If You Plant It, Will They Come? Flowering Phenology, Breeding System, and Pollination Ecology of the Threatened Texas Endemic Hibiscus dasycalyx in Natural and Experimental Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species, Study Sites, and Plant Morphology
2.2. Flowering Phenology
2.3. Floral Visitors and Pollen Loads
2.3.1. Floral Visitors
2.3.2. Pollen Receipt and Removal
2.4. Breeding System, Fruit Set, Seed Set, and Seed Germination
2.4.1. Breeding System Study Design, Fruit Set, and Seed Set
2.4.2. Seed Germination
2.5. Statistical Analysis and Visualization
3. Results
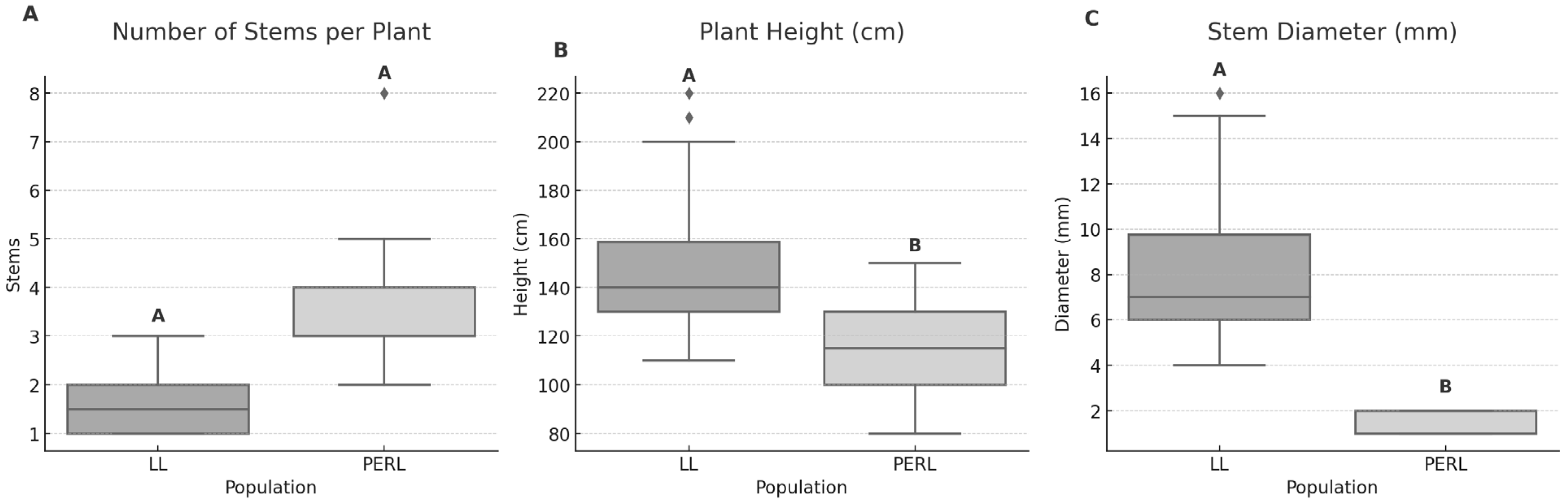
3.1. Site and Plant Characteristics
3.2. Flowering Phenology
3.3. Floral Visitors and Pollen Receipt and Removal
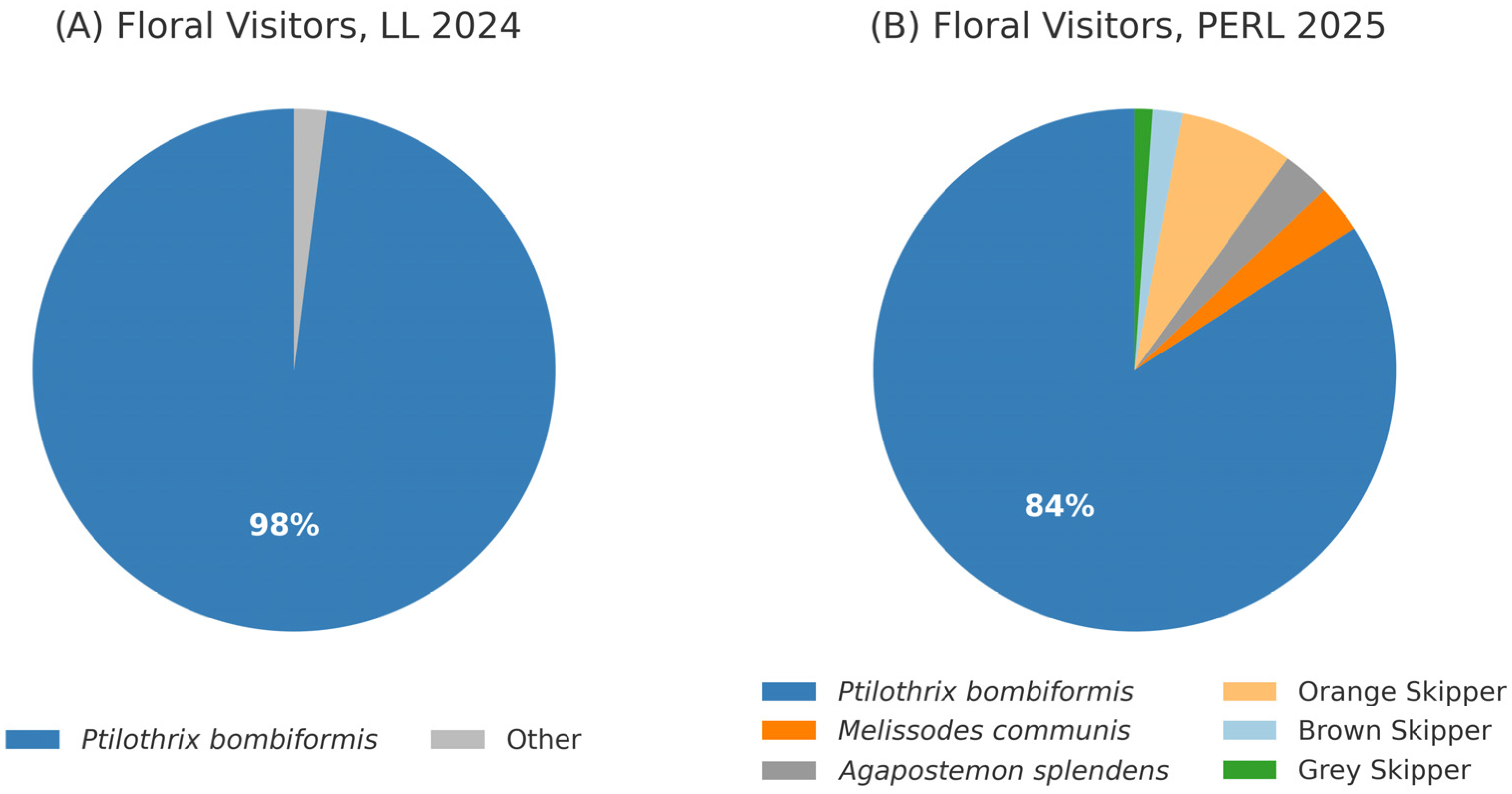
3.3.1. Floral Visitors
Floral Visitors to Natural Population LL (2024)
Floral Visitors to Created Population PERL (2024 and 2025)
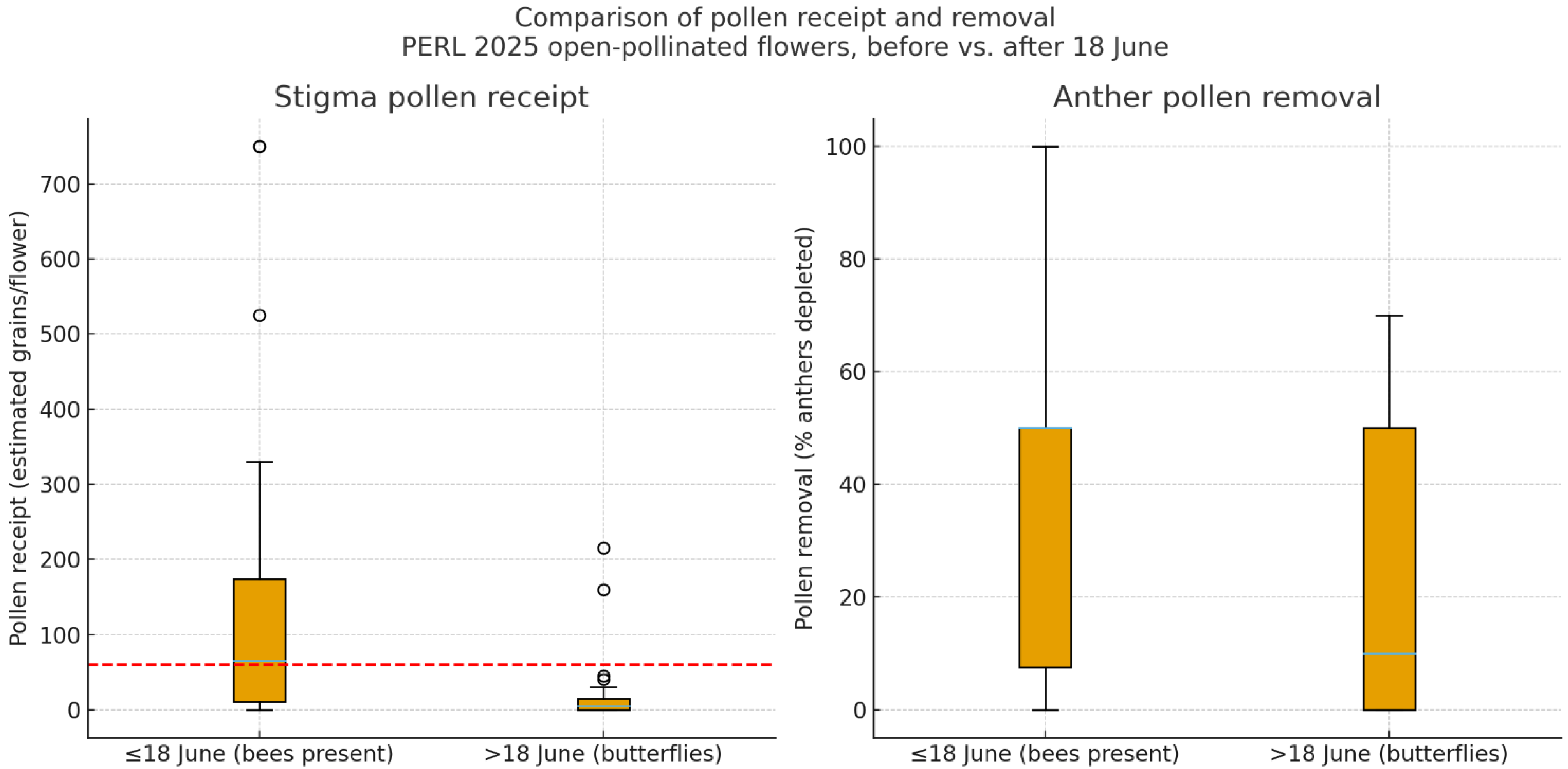
3.3.2. Pollen Receipt and Removal
Pollen Receipt
Pollen Removal
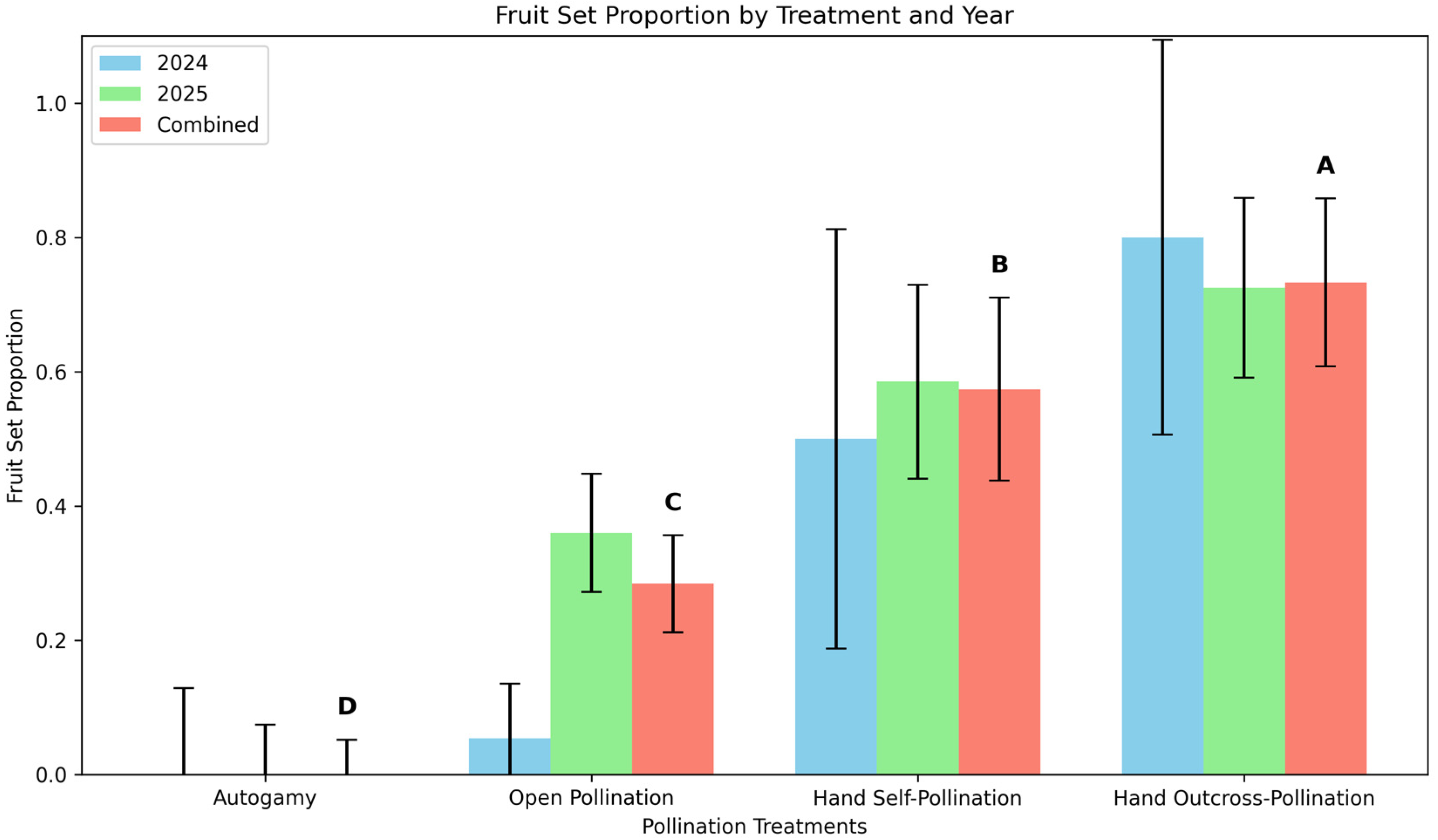
3.4. Breeding System Study
3.4.1. Fruit Set
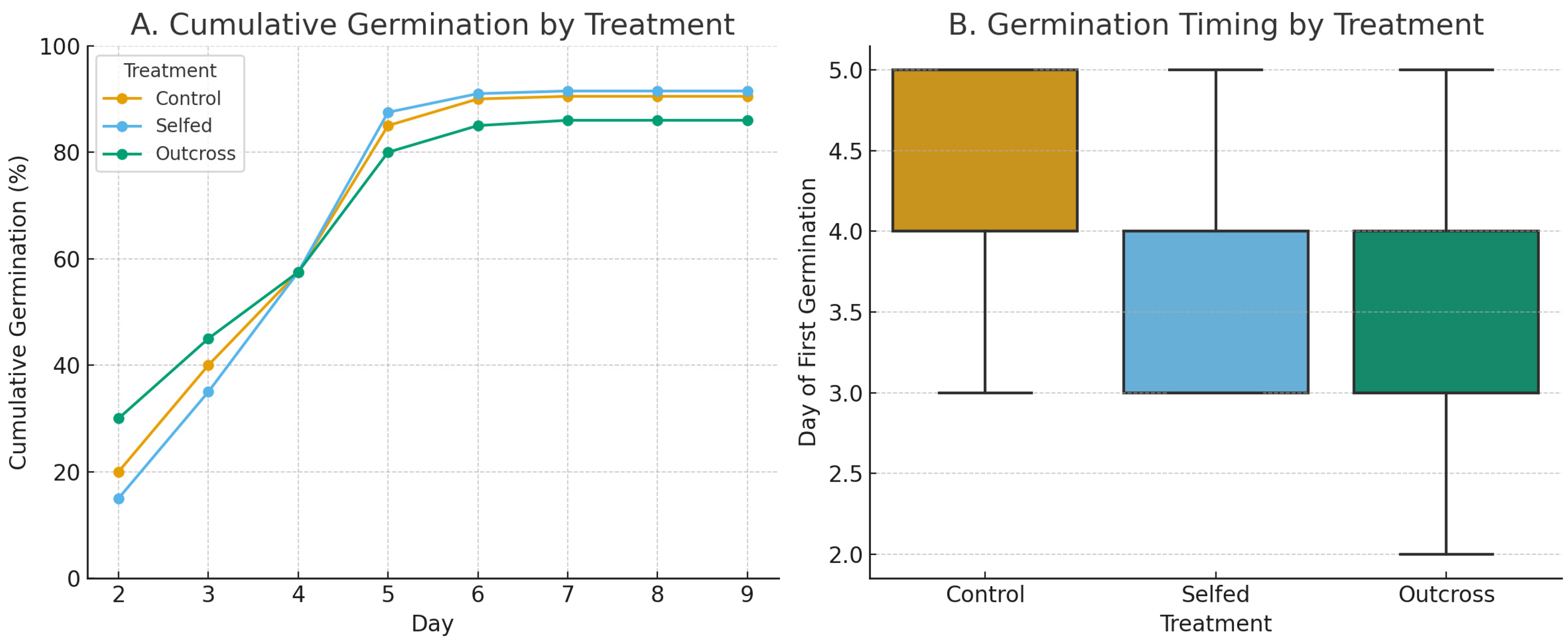
3.4.2. Seed Germination
Total Germination Percentage
Germination Speed
4. Discussion
4.1. Differences Between Natural and Created Sites
4.2. Floral Visitors, Behavior, and Pollination Efficiency
4.3. Breeding System
4.4. Seed Germination
4.5. Implications for Conservation and Restoration
5. Conclusions
- Establishing populations with adequate size and synchrony,
- Enhancing habitat features that attract and retain specialist pollinators,
- Monitoring reproductive metrics such as pollen deposition, fruit set, and seed viability.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathcke, B.; Lacey, E.P. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 1985, 16, 179–214. [Google Scholar] [CrossRef]
- Forrest, J.; Miller-Rushing, A.J. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3101–3112. [Google Scholar] [CrossRef]
- Lakra, N.; Dey, S.; Kumar, A.; Pandey, A.K. How floral phenology and breeding behaviour influence reproductive success by promoting cross-pollination of an endemic and endangered palm Bentinckia nicobarica (Arecaceae) in the niches of Andaman and Nicobar Islands of India. Arthropod-Plant Interact. 2025, 19, 10148. [Google Scholar] [CrossRef]
- Magrach, A.; Montoya, J.M. Stability in plant–pollinator communities across organizational levels: Present, gaps, and future. AoB Plants 2024, 16, plae026. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Ehrlén, J. Flower position within plants influences reproductive success both directly and via phenology. Am. J. Bot. 2024, 111, e16405. [Google Scholar] [CrossRef]
- Menz, M.H.M.; Phillips, R.D.; Winfree, R.; Kremen, C.; Aizen, M.A.; Johnson, S.D.; Dixon, K.W. Reconnecting plants and pollinators: Challenges in the restoration of pollination mutualisms. Trends Plant Sci. 2011, 16, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Steinger, T.; Müller-Schärer, H. The importance of plant provenance and genotypic diversity of seed material used for ecological restoration. Restor. Ecol. 2010, 18, 338–348. [Google Scholar] [CrossRef]
- Breed, M.F.; Harrison, P.A.; Blyth, C.; Byrne, M.; Gaget, V.; Gellie, N.J.C.; Lowe, A.J.; Munce, K.; Ottewell, K.M.; Thurgate, N.Y.; et al. The potential of genomics for restoring ecosystems and biodiversity. Nat. Rev. Genet. 2019, 20, 615–628. [Google Scholar] [CrossRef]
- Sipes, S.D.; Tepedino, V.J. Reproductive biology of the rare orchid Spiranthes diluvialis: Breeding system, pollination and implications for conservation. Conserv. Biol. 1995, 9, 929–938. [Google Scholar] [CrossRef]
- Kremen, C.; M′Gonigle, L.K. Small-scale restoration in intensive agricultural landscapes supports more specialized and less mobile pollinator species. J. Appl. Ecol. 2015, 52, 602–610. [Google Scholar] [CrossRef]
- Buisson, E.; Alvarado, S.T.; Le Stradic, S.; Morellato, L.P.C. Plant phenological research enhances ecological restoration. Restor. Ecol. 2017, 25, 164–171. [Google Scholar] [CrossRef]
- Glenny, W.; Kettenring, K.M.; Ogle, D.H.; Rohal, C.B.; Ricci, T.M.; Rohal, M.; Mealor, R.D.; Hinman, S.E. Plant selection for pollinator restoration in seminatural ecosystems. Front. Ecol. Environ. 2023, 21, 456–464. [Google Scholar] [CrossRef]
- Kline, C.; Joshi, N.K. Current trends in bee conservation and habitat restoration in anthropogenic habitats. Front. Ecol. Evol. 2024, 12, 1401233. [Google Scholar] [CrossRef]
- Gascoigne, J.; Berec, L.; Gregory, S.; Courchamp, F. Dangerously few liaisons: A review of mate-finding Allee effects. Popul. Ecol. 2009, 51, 355–372. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W. Pollinators, flowering plants, and conservation biology. BioScience 1997, 47, 297–307. [Google Scholar] [CrossRef]
- Bellis, J.; Albrecht, M.A.; Maschinski, J.; Osazuwa-Peters, O.; Stanley, T.; Heineman, K.D. Advancing the science and practice of rare plant conservation with the Center for Plant Conservation Reintroduction Database. Appl. Plant Sci. 2024, 12, e11583. [Google Scholar] [CrossRef]
- Liu, R.; Xie, D.; Li, M.; Ping, Y.; Li, D.; Dong, S.; Yu, Y.; Zhang, J.; Ning, Z. Selecting habitats to reintroduce the endangered species Ardisia gigantifolia (Primulaceae) based on growth and physiological traits. Glob. Ecol. Conserv. 2025, 57, e03388. [Google Scholar] [CrossRef]
- Trudgill, D. Past lessons and future requirements of reintroduction programmes for Cypripedium calceolus (Lady′s-slipper Orchid, Orchidaceae). Br. Ir. Bot. 2025, 7, 60–72. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, J.; Liao, Q.-C.; Zhou, Y.; Fu, X.-W.; Hu, Z.-H.; Hu, H.; Xu, G.-G.; Gao, T.-Y.; Zhang, S.-B. Reintroduction of an epiphytic orchid: Plant size matters. Glob. Ecol. Conserv. 2025, 58, e03496. [Google Scholar] [CrossRef]
- Stiling, P.; Duquesnel, J.; Fox, G. Prospects for the long-term persistence of a severely endangered plant, Consolea corallicola (Cactaceae). Conserv. Sci. Pract. 2025, 7, e70031. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, X.; Liu, Y.; Chen, J. Flower traits and breeding system of Rhododendron platypodum, an endangered plant species in China. PLoS ONE 2025, 20, e0319513. [Google Scholar] [CrossRef]
- Magray, R.A.; Ahmad, M.; Wani, A.A.; Dar, M.A.; Rather, M.A.; Ganie, S.A. How floral traits, pollinator behavior, and breeding strategy shape reproductive success in Phytolacca acinosa. Front. Plant Sci. 2025, 16, 1569420. [Google Scholar] [CrossRef] [PubMed]
- Kartesz, J.T. Biota of North America Program (BONAP). 2014. Available online: http://bonap.org (accessed on 3 November 2024).
- U.S. Fish and Wildlife Service (USFWS). Neches River Rose-Mallow (Hibiscus dasycalyx) Recovery Plan; U.S. Department of the Interior: Albuquerque, NM, USA, 2013.
- U.S. Fish and Wildlife Service (USFWS). Recovery Outline. Neches River Rose-Mallow (Hibiscus dasycalyx); U.S. Department of the Interior: Houston, TX, USA, 2018.
- Center for Plant Conservation. Hibiscus dasycalyx. 2024. Available online: https://saveplants.org (accessed on 25 July 2025).
- Poole, J.M.; Carr, W.R.; Price, D.M.; Singhurst, J.R. Rare Plants of Texas; Texas A&M University Press: College Station, TX, USA, 2008; 650p. [Google Scholar]
- Klips, R.A. Genetic affinity of the rare eastern Texas endemic Hibiscus dasycalyx (Malvaceae). Am. J. Bot. 1995, 82, 1463–1472. [Google Scholar] [CrossRef]
- Blanchard, O.J. Morphological and Hybridization Studies in Hibiscus sect. Muenchhusia. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1976. [Google Scholar]
- Wise, D.A.; Menzel, M.Y. Genetic affinities of the North American species of Hibiscus sect. Trionum. Brittonia 1977, 23, 425–437. [Google Scholar] [CrossRef]
- Small, R.L. Phylogeny of Hibiscus sect. Muenchhusia (Malvaceae) based on chloroplast rpl16 and ndhF, and nuclear ITS and GBSSI sequences. Syst. Bot. 2004, 29, 385–392. [Google Scholar] [CrossRef]
- Sain, M.P. Distinguishing the Neches River Rose Mallow, Hibiscus dasycalyx, from Its Congeners Using DNA Sequence Data and Niche Modeling Methods. Master’s Thesis, The University of Texas at Tyler, Tyler, TX, USA, 2015. [Google Scholar]
- Sain, M.P.; Norrell-Tober, J.; Barthel, K.; Seawright, M.; Blanton, A.; Hertweck, K.L.; Rushing, N. Multiple complementary studies clarify which co-occurring congener presents the greatest hybridization threat to a rare Texas endemic wildflower (Hibiscus dasycalyx: Malvaceae). J. Bot. Res. Inst. Tex. 2021, 15, 283–308. [Google Scholar] [CrossRef]
- Langlinais, M.E. Distribution and Soil Characteristics Associated with the Neches River Rose Mallow (Hibiscus dasycalyx). Electronic Theses and Dissertations 476. Master’s Thesis, Stephen F. Austin State University, Nacogdoches, TX, USA, 2022. Available online: https://scholarworks.sfasu.edu/etds/476 (accessed on 3 November 2024).
- Simpson, M.D. An Evaluation of Hibiscus moscheutos ssp. lasiocarpos and Ipomoea pandurata as Host Plants of the Specialist Bee Ptilothrix bombiformis (Apoidea: Emphorini) and the Role of Floral Scent Chemistry in Host-Plant Selection. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2009. [Google Scholar]
- Rust, R.W. The biology of Ptilothrix bombiformis (Hymenoptera: Anthophoridae). J. Kans. Entomol. Soc. 1980, 53, 427–436. Available online: https://www.jstor.org/stable/25084048 (accessed on 23 September 2025).
- Moskowitz, D. Observational habitat use of the rose mallow bee Ptilothrix bombiformis Hymenoptera: Apoidea in a suburban landscape. Urban Nat. 2023, 10, 1–8. [Google Scholar]
- Cane, J.H.; Sipes, S. Characterizing floral specialization by bees: Analytical methods and a revised lexicon for oligolecty. In Plant–Pollinator Interactions: From Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 99–122. [Google Scholar]
- Pascarella, J.B. An annotated checklist of the bee (Hymenoptera: Apoidea) faunal diversity in southeastern forest ecosystems of the USA. Trends Entomol. 2023, 19, 113–127. [Google Scholar]
- Flórez-Gómez, N.; Danforth, B. The North American bees of the genus Ptilothrix Cresson, 1878 (Hymenoptera, Apidae, Emphorini), with the description of two new species. J. Hymenopt. Res. 2023, 95, 275–293. [Google Scholar] [CrossRef]
- Augspurger, C.K. Phenology, flowering synchrony, and fruit set of six neotropical shrubs. Biotropica 1983, 15, 257–267. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.; Traveset, A. Effects of flowering phenology and synchrony on the reproductive success of a long-flowering shrub. AoB Plants 2016, 8, plw007. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.3.2; R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org (accessed on 3 November 2024).
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Minitab. Minitab Statistical Software, version 21.4.3; Minitab LLC: State College, PA, USA, 2024.
- Sampson, B.J.; Pounders, C.T.; Werle, C.T.; Mallette, T.R. Aggression between floral specialist bees enhances pollination of Hibiscus (section Trionum: Malvaceae). J. Pollin. Ecol. 2016, 18, 7–12. [Google Scholar] [CrossRef]
- Klips, R.A.; Snow, A.A. Delayed autonomous self-pollination in Hibiscus laevis (Malvaceae). Am. J. Bot. 1997, 84, 48–53. [Google Scholar] [CrossRef]
- Klips, R.A. Pollen competition as a reproductive isolating mechanism between two sympatric Hibiscus species (Malvaceae). Am. J. Bot. 1999, 86, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.A.; Spira, T.P. Differential pollen-tube growth rates and nonrandom fertilization in Hibiscus moscheutos. Am. J. Bot. 1991, 78, 1419–1426. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Vamosi, J.C.; Mazer, S.J.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mitchell, R.J.; Ashman, T.L. Pollen limitation of plant reproduction: Pattern and process. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 467–497. [Google Scholar] [CrossRef]
- Charlesworth, D.; Willis, J.H. The genetics of inbreeding depression. Nat. Rev. Genet. 2009, 10, 783–796. [Google Scholar] [CrossRef]
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, B.; Liu, X.; Li, F.; Zhu, Z.; Quan, Q.; Li, Y. Do larger pollinators have higher pollination efficiency for the generalized pollination plant Hibiscus mutabilis? Biology 2024, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Senapathi, D.; Fründ, J.; Albrecht, M.; Garratt, M.P.; Kleijn, D.; Pickles, B.J.; Potts, S.G.; An, J.; Andersson, G.K.; Bänsch, S.; et al. Wild insect diversity increases inter-annual stability in global crop pollinator communities. Proc. R. Soc. B 2021, 288, 20210212. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Eeraerts, M.; Osterman, J.; Beyer, N.; Hass, A.; Lundin, O.; Westphal, C. Crop diversification for pollinator conservation. Landsc. Ecol. 2025, 40, 19. [Google Scholar] [CrossRef]
- Aluri, J.S.R.; Kunuku, V.R.; Chappidi, P.R.; Kammarchedu, B.J.P. Pollination ecology of Hibiscus tiliaceus L. (Malvaceae), an evergreen tree species valuable in coastal and inland eco-restoration. Transylv. Rev. Syst. Ecol. Res. 2020, 22, 47–56. [Google Scholar] [CrossRef]
- Vaidya, K.R. Natural cross-pollination in roselle, Hibiscus sabdariffa L. (Malvaceae). Genet. Mol. Biol. 2000, 23, 667–669. [Google Scholar] [CrossRef]
- Tang, R.; Li, Y.; Xu, Y.; Schinnerl, J.; Sun, W.; Chen, G. In-situ and ex situ pollination biology of the four threatened plant species and the significance for conservation. Biodivers. Conserv. 2020, 29, 381–391. [Google Scholar] [CrossRef]

| Score | Pollen Grain Count |
|---|---|
| 0 | No grains |
| 1 | <10 grains |
| 2 | 10–50 grains |
| 3 | 50–100 grains |
| 4 | >100 grains |
| Variable | LL Mean ± SE | PERL Mean ± SE | t-Statistic | p-Value |
|---|---|---|---|---|
| Soil pH | 5.92 ± 0.09 | 5.50 ± 0.00 | 4.499 | 0.0003 |
| Soil Temperature (°C) | 24.78 ± 0.13 | 24.00 ± 0.00 | 6.018 | <0.0001 |
| Soil Moisture (score) | 4.00 ± 0.00 | 4.00 ± 0.00 | — | — |
| Light (score) | 5.20 ± 0.20 | 6.00 ± 0.00 | 4.000 | 0.0161 |
| Canopy Openness (%) | 88.19 ± 3.29 | 96.17 ± 3.83 | 1.578 | 0.1308 |
| Species | n | Handling Time (s) (Mean ± SE, Range) |
|---|---|---|
| Agapostemon splendens | 8 | 73.5 ± 25.6 (16–229) |
| Melissodes communis | 9 | 26.2 ± 7.26 (1–56) |
| Ptilothrix bombiformis | 122 | 14.1 ± 1.68 (1–134) |
| Behavior | n | Handling Time (s) (Mean ± SE, Range) |
|---|---|---|
| Nectaring | 7 | 5 (2–14) |
| Pollen gathering | 107 | 14.4 ± 1.81 (1–134) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascarella, J.B. If You Plant It, Will They Come? Flowering Phenology, Breeding System, and Pollination Ecology of the Threatened Texas Endemic Hibiscus dasycalyx in Natural and Experimental Populations. Sustainability 2025, 17, 9638. https://doi.org/10.3390/su17219638
Pascarella JB. If You Plant It, Will They Come? Flowering Phenology, Breeding System, and Pollination Ecology of the Threatened Texas Endemic Hibiscus dasycalyx in Natural and Experimental Populations. Sustainability. 2025; 17(21):9638. https://doi.org/10.3390/su17219638
Chicago/Turabian StylePascarella, John B. 2025. "If You Plant It, Will They Come? Flowering Phenology, Breeding System, and Pollination Ecology of the Threatened Texas Endemic Hibiscus dasycalyx in Natural and Experimental Populations" Sustainability 17, no. 21: 9638. https://doi.org/10.3390/su17219638
APA StylePascarella, J. B. (2025). If You Plant It, Will They Come? Flowering Phenology, Breeding System, and Pollination Ecology of the Threatened Texas Endemic Hibiscus dasycalyx in Natural and Experimental Populations. Sustainability, 17(21), 9638. https://doi.org/10.3390/su17219638






