Tu, H.; Tang, Y.; Xu, Y.; Guo, R.; Zhao, Z.; Yang, Z.; Wu, J. Synthesis of 4 H-Pyrazolo [3, 4-d] pyrimidin-4-one Hydrazine Derivatives as a Potential Inhibitor for the Self-Assembly of TMV Particles. J. Agric. Food Chem. 2024, 72, 2879–2887. [Google Scholar] [CrossRef]
- Adetunji, C.O. Applications of Biosurfactant in Agriculture; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Aboutorabi, M. A review on the biological control of plant diseases using various microorganisms. J. Res. Med. Dent. Sci. 2018, 6, 30–35. [Google Scholar]
- de Medeiros, A.O.; da Silva, M.d.G.C.; Converti, A.; de Almeida, F.C.G.; Sarubbo, L.A. Development of Natural Fungicidal Agricultural Defensives Using Microbial Glycolipid and Vegetable Oil Blends. Surfaces 2024, 7, 879–897. [Google Scholar] [CrossRef]
- Ali, Q.; Akhtar, T.; Zuhra, N.; Syarbiah, S.; Ega, A.A. Biotechnology for sustainable agriculture: Innovations in disease management. Plant Prot. 2024, 8, 803–816. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 122316. [Google Scholar] [CrossRef] [PubMed]
- Rajasimman, M.; Suganya, A.; Manivannan, P.; Pandian, A.M.K. Utilization of agroindustrial wastes with a high content of protein, carbohydrates, and fatty acid used for mass production of biosurfactant. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 127–146. [Google Scholar]
- Dhuldhaj, U.P.; Sayyed, R.; Bora, C.R. Application of Microbial Biosurfactants and Factors Needed for their Production: An Overview. In Microbial Surfactants in Pharmaceuticals and Cosmetics; CRC Press: Boca Raton, FL, USA, 2025; pp. 1–25. [Google Scholar]
- Valkenburg, A.D.; Teke, G.M.; van Rensburg, E.; Pott, R.W. Bioprocess development for microbial production and purification of cellobiose lipids by the smut fungus Ustilago maydis DSM 4500. Bioprocess Biosyst. Eng. 2025. [Google Scholar] [CrossRef] [PubMed]
- Nghia, N.K.; Thy, C.T.A.; Oanh, N.T.K.; Morton, L.W.; Demyan, M.S.; Tran, H.-T.; Giao, D.H.; Vien, D.M.; Toan, V.N.; Tecimen, H.B. Isolation of biosurfactant-producing bacteria from dioxin-contaminated soil and their biodegradation capacity to dibenzofuran. Biocatal. Agric. Biotechnol. 2025, 64, 103490. [Google Scholar] [CrossRef]
- Mehta, N.; Kaur, J.; Cameotra, S.S.; Mehta, S.K. Biosurfactants: A Viable Approach Towards Environmental Sustainability. In Role of Science and Technology for Sustainable Future: Volume 2—Applied Sciences and Technologies; Springer: Singapore, 2025; pp. 75–99. [Google Scholar]
- Kaleramana, P.; Sangwan, S.; Swami, P.; Kumar, M.; Singh, S.; Kumar, K. Biosurfactant-Mediated Synthesis of Nanosilver and Its Antagonistic Activity Towards Microbial Phytopathogens of Tomato (Solanum lycopersicum L.) Crop. BioNanoScience 2025, 15, 176. [Google Scholar] [CrossRef]
- Zamorano-González, C.A.; Ramírez-Trujillo, J.A.; Pilotzi-Xahuentitla, H.; Yáñez-Ocampo, G.; Hernández-Nuñéz, E.; Suárez-Rodríguez, R.; Orea-Flores, M.L.A.; Gómez-Rodríguez, O.; Espinosa-Zaragoza, S.; Rangel-Zaragoza, J.L. In Vitro Evaluation of the Biosurfactant Produced by Serratia ureilytica UTS with Antifungal and Nematicidal Activity Against Nacobbus aberrans. Curr. Microbiol. 2025, 82, 63. [Google Scholar] [CrossRef] [PubMed]
- Al-Araji, L.I.Y.; Rahman, R.N.Z.R.A.; Basri, M.; Salleh, A.B. Optimisation of rhamnolipids produced by Pseudomonas aeruginosa 181 using Response Surface Modeling. Ann. Microbiol. 2007, 57, 571–575. [Google Scholar] [CrossRef]
- Benincasa, M.; Contiero, J.; Manresa, M.; Moraes, I. Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J. Food Eng. 2002, 54, 283–288. [Google Scholar] [CrossRef]
- Hayes, D.G.; Solaiman, D.K.; Ashby, R.D. Biobased surfactants: Synthesis, properties, and applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Jamal, P.; Mir, S.; Alam, M.Z.; Nawawi, W.M.F.W. Isolation and selection of new biosurfactant producing bacteria from degraded palm kernel cake under liquid state fermentation. J. Oleo Sci. 2014, 63, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Abo Elsoud, M.M.A. Classification and production of microbial surfactants. In Microbial Biosurfactants: Preparation, Properties and Applications; Springer: Singapore, 2021; pp. 65–89. [Google Scholar]
- Pardhi, D.S.; Panchal, R.R.; Raval, V.H.; Joshi, R.G.; Poczai, P.; Almalki, W.H.; Rajput, K.N. Microbial surfactants: A journey from fundamentals to recent advances. Front. Microbiol. 2022, 13, 982603. [Google Scholar] [CrossRef]
- Christofi, N.; Ivshina, I. Microbial surfactants and their use in field studies of soil remediation. J. Appl. Microbiol. 2002, 93, 915–929. [Google Scholar] [CrossRef]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Han, Y.S. Biosurfactants: Production and potential application in insect pest management. Trends Entomol. 2018, 14, 79. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants: Minireview. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaabouni, S.; Ghribi, D. Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Shoeb, E.; Akhlaq, F.; Badar, U.; Akhter, J.; Imtiaz, S. Classification and industrial applications of biosurfactants. Acad. Res. Int. 2013, 4, 243. [Google Scholar]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Juang, R.-S.; Wei, Y.-H. Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem. Eng. J. 2015, 103, 158–169. [Google Scholar] [CrossRef]
- Rahman, P.K.; Gakpe, E. Production, characterisation and applications of biosurfactants—Review. Biotechnology 2008, 7, 360–370. [Google Scholar] [CrossRef]
- Das, N.; Mandal, S.K.; Das, D.; Madhavan, J.; Selvi, A. Recent Updates on the Role of Biosurfactants forRemediation of Various Pollutants. In Rhizomicrobiome Dynamics in Bioremediation; CRC Press: Boca Raton, FL, USA, 2021; pp. 180–197. [Google Scholar]
- Karlapudi, A.P.; Venkateswarulu, T.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Ramu Dirisala, V.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Saravanan, V. Biosurfactants-types, sources and applications. Res. J. Microbiol. 2015, 10, 181–192. [Google Scholar]
- Dhail, S. Isolation of potent biosurfactant producing bacteria from oil spilled marine water and marine sediments. Afr. J. Biotechnol. 2012, 11, 16751–16757. [Google Scholar]
- Saharan, B.; Sahu, R.; Sharma, D. A review on biosurfactants: Fermentation, current developments and perspectives. Genet. Eng. Biotechnol. J. 2011, 2011, 1–14. [Google Scholar]
- Raval, M.; Gund, S.; Shah, N.; Desai, R. Isolation and characterization of biosurfactant producing bacteria and their application as an antibacterial agent. Int. J. Pharma Bio Sci. 2017, 8, 302–310. [Google Scholar] [CrossRef]
- Hashemi, S.Z.; Fooladi, J.; Ebrahimipour, G.; Khodayari, S. Isolation and identification of crude oil degrading and biosurfactant producing bacteria from the oil-contaminated soils of Gachsaran. Appl. Food Biotechnol. 2016, 3, 83–89. [Google Scholar]
- Mahalingam, P.; Sampath, N. Isolation, characterization and identification of bacterial biosurfactant. Eur. J. Exp. Biol. 2014, 4, 59–64. [Google Scholar]
- Elazzazy, A.M.; Abdelmoneim, T.; Almaghrabi, O. Isolation and characterization of biosurfactant production under extreme environmental conditions by alkali-halo-thermophilic bacteria from Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 466–475. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef]
- Maheswari, N.; Parveen, L.F. Comparative study of biosurfactant by using Bacillus licheniformis and Trichoderma viride from paper waste contaminated soil. Int. J. Chem. Sci. 2012, 10, 1687–1697. [Google Scholar]
- Garfin, D.E. Isoelectric focusing. In Separation Science and Technology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 2, pp. 263–298. [Google Scholar]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13. [Google Scholar] [CrossRef]
- Raza, Z.A.; Rehman, A.; Khan, M.S.; Khalid, Z.M. Improved production of biosurfactant by a Pseudomonas aeruginosa mutant using vegetable oil refinery wastes. Biodegradation 2007, 18, 115–121. [Google Scholar] [CrossRef]
- Nurfarahin, A.H.; Mohamed, M.S.; Phang, L.Y. Culture medium development for microbial-derived surfactants production—An overview. Molecules 2018, 23, 1049. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, G.; Cameotra, S.S.; Chopra, H.K. Biosurfactants from fungi: A review. J. Pet. Environ. Biotechnol. 2013, 4, 160. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 2011, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Epstein, W. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 2003, 75, 293–320. [Google Scholar]
- Thaniyavarn, J.; Chongchin, A.; Wanitsuksombut, N.; Thaniyavarn, S.; Pinphanichakarn, P.; Leepipatpiboon, N.; Morikawa, M.; Kanaya, S. Biosurfactant production by Pseudomonas aeruginosa A41 using palm oil as carbon source. J. Gen. Appl. Microbiol. 2006, 52, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Thavasi, R.; Jayalakshmi, S.; Balasubramanian, T.; Banat, I.M. Production and characterization of a glycolipid biosurfactant from Bacillus megaterium using economically cheaper sources. World J. Microbiol. Biotechnol. 2008, 24, 917–925. [Google Scholar] [CrossRef]
- Auhim, H.S.; Mohamed, A.I. Effect of different environmental and nutritional factors on biosurfactant production from Azotobacter chroococcum. Int. J. Adv. Pharm. Biol. Chem. 2013, 2, 477–481. [Google Scholar]
- Jagtap, S.; Yavankar, S.; Pardesi, K.; Chopade, B. Production of bioemulsifier by Acinetobacter species isolated from healthy human skin. Indian J. Exp. Biol. 2010, 48, 70–76. [Google Scholar]
- Morais, I.; Cordeiro, A.; Teixeira, G.; Domingues, V.; Nardi, R.; Monteiro, A.; Alves, R.; Siqueira, E.; Santos, V. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P 6A and Lactobacillus gasseri P 65. Microb. Cell Factories 2017, 16, 155. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.K.; Kant, C.; Verma, H.; Kumar, D.; Singh, P.P.; Modi, A.; Droby, S.; Kesawat, M.S.; Alavilli, H. Microbial biosurfactant: A new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants 2021, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, K.; De Dobbelaere, I.; Sarrazyn, R.; Höfte, M. Control of Phytophthora cryptogea in the hydroponic forcing of witloof chicory with the rhamnolipid-based biosurfactant formulation PRO1. Plant Pathol. 2005, 54, 219–226. [Google Scholar] [CrossRef]
- Debode, J.; Maeyer, K.D.; Perneel, M.; Pannecoucque, J.; Backer, G.D.; Höfte, M. Biosurfactants are involved in the biological control of Verticillium microsclerotia by Pseudomonas spp. J. Appl. Microbiol. 2007, 103, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, M.; Bergstrand, K.-J.; Khalil, S.; Alsanius, B. Production of biosurfactants and antibiotics by fluorescent pseudomonads isolated from a closed hydroponic system equipped with a slow filter. Antonie van Leeuwenhoek 2008, 93, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Handique, P.J.; Deka, S. Rhamnolipid biosurfactant against Fusarium sacchari—The causal organism of pokkah boeng disease of sugarcane. J. Basic Microbiol. 2014, 54, 548–557. [Google Scholar] [CrossRef]
- Soltani Dashtbozorg, S.; Miao, S.; Ju, L.K. Rhamnolipids as environmentally friendly biopesticide against plant pathogen Phytophthora sojae. Environ. Prog. Sustain. Energy 2016, 35, 169–173. [Google Scholar] [CrossRef]
- Monnier, N.; Cordier, M.; Dahi, A.; Santoni, V.; Guénin, S.; Clément, C.; Sarazin, C.; Penaud, A.; Dorey, S.; Cordelier, S. Semipurified rhamnolipid mixes protect Brassica napus against Leptosphaeria maculans early infections. Phytopathology 2020, 110, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Gibbs, B.F. Types, production and applications of biosurfactants. Proc. Indian Natl. Sci. Acad. Part. B Rev. Tracts Biol. Sci. 2004, 70, 31–55. [Google Scholar]
- Chaprão, M.J.; Ferreira, I.N.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Moldes, A.B.; Paradelo, R.; Rubinos, D.; Devesa-Rey, R.; Cruz, J.M.; Barral, M.T. Ex situ treatment of hydrocarbon-contaminated soil using biosurfactants from Lactobacillus pentosus. J. Agric. Food Chem. 2011, 59, 9443–9447. [Google Scholar] [CrossRef]
- Lai, C.-C.; Huang, Y.-C.; Wei, Y.-H.; Chang, J.-S. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 2009, 167, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.G.; Nitschke, M.; Lépine, F.; Déziel, E.; Contiero, J. Structure, properties and applications of rhamnolipids produced by Pseudomonas aeruginosa L2-1 from cassava wastewater. Process Biochem. 2010, 45, 1511–1516. [Google Scholar] [CrossRef]
- Ferradji, F.Z.; Mnif, S.; Badis, A.; Rebbani, S.; Fodil, D.; Eddouaouda, K.; Sayadi, S. Naphthalene and crude oil degradation by biosurfactant producing Streptomyces spp. isolated from Mitidja plain soil (North of Algeria). Int. Biodeterior. Biodegrad. 2014, 86, 300–308. [Google Scholar] [CrossRef]
- Aşçı, Y.; Nurbaş, M.; Açıkel, Y.S. A comparative study for the sorption of Cd (II) by soils with different clay contents and mineralogy and the recovery of Cd (II) using rhamnolipid biosurfactant. J. Hazard. Mater. 2008, 154, 663–673. [Google Scholar] [CrossRef]
- Singh, P.; Cameotra, S.S. Enhancement of metal bioremediation by use of microbial surfactants. Biochem. Biophys. Res. Commun. 2004, 319, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Aşçı, Y.; Nurbaş, M.; Açıkel, Y.S. Investigation of sorption/desorption equilibria of heavy metal ions on/from quartz using rhamnolipid biosurfactant. J. Environ. Manag. 2010, 91, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.; Brasileiro, P.; Silveira, G.; Luna, J.; Rufino, R. Application of a low cost biosurfactant in the removal of heavy metals in soil. Chem. Eng. Trans. 2018, 64, 433–438. [Google Scholar]
- Chen, W.-J.; Hsiao, L.-C.; Chen, K.K.-Y. Metal desorption from copper (II)/nickel (II)-spiked kaolin as a soil component using plant-derived saponin biosurfactant. Process Biochem. 2008, 43, 488–498. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Klimiuk, E. Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere 2012, 86, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Homaei, A.; Patil, S.; Daverey, A. Microbial biosurfactants for oil spill remediation: Pitfalls and potentials. Appl. Microbiol. Biotechnol. 2019, 103, 27–37. [Google Scholar] [CrossRef]
- Moutinho, L.F.; Moura, F.R.; Silvestre, R.C.; Romão-Dumaresq, A.S. Microbial biosurfactants: A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnol. Prog. 2021, 37, e3093. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Hua, Y.; Chen, J.; Zhang, H.; Wang, H. Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl. Microbiol. Biotechnol. 2017, 101, 8309–8319. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Mazeyrat-Gourbeyre, F.; Baillieul, F.; Clément, C.; Ongena, M. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 2020, 8, 1014. [Google Scholar] [CrossRef]
- Stanaway, I.B.; Wallace, J.C.; Shojaie, A.; Griffith, W.C.; Hong, S.; Wilder, C.S.; Green, F.H.; Tsai, J.; Knight, M.; Workman, T. Human oral buccal microbiomes are associated with farmworker status and azinphos-methyl agricultural pesticide exposure. Appl. Environ. Microbiol. 2017, 83, e02149-16. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, D. Role of Biosurfactants in Agriculture and Soil Reclamation. In Biosurfactants: Greener Surface Active Agents for Sustainable Future: Microbial Surfactants; Springer: Singapore, 2021; pp. 145–174. [Google Scholar]
- Dalton, K.R.; Lee, M.; Wang, Z.; Zhao, S.; Parks, C.G.; Beane-Freeman, L.E.; Motsinger-Reif, A.A.; London, S.J. Occupational farm work activities influence workers’ indoor home microbiome. Environ. Res. 2024, 243, 117819. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Marecik, R.; Chrzanowski, Ł. Contributions of biosurfactants to natural or induced bioremediation. Appl. Microbiol. Biotechnol. 2013, 97, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.M.; Soberon-Chavez, G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl. Microbiol. Biotechnol. 2000, 54, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.; Karlson, U. Evaluation of bacterial strategies to promote the bioavailability of polycyclic aromatic hydrocarbons. Appl. Microbiol. Biotechnol. 2004, 63, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mukherji, S.; Mukherji, S. Surface hydrophobicity of petroleum hydrocarbon degrading Burkholderia strains and their interactions with NAPLs and surfaces. Colloids Surf. B Biointerfaces 2010, 78, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Brooijmans, R.J.; Pastink, M.I.; Siezen, R.J. Hydrocarbon-degrading bacteria: The oil-spill clean-up crew. Microb. Biotechnol. 2009, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Germida, J.; Frick, C.; Farrell, R. Phytoremediation of oil-contaminated soils. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2002; Volume 28, pp. 169–186. [Google Scholar]
- Tortella, G.R.; Diez, M.C.; Durán, N. Fungal diversity and use in decomposition of environmental pollutants. Crit. Rev. Microbiol. 2005, 31, 197–212. [Google Scholar] [CrossRef]
- Mulligan, C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef]
- Sun, W.; Cheng, K.; Sun, K.Y.; Ma, X. Microbially mediated remediation of contaminated sediments by heavy metals: A critical review. Curr. Pollut. Rep. 2021, 7, 201–212. [Google Scholar] [CrossRef]
- Raklami, A.; Meddich, A.; Oufdou, K.; Baslam, M. Plants—Microorganisms-based bioremediation for heavy metal cleanup: Recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int. J. Mol. Sci. 2022, 23, 5031. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization based remediation of As (III) contaminated soil by Sporosarcina ginsengisoli. J. Hazard. Mater. 2012, 201, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.A.; Rajpoot, I.K.; Gajjar, B.; Sachdeva, A. Comparative study of heavy metal bioremediation in soil by Bacillus subtilis and Saccharomyces cerevisiae. Indian J. Sci. Technol. 2016, 9. [Google Scholar] [CrossRef]
- Saha, L.; Tiwari, J.; Bauddh, K.; Ma, Y. Recent developments in microbe–plant-based bioremediation for tackling heavy metal-polluted soils. Front. Microbiol. 2021, 12, 731723. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Joia, J.; Sood, A.; Sood, R.; Sidhu, C.; Kaur, G. Microbes as potential tool for remediation of heavy metals: A review. J. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar] [CrossRef]
- Fauziah, S.; Agamuthu, P.; Hashim, R.; Izyani, A.; Emenike, C. Assessing the bioaugmentation potentials of individual isolates from landfill on metal-polluted soil. Environ. Earth Sci. 2017, 76, 40. [Google Scholar] [CrossRef]
- Pant, G.; Garlapati, D.; Agrawal, U.; Prasuna, R.G.; Mathimani, T.; Pugazhendhi, A. Biological approaches practised using genetically engineered microbes for a sustainable environment: A review. J. Hazard. Mater. 2021, 405, 124631. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Parthipan, P.; Huang, M.; Muthukumar, B.; Cheng, L.; Govarthanan, M.; Rajasekar, A. Enhanced biodegradation of hydrophobic organic pollutants by the bacterial consortium: Impact of enzymes and biosurfactants. Environ. Pollut. 2021, 289, 117956. [Google Scholar] [CrossRef]
- Koutinas, M.; Kyriakou, M.; Andreou, K.; Hadjicharalambous, M.; Kaliviotis, E.; Pasias, D.; Kazamias, G.; Varavvas, C.; Vyrides, I. Enhanced biodegradation and valorization of drilling wastewater via simultaneous production of biosurfactants and polyhydroxyalkanoates by Pseudomonas citronellolis SJTE-3. Bioresour. Technol. 2021, 340, 125679. [Google Scholar] [CrossRef] [PubMed]
- Hentati, D.; Chebbi, A.; Mahmoudi, A.; Hadrich, F.; Cheffi, M.; Frikha, I.; Sayadi, S.; Chamkha, M. Biodegradation of hydrocarbons and biosurfactants production by a newly halotolerant Pseudomonas sp. strain isolated from contaminated seawater. Biochem. Eng. J. 2021, 166, 107861. [Google Scholar] [CrossRef]
- Yalaoui-Guellal, D.; Fella-Temzi, S.; Djafri-Dib, S.; Brahmi, F.; Banat, I.M.; Madani, K. Biodegradation potential of crude petroleum by hydrocarbonoclastic bacteria isolated from Soummam wadi sediment and chemical-biological proprieties of their biosurfactants. J. Pet. Sci. Eng. 2020, 184, 106554. [Google Scholar] [CrossRef]
- Aransiola, S.; Ayams, J.; Abioye, O. Production of Biosurfactants Using Pseudomonas aeruginosa for Biodegradation of Herbicide. Int. J. Biotechnol. 2019, 8, 66–74. [Google Scholar]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.-S.; Ng, H.Y.; Wong, J.W.; Kim, S.-H. Production of biosurfactants from agro-industrial waste and waste cooking oil in a circular bioeconomy: An overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Modi, A.; Minipara, D.; Kumar, A. Microbial biosurfactants in management of organic waste. In Sustainable Environmental Clean-Up; Elsevier: Amsterdam, The Netherlands, 2021; pp. 211–230. [Google Scholar]
- Sharma, M.; Rathore, A.; Sharma, S.; Sadhu, V.; Reddy, K.R. Biosurfactants: Production Methods, Properties and Their Applications in Food Industry. In Sustainable Nanomaterials for Treatment and Diagnosis of Infectious Diseases; Scrivener Publishing LLC: Beverly, MA, USA, 2025; pp. 365–392. [Google Scholar]
- Yu, K.; Chai, B.; Zhuo, T.; Tang, Q.; Gao, X.; Wang, J.; He, L.; Lei, X.; Chen, B. Hydrostatic pressure drives microbe-mediated biodegradation of microplastics in surface sediments of deep reservoirs: Novel findings from hydrostatic pressure simulation experiments. Water Res. 2023, 242, 120185. [Google Scholar] [CrossRef] [PubMed]
- Tekere, M. Biosurfactant application and bioaugmentation for effective bioremediation of contaminated environment. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 323–339. [Google Scholar]
- Simões, L.A.; Fernandes, N.A.T.; dos Santos Junior, N.A.; Dias, D.R. Biosurfactants and their benefits for seeds. In Advancements in Biosurfactants Research; Springer: Cham, Switzerland, 2023; pp. 309–329. [Google Scholar]
- Patel, P.; Patel, R.; Mukherjee, A.; Munshi, N.S. Microbial biosurfactants for green agricultural technology. In Sustainable Agriculture Reviews 60: Microbial Processes in Agriculture; Springer: Cham, Switzerland, 2023; pp. 389–413. [Google Scholar]
- Daxini, A.; O’Donoghue, C.; Ryan, M.; Buckley, C.; Barnes, A.P.; Daly, K. Which factors influence farmers’ intentions to adopt nutrient management planning? J. Environ. Manag. 2018, 224, 350–360. [Google Scholar] [CrossRef]
- Bretzel, F.; Caudai, C.; Tassi, E.; Rosellini, I.; Scatena, M.; Pini, R. Culture and horticulture: Protecting soil quality in urban gardening. Sci. Total Environ. 2018, 644, 45–51. [Google Scholar] [CrossRef]
- Sah, D.; Rai, J.; Ghosh, A.; Chakraborty, M. A review on biosurfactant producing bacteria for remediation of petroleum contaminated soils. 3 Biotech 2022, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, D.; Elleuch, M.; Abdelkefi, L.; Ellouze-Chaabouni, S. Evaluation of larvicidal potency of Bacillus subtilis SPB1 biosurfactant against Ephestia kuehniella (Lepidoptera: Pyralidae) larvae and influence of abiotic factors on its insecticidal activity. J. Stored Prod. Res. 2012, 48, 68–72. [Google Scholar] [CrossRef]
- Yun, D.C.; Yang, S.Y.; Kim, Y.C.; Kim, I.S.; Kim, Y.H. Identification of surfactin as an aphicidal metabolite produced by Bacillus amyloliquefaciens G1. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 751–753. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, M.R.; Walia, R. Crop loss estimations due to plant-parasitic nematodes in major crops in India. Natl. Acad. Sci. Lett. 2020, 43, 409–412. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.; Askary, T.H. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest Control 2018, 28, 74. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, X.; Luo, L.; Ni, Y.; Yao, L.; Ni, W. Biostimulants in bioconversion compost of organic waste: A novel booster in sustainable agriculture. J. Clean. Prod. 2021, 319, 128704. [Google Scholar] [CrossRef]
- Kiran, G.S.; Hema, T.; Gandhimathi, R.; Selvin, J.; Thomas, T.A.; Ravji, T.R.; Natarajaseenivasan, K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B Biointerfaces 2009, 73, 250–256. [Google Scholar] [CrossRef] [PubMed]
- NR, A.S.; Luna, M.; Santiago, A.L.; Franco, L.O.; Silva, G.; de Souza, P.M.; Okada, K.; Albuquerque, C.; da Silva, C.; Campos-Takaki, G.M. Biosurfactant-and-bioemulsifier produced by a promising Cunninghamella echinulata isolated from Caatinga soil in the northeast of Brazil. Int. J. Mol. Sci. 2014, 15, 15377–15395. [Google Scholar] [CrossRef] [PubMed]
- Gautam, G.; Mishra, V.; Verma, P.; Pandey, A.K.; Negi, S. A cost effective strategy for production of bio-surfactant from locally isolated Penicillium chrysogenum SNP5 and its applications. J. Bioprocess. Biotech. 2014, 4, 1. [Google Scholar] [CrossRef]
- Campos, J.M.; Stamford, T.L.; Sarubbo, L.A. Production of a bioemulsifier with potential application in the food industry. Appl. Biochem. Biotechnol. 2014, 172, 3234–3252. [Google Scholar] [CrossRef] [PubMed]
- Zinjarde, S.S.; Pant, A. Emulsifier from a tropical marine yeast, Yarrowia lipolytica NCIM 3589. J. Basic Microbiol. 2002, 42, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Van Bogaert, I.N.; Saerens, K.; De Muynck, C.; Develter, D.; Soetaert, W.; Vandamme, E.J. Microbial production and application of sophorolipids. Appl. Microbiol. Biotechnol. 2007, 76, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, M.E.; Miller, R.M. Biosurfactants: Their identity and potential efficacy in the biological control of zoosporic plant pathogens. Plant Dis. 1997, 81, 4–12. [Google Scholar] [CrossRef]
- Kruijt, M.; Tran, H.; Raaijmakers, J.M. Functional, genetic and chemical characterization of biosurfactants produced by plant growth-promoting Pseudomonas putida 267. J. Appl. Microbiol. 2009, 107, 546–556. [Google Scholar] [CrossRef]
- Krzyzanowska, D.; Potrykus, M.; Golanowska, M.; Polonis, K.; Gwizdek-Wisniewska, A.; Lojkowska, E.; Jafra, S. Rhizosphere bacteria as potential biocontrol agents against soft rot caused by various Pectobacterium and Dickeya spp. strains. J. Plant Pathol. 2012, 94, 367–378. [Google Scholar]
- Velho, R.; Medina, L.; Segalin, J.; Brandelli, A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. 2011, 56, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Jenny, K.; Käppeli, O.; Fiechter, A. Biosurfactants from Bacillus licheniformis: Structural analysis and characterization. Appl. Microbiol. Biotechnol. 1991, 36, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.I.; Wang, J.; Mu, B. Isolation and characterization of a biosurfactant producing strain, Brevibacilis brevis HOB1. J. Ind. Microbiol. Biotechnol. 2008, 35, 1597–1604. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Zang, T.; Wei, J.; Wu, H.; Wei, C.; Qiu, G.; Li, F. A biosurfactant-producing Pseudomonas aeruginosa S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons. Bioresour. Technol. 2019, 281, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Shi, R.; Zhao, J.; Li, G.; Bai, X.; Han, S.; Zhang, Y. Heterologous production of Pseudomonas aeruginosa rhamnolipid under anaerobic conditions for microbial enhanced oil recovery. J. Appl. Microbiol. 2015, 118, 379–389. [Google Scholar] [CrossRef]
- Santa Anna, L.; Sebastian, G.; Menezes, E.; Alves, T.; Santos, A.; Pereira Jr, N.; Freire, D. Production of biosurfactants from Pseudomonas aeruginosa PA 1 isolated in oil environments. Braz. J. Chem. Eng. 2002, 19, 159–166. [Google Scholar] [CrossRef]
- Xu, M.; Fu, X.; Gao, Y.; Duan, L.; Xu, C.; Sun, W.; Li, Y.; Meng, X.; Xiao, X. Characterization of a biosurfactant-producing bacteria isolated from Marine environment: Surface activity, chemical characterization and biodegradation. J. Environ. Chem. Eng. 2020, 8, 104277. [Google Scholar] [CrossRef]
- Eddouaouda, K.; Mnif, S.; Badis, A.; Younes, S.B.; Cherif, S.; Ferhat, S.; Mhiri, N.; Chamkha, M.; Sayadi, S. Characterization of a novel biosurfactant produced by Staphylococcus sp. strain 1E with potential application on hydrocarbon bioremediation. J. Basic. Microbiol. 2012, 52, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Bertolini, P.; Pratella, G. Non-conventional methods for the control of post-harvest pear diseases. J. Appl. Microbiol. 2003, 94, 761–766. [Google Scholar] [CrossRef]
- Mari, M.; Guizzardi, M.; Pratella, G. Biological control of gray mold in pears by antagonistic bacteria. Biol. Control 1996, 7, 30–37. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Wattanaphon, H.; Kerdsin, A.; Thammacharoen, C.; Sangvanich, P.; Vangnai, A. A biosurfactant from Burkholderia cenocepacia BSP3 and its enhancement of pesticide solubilization. J. Appl. Microbiol. 2008, 105, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Nishio, E.; Ichiki, Y.; Tamura, H.; Morita, S.; Watanabe, K.; Yoshikawa, H. Isolation of bacterial strains that produce the endocrine disruptor, octylphenol diethoxylates, in paddy fields. Biosci. Biotechnol. Biochem. 2002, 66, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- D’Incau, E.; Spaudo, A.; Henry, S.; Ouvrard, S. Phytotoxic response of ryegrass (Lolium multiflorum L.) to extreme exposure to two anionic surfactants. Ecotoxicol. Environ. Saf. 2024, 288, 117320. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Kunimi, E.; Hirata, Y.; Muraoka, M.; Tsujino, H.; Arai, M.; Hirata, K.; Nagano, K. The Lower Toxicity and Wider Safety Range of Acidic Sophorolipid Compared to Surfactin and Rhamnolipid as Biosurfactants toward the HaCAT, THP-1, and RAW 264.7. BPB Rep. 2024, 7, 56–60. [Google Scholar] [CrossRef]
- Gunasekera, D.; Wijerathna, P.; Ratnasekera, D. Nanotechnology and Extremophiles: Agricultural Applications and Possibilities. In Extremophiles for Sustainable Agriculture and Soil Health Improvement; Springer: Cham, Switzerland, 2024; pp. 441–453. [Google Scholar]
- Cedrola, C.C.; Barbosa, F.G.; Marcelino, P.R.F.; da Silva, N.P.; Tavares, G.D.; Barradas, T.N.; da Silva, S.S.; Vilela, F.M.P. Unlocking the beauty benefits: Exploring biosurfactants from Scheffersomyces shehatae for cosmetics. J. Surfactants Deterg. 2024, 27, 801–811. [Google Scholar] [CrossRef]
- Silva, I.A.; Almeida, F.C.G.; Alves, R.N.; Cunha, M.C.; Oliveira, J.C.M.; Fernandes, M.L.B.; Sarubbo, L.A. Formulation of a Natural Detergent with a Biosurfactant Produced by Starmerella bombicola ATCC 22214 Cultivated in a Low-Cost Medium for Application in the Remediation of Coastal Environmental Compartments. Preprint 2024. [Google Scholar] [CrossRef]
- Roy, A.; Khan, M.R.; Mukherjee, A.K. Recent advances in the application of microbial biosurfactants in food industries: Opportunities and challenges. Food Control 2024, 163, 110465. [Google Scholar] [CrossRef]
- D’Almeida, A.P.; de Albuquerque, T.L.; Rocha, M.V.P. Recent advances in Emulsan production, purification, and application: Exploring bioemulsifiers unique potentials. Int. J. Biol. Macromol. 2024, 278, 133672. [Google Scholar] [CrossRef] [PubMed]
- Rabuffetti, M.; Sangiorgio, S.; Ballabio, G.; Pargoletti, E.; Raimondi, L.; Cappelletti, G.; Speranza, G. The BioSurf Project: A Biocatalytic Approach to the Synthesis of Bio-Based Surfactants Through the Valorization of Dairy Industry Waste. 2024. Available online: https://air.unimi.it/handle/2434/1062468 (accessed on 1 November 2024).
- Saini, K.; Murugesan, S.; Yadav, R.; Thakur, K.; Bharali, P.; Kumar, N.; Saxena, G. Microbial surfactants: The ecofriendly tools for sustainable bioremediation of petroleum and organic contaminants for environmental safety. In Development in Waste Water Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2025; pp. 15–43. [Google Scholar]
- Rizvi, H. Tenside, Surfactants, Detergents: A Critical Review on Scale-Up Strategies of Biosurfactant Production and Its Applications. 2024. Available online: https://digital.zlb.de/viewer/metadata/1335252347/ (accessed on 1 November 2024).
- Nasser, M.; Sharma, M.; Kaur, G. Advances in the production of biosurfactants as green ingredients in home and personal care products. Front. Chem. 2024, 12, 1382547. [Google Scholar] [CrossRef] [PubMed]
- Aqif, M.; Shah, M.U.H.; Khan, R.; Umar, M.; SajjadHaider; Razak, S.I.A.; Wahit, M.U.; Khan, S.U.-D.; Sivapragasam, M.; Ullah, S. Glycolipids biosurfactants production using low-cost substrates for environmental remediation: Progress, challenges, and future prospects. Environ. Sci. Pollut. Res. 2024, 31, 47475–47504. [Google Scholar] [CrossRef]
- Rangel, L.I.; Leveau, J.H. Applied microbiology of the phyllosphere. Appl. Microbiol. Biotechnol. 2024, 108, 211. [Google Scholar] [CrossRef] [PubMed]


| Experiment Conditions | Surfactant Type | Results | Reference |
|---|---|---|---|
| Review study | Microbial activity derived biosurfactants | Increasing feed digestibility, improving seed protection and fertility | [84] |
| Microbial sources: Pseudomonas, Bacillus, and Candida | Rhamnolipid, sophorolipid, and surfactin | Oil spill management | [85] |
| Analyze microbial biosurfactant production, focusing on the optimization of metabolic pathways and production | Rhamnolipids surfactin | Improved yield and reduced ATP | [86] |
| Review study | Rhamnolipids | Potential antimicrobials, immune modulators, virulence factors, and anticancer agent | [87] |
| Microbial sources: Pseudomonas, Burkholderia, and Bacillus species | Rhamnolipids and lipopeptides | Plant resistance to phytopathogens | [88] |
| Soil application study | Rhamnolipid and surfactin | Enhanced TPH release from soil | [75] |
| Soil washing study | Rhamnolipid solution | Desorption of cadmium and zinc | [80] |
| Heavy-metal-contaminated soil study | Surfactant produced by Pseudomonas aeruginosa | Enhanced efficiency of oil release from soil | [76] |
| Agricultural community cohort study | Rhamnolipid and other biosurfactants | Significant alterations in microbiome composition due to pesticide exposure | [89] |
| Investigation of biosurfactants for sustainable agriculture | Surfactin, lipopeptides | Improvement in nutrient availability and soil revitalization | [90] |
| Production of biosurfactants using agricultural residues | Rhamnolipids, sophorolipids | Potential in waste management and bioremediation applications | [17] |
| Study on farm work tasks | Multiple surfactants | Increased microbial diversity in indoor environments | [91] |
| Fungal Strains | Biosurfactants | Properties | References |
|---|---|---|---|
| Aspergillus ustus | Glycolipoprotein | Antimicrobial activity | [129] |
| Cunninghamella echinulata | Complex Carbohydrate/Protein/Lipid | Reducing and increasing the viscosity of hydrophobic substrates and their molecules | [130] |
| Penicillium chrysogenum SNP5 | Lipopeptide | Role in pharmaceuticals, as well as in the oil and petroleum industry | [131] |
| Candida utilis | Emulsifiers | Effective emulsifiers for various applications | [132] |
| Microsphaeropsis sp. | Eremophilane derivative | Antimicrobial properties | [133] |
| Candida bombicola | Sophorolipids | Emulsification, detergency, and potential therapeutic applications | [134] |
| Bacterial Strains | Biosurfactants | Properties | References |
|---|---|---|---|
| Pseudomonas aeruginosa S5 | Glycolipid | Removal of aromatic hydrocarbons | [141] |
| Pseudomonas aeruginosa | Rhamnolipid | Enhancement of oil recovery through anaerobic production | [142] |
| Bacillus subtilis A21 | Lipopeptide | Removal of heavy metals, petroleum hydrocarbons | [53] |
| Pseudomonas aeruginosa PA1 | Rhamnolipid | Capacity to use carbon sources | [143] |
| Paracoccus sp. MJ9 | Rhamnolipid | Increasing the solubility of hydrophobic compounds | [144] |
| Brevibacillus brevis HOB1 | Lipopeptide | Antibacterial activity, potential in biological control | [140] |
| Staphylococcus spp. | Biosurfactant | Inhibitory effect against Pseudomonas aeruginosa | [145] |
| Pseudomonas putida | Rhamnolipid | Zoospore lysis, inhibition of cucumber damping off disease | [136] |
| Pseudomonas fluorescens | Biosurfactant | Inhibition of fungal pathogens, plant disease management | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoli, S.; Asgari Lajayer, B.; Bagheri Novair, S.; Price, G.W. Unlocking the Potential of Biosurfactants in Agriculture: Novel Applications and Future Directions. Sustainability 2025, 17, 2110. https://doi.org/10.3390/su17052110
Abdoli S, Asgari Lajayer B, Bagheri Novair S, Price GW. Unlocking the Potential of Biosurfactants in Agriculture: Novel Applications and Future Directions. Sustainability. 2025; 17(5):2110. https://doi.org/10.3390/su17052110
Chicago/Turabian StyleAbdoli, Sima, Behnam Asgari Lajayer, Sepideh Bagheri Novair, and Gordon W. Price. 2025. "Unlocking the Potential of Biosurfactants in Agriculture: Novel Applications and Future Directions" Sustainability 17, no. 5: 2110. https://doi.org/10.3390/su17052110
APA StyleAbdoli, S., Asgari Lajayer, B., Bagheri Novair, S., & Price, G. W. (2025). Unlocking the Potential of Biosurfactants in Agriculture: Novel Applications and Future Directions. Sustainability, 17(5), 2110. https://doi.org/10.3390/su17052110








