Microbial Bioindicators for Monitoring the Impact of Emerging Contaminants on Soil Health in the European Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. The LUCAS Soil Dataset and Data Retrieval
2.2. Taxonomic Classification of Soil Microbial Communities
2.3. Microbial Communities Functional Annotation with PICRUSt2
2.4. Statistical Analysis and Visualization
2.5. Data Mining
3. Results
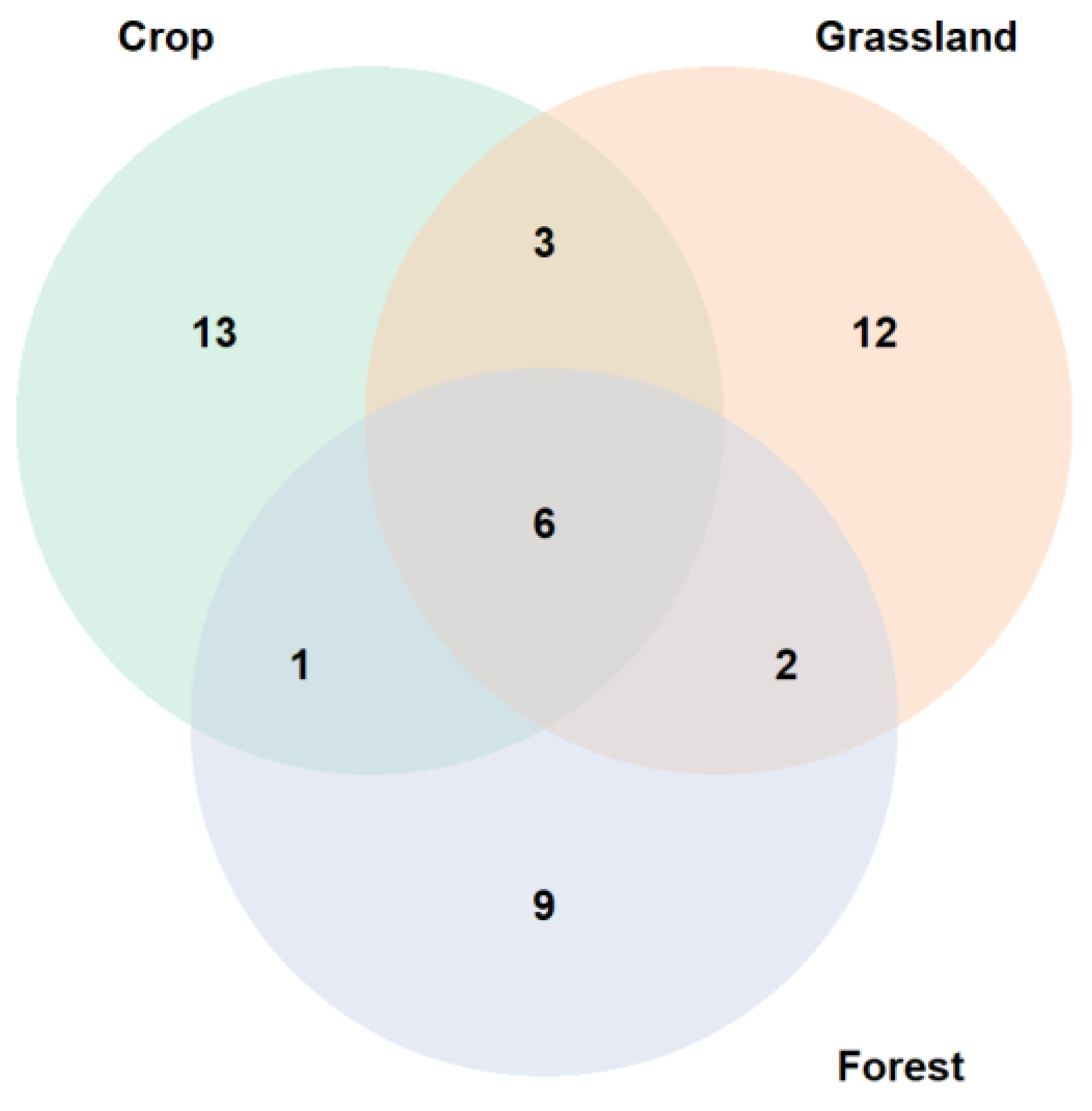
3.1. Microplastic Degradation Genes Occurrence in the European Framework
3.2. Identification of Potential Microbial Indicators
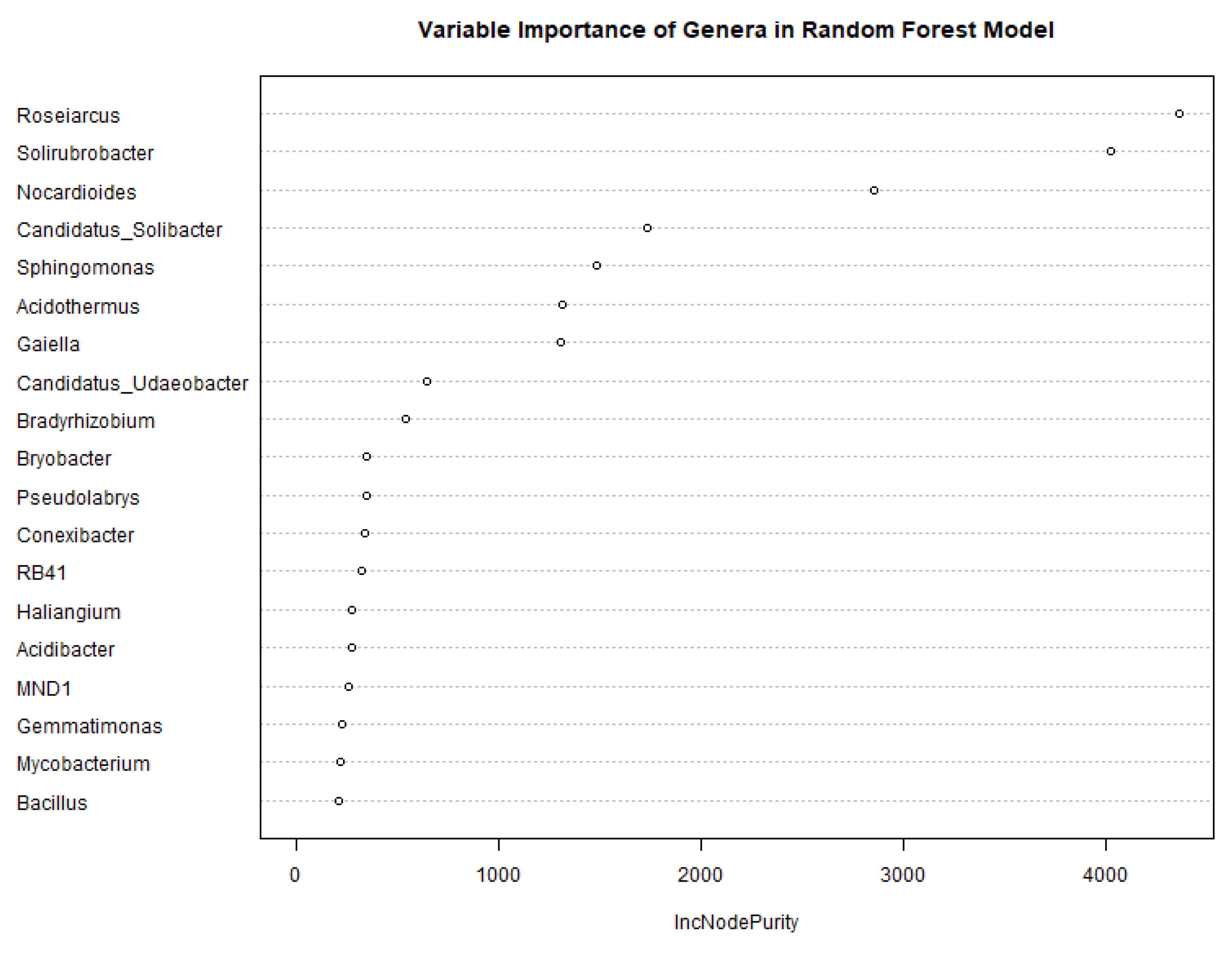
3.3. Random Forest
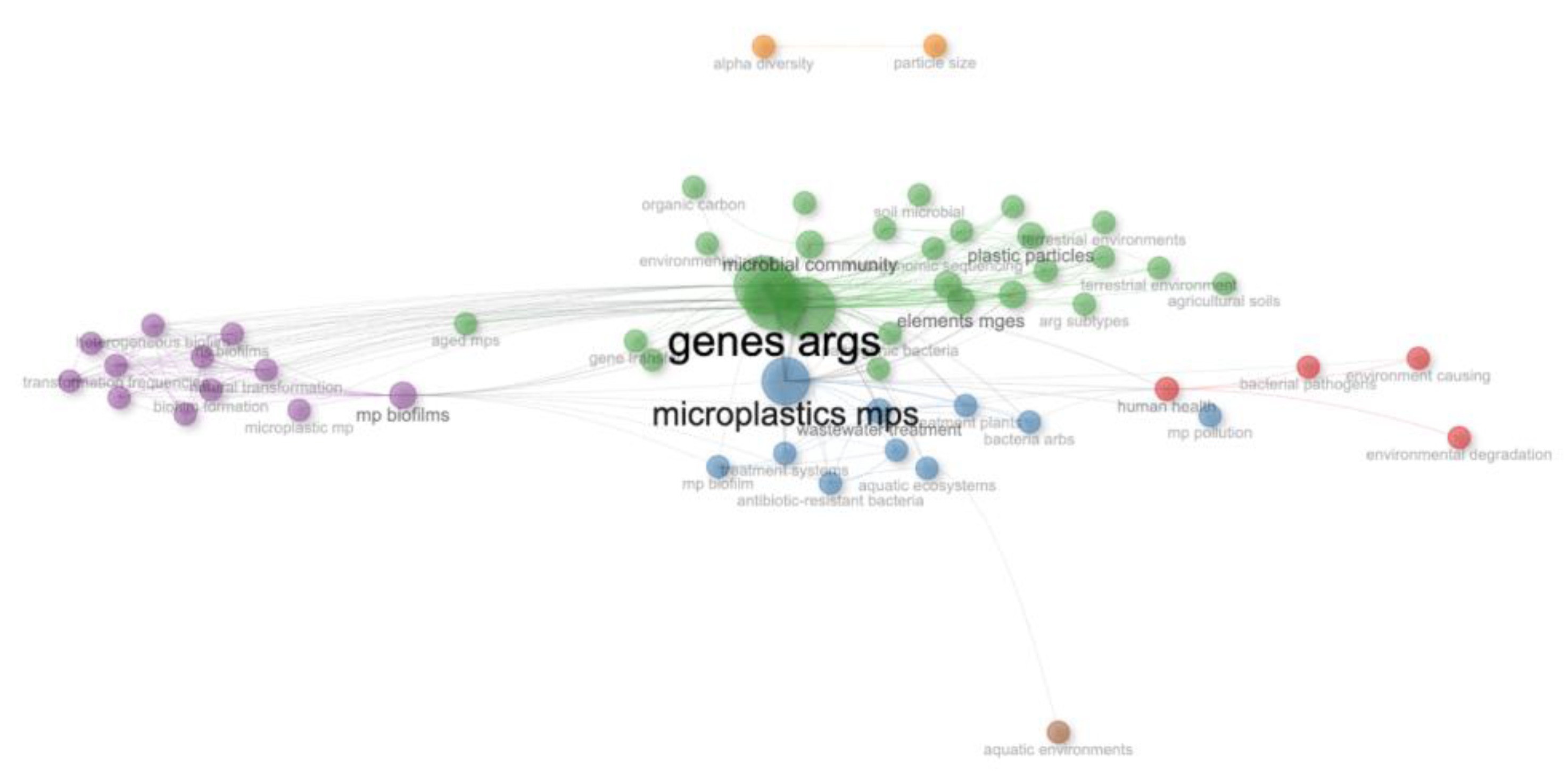
3.4. Data Mining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Telo Da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- European Commission EU Soil Directive. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=SWD:2023:0418:FIN:EN:PDF (accessed on 1 December 2024).
- Gardi, C.; Jeffery, S.; Saltelli, A. An Estimate of Potential Threats Levels to Soil Biodiversity in EU. Glob. Change Biol. 2013, 19, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; De Ruiter, P.C.; Van Der Putten, W.H.; Birkhofer, K.; Hemerik, L.; De Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive Agriculture Reduces Soil Biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in Soil Bacterial and Fungal Community Composition and Functional Groups during the Succession of Boreal Forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Cecilio Rebola, L.; Pandolfo Paz, C.; Valenzuela Gamarra, L.; Burslem, D.F.R.P. Land Use Intensity Determines Soil Properties and Biomass Recovery after Abandonment of Agricultural Land in an Amazonian Biodiversity Hotspot. Sci. Total Environ. 2021, 801, 149487. [Google Scholar] [CrossRef]
- Visca, A.; Di Gregorio, L.; Clagnan, E.; Bevivino, A. Sustainable Strategies: Nature-Based Solutions to Tackle Antibiotic Resistance Gene Proliferation and Improve Agricultural Productivity and Soil Quality. Environ. Res. 2024, 248, 118395. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Y.; Tan, W.; Zhang, Z. Microplastics as an Emerging Environmental Pollutant in Agricultural Soils: Effects on Ecosystems and Human Health. Front. Environ. Sci. 2022, 10, 855292. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics Using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jinjin, C.; Ji, R.; Ma, Y.; Yu, X. Microplastics in Agricultural Soils: Sources, Effects, and Their Fate. Curr. Opin. Environ. Sci. Health 2022, 25, 100311. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of Microplastics and Plastic Film Residues in the Soil Environment: A Critical Review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of Microplastics on Soil Properties: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef] [PubMed]
- Cusworth, S.J.; Davies, W.J.; McAinsh, M.R.; Gregory, A.S.; Storkey, J.; Stevens, C.J. Agricultural Fertilisers Contribute Substantially to Microplastic Concentrations in UK Soils. Commun. Earth Environ. 2024, 5, 7. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Q.; Yan, C.; Mancl, K.; Gong, D.; He, J.; Mei, X. Macro-and/or Microplastics as an Emerging Threat Effect Crop Growth and Soil Health. Resour. Conserv. Recycl. 2022, 186, 106549. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Singh, L.; Bolan, N.S.; Saha, M. Microplastics as an Emerging Source of Particulate Air Pollution: A Critical Review. J. Hazard. Mater. 2021, 418, 126245. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C. Present and Future Opportunities in the Use of Atomic Force Microscopy to Address the Physico-Chemical Properties of Aquatic Ecosystems at the Nanoscale Level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Atugoda, T.; Piyumali, H.; Liyanage, S.; Mahatantila, K.; Vithanage, M. Fate and Behavior of Microplastics in Freshwater Systems. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M.F., Mouneyrac, C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 781–811. ISBN 978-3-030-39040-2. [Google Scholar]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an Emerging Threat to Terrestrial Ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Onandia, G.; Maaß, S.; Zhao, T.; Rillig, M.C. Effects of Microplastics and Drought on Soil Ecosystem Functions and Multifunctionality. J. Appl. Ecol. 2021, 58, 988–996. [Google Scholar] [CrossRef]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Rodríguez-López, L.; Arias-Estévez, M.; Rodríguez-Seijo, A. Microplastics as Antibiotic Resistance Genes Carriers on Agricultural Soils: A Call for Research. Pedosphere 2024, in press. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Zhang, C.-M.; Yuan, Q.-Q.; Wu, K. New Insight into the Effect of Microplastics on Antibiotic Resistance and Bacterial Community of Biofilm. Chemosphere 2023, 335, 139151. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Q.; Zhao, E.; Li, X.; Xiong, X.; Wu, C. Biofilm Formation on Microplastics and Interactions with Antibiotics, Antibiotic Resistance Genes and Pathogens in Aquatic Environment. Eco-Environ. Health 2024, 3, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; De, J.; Sur, S.; Banerjee, P. Interaction of Microbes with Microplastics and Nanoplastics in the Agroecosystems—Impact on Antimicrobial Resistance. Pathogens 2023, 12, 888. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, N.; Pan, J.; An, Q.; Li, X.; Sun, S.; Chen, C.; Zhu, H.; Li, Z.; Ji, Y. Distribution and Major Driving Elements of Antibiotic Resistance Genes in the Soil-Vegetable System under Microplastic Stress. Sci. Total Environ. 2024, 906, 167619. [Google Scholar] [CrossRef]
- Caruso, G. Microplastics as Vectors of Contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, X.; Tang, Z.; Fu, J.; Chen, F.; Zhao, Y.; Ruan, L.; Yang, Y. Downward Transport of Naturally-Aged Light Microplastics in Natural Loamy Sand and the Implication to the Dissemination of Antibiotic Resistance Genes. Environ. Pollut. 2020, 262, 114270. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Visca, A.; Caracciolo, A.B. Antibiotics as Emerging Pollutants of Soil Ecosystems. In Frontier Studies in Soil Science; Núñez-Delgado, A., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 21–41. ISBN 978-3-031-50502-7. [Google Scholar]
- Hartmann, M.; Six, J. Soil Structure and Microbiome Functions in Agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE Microplastic Films Alter Microbial Community Composition and Enzymatic Activities in Soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of Soil Enzyme Activities and Bacterial Communities to the Accumulation of Microplastics in an Acid Cropped Soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The Microplastisphere: Biodegradable Microplastics Addition Alters Soil Microbial Community Structure and Function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Visca, A.; Rauseo, J.; Spataro, F.; Patrolecco, L.; Grenni, P.; Massini, G.; Mazzurco Miritana, V.; Barra Caracciolo, A. Antibiotics and Antibiotic Resistance Genes in Anaerobic Digesters and Predicted Concentrations in Agroecosystems. J. Environ. Manag. 2022, 301, 113891. [Google Scholar] [CrossRef] [PubMed]
- Chacón, L.; Reyes, L.; Rivera-Montero, L.; Barrantes, K. Transport, Fate, and Bioavailability of Emerging Pollutants in Soil, Sediment, and Wastewater Treatment Plants: Potential Environmental Impacts. In Emerging Contaminants in the Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 111–136. ISBN 978-0-323-85160-2. [Google Scholar]
- Sungur, Ş. Pharmaceutical and Personal Care Products in the Environment: Occurrence and Impact on the Functioning of the Ecosystem. In Emerging Contaminants in the Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–157. ISBN 978-0-323-85160-2. [Google Scholar]
- Trufanov, D.; Akimenko, Y.; Kolesnikov, S.; Kazeev, K. Effects of Veterinary Antibiotics on the Soil Properties. In Emerging Contaminants; Elsevier: Amsterdam, The Netherlands, 2024; pp. 249–265. ISBN 978-0-443-18985-2. [Google Scholar]
- Kim, D.-W.; Cha, C.-J. Antibiotic Resistome from the One-Health Perspective: Understanding and Controlling Antimicrobial Resistance Transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Miłobedzka, A.; Ferreira, C.; Vaz-Moreira, I.; Calderón-Franco, D.; Gorecki, A.; Purkrtova, S.; Bartacek, J.; Dziewit, L.; Singleton, C.M.; Nielsen, P.H.; et al. Monitoring Antibiotic Resistance Genes in Wastewater Environments: The Challenges of Filling a Gap in the One-Health Cycle. J. Hazard. Mater. 2022, 424, 127407. [Google Scholar] [CrossRef]
- Gago, J.; Booth, A.M.; Tiller, R.; Maes, T.; Larreta, J. Microplastics Pollution and Regulation. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M.F., Mouneyrac, C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1071–1096. ISBN 978-3-030-39040-2. [Google Scholar]
- Manaia, C.M. Framework for Establishing Regulatory Guidelines to Control Antibiotic Resistance in Treated Effluents. Crit. Rev. Environ. Sci. Technol. 2023, 53, 754–779. [Google Scholar] [CrossRef]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A Review on Effective Soil Health Bio-Indicators for Ecosystem Restoration and Sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef] [PubMed]
- Saljnikov, E.; Lavrishchev, A.; Römbke, J.; Rinklebe, J.; Scherber, C.; Wilke, B.-M.; Tóth, T.; Blum, W.E.H.; Behrendt, U.; Eulenstein, F.; et al. Understanding and Monitoring Chemical and Biological Soil Degradation. In Advances in Understanding Soil Degradation; Saljnikov, E., Mueller, L., Lavrishchev, A., Eulenstein, F., Eds.; Innovations in Landscape Research; Springer International Publishing: Cham, Switzerland, 2022; pp. 75–124. ISBN 978-3-030-85681-6. [Google Scholar]
- Changey, F.; Nunan, N.; Herrmann, A.M.; Lerch, T.Z. Catching Change in Microbial Diversity Indicators under Different Soil Organic Matter Managements: Higher Taxonomic Resolution, Better Discrimination? Ecol. Indic. 2022, 139, 108897. [Google Scholar] [CrossRef]
- Chang, T.; Feng, G.; Paul, V.; Adeli, A.; Brooks, J.P. Soil Health Assessment Methods: Progress, Applications and Comparison. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2022; Volume 172, pp. 129–210. ISBN 978-0-323-98953-4. [Google Scholar]
- Raghavendra, M.; Sharma, M.P.; Ramesh, A.; Richa, A.; Billore, S.D.; Verma, R.K. Soil Health Indicators: Methods and Applications. In Soil Analysis: Recent Trends and Applications; Rakshit, A., Ghosh, S., Chakraborty, S., Philip, V., Datta, A., Eds.; Springer: Singapore, 2020; pp. 221–253. ISBN 9789811520389. [Google Scholar]
- Labouyrie, M.; Ballabio, C.; Romero, F.; Panagos, P.; Jones, A.; Schmid, M.W.; Mikryukov, V.; Dulya, O.; Tedersoo, L.; Bahram, M.; et al. Patterns in Soil Microbial Diversity across Europe. Nat. Commun. 2023, 14, 3311. [Google Scholar] [CrossRef] [PubMed]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. Emu: Species-Level Microbial Community Profiling of Full-Length 16S rRNA Oxford Nanopore Sequencing Data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Orgiazzi, A.; Ballabio, C.; Panagos, P.; Jones, A.; Fernández-Ugalde, O. LUCAS Soil, the Largest Expandable Soil Dataset for Europe: A Review. Eur. J. Soil. Sci. 2018, 69, 140–153. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. PlasticDB: A Database of Microorganisms and Proteins Linked to Plastic Biodegradation. Database 2022, 2022, baac008. [Google Scholar] [CrossRef]
- Dulya, O.; Mikryukov, V.; Shchepkin, D.V.; Pent, M.; Tamm, H.; Guazzini, M.; Panagos, P.; Jones, A.; Orgiazzi, A.; Marroni, F.; et al. A Trait-Based Ecological Perspective on the Soil Microbial Antibiotic-Related Genetic Machinery. Environ. Int. 2024, 190, 108917. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Simjee, S.; Ippolito, G. European Regulations on Prevention Use of Antimicrobials from January 2022. Braz. J. Vet. Med. 2022, 44, e000822. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Liu, Y.; Wang, X.; Zhang, B.; Zhang, W.; Chen, T.; Liu, G.; Xue, L.; Cui, X. Nocardioides: “Specialists” for Hard-to-Degrade Pollutants in the Environment. Molecules 2023, 28, 7433. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-M.; Mou, T.; Sun, Y.; Su, J.; Yu, L.-Y.; Zhang, Y.-Q. Environmental Distribution and Genomic Characteristics of Solirubrobacter, with Proposal of Two Novel Species. Front. Microbiol. 2023, 14, 1267771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Juneau, P.; Huang, R.; He, Z.; Sun, B.; Zhou, J.; Liang, Y. Coexistence between Antibiotic Resistance Genes and Metal Resistance Genes in Manure-Fertilized Soils. Geoderma 2021, 382, 114760. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, K.; Liu, C. Low-Density Polyethylene Enhances the Disturbance of Microbiome and Antibiotic Resistance Genes Transfer in Soil-Earthworm System Induced by Pyraclostrobin. J. Hazard. Mater. 2024, 465, 133459. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Tong, T.; Miao, S.; Huang, J.; Xie, S. Sulfadiazine Degradation in Soils: Dynamics, Functional Gene, Antibiotic Resistance Genes and Microbial Community. Sci. Total Environ. 2019, 691, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, G.; Yu, Y. Freeze-Thaw Aged Polyethylene and Polypropylene Microplastics Alter Enzyme Activity and Microbial Community Composition in Soil. J. Hazard. Mater. 2024, 470, 134249. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Na, J.; An, D.; Jung, J. Role of Benzophenone-3 Additive in Chronic Toxicity of Polyethylene Microplastic Fragments to Daphnia Magna. Sci. Total Environ. 2021, 800, 149638. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Guo, L.; Ye, C.; Zhu, J.; Zhang, M.; Yu, X. Accumulation of Antibiotic Resistance Genes in Full-Scale Drinking Water Biological Activated Carbon (BAC) Filters during Backwash Cycles. Water Res. 2021, 190, 116744. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Harkes, P.; Van Steenbrugge, J.J.M.; Geissen, V. Effects of Microplastics on Common Bean Rhizosphere Bacterial Communities. Appl. Soil Ecol. 2023, 181, 104649. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, Y.; Doyle, E.; Zhu, C.; Jin, X.; Chen, Z.; Wang, C.; He, H.; Zhou, D.; Gu, C. Microplastics Altered Soil Microbiome and Nitrogen Cycling: The Role of Phthalate Plasticizer. J. Hazard. Mater. 2022, 427, 127944. [Google Scholar] [CrossRef]
- Meng, M.; Li, Y.; Yao, H. Plasmid-Mediated Transfer of Antibiotic Resistance Genes in Soil. Antibiotics 2022, 11, 525. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Judge, M.F.; Maróti, G.; Becsei, Á.; Spisák, S.; Solymosi, N. Mobile Antimicrobial Resistance Genes in Probiotics. Antibiotics 2021, 10, 1287. [Google Scholar] [CrossRef]
- Ul Haq, H.; Huang, W.; Li, Y.; Zhang, T.; Ma, S.; Zhang, Y.; Song, Y.; Lin, D.; Tian, B. Genetic and Genomic Characterization of Multidrug Resistant Bacillus Subtilis M3 Isolated from an Activated Sludge Reactor Treating Wastewater. Biologia 2022, 77, 1151–1160. [Google Scholar] [CrossRef]
- Berbers, B.; Saltykova, A.; Garcia-Graells, C.; Philipp, P.; Arella, F.; Marchal, K.; Winand, R.; Vanneste, K.; Roosens, N.H.C.; De Keersmaecker, S.C.J. Combining Short and Long Read Sequencing to Characterize Antimicrobial Resistance Genes on Plasmids Applied to an Unauthorized Genetically Modified Bacillus. Sci. Rep. 2020, 10, 4310. [Google Scholar] [CrossRef]
- Rismondo, J.; Schulz, L.M. Not Just Transporters: Alternative Functions of ABC Transporters in Bacillus Subtilis and Listeria Monocytogenes. Microorganisms 2021, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Li, W.; Liu, Y.; Yang, K.; Wu, M.; Zhou, H. Effect of Polystyrene Microplastics of Different Sizes to Escherichia Coli and Bacillus Cereus. Bull. Environ. Contam. Toxicol. 2021, 107, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus Strains Isolated from Mangrove Ecosystems in Peninsular Malaysia for Microplastic Degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Vimala, P.P.; Mathew, L. Biodegradation of Polyethylene Using Bacillus Subtilis. Procedia Technol. 2016, 24, 232–239. [Google Scholar] [CrossRef]
- Chen, L.; Chen, S.; Zhang, Y.; Long, Y.; Kong, X.; Wang, S.; Li, L.; Wang, F.; Sun, Y.; Xu, A. Co-Occurrence Network of Microbial Communities Affected by Application of Anaerobic Fermentation Residues during Phytoremediation of Ionic Rare Earth Tailings Area. Sci. Total Environ. 2023, 856, 159223. [Google Scholar] [CrossRef]
- Rogiers, T.; Claesen, J.; Van Gompel, A.; Vanhoudt, N.; Mysara, M.; Williamson, A.; Leys, N.; Van Houdt, R.; Boon, N.; Mijnendonckx, K. Soil Microbial Community Structure and Functionality Changes in Response to Long-term Metal and Radionuclide Pollution. Environ. Microbiol. 2021, 23, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Jara-Servin, A.; Mejia, G.; Romero, M.F.; Peimbert, M.; Alcaraz, L.D. Unravelling the Genomic and Environmental Diversity of the Ubiquitous Solirubrobacter. Environ. Microbiol. 2024, 26, e16685. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, J.; Jia, T.; Chai, B. The Relationships between Soil Physicochemical Properties, Bacterial Communities and Polycyclic Aromatic Hydrocarbon Concentrations in Soils Proximal to Coking Plants. Environ. Pollut. 2022, 298, 118823. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Y.; Liu, L.; Wang, L.; Tang, J. Interaction between Microplastic Biofilm Formation and Antibiotics: Effect of Microplastic Biofilm and Its Driving Mechanisms on Antibiotic Resistance Gene. J. Hazard. Mater. 2023, 459, 132099. [Google Scholar] [CrossRef]
- Liu, X.; Fang, L.; Yan, X.; Gardea-Torresdey, J.L.; Gao, Y.; Zhou, X.; Yan, B. Surface Functional Groups and Biofilm Formation on Microplastics: Environmental Implications. Sci. Total Environ. 2023, 903, 166585. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tu, Y.; Chen, C.; Wang, F.; Yang, Y.; Hu, Y. Biofilms on Microplastic Surfaces and Their Effect on Pollutant Adsorption in the Aquatic Environment. J. Mater. Cycles Waste Manag. 2024, 26, 3303–3323. [Google Scholar] [CrossRef]
- Liu, X.; Wei, H.; Ahmad, S.; Wang, R.; Gao, P.; Chen, J.; Song, Y.; Liu, C.; Ding, N.; Tang, J. Effects and Mechanism of Microplastics on Abundance and Transfer of Antibiotic Resistance Genes in the Environment—A Critical Review. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1852–1874. [Google Scholar] [CrossRef]
- Petersen, F.; Hubbart, J.A. The Occurrence and Transport of Microplastics: The State of the Science. Sci. Total Environ. 2021, 758, 143936. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xiong, X.; Zhang, Y.; Wu, C.; Xu, X.; Sun, C.; Shi, H. Global Transportation of Plastics and Microplastics: A Critical Review of Pathways and Influences. Sci. Total Environ. 2022, 831, 154884. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of Microplastics and Antibiotic Resistance Genes and Their Effects on the Aquaculture Environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Z.; Zhu, J.; Shi, J.; Wei, H.; Xie, B.; Shi, H. Microplastics Act as Vectors for Antibiotic Resistance Genes in Landfill Leachate: The Enhanced Roles of the Long-Term Aging Process. Environ. Pollut. 2021, 270, 116278. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visca, A.; Di Gregorio, L.; Costanzo, M.; Clagnan, E.; Nolfi, L.; Bernini, R.; Orgiazzi, A.; Jones, A.; Vitali, F.; Mocali, S.; et al. Microbial Bioindicators for Monitoring the Impact of Emerging Contaminants on Soil Health in the European Framework. Sustainability 2025, 17, 1093. https://doi.org/10.3390/su17031093
Visca A, Di Gregorio L, Costanzo M, Clagnan E, Nolfi L, Bernini R, Orgiazzi A, Jones A, Vitali F, Mocali S, et al. Microbial Bioindicators for Monitoring the Impact of Emerging Contaminants on Soil Health in the European Framework. Sustainability. 2025; 17(3):1093. https://doi.org/10.3390/su17031093
Chicago/Turabian StyleVisca, Andrea, Luciana Di Gregorio, Manuela Costanzo, Elisa Clagnan, Lorenzo Nolfi, Roberta Bernini, Alberto Orgiazzi, Arwyn Jones, Francesco Vitali, Stefano Mocali, and et al. 2025. "Microbial Bioindicators for Monitoring the Impact of Emerging Contaminants on Soil Health in the European Framework" Sustainability 17, no. 3: 1093. https://doi.org/10.3390/su17031093
APA StyleVisca, A., Di Gregorio, L., Costanzo, M., Clagnan, E., Nolfi, L., Bernini, R., Orgiazzi, A., Jones, A., Vitali, F., Mocali, S., & Bevivino, A. (2025). Microbial Bioindicators for Monitoring the Impact of Emerging Contaminants on Soil Health in the European Framework. Sustainability, 17(3), 1093. https://doi.org/10.3390/su17031093









