Valorisation of Red Mud: Disclosing the Potential of an Abundant Residue
Abstract
1. Introduction
2. Material and Methodology
2.1. Materials
2.2. Methodology
2.2.1. Characterization Techniques
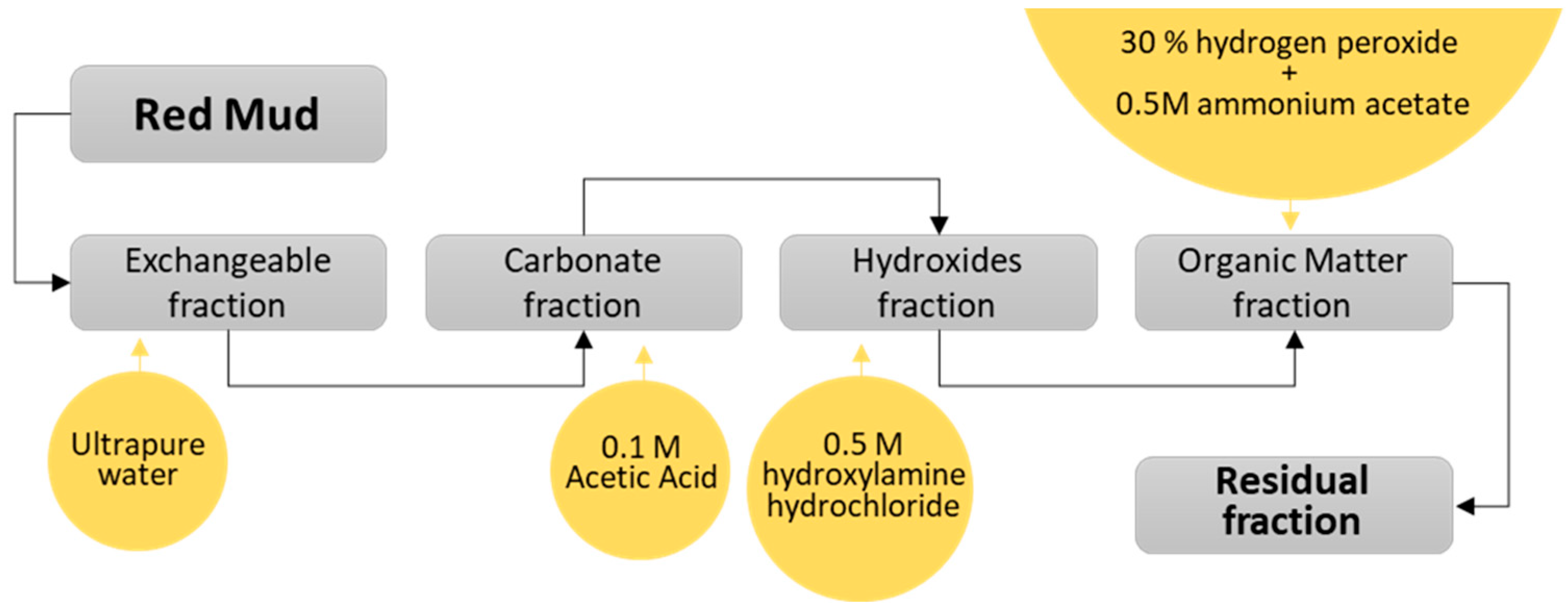
2.2.2. Sequential Extraction Procedure
- Exchangeable fraction (Step 1): 5 g of RM sample were weighed and stirred for 16 h at room temperature with 200 mL of ultrapure water and covered with watch glasses.
- Carbonate fraction (Step 2): The residue obtained in Stage 1 (Residue 1) was stirred for 16 h at room temperature with 200 mL of 0.1 M acetic acid (CH3COOH), covering the beakers with watch glasses.
- Hydroxides fraction (Step 3): The residue obtained in Step 2 (Residue 2) was stirred for 16 h at room temperature with 200 mL of 0.5 M hydroxylamine hydrochloride (NH2OH·HCl; pH = 2–3), covering the beakers with watch glasses.
- Organic matter fraction (Step 4): The residue obtained in Step 3 (Residue 3) was stirred for 1 h at room temperature with 50 mL of 30% hydrogen peroxide (H2O2) covering the beaker with watch glasses. The watch glasses were removed, and the solution was again stirred for 1 h in a water bath at 85 ± 5 °C. Subsequently, a further 50 mL of 30% H2O2 was added. This solution was covered with a watch glass, and the agitation was stopped. The water bath was kept at the same temperature for another hour. Finally, the solution was removed from the water bath, and 250 mL of 0.5 M ammonium acetate (NH4CH3COO) was added and stirred at room temperature for 16 h [30].
- Residual fraction (Step 5): The residual fraction from Step 4 (Residue 4) was subjected to an alkaline fusion.
2.2.3. Life Cycle Assessment
3. Results
3.1. Physicochemical Characterisation of RM
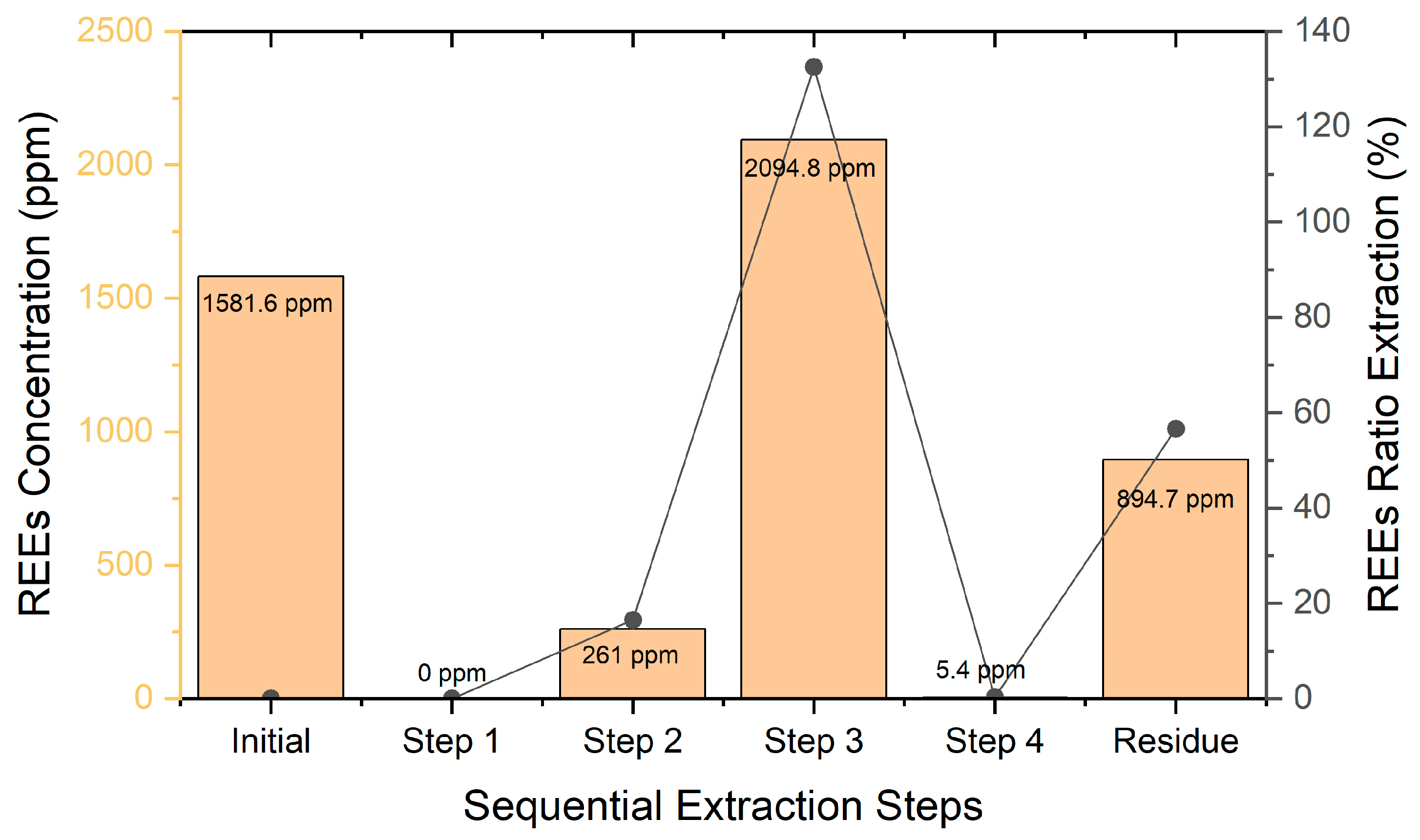
3.2. Sequential Extraction
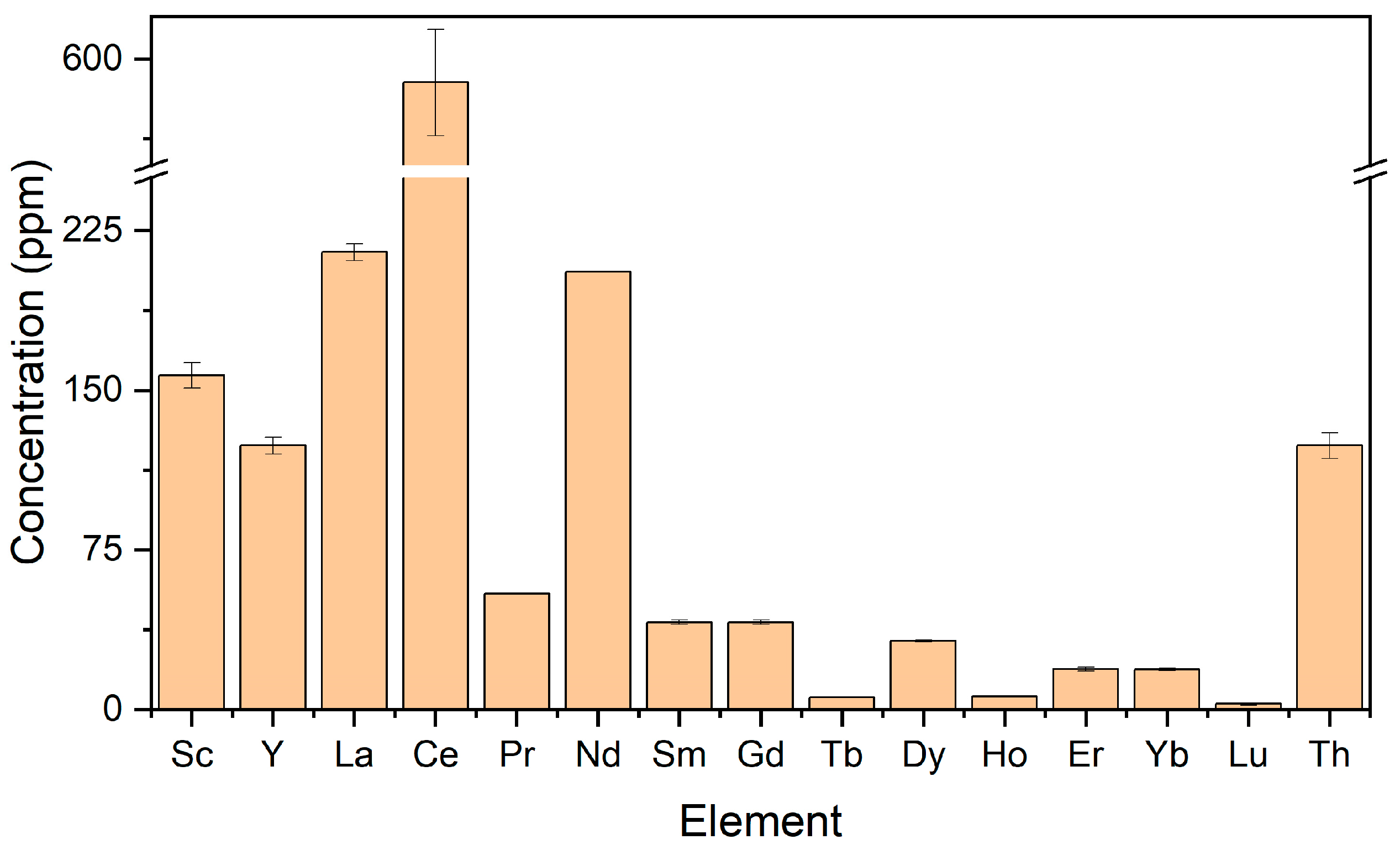
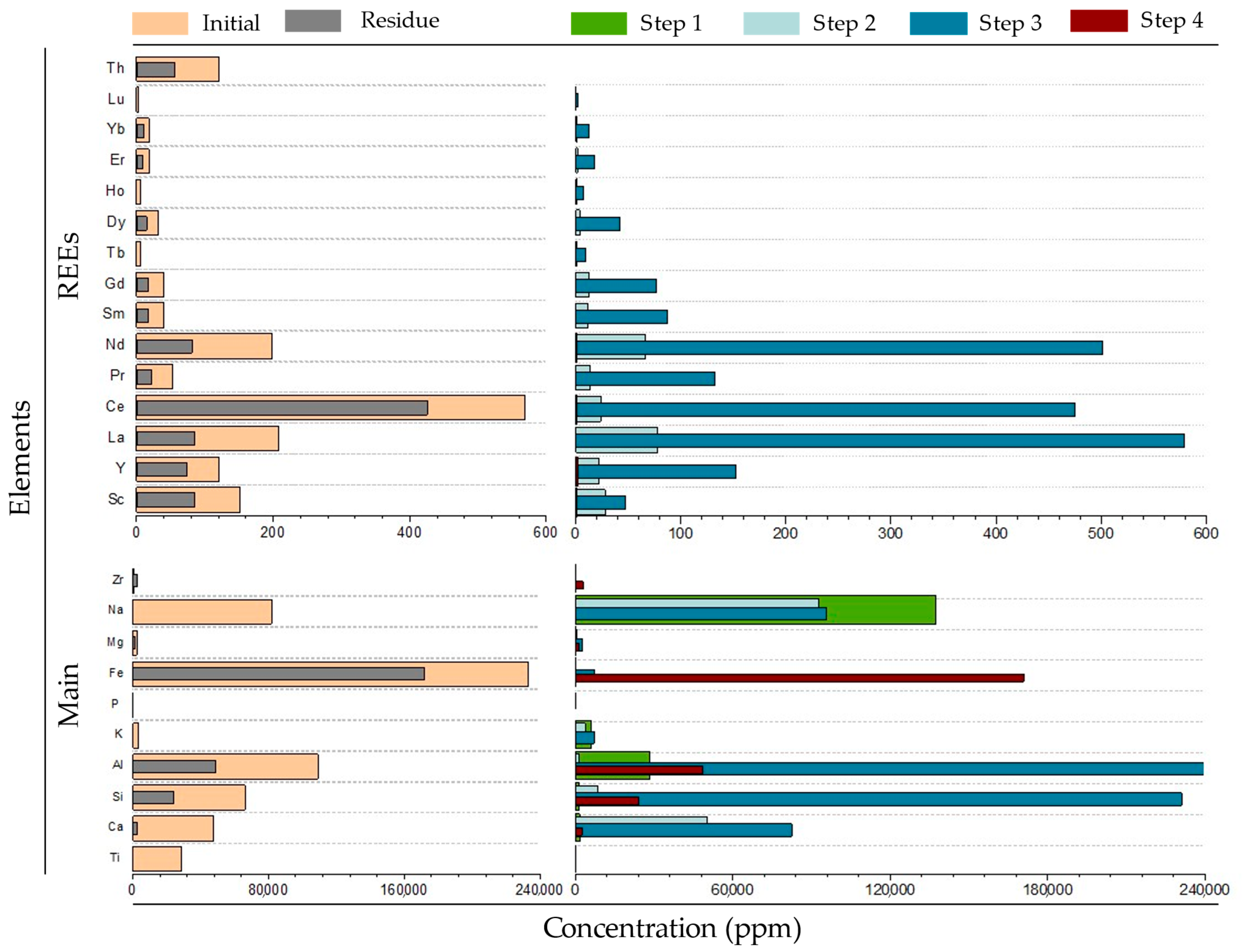
3.2.1. Extraction of REE and Other Valuable Elements
3.2.2. RM Residue After Sequential Extraction
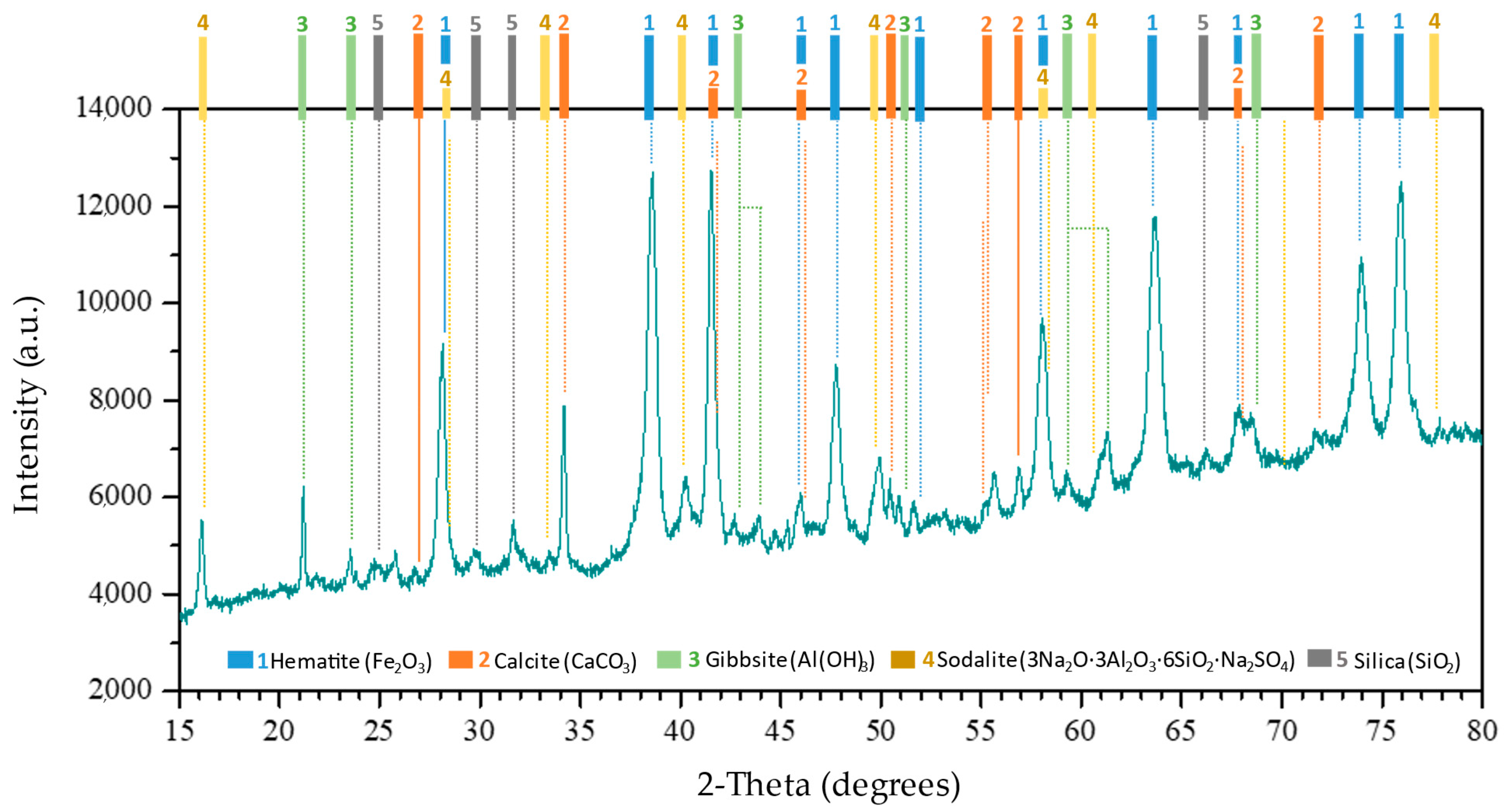
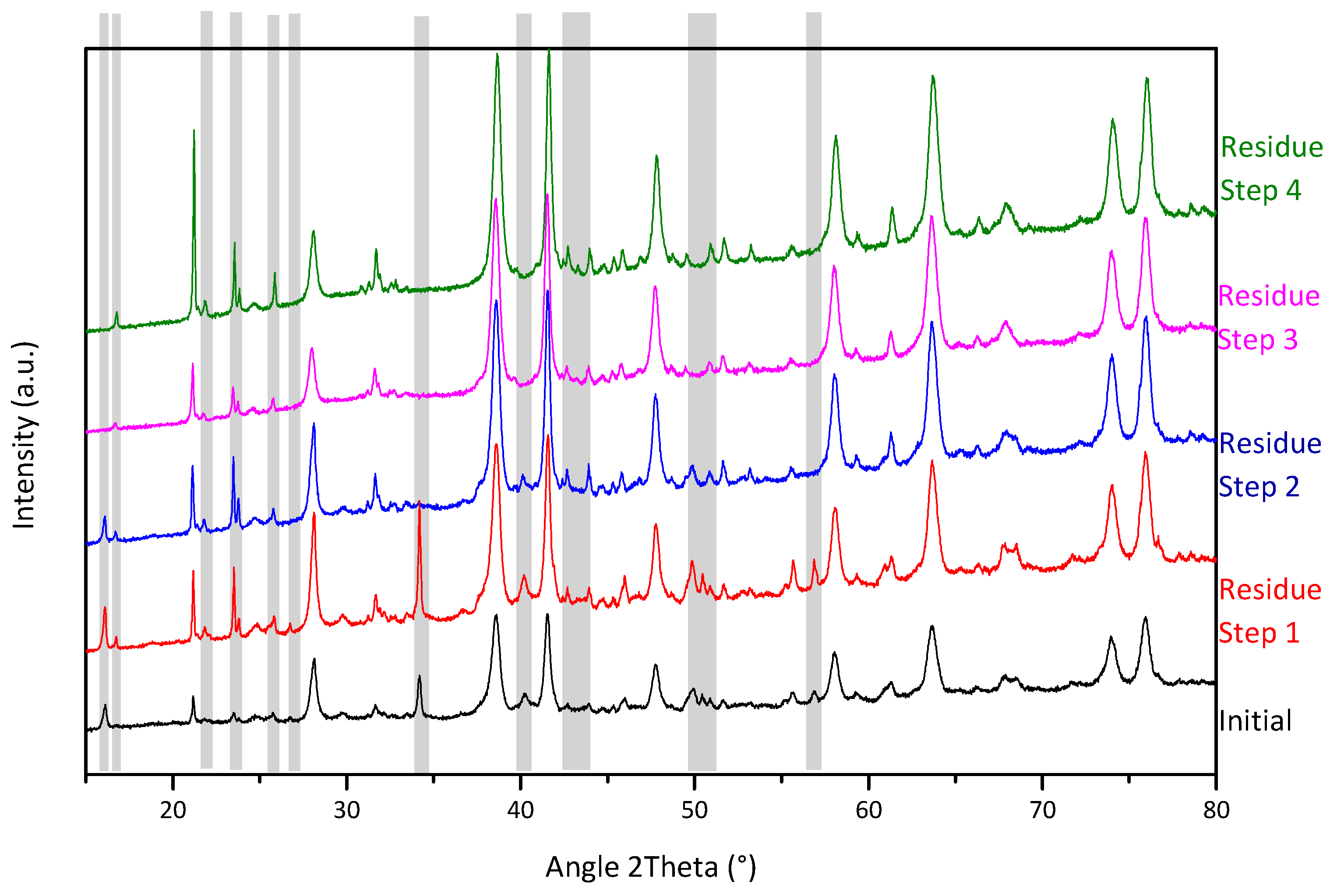
3.2.3. Crystallographic Phases Evolution During Sequential Extraction
4. Discussion
4.1. Potential Prospectivity of REE from RM
| Ref | Ore Type | Region | Location | LREE | HREE | Others | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | Sc | ∑ REE | ∑ LREE | ∑ HREE | ∑ Y + Sc | ||||
| [27] | Average in Earth’s crust | Global | - | 39 | 67 | 9 | 42 | 7 | 2 | 6 | 1 | 5 | 1 | 4 | 1 | 3 | 0.8 | 33 | 22 | 242 | 165 | 22 | 55 |
| [68] | Bayan Obo Sediments | China | Bayan Obo region | 33 | 63 | 8 | 29 | 5 | 1 | 5 | 1 | 4 | 1 | 3 | 0 | 3 | 0 | 24 | - | 179 | 139 | 17 | 24 |
| [69] | Iron-rich/dolomite | China | Bayan Obo ore body | 6105 | 10,134 | 1206 | 5490 | 360 | 75 | 253 | 23 | 61 | 7 | 19 | 1 | 4 | 1 | 142 | - | 23,882 | 23,371 | 369 | 142 |
| [70] | Iron-rich/dolomite | China | Bayan Obo ore body | 8869 | 34,430 | 552 | 5229 | 367 | 61 | 165 | 2 | 12 | 11 | 32 | 2 | 9 | 1 | 134 | - | 49,875 | 49,507 | 234 | 134 |
| [70] | Iron-rich/dolomite | China | Bayan Obo ore body | 4605 | 17,744 | 345 | 3986 | 285 | 61 | 130 | 1 | 10 | 2 | 7 | 0 | 1 | 0 | 91 | - | 27,268 | 27,025 | 153 | 91 |
| [70] | Iron-rich/dolomite | China | Bayan Obo ore body | 1279 | 7977 | 152 | 2100 | 259 | 39 | 182 | 1 | 4 | 2 | 8 | 0 | 2 | 0 | 181 | - | 12,186 | 11,805 | 200 | 181 |
| [70] | Iron-rich/dolomite | China | Bayan Obo ore body | 9380 | 32,558 | 497 | 4414 | 328 | 73 | 121 | 3 | 17 | 3 | 10 | 1 | 3 | 0 | 110 | - | 47,518 | 47,250 | 158 | 110 |
| [71] | Carbonatites | USA | Mountain pass belt | 303 | 658 | 83 | 330 | 64 | 14 | 41 | 4 | 18 | 3 | 5 | 1 | 3 | 1 | 69 | 18 | 1615 | 1528 | 76 | 87 |
| [72] | Carbonatites | USA | Mountain pass belt | 235 | 465 | 52 | 197 | 33 | 7 | 23 | 2 | 10 | 2 | 4 | 0 | 3 | 0 | 47 | 12 | 1093 | 1034 | 45 | 59 |
| [73] | Ion-adsorption clays | Madagascar | - | 908 | 276 | 156 | 506 | 96 | 7 | 60 | 10 | 26 | 6 | 62 | 3 | 10 | 2.4 | 166 | - | 2294 | 1949 | 179 | 166 |
| [73] | Ion-adsorption clays | South-East Asia | - | 525 | 130 | 110 | 405 | 110 | 5 | 140 | 30 | 180 | 35 | 315 | 30 | 95 | 12.4 | 1020 | - | 3143 | 1285 | 837 | 1020 |
| [73] | Ion-adsorption clays | Brazil | - | 450 | 120 | 100 | 290 | 60 | 20 | 100 | 60 | 220 | 70 | 210 | 50 | 260 | 50 | 1200 | - | 3260 | 1040 | 1020 | 1200 |
| [74] | Iron ore | Sweden | Kiirunavaara | 252 | 782 | 101 | 524 | 122 | 15 | 157 | 23 | 130 | 25 | 76 | 9 | 66 | 8 | 802 | 4 | 3097 | 1796 | 495 | 806 |
| [74] | Iron ore | Sweden | Kiirunavaara | 544 | 1650 | 188 | 846 | 151 | 22 | 163 | 23 | 108 | 20 | 59 | 7 | 51 | 6 | 638 | 3 | 4480 | 3401 | 438 | 641 |
| Average REE (ppm) in conventional ores | 2788 | 8910 | 295 | 2026 | 186 | 33 | 128 | 15 | 66 | 16 | 67 | 9 | 42 | 7 | 383 | 12 | 14,976 | 14,249 | 350 | 386 | |||
| Ref | Ore Type | Region | Location | LREE | HREE | Others | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | Sc | ∑ REE | ∑ LREE | ∑ HREE | ∑ Y + Sc | ||||
| [3] | RM | Greece | - | 156 | 483 | 32 | 125 | 25 | 5 | 23 | - | 24 | - | 14 | - | 14 | - | 115 | 134 | 1150 | 827 | 75 | 249 |
| [75] | RM | Greece | Agios Nikolaos | 114 | 368 | 28 | 99 | 21 | 5 | 22 | 4 | 17 | 4 | 14 | 2 | 14 | 2 | 76 | 121 | 910 | 635 | 78 | 197 |
| [76] | RM | Greece | 149 | 418 | 26 | 115 | 29 | 5 | 23 | - | 13 | 4 | 17 | - | 16 | 2 | 91 | 128 | 1036 | 742 | 76 | 219 | |
| [77] | RM | Greece | AoG plant-Boeotia | 130 | 480 | 29 | 107 | 19 | 5 | 22 | 3 | 20 | 4 | 13 | 2 | 14 | 2 | 108 | - | 959 | 770 | 81 | 108 |
| [78] | RM | Greece | Parnassus-Giona | 127 | 409 | 28 | 103 | 20 | 4 | 18 | 2 | 19 | 3 | 11 | 2 | 13 | 2 | 98 | - | 859 | 691 | 70 | 98 |
| [61] | RM | Greece | Parnassus-Giona | 151 | 422 | 26 | 121 | 29 | 5 | 23 | - | 14 | 4 | 17 | - | 16 | 2 | 93 | - | 923 | 754 | 76 | 93 |
| [79] | RM | China | - | 416 | 842 | 95 | 341 | 64 | 110 | 56 | 184 | 48 | 25 | 28 | 14 | 28 | 14 | 266 | 158 | 2689 | 1868 | 397 | 424 |
| [79] | RM | India | - | 112 | 191 | 18 | 48 | 9 | 2 | 7 | - | 4 | - | 1 | - | 2 | - | 13 | 58 | 465 | 380 | 14 | 71 |
| [80] | RM | Jamaica | - | 287 | 366 | 74 | 69 | 0 | 0 | 37 | 0 | 38 | 6 | 21 | 14 | 17 | 2 | 373 | 55 | 1359 | 797 | 134 | 429 |
| [81] | RM | Jamaica | Alcan Plant | 626 | 714 | - | 295 | 80 | 15 | - | 14 | 83 | - | - | - | 34 | 6 | - | 162 | 2029 | 1730 | 137 | 162 |
| [82] | RM | Russia | Rusal Plant | 234 | 508 | 58 | 222 | 43 | 8 | 44 | 5 | 31 | 6 | 17 | 2 | 15 | 2 | 145 | 86 | 1425 | 1072 | 122 | 231 |
| [83] | RM | Montenegro | Podgorica | 303 | 558 | 58 | 215 | 40 | 8 | 36 | 6 | 33 | 7 | 20 | 3 | 20 | 3 | 180 | 105 | 1594 | 1183 | 127 | 284 |
| [66] | RM | Montenegro | Podgorica | 292 | 539 | 56 | 208 | 39 | 8 | 35 | 5 | 32 | 7 | 19 | 3 | 19 | 3 | 174 | 102 | 1541 | 1142 | 123 | 276 |
| [66] | RM | Slovenia | Kidričevo | 182 | 363 | 33 | 116 | 21 | 4 | 19 | 3 | 22 | 5 | 15 | 2 | 15 | 2 | 131 | 85 | 1020 | 719 | 85 | 216 |
| [66] | RM | Hungary | Almásfüzíto | 241 | 426 | 54 | 199 | 38 | 7 | 33 | 5 | 29 | 6 | 16 | 3 | 16 | 3 | 155 | 77 | 1308 | 965 | 111 | 232 |
| [66] | RM | Hungary | Ajka | 210 | 429 | 46 | 171 | 32 | 6 | 27 | 4 | 24 | 5 | 14 | 2 | 14 | 2 | 136 | n/a | 1122 | 894 | 92 | 136 |
| [84] | RM | Hungary | Ajka | 114 | 368 | - | 99 | - | - | - | - | - | - | - | - | - | - | 68 | 54 | 703 | 581 | 0 | 122 |
| [61] | RM | Türkiye | Seydişehir | 219 | 616 | 62 | 210 | 42 | 9 | 6 | 34 | 31 | 6 | 17 | 3 | 18 | 3 | 145 | - | 1420 | 1157 | 118 | 145 |
| [28] | RM | Türkiye | Seydişehir | 209 | 406 | 99 | 186 | 54 | 1 | 26 | 12 | 31 | 6 | 15 | - | 17 | 9 | 135 | 104 | 1309 | 955 | 115 | 239 |
| * | RM | Türkiye | Seydişehir | 215 | 589 | 54 | 206 | 41 | - | 41 | 6 | 32 | 6 | 19 | - | 19 | 3 | 124 | 157 | 1512 | 1105 | 126 | 281 |
| Average REE (ppm) in RM | 224 | 475 | 49 | 163 | 34 | 12 | 28 | 20 | 29 | 7 | 16 | 4 | 17 | 4 | 138 | 106 | 1267 | 948 | 108 | 211 | |||
Feasibility of Extracting Main Targets from RM
4.2. Sustainability of RM Valorisation
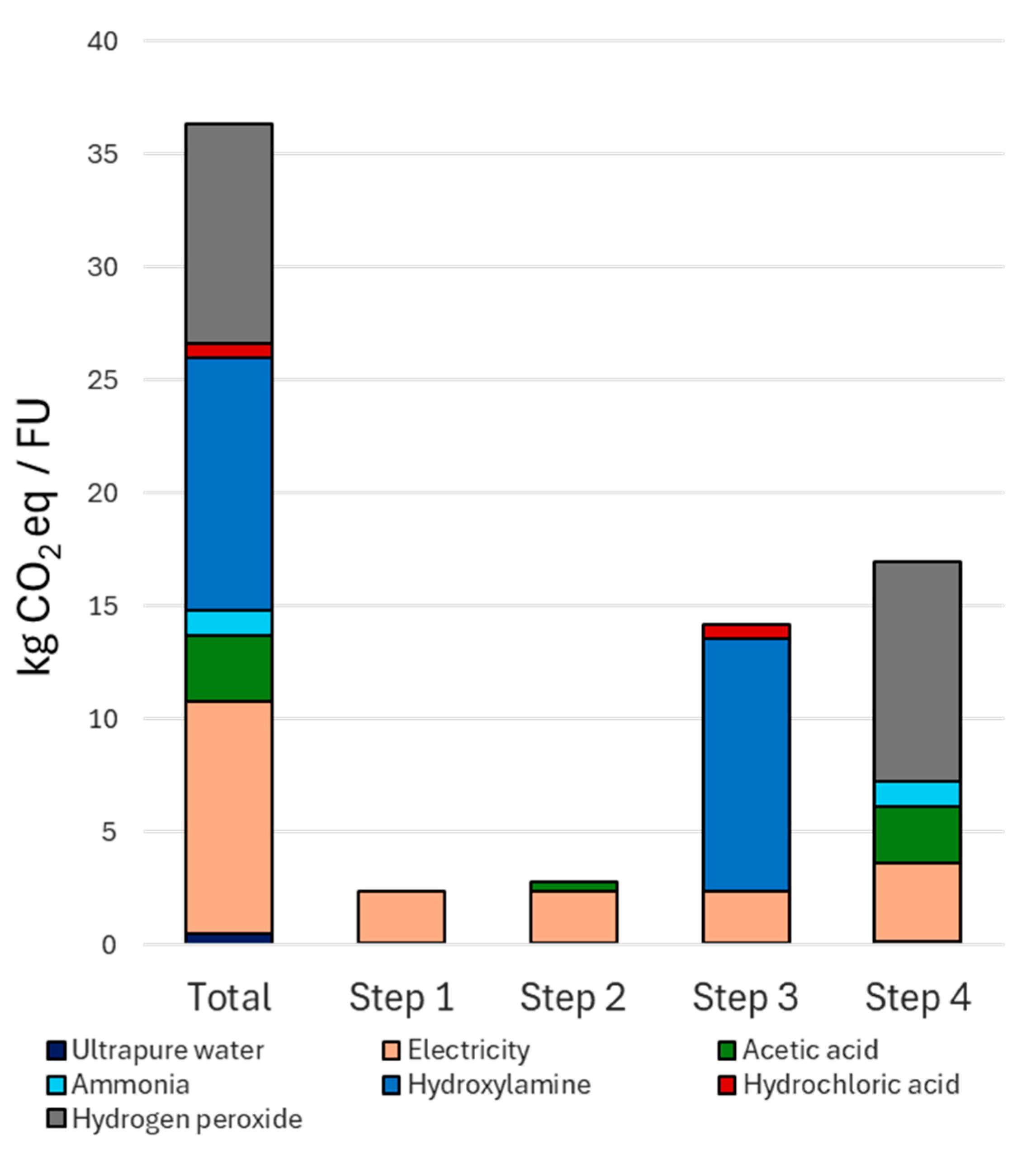
4.3. Global Warming Potential of the Sequential Extraction Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agrawal, S.; Dhawan, N. Evaluation of red mud as a polymetallic source—A review. Miner. Eng. 2021, 171, 107084. [Google Scholar] [CrossRef]
- Svobodova-Sedlackova, A.; Calderón, A.; Fernandez, A.I.; Chimenos, J.M.; Berlanga, C.; Yücel, O.; Barreneche, C.; Rodriguez, R. Mapping the research landscape of bauxite by-products (red mud): An evolutionary perspective from 1995 to 2022. Heliyon 2024, 10, e24943. [Google Scholar] [CrossRef]
- Balomenos, E.; Davris, P.; Pontikes, Y.; Panias, D. Mud2Metal: Lessons Learned on the Path for Complete Utilization of Bauxite Residue Through Industrial Symbiosis. J. Sustain. Metall. 2016, 3, 551–560. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Akcil, A.; Lee, J.C. Red mud valorization an industrial waste circular economy challenge; review over processes and their chemistry. Crit. Rev. Environ. Sci. Technol. 2020, 52, 520–570. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A review on comprehensive utilization of red mud and prospect analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, Á.D.; Molnár, M.; Feigl, V.; Ujaczki, É. Advances in Understanding Environmental Risks of Red Mud After the Ajka Spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, F.; Kong, X.; Wu, C.; Huang, L.; Huang, N.; Hartley, W. A review of the characterization and revegetation of bauxite residues (Red mud). Environ. Sci. Pollut. Res. 2016, 23, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Santini, T.C.; Fey, M.V. Spontaneous vegetation encroachment upon bauxite residue (red mud) as an indicator and facilitator of in situ remediation processes. Environ. Sci. Technol. 2013, 47, 12089–12096. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Li, Z.; Lei, J.; Yin, K.; Zhang, Z.; Xie, N. Upcycling use of red mud-based solid waste in engineered cementitious composites: Properties, activation mechanism, and life-cycle assessment. J. Clean. Prod. 2024, 447, 141504. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Adelfio, L.; Sgarbossa, F.; Leone, R.; La Scalia, G. Life Cycle Assessment of Red Mud-Based Geopolymer Production at Industrial Scale, in Advances in Production Management Systems. In IFIP Advances in Information and Communication Technology; Alfnes, E., Romsdal, A., Strandhagen, J.O., von Cieminski, G., Romero, D., Eds.; Springer Nature: Cham, Switzerland, 2023; Volume 692, pp. 593–606. [Google Scholar] [CrossRef]
- Swain, B.; Lee, C.G.; Park, J.R. Assessment of bauxite residue as secondary resource for rare earth metal and valorization challenges: A perspective. Resour. Conserv. Recycl. Adv. 2022, 14, 200078. [Google Scholar] [CrossRef]
- Bonomi, C.; Cardenia, C.; Yin, P.T.W.; Panias, D. Review of Technologies in the Recovery of Iron, Aluminium, Titanium and Rare Earth Elements from Bauxite Residue (Red Mud). In Proceedings of the International Symposium on Enhanced Landfill Mining, Lisboa, Portugal, 8–10 February 2016. [Google Scholar]
- Swain, B. Challenges and Opportunities for Sustainable Valorization of Rare Earth Metals from Anthropogenic Waste; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Salman, A.D.; Juzsakova, T.; Rédey, Á.; Le, P.-C.; Nguyen, X.C.; Domokos, E.; Abdullah, T.A.; Vagvolgyi, V.; Chang, S.W.; Nguyen, D.D. Enhancing the Recovery of Rare Earth Elements from Red Mud. Chem. Eng. Technol. 2021, 44, 1768–1774. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Element Deposits: Sources, and Exploration Strategies. J. Geol. Soc. India 2022, 98, 1210–1216. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2024: U.S. Geological Survey. USGS. Available online: https://www.usgs.gov/centers/national-minerals-information-center/rare-earths-statistics-and-information (accessed on 13 September 2024).
- Balaram, V. Sources and applications of rare earth elements. In Environmental Technologies to Treat Rare Earth Element Pollution: Principles and Engineering; IWA Publishing: London, UK, 2022; pp. 75–113. [Google Scholar] [CrossRef]
- Eurostat. “Trade in Rare Earth Elements Increases in 2022”, EU. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20231113-1 (accessed on 6 September 2024).
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare Earth Elements: Industrial Applications and Economic Dependency of Europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef]
- European Raw Materials Alliance. Rare Earth Magnets and Motors: A European Call for Action. European Raw Materials Alliance: Berlin, Germany, 2021; Available online: https://eit.europa.eu/sites/default/files/2021_09-24_ree_cluster_report2.pdf (accessed on 4 February 2025).
- Swain, B. Red Mud: An Environmental Challenge But Overlooked Treasure for Critical Rare Earth Metals; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Çelebi, E.E. Determination of metal fractions and rare earth anomalies in red mud: The case of bauxite mining district of Seydişehir (Turkey). Environ. Earth Sci. 2024, 83, 93. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Pérez-Martínez, S.; Giro-Paloma, J.; Maldonado-Alameda, A.; Formosa, J.; Queralt, I.; Chimenos, J.M. Characterisation and partition of valuable metals from WEEE in weathered municipal solid waste incineration bottom ash, with a view to recovering. J. Clean. Prod. 2019, 218, 61–68. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006; Volume 14040.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006; Volume 14044.
- GaBi Software, version 10.7.1.28; Sphera Solutions GmbH, LCA for Experts Software and Database for Life Cycle Engineering: Stuttgart, Germany, 2023.
- Wernet, G.B.C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. Ecoinvent, Version 3.9.1; Ecoinvent: Zurich, Switzerland, 2016.
- Hischier, R.; Jungbluth, N.; Althaus, H.-J.; Hischier, R.; Doka, G.; Bauer, C.; Dones, R.; Nemecek, T.; Hellweg, S.; Humbert, S.; et al. Implementation of Life Cycle Impact Assessment Methods. Ecoinvent Report No. 3, v2.2.; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2010. [Google Scholar]
- Grzesik, K.; Bieda, B.; Kozakiewicz, R.; Kossakowska, K. Goal and scope and its evolution for life cycle assessment of rare earth elements recovery from secondary sources. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, International Multidisciplinary Scientific Geoconference, Albena, Bulgaria, 27 June–6 July 2017; pp. 107–114. [Google Scholar] [CrossRef]
- Gu, H.; Wang, N.; Hargreaves, J.S.J. Sequential Extraction of Valuable Trace Elements from Bayer Process-Derived Waste Red Mud Samples. J. Sustain. Metall. 2018, 4, 147–154. [Google Scholar] [CrossRef]
- Erçağ, E.; Apak, R. Furnace smelting and extractive metallurgy of red mud: Recovery of TiO2, Al2O3 and pig iron. J. Chem. Technol. Biotechnol. 1997, 70, 241–246. [Google Scholar] [CrossRef]
- Şayan, E.; Bayramoğlu, M. Statistical Modelling of Sulphuric Acid Leaching of TiO2, Fe2O3 and A12O3 from Red Mud. Process Saf. Environ. Prot. 2001, 79, 291–296. [Google Scholar] [CrossRef]
- Alp, A.; Goral, M.S. The influence of soda additive on the thermal properties of red mud. J. Therm. Anal. Calorim. 2003, 73, 201–207. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, W.; Zhang, Y.; Xue, C.; Xu, Z.; Gao, Q.; Zhao, Q. Low carbon binder preparation from slag-red mud activated by MSWI fly ash-carbide slag: Hydration characteristics and heavy metals’ solidification behavior. J. Clean. Prod. 2022, 374, 134007. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, B. Development of unsintered construction materials from red mud wastes produced in the sintering alumina process. Constr. Build. Mater. 2008, 22, 2299–2307. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Sun, H.; Li, L. Pozzolanic behaviour of compound-activated red mud-coal gangue mixture. Cem. Concr. Res. 2011, 41, 270–278. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Corpas-Iglesias, F.A.; Martínez-Martínez, S.; Artiaga, R.; Pascual-Cosp, J. Manufacturing new ceramic materials from clay and red mud derived from the aluminium industry. Constr. Build. Mater. 2012, 35, 656–665. [Google Scholar] [CrossRef]
- Lv, Z.; Pan, X.; Pei, J.; Xu, D.; Yu, H. Hydrothermal preparation of lightweight calcium silicate powder from alumina-leaching residue of low-calcium sintering red mud. Ceram. Int. 2022, 48, 33835–33847. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek ‘red mud’ residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process. J. Hazard. Mater. 2013, 254–255, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.; Oustadakis, P. Red mud addition in the raw meal for the production of Portland cement clinker. J. Hazard. Mater. 2004, 116, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Abhilash; Sinha, S.; Sinha, M.K.; Pandey, B.D. Extraction of lanthanum and cerium from Indian red mud. Int. J. Miner. Process. 2014, 127, 70–73. [Google Scholar] [CrossRef]
- Sinha, S.; Abhilash; Meshram, P.; Pandey, B.D. Metallurgical processes for the recovery and recycling of lanthanum from various resources—A review. Hydrometallurgy 2016, 160, 47–59. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Other Valuable Metals from Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Li, Y.K.; Ke, C.H.; She, H.Q.; Wang, D.H.; Xu, C.; Wang, A.J.; Li, R.-P.; Peng, Z.-D.; Zhu, Z.-Y.; Yang, K.-F.; et al. Geology and mineralization of the Bayan Obo supergiant carbonatite-type REE-Nb-Fe deposit in Inner Mongolia, China: A review. China Geol. 2023, 6, 716–750. [Google Scholar]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Castor, S.B. Rare earth deposits of North America. In Resource Geology; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 337–347. [Google Scholar] [CrossRef]
- Burron, I. Mountain Pass: America’s Once and Future Rare Earth King. Geology for Investors, 1 February 2023. Available online: https://www.geologyforinvestors.com/mountain-pass-americas-once-and-future-rare-earth-king/ (accessed on 12 July 2024).
- Mahoney, N. US Wants to Reclaim Critical rare Earth Supply Chain. Available online: https://www.freightwaves.com/news/us-wants-to-reclaim-critical-rare-earth-supply-chain (accessed on 12 July 2024).
- Fan, H.R.; Yang, K.F.; Hu, F.F.; Liu, S.; Wang, K.Y. The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Uyanik, C.; Kocak, K.; Doyen, A.D. The Bauxite deposits of Seydişehir region (Mortaş and Doğankuzu deposits); Their geological, mineralogical and geochemical characteristics. Acta Geobalc. 2016, 2, 21–26. [Google Scholar] [CrossRef]
- Deady, É.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Miner. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Tuduri, J.; Charles, N.; Vaxelaire, S.; Dubois, D.; Fargier, H. Material flow analysis applied to rare earth elements in Europe. J. Clean. Prod. 2015, 107, 215–228. [Google Scholar] [CrossRef]
- Machacek, E.; Kalvig, P. Assessing advanced rare earth element-bearing deposits for industrial demand in the EU. Resour. Policy 2016, 49, 186–203. [Google Scholar] [CrossRef]
- Phoung, S.; Williams, E.; Gaustad, G.; Gupta, A. Exploring global supply and demand of scandium oxide in 2030. J. Clean. Prod. 2023, 401, 136673. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Tomašić, N. Bauxite and Bauxite Residue as a Potential Resource of REE in the ESEE Region; University of Zagreb Faculty of Science: Zabreb, Croatia, 2020. [Google Scholar]
- Pavel, C.C.; Lacal-Arántegui, R.; Marmier, A.; Schüler, D.; Tzimas, E.; Buchert, M.; Jenseit, W.; Blagoeva, D. Substitution strategies for reducing the use of rare earths in wind turbines. Resour. Policy 2017, 52, 349–357. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Nie, L.; McKinley, J.M.; Liu, H.; Zhang, B.; Han, Z. Geochemical background and dispersion pattern of the world’s largest REE deposit of Bayan Obo, China. J. Geochem. Explor. 2020, 215, 106545. [Google Scholar] [CrossRef]
- Lai, X.; Yang, X. Geochemical characteristics of the Bayan Obo giant REE-Nb-Fe deposit: Constraints on its genesis. J. S. Am. Earth Sci. 2013, 41, 99–112. [Google Scholar] [CrossRef]
- Hou, X.Z.; Yang, Z.F.; Wang, Z.J. The occurrence characteristics and recovery potential of middle-heavy rare earth elements in the Bayan Obo deposit, Northern China. Ore Geol. Rev. 2020, 126, 103737. [Google Scholar] [CrossRef]
- Watts, K.E.; Haxel, G.B.; Miller, D.M. Temporal and petrogenetic links between mesoproterozoic alkaline and carbonatite magmas at Mountain Pass, California. Econ. Geol. 2022, 117, 1–23. [Google Scholar] [CrossRef]
- Poletti, J.E.; Cottle, J.M.; Hagen-Peter, G.A.; Lackey, J.S. Petrochronological constraints on the origin of the Mountain Pass ultrapotassic and carbonatite intrusive suite, California. J. Pet. 2016, 57, 1555–1598. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. An overview of rare-earth recovery by ion-exchange leaching from ion-adsorption clays of various origins. Miner Mag 2016, 80, 63–76. [Google Scholar] [CrossRef]
- Frietsch, R. Ore Geology Reviews Rare earth elements in apatite and magnetite in Kiruna-type iron ores and some other iron ore types. Ore Geol. Rev. 1995, 9, 489–510. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Ochsenkiihn-Petropulu, M.; Lyberopulu, T.; Paris, G. Direct determination of lanthanides, yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.E.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Rare earth element phases in bauxite residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef]
- Deady, É.; Mouchos, E.; Goodenough, K.; Williamson, B.; Wall, F. Rare Earth Elements in Karst-Bauxites: A Novel Untapped European Resource? In Proceedings of the ERES 2014: 1st Conference on European Rare Earth Resources, Milos, Greece, 4–7 September 2014. [Google Scholar]
- Ujaczki, É.; Feigl, V.; Molnár, M.; Cusack, P.; Curtin, T.; Courtney, R.; O’Donoghue, L.; Davris, P.; Hugi, C.; Evangelou, M.W.; et al. Re-using bauxite residues: Benefits beyond (critical raw) material recovery. J. Chem. Technol. Biotechnol. 2018, 93, 2498–2510. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Kazantzis, N.K.; Emmert, M.H. Process for Scandium Recovery from Jamaican Bauxite Residue: A Probabilistic Economic Assessment. Mater. Today Proc. 2019, 9, 578–586. [Google Scholar] [CrossRef]
- Wagh, A.S.; Pinnock, W.R. Occurrence of Scandium and Rare Earth Elements in Jamaican Bauxite Waste. Econ. Geol. 1987, 82, 757–761. [Google Scholar] [CrossRef]
- Boyarintsev, A.V.; Aung, H.Y.; Stepanov, S.I.; Shoustikov, A.A.; Ivanov, P.I.; Giganov, V.G. Evaluation of Main Factors for Improvement of the Scandium Leaching Process from Russian Bauxite Residue (Red Mud) in Carbonate Media. ACS Omega 2022, 7, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Radusinović, S.; Papadopoulos, A. The potential for REE and associated critical metals in karstic bauxites and bauxite residue of Montenegro. Minerals 2021, 11, 975. [Google Scholar] [CrossRef]
- Ujaczki, É.; Zimmermann, Y.-S.; Feigl, V.; Lenz, M. Recovery of Rare Earth Elements from Hungarian Red Mud with Combined Acid Leaching and Liquid Extraction. 2015. Available online: https://www.researchgate.net/publication/282672503 (accessed on 5 December 2024).
- Decarlo, S.; Goodman, S. Russia and Scandium’s Scant Scale. 2022. Available online: https://www.usitc.gov/publications/332/executive_briefings/ebot_russia_and_scandium_scant_scale.pdf (accessed on 5 February 2025).
- Narayanan, R.P.; Ma, L.C.; Kazantzis, N.K.; Emmert, M.H. Cost Analysis as a Tool for the Development of Sc Recovery Processes from Bauxite Residue (Red Mud). ACS Sustain. Chem. Eng. 2018, 6, 5333–5341. [Google Scholar] [CrossRef]
- Ochsenkiihn-Petropulu, M.; Lyberopulu, T.; Ochsenkiihn’, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud leaching by selective. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Russian Aluminum Association (RAA). Aluminium-Scandium Alloys. Available online: https://www.aluminas.ru/en/projects/aluminum_scandium_alloys/?sphrase_id=33349 (accessed on 9 July 2024).
- UC RUSAL. RUSAL Sustainability Report 2014. 2015. Available online: https://www.responsibilityreports.com/HostedData/ResponsibilityReportArchive/r/rusal_2014.pdf (accessed on 9 July 2024).
- UC RUSAL. RUSAL Sustainability Report 2017. 2018. Available online: https://www.rusal.ru/upload/iblock/247/247fe7043002c65d0835e463cd1d4b92.PDF (accessed on 9 July 2024).
- Petrakova, O.V.; Panov, A.V.; Gorbachev, S.N.; Klimentenok, G.N.; Perestoronin, A.V.; Vishnyakov, S.E.; Anashkin, V.S. Improved efficiency of Red Mud processing through Scandium Oxide Recovery. In Light Metals 2015; Wiley: Hoboken, NJ, USA, 2015; pp. 93–96. [Google Scholar]
- UC RUSAL. RUSAL Sustainability Report 2016. 2017. Available online: https://www.rusal.ru/upload/iblock/68d/68d57844daba39d2a13ec4fe73b2c07a.pdf (accessed on 9 July 2024).
- Zhang, N.; Li, H.X.; Liu, X.M. Recovery of scandium from bauxite residue—Red mud: A review. Rare Met. 2016, 35, 887–900. [Google Scholar] [CrossRef]
- Pan, X.; Wu, H.; Lv, Z.; Yu, H.; Tu, G. Recovery of valuable metals from red mud: A comprehensive review. Sci. Total Environ. 2023, 904, 166686. [Google Scholar] [CrossRef]
- Rivera, A. Innovative Technologies for Rare Earth Elements Recovery from Bauxite Residue, Leuven. 2019. Available online: https://lirias.kuleuven.be/2334468?limo=0&trk=public_post_comment-text (accessed on 25 September 2024).
- Cížková, M.; Mezricky, D.; Rucki, M.; Tóth, T.M.; Náhlík, V.; Lanta, V.; Bišová, K.; Zachleder, V.; Vítová, M. Bio-mining of lanthanides from red mud by green microalgae. Molecules 2019, 24, 1356. [Google Scholar] [CrossRef]
- Jouini, M.; Royer-Lavallée, A.; Pabst, T.; Chung, E.; Kim, R.; Cheong, Y.W.; Neculita, C.M. Sustainable Production of Rare Earth Elements from Mine Waste and Geoethics. Minerals 2020, 12, 809. [Google Scholar] [CrossRef]
- Joyce, P.J. Environmental Considerations in the Zero-Waste Valorisation of Bauxite Residue: A Life Cycle Perspective. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2018. [Google Scholar]
- Panda, S.; Costa, R.B.; Shah, S.S.; Mishra, S.; Bevilaqua, D.; Akcil, A. Biotechnological trends and market impact on the recovery of rare earth elements from bauxite residue (red mud)—A review. Resour. Conserv. Recycl. 2021, 171, 105645. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Fernández, Á.M.; Torres, V.M. Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review. J. Sustain. Metall. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Joyce, P.J.; Björklund, A. Using Life Cycle Thinking to Assess the Sustainability Benefits of Complex Valorization Pathways for Bauxite Residue. J. Sustain. Metall. 2019, 5, 69–84. [Google Scholar] [CrossRef]
- Koltun, P.; Tharumarajah, A. Life Cycle Impact of Rare Earth Elements. ISRN Metall. 2014, 2014, 907536. [Google Scholar] [CrossRef]
- Deng, H.; Kendall, A. Life cycle assessment with primary data on heavy rare earth oxides from ion-adsorption clays. Int. J. Life Cycle Assess. 2019, 24, 1643–1652. [Google Scholar] [CrossRef]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of valuable elements from red mud with a focus on using liquid media—A review. Recycling 2021, 6, 38. [Google Scholar] [CrossRef]
| Step of the Leaching Process | Flows | Type | Value | Unit |
|---|---|---|---|---|
| 1 | Raw Materials | |||
| RM | Input | 1 | kg | |
| Ultrapure water | Input | 40 | l | |
| Leaches | Output | 0.25 | kg | |
| RM residue (1) | Output | 0.75 | kg | |
| Energy | ||||
| Mixing | Electricity | 12,800 | Wh | |
| Filtering | Electricity | 2310 | Wh | |
| 2 | Raw Materials | |||
| RM residue (1) | Input | 0.75 | kg | |
| Ultrapure water | Input | 40 | l | |
| Acetic acid 0.1 M | Input | 0.24 | kg | |
| Leaches | Output | 0.15 | kg | |
| RM residue (2) | Output | 0.60 | kg | |
| Energy | ||||
| Mixing | Electricity | 12,800 | Wh | |
| Filtering | Electricity | 2310 | Wh | |
| 3 | Raw Materials | |||
| RM residue (2) | Input | 0.60 | kg | |
| Ultrapure water | Input | 40 | l | |
| Hydroxylamine | Inputs for hydroxylamine hydrochloride 0.5 M | 0.66 | kg | |
| Hydrochloride | 0.73 | kg | ||
| Leaches | Output | 0.20 | kg | |
| RM residue (3) | Output | 0.40 | kg | |
| Energy | ||||
| Mixing | Electricity | 12,800 | Wh | |
| Filtering | Electricity | 2310 | Wh | |
| 4 | Raw Materials | |||
| RM residue (3) | Input | 0.40 | kg | |
| Ultrapure water | Input | 61.30 | l | |
| Hydrogen peroxide | Input | 8.70 | kg | |
| Ammonia | Inputs for ammonium acetate 0.5 M | 0.43 | kg | |
| Acetic acid | 1.50 | kg | ||
| Leaches | Output | 0.12 | kg | |
| RM residue (4) | Output | 0.28 | kg | |
| Energy | ||||
| Mixing | Electricity | 16,800 | Wh | |
| Mixing at 85 °C | Electricity | 2000 | Wh | |
| Heating at 85 °C | Electricity | 1500 | Wh | |
| Filtering | Electricity | 2310 | Wh |
| Sample | Fe2O3 | TiO2 | CaO | K2O | P2O5 | SiO2 | Al2O3 | MgO | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| RM 1 | 33.31 | 4.81 | 6.58 | 0.38 | 0.05 | 14.03 | 20.64 | 0.36 | 8.60 |
| RM 2 | 33.23 | 4.83 | 6.74 | 0.38 | 0.05 | 14.26 | 20.50 | 0.36 | 8.62 |
| RM 3 | 33.31 | 4.72 | 6.16 | 0.38 | 0.05 | 13.70 | 20.49 | 0.33 | 8.45 |
| RM 4 | 33.48 | 4.74 | 6.71 | 0.37 | 0.05 | 13.69 | 20.60 | 0.35 | 8.40 |
| Average | 33.3 ± 0.1 | 4.78 ± 0.04 | 6.55 ± 0.2 | 0.38 ± 0.01 | 0.05 ± 0.00 | 13.9 ± 0.2 | 20.6 ± 0.1 | 0.35 ± 0.01 | 8.5 ± 0.1 |
| Element | ppm | Element | ppm | Element | ppm | Element | ppm |
|---|---|---|---|---|---|---|---|
| Zr | 772 ± 12 | Sc | 142 ± 2 | Co | 29 ± 1 | Ag | 27 ± 1 |
| Cr | 718 ± 16 | Mn | 168 ± 1 | Br | 9.0 ± 0.1 | Ba | 26 ± 2 |
| Ce | 638 ± 13 | Pb | 122 ± 3 | Tl | 5.1 ± 0.3 | Sn | 18 ± 1 |
| Ni | 461 ± 6 | Y | 101 ± 1 | Cu | 31 ± 1 | Ga | 10 ± 2 |
| V | 474 ± 2 | Nb | 76 ± 1 | Mo | 28 ± 1 | Bi | 12.1 ± 0.1 |
| La | 205 ± 2 | Th | 79 ± 1 | Rb | 24.3 ± 0.1 | Se | 2 ± 1 |
| Nd | 188 ± 6 | Sr | 53 ± 1 | Cd | 29.5 ± 04 | ||
| As | 151 ± 3 | Sm | 34 ± 4 | W | 28 ± 1 |
| Element | Residue μg/g | Δ (μg/g) | Element | Initial μg/g | Δ (μg/g) | Element | Residue μg/g | Δ (μg/g) |
|---|---|---|---|---|---|---|---|---|
| Zr | 2999 | 288.2 | Rb | 24.3 | −100.0 | Ce | 426 | −27.7 |
| Cr | 453 | −36.9 | W | 27.5 | −100.0 | Gd | 17 | −54.1 |
| Ni | 326 | −29.3 | Ba | 26.3 | 177.1 | Sc | 85 | −45.9 |
| Nd | 81 | −60.6 | Sn | 17.9 | 95.4 | Yb | 10 | −47.1 |
| As | 67 | −55.7 | U | 12.6 | −52.3 | Y | 73 | −41.1 |
| Ga | 24 | −39.8 | Co | 29.2 | 36.9 | Th | 56 | −54.8 |
| Pb | 166 | 35.6 | REE | Pr | 22 | −59.6 | ||
| Nb | 24 | −69.0 | La | 215.0 | −60.5 | Sm | 17 | −58.5 |
| Sr | 26 | −51.3 | Nd | 205.6 | −60.6 | Tb | 0 | −100.0 |
| Cu | 31 | 1.6 | Lu | 2.7 | −100.0 | Ho | 0 | −100.0 |
| Mo | 15 | −46.6 | Er | 19.0 | −52.6 | Dy | 15 | −53.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vielma, C.A.; Svobodova-Sedlackova, A.; Chimenos, J.M.; Fernández, A.I.; Berlanga, C.; Rodriguez, R.; Barreneche, C. Valorisation of Red Mud: Disclosing the Potential of an Abundant Residue. Sustainability 2025, 17, 1849. https://doi.org/10.3390/su17051849
Vielma CA, Svobodova-Sedlackova A, Chimenos JM, Fernández AI, Berlanga C, Rodriguez R, Barreneche C. Valorisation of Red Mud: Disclosing the Potential of an Abundant Residue. Sustainability. 2025; 17(5):1849. https://doi.org/10.3390/su17051849
Chicago/Turabian StyleVielma, Carlos A., Adela Svobodova-Sedlackova, Josep Maria Chimenos, Ana Inés Fernández, Carlos Berlanga, Rafael Rodriguez, and Camila Barreneche. 2025. "Valorisation of Red Mud: Disclosing the Potential of an Abundant Residue" Sustainability 17, no. 5: 1849. https://doi.org/10.3390/su17051849
APA StyleVielma, C. A., Svobodova-Sedlackova, A., Chimenos, J. M., Fernández, A. I., Berlanga, C., Rodriguez, R., & Barreneche, C. (2025). Valorisation of Red Mud: Disclosing the Potential of an Abundant Residue. Sustainability, 17(5), 1849. https://doi.org/10.3390/su17051849










