Enhancing Utilization of Municipal Solid Waste Bottom Ash by the Stabilization of Heavy Metals
Abstract
1. Introduction
2. Methods
2.1. Ash Sampling
2.2. Ash Treatment Experiments (CO2, H2O and CO2/H2O Combination)
- A—industrial carbon dioxide (CO2; added 100% ash weight);
- B—water shower (H2O, addition of 10% ash weight);
- C—a combination of a water shower (10% ash weight) and gas (CO2).
2.3. Thermodynamic Calculations
2.4. Preparation of Ash Sample Leachates
2.5. Analyses of Physical–Chemical Parameters of Leachate Quality
3. Results
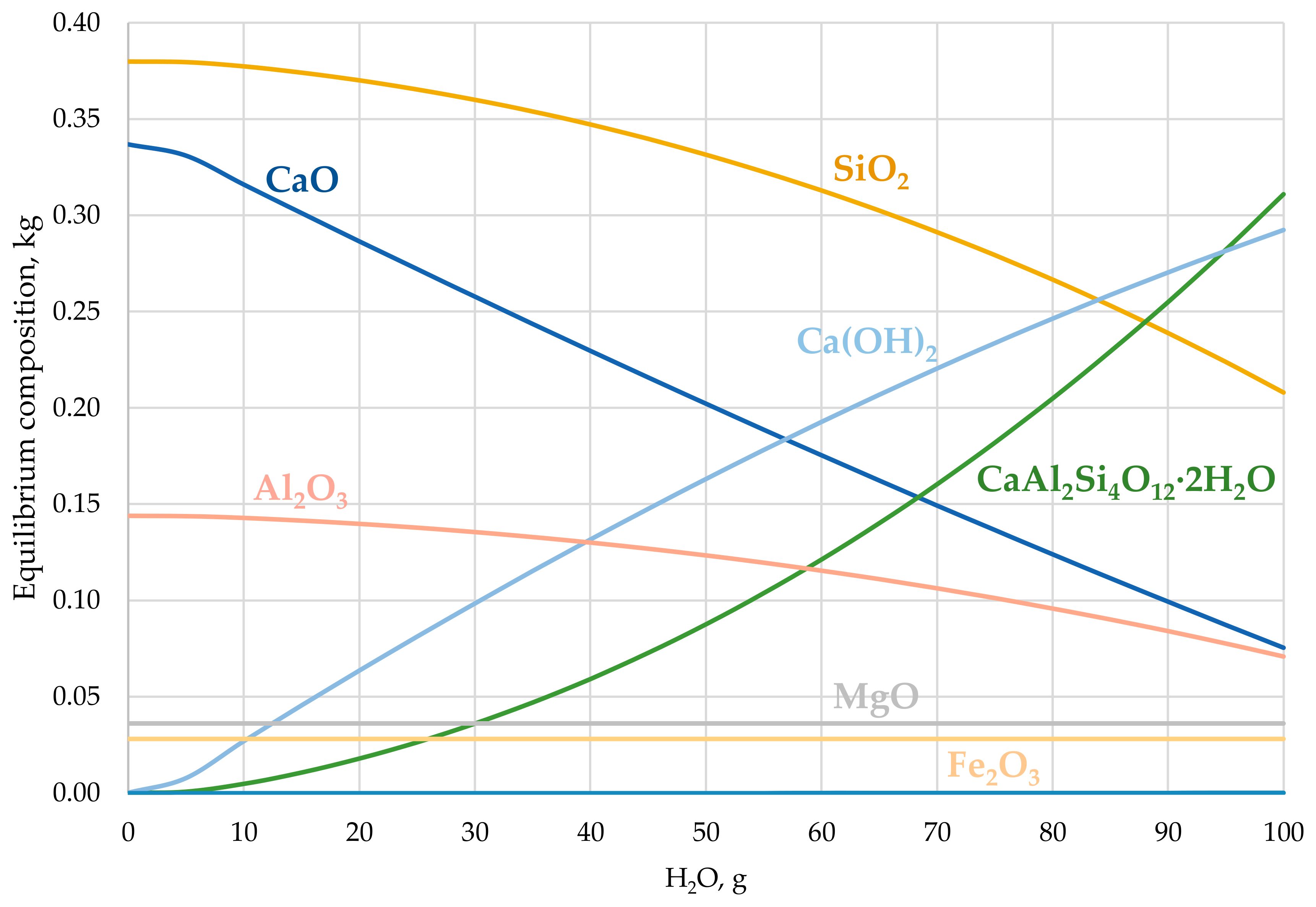
3.1. Thermodynamic Calculation Results
3.1.1. Treatment with CO2
3.1.2. Treatment with H2O
3.1.3. Treatment with H2O and CO2
3.2. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; Available online: http://hdl.handle.net/10986/30317 (accessed on 27 December 2024).
- Baccini, P.; Brunner, P.H. Metabolism of the Anthroposphere—Analysis, Evaluation, Design; The MIT Press: Cambridge, MA, USA, 2012; p. 228. [Google Scholar] [CrossRef]
- ACT Environmental Services. A Complete Guide to Solid Waste Incineration. Available online: https://www.actenviro.com/solid-waste-incineration/ (accessed on 27 December 2024).
- U.S. U.S. Energy Information Administration. Waste-to-Energy (Municipal Solid Waste). Available online: https://www.eia.gov/energyexplained/biomass/waste-to-energy.php (accessed on 10 December 2024).
- CEWEP. Waste-to-Energy Plants in Europe in 2021. Available online: https://www.cewep.eu/waste-to-energy-plants-in-europe-in-2021/ (accessed on 10 October 2024).
- Esoprog GmbH. Waste to Energy 2023/2024, Report. Available online: www.ecoprog.com (accessed on 10 October 2024).
- Valentim, B.; Guedes, A.; Kuźniarska-Biernacka, I.; Dias, J.; Predeanu, G. Variation in the Composition of Municipal Solid Waste Incineration Ash. Minerals 2024, 14, 1146. [Google Scholar] [CrossRef]
- Węgliński, S.; Martysz, G. Utilization of Municipal Solid Waste Incineration Bottom Ash and Fine Sand in Road Construction. Sustainability 2024, 16, 1865. [Google Scholar] [CrossRef]
- Atstaja, D.; Cudecka-Purina, N.; Koval, V.; Kuzmina, J.; Butkevics, J.; Hrinchenko, H. Waste-to-Energy in the Circular Economy Transition and Development of Resource-Efficient Business Models. Energies 2024, 17, 4188. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Lynn, C.J.; Dhir, R.K. Environmental Impacts of the Use of Bottom Ashes from Municipal Solid Waste Incineration: A Review. Resour. Conserv. Recycl. 2019, 140, 23–35. [Google Scholar] [CrossRef]
- Xue, M.; Hu, W.; Huanyu, L.; Fu, Y. Beneficial Reuse of Municipal Solid Waste Incineration Bottom Slag in Civil Engineering. Eng. Technol. Appl. Sci. Res. 2022, 12, 8306–8310. [Google Scholar] [CrossRef]
- Waste Management World. Fly Ash Recycling: Swedish Cleantech Company Treats Fly Ash for Full Circularity of Waste-to-Energy. Available online: https://waste-management-world.com/resource-use/fly-ash-recycling-for-full-circularity-of-waste-to-energy/ (accessed on 16 May 2024).
- ESWET. Giving Ash a New Life: Waste-to-Energy and Material Recovery. Available online: https://eswet.eu/giving-ash-a-new-life-waste-to-energy-and-material-recovery/ (accessed on 16 May 2024).
- European Commission. Commission Decision of 3 May 2000 Replacing Decision 94/3/EC Establishing a List of Wastes Pursuant to Article 1(a) of Council Directive 75/442/EEC on Waste and Council Decision 94/904/EC Establishing a List of Hazardous Waste Pursuant to Article 1(4) of Council Directive 91/689/EEC on Hazardous Waste; Document 02000D0532-20231206; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- European Parliament; Council of the European Union. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; European Union: Brussels, Belgium, 2008. [Google Scholar]
- Council of the European Union. Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste; European Union: Brussels, Belgium, 1999. [Google Scholar]
- Van Gerven, T.; Van Keer, E.; Arickx, S.; Jaspers, M.; Wauters, G.; Vandecasteele, C. Carbonation of MSWI-bottom ash to decrease heavy metal leaching, in view of recycling. Waste Manag. 2005, 25, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, K.; Brück, F.; Mansfeldt, T.; Weigand, H. Full-scale accelerated carbonation of waste incinerator bottom ash under continuous-feed conditions. Waste Manag. 2021, 125, 40–48. [Google Scholar] [CrossRef] [PubMed]
- PCC Group. Elements in the Earth’s Crust. Available online: https://www.products.pcc.eu/en/academy/elements-in-the-earths-crust/ (accessed on 16 May 2024).
- Tang, P.; Florea, M.V.A.; Spiesz, P.; Brouwers, H.J.H. Characteristics and Application Potential of Municipal Solid Waste Incineration (MSWI) Bottom Ashes from Two Waste-to-Energy Plants. Constr. Build. Mater. 2015, 83, 77–94. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Phoungthong, K.; Shi, D.X.; Shen, W.H.; Shao, L.M.; He, P.J. Leaching characteristics of calcium-based compounds in MSWI Residues: From the viewpoint of clogging risk. Waste Manag. 2015, 42, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Lin, H.-H.; Tang, Y.-C.; Shen, Y.-H. Recovery of Calcium from Reaction Fly Ash. Sustainability 2023, 15, 2428. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Fernández, A.I.; Miralles, L.; Segarra, M.; Espiell, F. Short-term natural weathering of MSWI bottom ash as a function of particle size. Waste Manag. 2003, 23, 887–895. [Google Scholar] [CrossRef] [PubMed]
- EN 12457-4:2002; Characterisation of Waste—Leaching—Conformity Test for Leaching of Granulated Waste and Sludge—Part 4: Single-Stage Batch Test at a Liquid/Solid Ratio of 10 L/kg for Materials with Particle Sizes Below 10 mm (Without Crushing or with Crushing). CEN/TC 444—Environmental Characterisation: Brussels, Belgium, 2002.
- EN 14899:2006; Characterization of Waste—Sampling of Waste Materials—Framework for the Preparation and Application of a Sampling Plan. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2006. Available online: https://standards.iteh.ai/catalog/standards/sist/132f21f0-38c3-49cd-b94e-fbd1792a3825/sist-en-14899-2006 (accessed on 13 October 2024).
- Fällman, A.-M. Leaching of chromium from steel slag in laboratory and field tests—A solubility-controlled process? Stud. Environ. Sci. 1997, 71, 531–540. [Google Scholar] [CrossRef]
- Choi, S.-K.; Lee, S.; Song, Y.-K.; Moon, H.-S. Leaching characteristics of selected Korean fly ashes and its implications for the groundwater composition near the ash disposal mound. Fuel 2002, 81, 1083–1090. [Google Scholar] [CrossRef]
- European Commission DG ENV. E3. Heavy Metals in Waste, Final Report, Project ENV.E.3/ETU/2000/0058; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Izquierdo, M.; Querol, X. Leaching Behaviour of Elements from Coal Combustion Fly Ash: An Overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef]
- DIN 38404-4:1976-12; German Standard Methods for Analysing of Water, Waste Water and Sludge—Physical and Physical-chemical Parameters (Group C)—Determination of Temperature (C4). German Institute for Standardization: Berlin, Germany, 1976. [CrossRef]
- ISO 10523:2008; Water Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2008.
- EN 27888:1993; Water Quality—Determination of Electrical Conductivity (ISO 7888:1985). European Committee for Standardization: Brussels, Belgium, 1993.
- EN 14346:2006; Characterization of Waste—Calculation of Dry Matter by Determination of Dry Residue or Water Content. European Committee for Standardization: Brussels, Belgium, 2006.
- EN 15169:2007; Characterization of Waste—Determination of Loss on Ignition in Waste, Sludge and Sediments. European Committee for Standardization: Brussels, Belgium, 2007.
- Radovanović, D.; Kamberović, Ž.; Korać, M.; Rogan, J. Solidified Structure and Leaching Properties of Metallurgical Wastewater Treatment Sludge after Solidification/Stabilization Process. J. Environ. Sci. Health Part A 2016, 51, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Petronijević, N.; Alivojvodić, V.; Sokić, M.; Marković, B.; Stanković, S.; Radovanović, D. Sustainable Mining towards Accomplishing Circular Economy Principles. J. Appl. Eng. Sci. 2020, 18, 493–499. [Google Scholar] [CrossRef]
- Republic of Slovenia. Republic of Slovenia. Regulation on Landfills [Uredba o odpadkih]. Official Gazette of the Republic of Slovenia, no. 10/14, 54/15, 36/16, 37/18, 13/21 and 44/22—ZVO-2. Available online: https://pisrs.si/pregledPredpisa?id=URED701 (accessed on 25 November 2024). (In Slovenian).









| Macroconstituents | Content (wt.%) |
|---|---|
| SiO2 | 37.98 |
| CaO | 33.69 |
| Al2O3 | 14.39 |
| MgO | 3.60 |
| Fe2O3 | 2.80 |
| Microconstituents | Content (g/kg) |
| PbO | 1.00 |
| BaO | 1.00 |
| Parameter | Method |
|---|---|
| Temperature (°C) | DIN 38404-4 [30] |
| pH value after 24 h/(leachate) | ISO 10523 [31] |
| Conductivity (mS/cm) after 24 h/(leachate) | EN 27888 [32] |
| Dry solids share (mg/L) | EN 14346 [33] and EN 15169 [34] |
| Acid capacity Ks 4.3 (mmol/L) | HL LCK 362 (0.5–8.0 mmol/L) |
| Barium (mg/L Ba) | HL PP 8014 (2–100 mg/L Ba) |
| Lead (mg/L Pb) | AAS |
| Chrom total (mg/L Cr) | AAS |
| Chloride (mg Cl/L) | HL LCK 311 (70–1000 mg/L Cl) |
| Sulfate (mg SO42−/L) | MN 086 (10–200 mg/L SO42−) |
| Compound | Content [kg] |
|---|---|
| SiO2 | 0.2078 |
| CaO | 0.0754 |
| CaAl2Si4O12 · 2H2O | 0.3109 |
| Ca(OH)2 | 0.2925 |
| Al2O3 | 0.0709 |
| MgO | 0.0360 |
| Fe2O3 | 0.0280 |
| TiO2 | 0.0120 |
| NaOH | 0.0116 |
| CuO | 0.0090 |
| KOH | 0.0024 |
| Ba(OH)2 | 0.0011 |
| PbO | 0.0010 |
| ZnO | 0.0010 |
| Ca3(Al2Si2O8)3 · CaCO3 | 0.0004 |
| Storage Conditions | Anaerobic | ||
|---|---|---|---|
| Ash Stabilization | None | ||
| Ash Aging | Start Sample | 15 Days | 30 Days |
| Date of Analysis | 26 November | 3 December | 18 December |
| Temperature (°C) | 21.3 | 22.3 | 21.2 |
| pH value after 24 h/(leachate) | 12.22 | 12.17 | 12.28 |
| Conductivity (mS/cm) after 24 h/(leachate) | 10.29 | 9.8 | 10.07 |
| Dry solids share (mg/L) | 99.686 | 99.686 | 99.612 |
| Acid capacity Ks 4.3 (mmol/L) | 35.65 | 21.7 | 27.45 |
| Barium (mg/L Ba) | 29.00 | 27.5 | 24.5 |
| Lead (mg/L Pb) | 0.266 | 0.374 | 0.309 |
| Chrom total (mg/L Cr) | 0.104 | 0.097 | 0.118 |
| Chloride (mg Cl/L) | 1775.0 | 1525.0 | 1227.5 |
| Sulfate (mg SO42−/L) | <10 | <10 | <10 |
| Barium (mg/kg d.s.) | 290.91 | 275.87 | 245.95 |
| Lead (mg/kg d.s.) | 2.668 | 3.752 | 3.102 |
| Chrom total (mg/kg d.s.) | 1.043 | 0.973 | 1.185 |
| Chloride (mg/kg d.s.) | 17,806 | 15,298 | 12,323 |
| Sulfate (mg/kg d.s.) | <100 | <100 | <100 |
| Storage Conditions | Aerobic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ash Stabilization | None | CO2 | H2O | CO2/H2O | ||||||
| Ash Aging | 0 Days | 0 Days | 2 Days | 5 Days | 0 Days | 2 Days | 5 Days | 0 Days | 2 Days | 5 Days |
| Date of Analysis | 25 Nov. | 25 Nov. | 27 Nov. | 30 Nov | 25 Nov. | 27 Nov. | 30 Nov. | 25 Nov. | 27 Nov. | 30 Nov. |
| Parameter | ||||||||||
| Temperature (°C) | 21.1 | 22.3 | 20.4 | 19.4 | 22.0 | 21.7 | 20.4 | 22.2 | 21.0 | 20.1 |
| pH value after 24 h/(leachate) | 11.43 | 11.17 | 11.76 | 11.76 | 12.22 | 12.02 | 12.177 | 12.02 | 11.17 | 11.16 |
| Conductivity (mS/cm) after 24 h/(leachate) | 5.81 | 5.33 | 5.90 | 5.37 | 9.30 | 7.87 | 8.64 | 7.63 | 5.13 | 5.03 |
| Dry solids share (mg/L) | 99.789 | 99.605 | 99.605 | 99.605 | 94.012 | 94.012 | 94.012 | 94.128 | 94.128 | 94.128 |
| Acid capacity Ks 4.3 (mmol/L) | 18.80 | 19.90 | 19.00 | 18.75 | 27.17 | 21.35 | 17.20 | 13.15 | 21.65 | 17.60 |
| Barium (mg/L Ba) | 26.00 | 26.50 | 25.00 | 27.500 | 29.00 | 20.00 | 20.60 | 28.50 | 14.50 | 14.750 |
| Lead (mg/L Pb) | 0.797 | 0.795 | 0.419 | 0.490 | 0.553 | 0.753 | 0.593 | 0.452 | 0.457 | 0.493 |
| Chrom total (mg/L Cr) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.0016 | <0.001 | <0.001 |
| Chloride (mg Cl/L) | 1270.0 | 1395.0 | 1320.0 | 1215.000 | 920.0 | 1262.5 | 1270.0 | 1447.5 | 1421.0 | 1240.000 |
| Sulfate (mg SO42−/L) | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Storage Conditions | Anaerobic | Aerobic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ash Stabilization | None | None | CO2 | H2O | CO2/H2O | |||||||
| Ash Aging | 0 Days | 0 Days | 0 Days | 2 Days | 5 Days | 0 Days | 2 Days | 5 Days | 0 Days | 2 Days | 5 Days | |
| Date of analysis | 25 Nov. | 25 Nov. | 25 Nov. | 27 Nov. | 30 Nov. | 25 Nov. | 27 Nov. | 30 Nov. | 25 Nov. | 27 Nov. | 30 Nov. | |
| Parameter | ELV * | Reference Sample | Start Sample | |||||||||
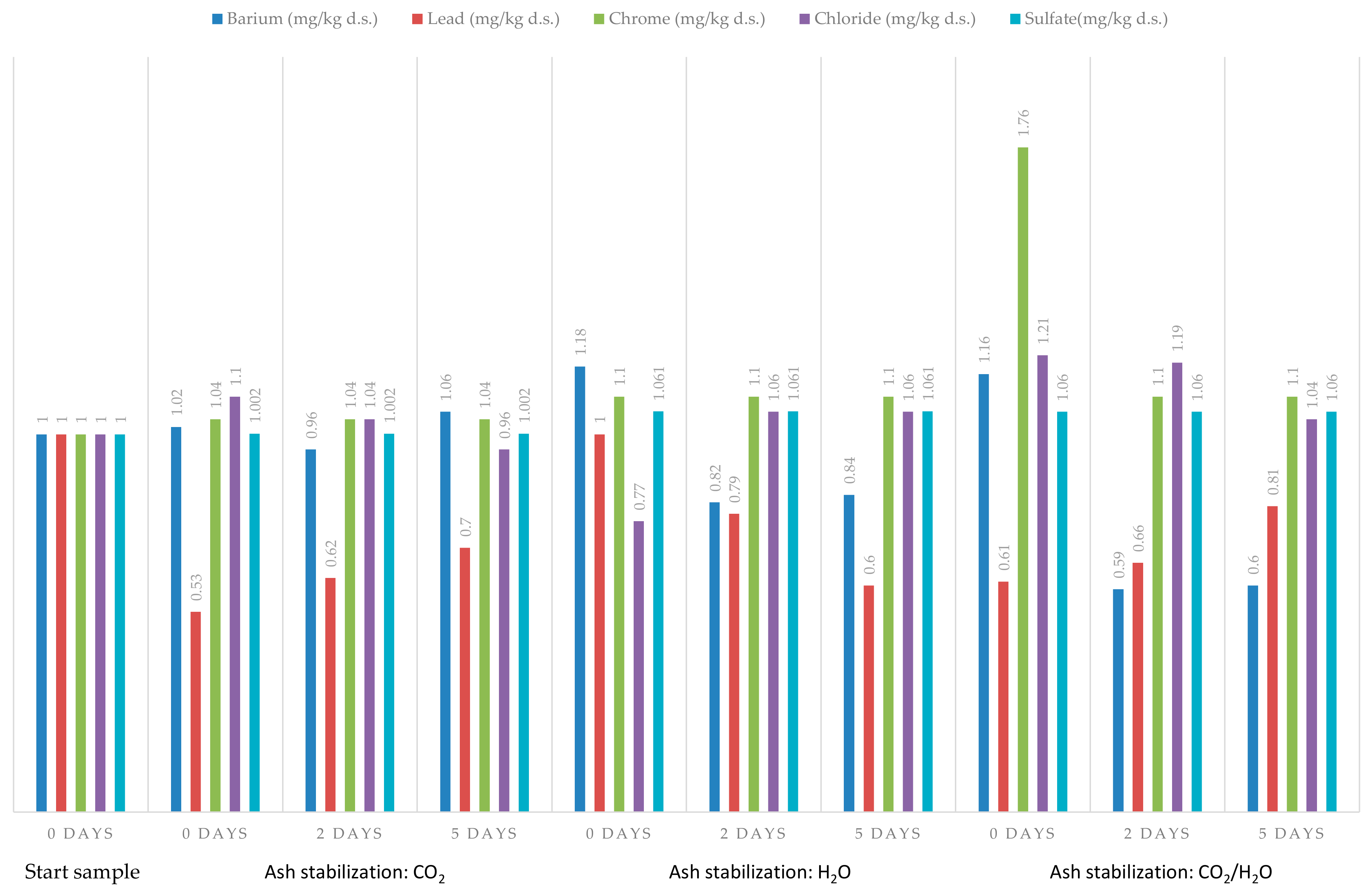
| 1 Barium (mg/kg d.s.) | 100 | 210.91 | 188.90 | 192.89 | 181.97 | 200.17 | 223.64 | 154.24 | 158.86 | 219.51 | 111.68 | 113.61 |
| 2 Barium | 1.12 | 1.00 | 1.02 | 0.96 | 1.06 | 1.18 | 0.82 | 0.84 | 1.16 | 0.59 | 0.60 | |
| 3 Barium | 1.00 | 0.90 | 0.91 | 0.86 | 0.95 | 1.06 | 0.73 | 0.75 | 1.04 | 0.53 | 0.54 | |
| 1 Lead (mg/kg d.s.) | 10 | 5.75 | 5.78 | 3.05 | 3.57 | 4.03 | 5.81 | 4.57 | 3.49 | 3.52 | 3.80 | 4.68 |
| 2 Lead | 0.99 | 1.00 | 0.53 | 0.62 | 0.70 | 1.00 | 0.79 | 0.60 | 0.61 | 0.66 | 0.81 | |
| 3 Lead | 1.00 | 1.00 | 0.53 | 0.62 | 0.70 | 1.01 | 0.80 | 0.61 | 0.61 | 0.66 | 0.81 | |
| 1 Chrome (mg/kg d.s.) | 10 | 0.160 | 0.007 | 0.007 | 0.007 | 0.007 | 0.008 | 0.008 | 0.008 | 0.012 | 0.008 | 0.008 |
| 2 Chrome | 22.90 | 1.00 | 1.04 | 1.04 | 1.04 | 1.10 | 1.10 | 1.10 | 1.76 | 1.10 | 1.10 | |
| 3 Chrome | 1.000 | 0.045 | 0.045 | 0.045 | 0.045 | 0.048 | 0.048 | 0.048 | 0.077 | 0.048 | 0.048 | |
| 1 Chloride (mg/kg d.s.) | 15,000 | 12,909 | 9227 | 10,154 | 9608 | 8844 | 7095 | 9736 | 9794 | 11,149 | 10,945 | 9551 |
| 2 Chloride | 1.40 | 1.00 | 1.10 | 1.04 | 0.96 | 0.77 | 1.06 | 1.06 | 1.21 | 1.19 | 1.04 | |
| 3 Chloride | 1.00 | 0.71 | 0.79 | 0.74 | 0.69 | 0.55 | 0.75 | 0.76 | 0.86 | 0.85 | 0.74 | |
| 1 Sulfate (mg/kg d.s.) | 20,000 | 72.73 | 72.65 | 72.79 | 72.79 | 72.79 | 77.12 | 77.12 | 77.12 | 77.02 | 77.02 | 77.02 |
| 2 Sulfate | 1.001 | 1.000 | 1.002 | 1.002 | 1.002 | 1.061 | 1.061 | 1.061 | 1.060 | 1.060 | 1.060 | |
| 3 Sulfate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.06 | 1.06 | 1.06 | 1.06 | 1.06 | 1.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokalj, F.; Alivojvodić, V.; Lešnik, L.; Petronijević, N.; Radovanović, D.; Samec, N. Enhancing Utilization of Municipal Solid Waste Bottom Ash by the Stabilization of Heavy Metals. Sustainability 2025, 17, 1078. https://doi.org/10.3390/su17031078
Kokalj F, Alivojvodić V, Lešnik L, Petronijević N, Radovanović D, Samec N. Enhancing Utilization of Municipal Solid Waste Bottom Ash by the Stabilization of Heavy Metals. Sustainability. 2025; 17(3):1078. https://doi.org/10.3390/su17031078
Chicago/Turabian StyleKokalj, Filip, Vesna Alivojvodić, Luka Lešnik, Nela Petronijević, Dragana Radovanović, and Niko Samec. 2025. "Enhancing Utilization of Municipal Solid Waste Bottom Ash by the Stabilization of Heavy Metals" Sustainability 17, no. 3: 1078. https://doi.org/10.3390/su17031078
APA StyleKokalj, F., Alivojvodić, V., Lešnik, L., Petronijević, N., Radovanović, D., & Samec, N. (2025). Enhancing Utilization of Municipal Solid Waste Bottom Ash by the Stabilization of Heavy Metals. Sustainability, 17(3), 1078. https://doi.org/10.3390/su17031078







