Abstract
Employing crop cultivars with low cadmium (Cd) accumulation and high yield is an effective strategy for the sustainable and safe utilization of Cd-contaminated farmland. However, the current understanding of peanut cultivars, particularly under field conditions, is limited. This study identified low-Cd cultivars and their rhizosphere microbial characteristics in acidic and alkaline fields with moderate Cd contamination. The results indicated that cultivars LH11, FH1, LH14, and YH9414 exhibited low Cd accumulation and high yield, with kernel Cd content reduced by 27.27% to 47.28% and yield increased by 9.27% to 14.17% compared with cultivar SLH. Among them, FH1 was validated to achieve safe production in two fields. A unique microbial community was formed by the recruitment of diverse microbes, such as Alphaproteobacteria, Acidobacteria, Gemmatimonadetes, and Chloroflexi, to the rhizosphere soil of FH1, which might be associated with Cd immobilization and the promotion of plant growth. Functional predictions further validated these findings, revealing enhanced functional pathways in the FH1 rhizosphere related to microbial proliferation, Cd stabilization, and detoxification. This study provides valuable germplasm resources for safe agriculture of Cd-polluted soils and elucidates the rhizosphere microbial characteristics of different peanut cultivars under field conditions. These findings are important for the targeted management of contaminated farmland and ensuring safe food production.
1. Introduction
Cadmium (Cd) is a nonessential element with significant toxicity risks to human. The increase in industrial activities and widespread agrochemical use have greatly aggravated Cd contamination in soils [1,2]. Cd can be taken up by plants through their root system, affecting normal growth, and severely hindering the advancement of sustainable agriculture [3,4]. Cd in crops can enter animals and humans through the food chain, leading to various health issues [3]. Consequently, various strategies have been adopted to minimize Cd content in the edible parts of crops [5,6,7]. Among these strategies, low-Cd-accumulating cultivars (LACs) stand out due to their advantages of sustainability, no secondary contamination, and low cost [8]. The adoption of LACs effectively reduces the Cd content in the grains of crops such as rice, wheat, and soybean [9,10,11]. However, research on peanuts remains insufficient, and the selection of suitable peanut cultivars for Cd-contaminated farmland is still significantly limited.
Peanut (Arachis hypogaea L.) is a widely cultivated oil crop with nutrient-rich kernels that is known for its high protein content, healthy fat content, and diverse culinary applications [12]. In China, peanuts hold a crucial position in food and oil production as well as in agricultural exports. According to the China Statistical Yearbook 2024, China’s peanut planting area in 2023 reached 4798 thousand hectares, with an annual production of 19.231 million tons. However, peanuts have been found to be contaminated with varying levels of potentially toxic elements [13,14]. Studies have indicated that peanuts have greater accumulation capacity for Cd than other potentially toxic elements, and a greater capacity in comparison with other crops in polluted areas [15,16]. This phenomenon poses a consumption risk when peanuts are grown in Cd-contaminated soil. Increasing evidence suggests that Cd accumulation differs significantly among peanut cultivars [17,18,19]. This makes it possible to screen LACs of peanuts. In previous studies, we have made some progress in screening peanut cultivars with different Cd-accumulation characteristics under acidic soil conditions [18,20]. However, given the potential uncertainty of Cd-accumulation characteristics across different environmental conditions, further screening of cultivars in diverse environments is necessary. More critically, most efforts to screen peanut cultivars have relied primarily on pot experiments, with limited attention given to field-based screening in real growing environments [20,21,22]. Moreover, in actual production, yield is directly related to farmers’ economic benefits. Therefore, conducting in-depth studies on peanut cultivars with both low Cd accumulation and high yield under actual field conditions is essential for safeguarding agricultural production and promoting the sustainability of agricultural systems.
LACs typically resist Cd in polluted soil by activating their defense mechanisms and regulating their interaction with the environment [23]. The rhizosphere, as a complex zone where plant roots, soil, and microbes interact, plays a crucial role in the accumulation of Cd [24]. Peanuts primarily absorb Cd from the soil through their roots, and peanut cultivars with varying Cd accumulation characteristics may present significant differences in rhizosphere environments [25]. Advances in high-throughput sequencing technologies and analytical approaches have offered powerful tools for the study of rhizosphere microorganisms [26]. Studies on other crops have demonstrated that differences in root exudates among cultivars can regulate the composition and function of rhizosphere bacterial communities [27,28]. To cope with Cd stress, LACs may enhance plant Cd tolerance and promote growth by recruiting beneficial microorganisms [23]. Some microorganisms have been found to directly or indirectly participate in Cd stabilization, thereby reducing Cd uptake by plants [28]. However, the rhizosphere soil microbial characteristics of low-Cd-accumulation peanut cultivars remain poorly understood. An understanding of the rhizosphere microbial characteristics of peanuts is crucial for advancing peanut cultivar development and the targeted regulation of Cd uptake in crops.
This study investigated the Cd-accumulation characteristics and rhizosphere microenvironment of different peanut cultivars with the following aims: (1) to identify valuable high-yield LACs of peanuts for farmland with low-to-moderate-Cd pollution; (2) to discover differences in the rhizosphere microenvironment among peanut cultivars; and (3) to elucidate the rhizosphere microorganisms and their functional characteristics in relation to LACs. In summary, this study offers germplasm resources for the safe utilization of Cd-polluted farmland and offers a scientific basis for targeted regulation, which will strongly safeguard human health and promote sustainable agriculture.
2. Materials and Methods
2.1. Experimental Setup and Sample Collection
Field trials were conducted over two years in two fields with different soil properties in Shandong Province, China. The soil texture of Field I was silty loam, with a pH of 8.22 ± 0.04, electrical conductivity (EC) of 150 ± 11 μs cm−1, soil organic matter (SOM) content of 34.51 ± 1.96 g kg−1, and total Cd content of 1.39 ± 0.20 mg kg−1. The soil texture of experimental Field II was silty clay loam, with a pH of 5.99 ± 0.79, EC of 45 ± 7 μs cm−1, SOM content of 25.29 ± 2.66 g kg−1, and total Cd content of 0.36 ± 0.09 mg kg−1. According to GB15618-2018 [29], both fields were at risk of moderate soil contamination. In the first year, a screening experiment involving 30 peanut cultivars was conducted in Field I. Detailed information on the peanut cultivars is listed in Supplementary Table S1. The experimental farmland was divided into 90 experimental plots (2 m by 3 m each) in a completely randomized design, with each of the 30 peanut cultivars replicated three times. Based on previous studies and the results from Field I, the low-Cd cultivar FH1 and the high-Cd cultivar SLH were selected to validate Cd accumulation in Field II. Each peanut cultivar was replicated three times in a randomized trial design, with Field II containing six experimental plots (3.25 m × 2.5 m each). Each experimental plot in each field was configured with two ridges, with two peanut seeds planted per hole on each side of the ridge. The plots were spaced 0.5 m apart and surrounded by a 2 m buffer zone to minimize interference from adjacent plots. Both experiments used specialized peanut fertilizer as a base fertilizer prior to planting, in accordance with local agricultural practices for peanut management.
After peanut ripening, peanut and corresponding soil samples were collected synchronously via the five-point sampling method (NY/T 395-2012) [30]. Ten peanut plants were harvested from each experimental plot, and their pods were carefully collected. After shaking off the bulk soil from the peanut root zone, the soil remaining attached to the roots was collected [31]. A part of each sample was air-dried to determine the soil properties. The rest was transported under dry ice conditions and preserved at −80 °C for subsequent microbial analyses.
2.2. Sample Preparation and Analytical Methods
The peanut pods were cleaned and air-dried, and their kernels were collected for yield determination. The kernels were dried in an oven at 65 °C until they reached a constant weight, and then ground into powder. Approximately 0.3000 g of the sample was digested with an HNO3–HClO4 mixture (3:1, v/v) [20]. Following the method of GB 5009.15–2014 [32], the digested solution was then diluted to a final volume of 25 mL and filtered to determine the Cd concentration via an iCE 3500 atomic absorption spectrometer (Thermo Scientific, USA). Reference materials GBW(E)100718 (peanut) and P14239 (beans) were used for quality control, along with blanks.
The air-dried soils were ground through a 10-mesh nylon sieve to determine the soil pH and EC. The soil pH (HJ 962–2018) [33] and EC [34] were measured via a pH meter and a conductivity meter, respectively. The soil samples were subsequently ground and passed through a 60-mesh sieve. A portion of the samples was used to determine the SOM, while the remainder was further ground through a 100-mesh sieve for total Cd analysis. The SOM was measured with reference to the method in NY/T 1121.6-2006 [35]. The total Cd was determined as described by Bai et al. (2023a) [36] and GB/T 17141-1997 [37]. In brief, approximately 0.2000 g of sample was digested in a fully automatic graphite digestion apparatus with sequential additions of 10 mL HNO3, 5 mL HF, and 2 mL HClO4. After digestion, the solution was diluted to a final volume of 25 mL and filtered, and the Cd concentration in the filtrate was determined via the iCE 3500 atomic absorption spectrometer [36]. Soil standard substances and blank samples were utilized for quality control during the soil analysis procedure. The soil standards used were GBW07416b (ASA-5b) for the pH, EC, and SOM, and GBW07982 (GSS-40) and GBW07555 (GSS-64) for the total Cd.
Soil samples preserved at −80 °C were used for 16S rRNA gene sequencing. First, the soil bacterial DNA was extracted via the TIANamp Soil DNA Kit. The V4 region of the bacterial 16S rRNA gene was amplified via PCR using the primer pairs of 515F/806R (515F: 5′-GTGCCAGCMGCCGCGGTAA-3′, 806R: 5′-GGACTACHVGGGTWTCTAAT-3′) [38]. The PCR conditions involved initial denaturation at 98 °C for 1 min, followed by 30 cycles of 98 °C for 10 s, 50 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min. The PCR amplicons were purified via magnetic beads and recovered with a Universal DNA Purification Kit (TIANGEN, Beijing, China). Libraries were constructed via the NEBNext® UltraTM II DNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA). The quantified libraries were then sequenced via paired-end (PE250) technology on the NovaSeq 6000 platform at Novogene Co., Ltd. (Beijing, China).
2.3. Statistical and Bioinformatic Analyses
The bioconcentration factor (BCF) of peanuts was calculated in Microsoft Excel 2019 as the ratio of the Cd content in the kernels to that in the soil. A higher BCF indicates a greater accumulation capacity and increased consumption risk. The peanut cultivars were classified according to their BCF values via the hierarchical clustering method implemented in SPSS 27.0. Normality tests were performed via SPSS 27.0, followed by either Student’s t-test (parametric) or the Wilcoxon rank-sum test (nonparametric) to compare differences in various indicators between the two cultivars. Spearman’s correlation analysis was conducted via R software (v.4.3.3) to elucidate the relationships between environmental factors and Cd-accumulation characteristics.
The microbiome bioinformatics data were analyzed via EasyAmplicon [39], with sequences processed via VSEARCH [40] and USEARCH [41] for merging, primer removal, quality control, and dereplication. Denoising was conducted via UNOISE3 to generate amplicon sequence variants (ASVs) (Supplementary Table S3). Taxonomic annotation was performed via the Silva database [42,43], with the removal of chimeras, mitochondria, chloroplasts, and archaea (Supplementary Table S3c). Principal coordinate analysis (PCoA) with Bray–Curtis distances was conducted to analyze bacterial differences between samples, with statistical testing performed via permutational multivariate analysis of variance (PERMANOVA). Alpha diversity was analyzed via the “vegan” package [44]. Differential ASVs were analyzed using the “EdgeR” package (Benjamini–Hochberg false discovery rate (FDR) adjusted p < 0.01). Biomarkers distinguishing the peanut cultivars were identified via the linear discriminant analysis effect size (LEfSe) on the “imageGP” online platform [45]. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) analysis was conducted on the “Wekemo Bioincloud” online platform [46]. Subsequent analyses of the output profiles from PICRUSt2 were conducted via the “ggpicrust2” package [47]. Differential abundance analysis (FDR adjusted p value < 0.05) was carried out via DESeq2. All the statistical and bioinformatics results were visualized via Origin 2021 and R software (v.4.3.3).
3. Results
3.1. Identification of High- and Low-Cadmium-Accumulating Peanut Cultivars Based on Bioconcentration Factors
This study investigated the Cd-accumulation characteristics of 30 peanut cultivars in Field I, an alkaline Cd-contaminated farmland (Supplementary Figure S1). The results revealed variations in the Cd content in the kernels and yield characteristics among the peanut cultivars (Figure 1a,b). The Cd content in the kernels ranged from 0.11 mg kg−1 to 0.27 mg kg−1, remaining below the standard limit (0.5 mg kg−1, GB 2762-2022 [48]) (Figure 1b). Correlation analysis indicated that the Cd content in the peanut kernels was significantly positively correlated with the total Cd content in the soil (Figure 1c). However, the total Cd content in the soil varied across the experimental plots, ranging from 0.98 mg kg−1 to 1.77 mg kg−1 (Supplementary Table S2a). Therefore, the BCF of peanuts in each experimental plot was calculated to screen the cultivars. Cluster analysis of the BCF values was performed to classify the cultivars into three categories. The results revealed that YH9414, WH8, LH14, SNH26, LH11, YH8, HY32, HY16, HH1, FH1, and 9616 were classified as low-Cd-accumulating cultivars, whereas HY25, SF1580, HY20, YWF, BS1016, LH8, and SLH were classified as high-Cd-accumulating cultivars. The remaining cultivars were classified as medium-Cd (Figure 1d). Peanut cultivars in the low-Cd group presented BCF values below 0.126, whereas those in the high-Cd group had BCF values above 0.160 (Supplementary Table S2b). In combination with yield characteristics (Figure 1a), LH11, FH1, LH14, and YH9414 were screened as low-Cd and high-yield cultivars and were recommended for cultivation in alkaline fields with mild to moderate Cd contamination. Compared with cultivar SLH, the Cd content in the kernels of these cultivars was reduced by 27.27% to 47.28%, and the yield increased by 9.27% to 14.17%.
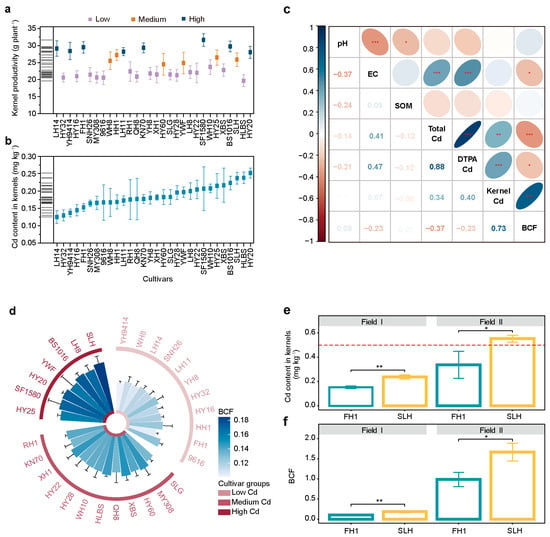
Figure 1.
Identification of peanut cultivars with low-Cd-accumulation characteristics. (a) Yield characteristics and hierarchical clustering of 30 peanut cultivars in Field I. (b) Cd content in the kernels of 30 peanut cultivars in Field I. (c) Spearman correlation analysis between the cadmium-accumulation characteristics and soil environmental factors (* p < 0.05, ** p < 0.01, *** p < 0.001). (d) Bioconcentration factor (BCF) and hierarchical clustering of 30 peanut cultivars in Field I. (e) The Cd content in kernels and (f) BCF of FH1 were significantly lower than those of SLH (* p < 0.05, ** p < 0.01; t-test). The red line represents the limit value of 0.5 mg kg−1 in GB 2762-2022 [48].
FH1, identified as a low-Cd-accumulating cultivar, and SLH, identified as a high-Cd accumulator, were studied under different environmental conditions to determine their Cd-accumulation stability. The results showed that the kernel Cd content and BCF of both cultivars were higher in Field II than in Field I. In Field I, FH1 had a significantly lower kernel Cd content and BCF than did SLH (Figure 1e,f). In Field II, the results indicated that the average BCF of FH1 was 0.99, which was significantly lower than that of SLH (Figure 1f). The kernel Cd content of SLH exceeded the standard limit (0.5 mg kg−1, GB 2762-2022 [48]) and was significantly greater than that of FH1 (Figure 1e). The average Cd content in FH1 kernels was 0.34 mg kg−1, indicating safe production in this farmland. In both Field I and Field II, FH1 consistently presented a lower kernel Cd content and BCF than SLH did. These findings make the two cultivars promising candidates for further investigation of the characteristics of rhizosphere microbial differences.
3.2. Differences in Rhizosphere Bacterial Communities Between Peanut Cultivars
3.2.1. Bacterial Diversity and Composition Differences
The rhizosphere bacterial communities of FH1 (low-Cd) and SLH (high-Cd) cultivars were analyzed to explore diversity and composition differences. The rarefaction curve leveled off, suggesting that the sequencing depth was sufficient for capturing the bacterial diversity (Supplementary Figure S2a). PCoA and PERMANOVA revealed significant distinctions in the community structures of FH1 and SLH (Figure 2c). The ACE, Shannon, and Chao1 indices demonstrated that the α diversity in FH1 was greater than that in SLH, reaching statistical significance in Field I (Figure 2a,b and Supplementary Figure S2b). These findings indicated that the FH1 rhizosphere recruited more microbial species than did the SLH rhizosphere. Furthermore, FH1 showed no significant differences in the Shannon and ACE indices between fields, while SLH exhibited significant differences (p < 0.05). The dominant bacterial phyla (with relative abundance > 1%) are shown in Figure 2d. Results indicated that these phyla accounted for more than 95% of the total abundance. Proteobacteria, Acidobacteria, Actinobacteria, Chloroflexi, Planctomycetes, Bacteroidetes, and Gemmatimonadetes were identified as the major dominant bacterial phyla in the rhizosphere of FH1 and SLH across both fields. Additionally, in Field I, the dominant bacterial phyla of FH1 also included Nitrospirae and Verrucomicrobia, while Firmicutes were dominant in SLH. In Field II, the dominant bacterial phyla of FH1 also included Verrucomicrobia, Nitrospirae, Saccharibacteria, and Armatimonadetes, whereas SLH included Verrucomicrobia, Firmicutes, Armatimonadetes, and Saccharibacteria.
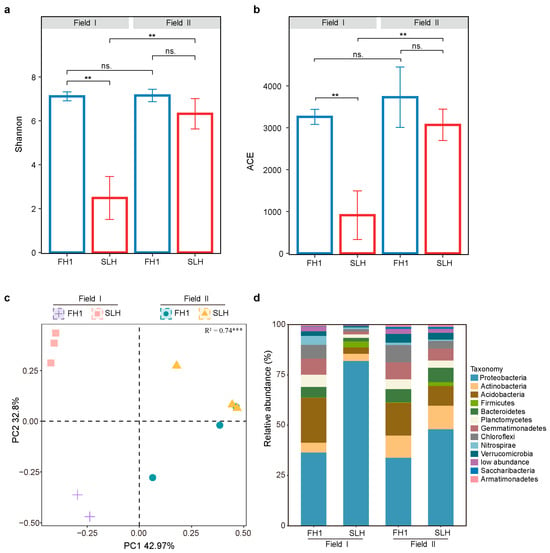
Figure 2.
Diversity and composition of bacterial communities in the FH1 and SLH rhizospheres. (a) Shannon and (b) ACE indices representing the α diversity of bacterial communities in the FH1 and SLH rhizospheres across two fields. Significance levels: ns.: p > 0.05 and ** p < 0.01. (c) PCoA with Bray–Curtis distances revealing the separation of rhizosphere microorganisms between FH1 and SLH across two fields (*** p < 0.001, PERMANOVA by Adonis). (d) Phylum-level distribution of microbial communities in the FH1 and SLH rhizospheres across two fields.
There were differences in the abundance of the dominant bacterial phyla between different peanut cultivars in the two fields (Figure 3). For FH1, the relative abundance of Actinobacteria was more abundant in Field II, while for SLH, the relative abundance of Proteobacteria was more abundant in Field I, and Gemmatimonadetes was more abundant in Field II. The results showed that, regardless of whether in Field I or Field II, the relative abundance of Proteobacteria in the FH1 rhizosphere was lower than that of SLH, while the relative abundances of Acidobacteria, Actinobacteria, Chloroflexi, Planctomycetes, and Gemmatimonadetes were higher in FH1. In Field I, significant differences (p < 0.05) in the relative abundances of bacterial phyla between the two cultivars were observed for Proteobacteria (FH1: 36.43 ± 2.06%, SLH: 81.93 ± 12.12%), Acidobacteria (FH1: 22.37 ± 1.02%, SLH:3.25 ± 2.12%), Bacteroidetes (FH1: 5.31 ± 1.26%, SLH: 1.89 ± 0.48%), Chloroflexi (FH1: 6.96 ± 1.08%, SLH: 1.36 ± 1.05%), Planctomycetes (FH1: 5.95 ± 0.78%, SLH: 1.56 ± 0.99%), and Gemmatimonadetes (FH1: 8.05 ± 0.19%, SLH: 1.39 ± 0.76%). In Field II, a significant difference was observed for Chloroflexi (FH1: 8.62 ± 1.98%, SLH:3.96 ± 1.53%).
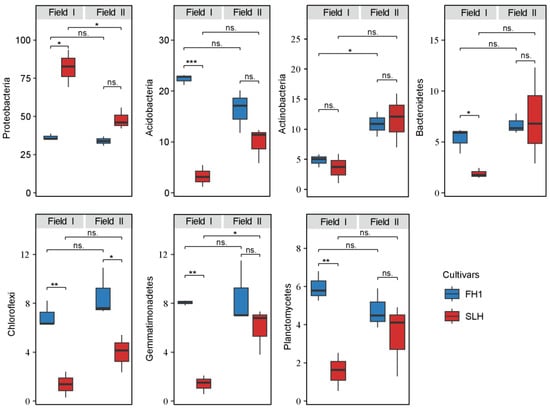
Figure 3.
Differences in the abundance of dominant bacterial phyla in the rhizosphere of different peanut cultivars. Significance levels: ns.: p > 0.05, * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.2.2. Bacterial Composition Differences at the ASV Level
The differences at the ASV level between the FH1 and SLH bacterial communities were analyzed to highlight specific compositional variations. First, the volcano plot demonstrated the number of ASVs enriched in the rhizosphere of FH1 and SLH (FDR adjusted p < 0.01). The number of ASVs enriched in the FH1 rhizosphere greatly exceeded that enriched in the SLH rhizosphere in both Field I and Field II (Figure 4a,b and Supplementary Table S4a,b). Second, the taxonomy of the enriched ASVs was analyzed and illustrated via Manhattan plots (Figure 4c,d). The results showed that in Field I, a total of 229 bacterial ASVs were enriched in the FH1 rhizosphere. The predominant microbial taxa (with more than 3 ASVs) were Proteobacteria (71 ASVs), Acidobacteria (53 ASVs), Chloroflexi (21 ASVs), Actinobacteria (19 ASVs), Gemmatimonadetes (19 ASVs), Planctomycetes (13 ASVs), Bacteroidetes (12 ASVs), Nitrospirae (7 ASVs), and Armatimonadetes (5 ASVs). In the SLH rhizosphere, a total of 54 bacterial ASVs were enriched, with the predominant microbial taxa being Firmicutes (16 ASVs), Proteobacteria (15 ASVs), Actinobacteria (10 ASVs), and Bacteroidetes (4 ASVs). In Field II, a total of 125 bacterial ASVs were enriched in the FH1 rhizosphere. The dominant microbial taxa were Acidobacteria (43 ASVs), Proteobacteria (31 ASVs), Chloroflexi (13 ASVs), Gemmatimonadetes (11 ASVs), Bacteroidetes (8 ASVs), Verrucomicrobia (6 ASVs), and Planctomycetes (4 ASVs). In the SLH rhizosphere, a total of 22 bacterial ASVs were enriched, with the predominant microbial taxa being Proteobacteria (10 ASVs), Bacteroidetes (6 ASVs), and Firmicutes (4 ASVs).
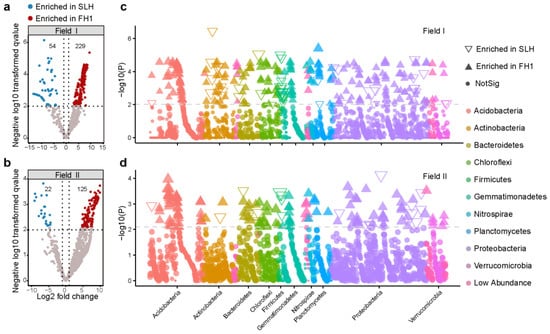
Figure 4.
Differences in the abundances of rhizosphere bacteria between FH1 and SLH at the ASV level. (a–d) Volcano and Manhattan plots displaying the number and taxonomic annotations of enriched ASVs in the FH1 and SLH rhizospheres in Field I (a,c) and Field II (b,d), respectively. Each dot or triangle represents an ASV, with differential analysis results performed via edgeR. Different colors in the volcano plot and various triangular shapes in the Manhattan plot represent enriched ASVs in FH1 or SLH rhizospheres (FDR-adjusted p < 0.01). The ASVs in the Manhattan plot are colored according to their phylum level.
3.3. Biomarkers Among Different Cultivars
Linear discriminant analysis was used to estimate the relative influence of species that exhibited statistically significant differences between FH1 and SLH (Figure 5 and Supplementary Table S5). A cladogram was constructed to illustrate these differential species and their evolutionary relationships (Supplementary Figure S3). Specifically, in Field I, Gemmatimonadetes (from phylum to family Gemmatimonadaceae), Acidobacteria (from phylum to order Subgroup_6), and Alphaproteobacteria (class) were enriched in the FH1 rhizosphere, whereas Proteobacteria (from phylum to genus Achromobacter and genus Escherichia_Shigella) were enriched in the SLH rhizosphere. In Field II, Nitrosomonadales (order), Nitrosomonadaceae (family), Subgroup_6 (order), and Chloroflexi (phylum) were enriched in the FH1 rhizosphere, whereas Proteobacteria (from phylum to genus Burkholderia) and Gammaproteobacteria (class) were enriched in the SLH rhizosphere. In both fields, Subgroup_6 (order) was enriched in the FH1 rhizosphere, whereas Proteobacteria (from phylum to order Burkholderiales) and Gammaproteobacteria (class) were enriched in the SLH rhizosphere.
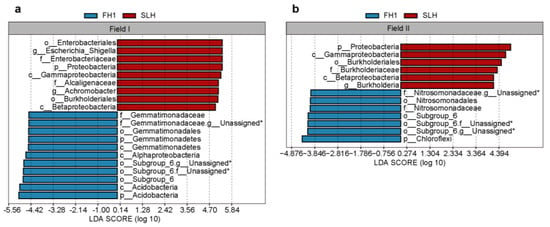
Figure 5.
Biomarkers in the rhizosphere of FH1 and SLH in Field I (a) and Field II (b). Microbes marked with an asterisk (*) are unassigned at that taxonomic level and indicate their highest assigned taxonomic level.
3.4. Functional Prediction Among Different Cultivars
To explore the functional potential of soil bacterial communities, we employed PICRUSt2, an advanced bioinformatics tool, to enhance functional prediction analysis on the basis of 16S rRNA gene amplicon data [49]. In this study, a total of 7303 functions were predicted and arranged into a three-level hierarchy of related functional pathways via the KEGG database (Supplementary Table S6a,b). The first classification level consisted of 6 functional pathways, which included 47 s-level functional pathways (Figure 6a and Supplementary Figure S4). PCoA revealed functional separation among the rhizosphere bacterial communities of different peanut cultivars (Figure 6b). The analysis revealed 262 and 68 differential functional pathways between FH1 and SLH in Field I and Field II, respectively (BH correction, p < 0.05) (Supplementary Table S6c,d). The overlap of these differential functional pathways in FH1 or SLH across both fields is shown in Supplementary Table S6e. Figure 6c highlights the overlapping differential pathways with an average relative abundance exceeding 0.4%, specifically those related to environmental information processing, metabolism, and genetic information. Among these pathways, those with relatively high relative abundances of SLH across both fields included ABC transporters, sulfur metabolism, beta-Alanine metabolism, and limonene and pinene degradation. In FH1, significant differences were observed in several key genetic information and metabolic pathways. Specifically, the genetic information pathways included DNA replication and repair (encompassing homologous recombination, nucleotide excision repair, and mismatch repair), RNA degradation, protein export, aminoacyl-tRNA biosynthesis, and ribosome (Figure 6c and Supplementary Table S6e). In terms of metabolic, functional pathways involved in the synthesis of amino acids and secondary metabolites, carbohydrate metabolism, energy metabolism, and the metabolism of cofactors and vitamins were significantly enriched in FH1 (Figure 6c and Supplementary Table S6e). Additionally, the overlap of FH1-enriched ASVs (Supplementary Figure S5a) or SLH-enriched ASVs (Supplementary Figure S5b) was detected across both fields. The correlation analysis revealed a consistent relationship between the relative abundance of overlapping functional pathways and ASVs (Supplementary Figure S5c), which will be a key focus of future research.
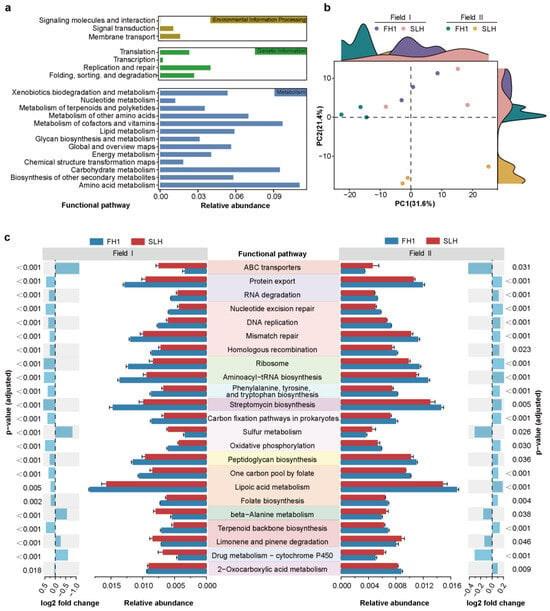
Figure 6.
Predicted functions of the bacterial communities in the FH1 and SLH rhizospheres. (a) Integrated overview of functional pathways at level 2 predicted by PICRUSt2. The data represent the average relative abundance across all samples. Different colors correspond to their respective functional pathways at the first-level classification, with the names displayed in the upper right corner of the rectangle. (b) Principal component analysis (PCA) revealing the variations in the abundance of microbial functional pathways in the FH1 and SLH rhizospheres across two fields. (c) Differential abundance of predicted functional pathways between the FH1 and SLH rhizospheres, highlighting overlapping features across the two fields. The error bar plots and corresponding labels display a direct comparison of pathway abundance at level 3 between the FH1 and SLH rhizospheres (DESeq2, adjusted p value < 0.05). The side bar plots display log2-fold changes in pathway abundance.
4. Discussion
4.1. Identification and Application of Low-Cadmium Cultivars for Safe Agriculture on Cd-Polluted Farmland
The selection of crop cultivars is crucial for ensuring safe agricultural production in contaminated soils. Our findings confirm that the use of the BCF as a basis for identifying low-Cd cultivars in field environments is scientifically justified (Figure 1). This is primarily due to soil heterogeneity; even within the same field (Field I), variability in soil properties was observed among the experimental plots (Supplementary Figure S1). Notably, spatial variation in the soil Cd content significantly influenced Cd accumulation in peanut kernels (Figure 1c). Therefore, assessing cultivars solely on the basis of kernel Cd content may be limiting. This observation is consistent with recent findings, emphasizing the importance of using the BCF as a key parameter for evaluating Cd accumulation in different cultivars across varying soil conditions, as it could somewhat mitigate the influence of total soil cadmium variation [50,51]. Cluster analysis further supports the scientific rigor and effectiveness of cultivar screening, a method widely applied to staple crops such as rice and wheat [52,53]. By integrating cluster analysis results of the BCF and yield characteristics (Figure 1a,d), this study offers cultivar screening strategies and valuable resources for optimizing safe peanut production in Cd-contaminated soils.
Our results further validated FH1 as an LAC with strong potential for practical application. FH1 was previously identified as an LAC in acidic pot experiments [20] and has been studied under pot or hydroponic conditions; however, its field application remains limited [54,55,56]. In this study, FH1 presented both low Cd accumulation and high yield in Field I (Figure 1a,b,d). The yield performance was superior to that in previous reports on FH1 [20], likely due to resource limitations and the influence of exogenously applied Cd in pot experiments. This implies potential discrepancies between pot experiments and field performance, emphasizing the importance of field-based validation for accurate cultivar evaluation. In the acidic soil of Field II, FH1 consistently presented a low-Cd-accumulating advantage, with a significantly lower BCF than did SLH (Figure 1f), indicating its potential for safe production in Cd-contaminated soils. However, FH1 exhibited significant differences in the kernel Cd content and BCF between the two fields. This variation is primarily attributed to the distinct soil properties of the two fields, particularly soil pH [57]. Previous studies have shown that the crop BCF and kernel Cd content generally decrease as soil pH increases, which aligns with our findings [36]. These findings highlight the necessity of multi-site field validation across diverse soil types and the exploration of thresholds for safe production.
The low Cd accumulation observed in FH1 may be attributed to several potential mechanisms, including the specific root traits, metal ion transport mechanisms, and interactions with rhizosphere microorganisms [23,58,59]. Studies have shown that the biomass and fine root structure of peanuts influence Cd accumulation, with LACs tending to have greater biomass and fewer fine roots [60,61]. Moreover, LACs may possess specific mechanisms that limit Cd translocation, as demonstrated by FH1, which showed lower root-to-shoot Cd translocation than SLH did [9,62]. Rhizosphere microorganisms play a crucial role in regulating Cd accumulation in crops. Therefore, further exploration of the diversity and functional characteristics of rhizosphere microorganisms in FH1 and SLH will provide a theoretical basis for understanding the ecological mechanisms of LACs.
4.2. Differences in Bacterial Diversity and Composition Between Peanut Cultivars
Exploring the potential contributions of rhizosphere microorganisms in peanut Cd accumulation under field conditions can contribute to the scientific regulation of the rhizosphere environment [63]. The diversity and composition of rhizosphere microorganisms are jointly shaped and influenced by the soil environment and plant cultivars [27,64]. The results of this study revealed significant differences in the microbial community characteristics of rhizosphere soils between the FH1 and SLH, with distinct responses observed for the two cultivars under different field conditions. In both Field I and Field II, the Shannon, ACE, and Chao1 indices of rhizosphere microorganisms in FH1 were consistently higher than those in SLH. This indicates that the rhizosphere of FH1 comprises more diverse and abundant microorganisms, in agreement with results from similar previous research [65,66]. Increased microbial diversity is known to favor the stability of community structure [67]. Furthermore, the FH1 rhizosphere presented a greater number of enriched ASVs (Figure 4a,b). These findings confirmed that the FH1 rhizosphere comprised a wider variety of microorganisms, which was proposed as a potential Cd-resistance mechanism in LACs. The bacterial diversity in FH1 rhizospheres exhibited no significant differences across the two field environments, potentially indicating that FH1 possesses strong environmental adaptability and microbial regulatory capacity. This stability may be associated with the specific root exudates of FH1, which consistently promote the enrichment of a diverse and abundant microbial community in the rhizosphere, regardless of environmental changes [68]. Compared with FH1, the lower bacterial diversity in the SLH rhizosphere might be attributed to the selective recruitment of specific Cd-tolerant microbial taxa by root exudates, such as members of the phylum Proteobacteria [68,69]. This selective recruitment process appears to be sensitive to soil conditions, leading to significant variations in bacterial diversity within SLH rhizospheres across the two field environments [64,69].
The bacterial communities in the FH1 and SLH rhizospheres presented significant compositional differences across both fields (Figure 2 and Figure 3). These results indicated that both field environments and crop cultivars significantly influenced the composition of rhizosphere microbial communities. The variation in dominant bacterial taxa for the same peanut cultivar (FH1 or SLH) across different fields may reflect a synergistic interaction between soil environment and specific microbial communities [57]. Between the two cultivars, the low-Cd cultivar FH1 tended to recruit beneficial microorganisms, forming a distinct microbial community. The enrichment of beneficial microorganisms such as Gemmatimonadetes, Chloroflexi, and Alphaproteobacteria in the FH1 rhizosphere aligns with findings from studies on LACs of wheat [66]. In addition, Acidobacteria (phylum), Subgroup_6 (order), Nitrosomonadaceae (family), and Nitrosomonadales (order) were also enriched in the FH1 rhizosphere (Figure 5). These potentially beneficial bacteria are commonly associated with growth promotion and Cd resistance in plants [67,70]. For example, Alphaproteobacteria have been reported to be positively correlated with wheat biomass and negatively correlated with the grain Cd concentration [64]. Key microbial taxa such as Acidobacteria, Chloroflexi, Planctomycetes, Gemmatimonadetes, and Subgroup_6 within the Acidobacteria phylum play a crucial role in enhancing crop productivity [71,72]. It has been found that certain microorganisms participated in Cd stabilization through complexation and adsorption mechanisms, thereby improving crop resistance to Cd stress and supporting plant growth under heavy metal-contaminated conditions [72,73,74]. This process varies depending on the specific genus or species of microorganism involved. Among them, Acidobacteria are recognized as important players in iron (Fe) cycling, and their abundance may influence Cd bioavailability in rice rhizosphere soil, potentially reducing Cd content in grains [75,76]. Earlier research has indicated that Fe conditions are also key factors influencing Cd accumulation in peanuts [77]. However, the underlying mechanisms by which Acidobacteria influence Cd accumulation in peanuts require further research.
On the other hand, the FH1 rhizosphere exhibited a lower abundance of microorganisms potentially involved in Cd activation, such as members of Proteobacteria. Although the number of ASVs annotated as Proteobacteria was greater in FH1 than that in SLH (Figure 4c,d), their overall abundance was lower (Figure 3). These findings suggest that FH1 selectively recruits a more diverse yet lower-abundance array of Proteobacteria. Studies in other crops have shown that a reduced abundance of Proteobacteria is correlated with lower Cd accumulation [71], consistent with the findings of this study. LEfSe analysis further revealed that the abundances of Gammaproteobacteria and Betaproteobacteria were notably lower in the FH1 rhizosphere than in the SLH rhizosphere (Figure 5). This result aligns with findings from studies on wheat and rice, which reported that members of Gammaproteobacteria and Betaproteobacteria were more abundant in high-Cd cultivars compared with low-Cd cultivars [66,68]. This may be attributed to the role of certain members in Cd activation. For example, the genus Burkholderia (belonging to the Betaproteobacteria) is abundantly recruited in the rhizosphere of high-Cd plant cultivars, which aligns with the findings for SLH in this study [78]. Previous research has demonstrated that members of this genus can colonize the root system to facilitate plant growth and Cd absorption through the secretion of IAA and siderophores and the overexpression of Cd transporter proteins [79,80]. Additionally, the genus Achromobacter (belonging to Betaproteobacteria), which was enriched in the SLH rhizosphere, has also been found to be enriched in the rhizosphere of high-Cd crops such as S. alfredii [81]. This genus is considered a key player in promoting plant growth and enhancing Cd tolerance [81].
The rhizosphere microbial community characteristics of the low-Cd cultivar FH1 could be summarized as follows: (1) the formation of a diverse microbial community; (2) the recruitment of beneficial microorganisms that promote plant growth in Cd-contaminated environments; and (3) the selective enrichment of Cd-stabilizing microbes, which enhance plant resistance to Cd stress. Future studies should integrate field and controlled experiments for multidimensional investigation to deeply elucidate the mechanisms of rhizosphere microorganisms in low-Cd accumulation in peanuts.
4.3. Predicted Functional Characteristics of the Bacterial Community in the Rhizosphere of the Low-Cd Cultivar FH1
Differences in KEGG pathways associated with functional genes in bacterial communities reflect underlying biomolecular mechanisms in response to specific environments [82]. This study demonstrated that the predicted functional profiles of rhizosphere bacteria differed among peanut cultivars (Figure 6b). These differences may result from the unique rhizosphere microenvironments shaped by root secretions, suggesting that the two cultivars may detoxify Cd through different mechanisms [83]. The number of functional classifications significantly enriched in the FH1 rhizosphere was greater than that enriched in the SLH rhizosphere (Figure 6c and Supplementary Table S6e). In FH1, the enriched pathways related to carbohydrate and energy metabolism as well as genetic information processing play crucial roles in microbial cell growth and reproduction [82]. The enhancement of these pathways in the FH1 rhizosphere may suggest an overall proliferation of microorganisms, which is consistent with the observed trends in bacterial diversity and community composition (Figure 2 and Figure 3). Furthermore, peptidoglycan biosynthesis, amino acid metabolism, and other pathways may be key pathways involved in Cd stabilization and detoxification in the FH1 rhizosphere (Figure 6c). Peptidoglycan biosynthesis is associated with the production of extracellular polymeric substances (EPSs) in microbial cells, which may contribute to Cd adhesion [84]. EPS, a complex biopolymer, contains a matrix rich in functional groups capable of sequestering trace metal ions such as Cd2+, thereby limiting their uptake by plants from the soil [85]. Amino acids have been shown to contribute to soil Cd stabilization by forming complexes with Cd, which facilitate its immobilization [86]. Amino acid metabolism can regulate reactive oxygen species (ROS) homeostasis and increase Cd tolerance when microbes accumulate excess intracellular Cd [82]. In addition, pathways such as terpenoid backbone biosynthesis, folate biosynthesis, and lipoic acid metabolism are crucial for enhancing crop resistance to environmental stress [87,88,89].
Conversely, FH1 showed downregulated functional pathways associated with ABC transporters (Figure 6c), which is consistent with previous findings [64,90]. ABC transporters play crucial roles in mediating the transmembrane transport of various substrates, including essential nutrients and metals [91]. Studies by Fajardo et al. (2019) [92] and Li et al. (2021) [93] further emphasized the critical role of bacterial ABC transporters in Cd transport under stress conditions. In conclusion, the upregulation of mechanisms related to Cd immobilization, detoxification, and energy supply, coupled with the downregulation of Cd transport-related pathways, may represent key functional adaptations of FH1 to its specific microenvironment. Future research should employ techniques such as metagenomics to characterize the taxonomic functions of the rhizosphere microbiota more accurately.
5. Conclusions
This study identified FH1 as a low-Cd-accumulating and high-yield peanut cultivar capable of achieving safe production in both alkaline and acidic Cd-contaminated fields. FH1 recruited a diverse array of rhizosphere microorganisms, forming a unique microbial community. The enriched beneficial microbes, including Alphaproteobacteria, Acidobacteria, Chloroflexi, and Gemmatimonadetes, likely contributed to Cd immobilization, detoxification, and plant growth. In contrast, the abundance of potentially Cd-activating microbes such as Gammaproteobacteria and Betaproteobacteria was reduced. Functional predictions further suggested that the low Cd accumulation in FH1 may be attributed to the upregulation of pathways related to Cd immobilization, detoxification, and energy metabolism, coupled with the downregulation of Cd transport-related pathways. In summary, FH1 represents a promising cultivar for mitigating Cd risk in agriculture, with distinct rhizosphere microbial interactions that provide valuable insights into sustainable crop management in contaminated soils. Future research should prioritize in-depth metagenomic analyses to further elucidate the functional mechanisms underpinning these beneficial microbial interactions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su17020626/s1. Supplementary Figure S1: Spatial arrangement of the soil properties and peanut cultivars in the experimental plots in Field I; Supplementary Figure S2: Rarefaction curve (a) and alpha-diversity Chao1 index (b) of microbial communities in the FH1 and SLH rhizospheres; Supplementary Figure S3: Biomarkers in the rhizosphere of FH1 and SLH in Field I (a) and Field II (b); Supplementary Figure S4: Integrated overview of functional pathways at level 2 predicted by PICRUSt2; Supplementary Figure S5: The ASVs enriched in the FH1 or SLH rhizospheres in the two fields and Spearman correlation with the predicted functions; Supplementary Table S1: Basic soil properties and peanut cultivars planted in two experimental fields; Supplementary Table S2: Analysis of the properties of soil and peanut samples from each experimental plot in two fields; Supplementary Table S3: Sample information, representative sequences of ASVs, ASVs table, and taxonomy annotation; Supplementary Table S4: Differential analysis at ASVs level between FH1 and SLH in two fields; Supplementary Table S5: Information on rhizosphere biomarkers of FH1 and SLH in two fields; Supplementary Table S6: Annotation results and differential analysis of functional pathways of FH1 and SLH in two fields.
Author Contributions
Conceptualization, K.Z. and J.D.; methodology, K.Z., S.L., H.L. and J.D.; software, K.Z., S.L. and J.D.; validation, S.L. and L.B.; formal analysis, K.Z.; investigation, K.Z., X.D., X.L. and L.B.; resources, X.D., X.L., H.L. and J.D.; data curation, K.Z. and J.D.; writing—original draft preparation, K.Z.; writing—review and editing, H.L., L.B. and J.D.; visualization, K.Z., L.B. and J.D.; supervision, L.B. and J.D.; project administration, J.D.; funding acquisition, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 41977144; the Agricultural Major Technology Collaborative Promotion Plan of Shandong Province, grant number SDNYXTTG-2022-22; the Research and Development Plan Initiated Projects of Jining, grant number 2023NYNS016.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, P.; Chen, H.P.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, Y.; Liu, Y.J.; Li, K.; Xiao, L.; Li, M.Y.; Tian, Y.; Wu, F. Assessment of anthropogenic sources of potentially toxic elements in soil from arable land using multivariate statistical analysis and random forest analysis. Sustainability 2020, 12, 8538. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotox. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Qin, S.Y.; Liu, H.E.; Nie, Z.J.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Hu, J.H.; Chen, G.L.; Xu, K.; Wang, J. Cadmium in cereal crops: Uptake and transport mechanisms and minimizing strategies. J. Agric. Food Chem. 2022, 70, 5961–5974. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Liu, H.Y.; Gao, M.; Zhou, J.; Zhou, J. Effects of soil amendments, foliar sprayings of silicon and selenium and their combinations on the reduction of cadmium accumulation in rice. Pedosphere 2022, 32, 649–659. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.F.; Xu, Y.M.; Qin, X.; Huang, Q.Q.; Wang, L.; Sun, Y.B. Remediation of heavy metal-polluted agricultural soils using clay minerals: A review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.Y.; Liao, X.Y.; Li, X.H.; Zheng, S.N.; Zhao, F.H. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard. Mater. 2021, 413, 125427. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Ding, S.; Li, X.L.; Ning, C.L.; Liu, H.; Sun, M.; Liu, D.M.; Zhang, K.; Li, S.S.; Yu, X.J.; et al. Low-cadmium wheat cultivars limit the enrichment, transport and accumulation of cadmium. Agronomy 2024, 14, 1191. [Google Scholar] [CrossRef]
- Zhan, J.; Twardowska, I.; Wang, S.Q.; Wei, S.H.; Chen, Y.Q.; Ljupco, M. Prospective sustainable production of safe food for growing population based on the soybean (Glycine max L. Merr.) crops under Cd soil contamination stress. J. Clean. Prod. 2019, 212, 22–36. [Google Scholar] [CrossRef]
- Feng, K.X.; Li, J.X.; Yang, Y.C.; Li, Z.; Wu, W.G. Cadmium absorption in various genotypes of rice under cadmium stress. Int. J. Mol. Sci. 2023, 24, 8019. [Google Scholar] [CrossRef]
- Zhang, M.X.; Wang, O.; Cai, S.B.; Zhao, L.; Zhao, L. Composition, functional properties, health benefits and applications of oilseed proteins: A systematic review. Food Res. Int. 2023, 171, 113061. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.L.; Zhang, C.X.; Zhang, X.J.; Wang, G.; Li, L.; Geng, H.R.; Liu, Y.; Nie, C.R. Survey of aflatoxin B1 and heavy metal contamination in peanut and peanut soil in China during 2017–2018. Food Control 2020, 118, 107372. [Google Scholar] [CrossRef]
- Dong, Y.J.; Wang, L.; Cai, D.; Zhang, C.S.; Zhao, S.C. Risk assessment on dietary exposure to aflatoxin B1, heavy metals and phthalates in peanuts, a case study of Shandong province, China. J. Food Compos. Anal. 2023, 120, 105359. [Google Scholar] [CrossRef]
- Dou, X.L.; Li, B.; Cui, J.H.; Li, G.C.; Wang, Y.H. Assessment of heavy metal pollution and risk of farmland soil and agricultural products around a smelter in Liaoning. J. Agro-Environ. Sci. 2020, 39, 2249–2258. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, Z.F.; Zhang, Q.Z.; Ji, W.B.; Guan, D.X.; Liu, X.; Yu, T.; Wang, L.; Zhuo, X.X.; Ji, J.F. Transferability of heavy metal(loid)s from karstic soils with high geochemical background to peanut seeds. Environ. Pollut. 2022, 299, 118819. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.G.; Liu, D.W.; Li, L.; Wan, S.B.; Zhang, H.; Zhang, L.Q.; Zou, D.S. Cadmium concentration and translocation in paddy fields with different peanut varieties. Chin. J. Oil Crop Sci. 2019, 41, 568–576. [Google Scholar] [CrossRef]
- Zhang, K.; Song, S.X.; Li, S.S.; Bai, L.Y.; Liu, H.; Sun, M.; Yu, X.J.; Dai, J.L. Evaluation of cadmium phytoextraction potential of peanut and the rhizospheric properties of specific cultivars. J. Clean. Prod. 2024, 452, 142228. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Z.H.; Ma, X.L.; Cao, D.; Zhao, K.K.; Zhao, K.; Ma, Q.; Gong, F.P.; Li, Z.F.; Qiu, D.; et al. Natural resistance-associated macrophage proteins are involved in tolerance to heavy metal Cd2+ toxicity and resistance to bacterial wilt of peanut (Arachis hypogaea L.). Plant Physiol. Biochem. 2024, 207, 108411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ding, S.; Yan, Y.; Huang, X.M.; Li, S.S.; Zhao, W.N.; Chen, X.G.; Dai, J.L. Screening of peanut cultivars with low-cadmium accumulation assisted by cadmium resistance: Promoting safe utilization of cadmium contaminated soils. Appl. Soil Ecol. 2024, 193, 105109. [Google Scholar] [CrossRef]
- Shi, G.R.; Su, G.Q.; Lu, Z.W.; Liu, C.F.; Wang, X.M. Relationship between biomass, seed components and seed Cd concentration in various peanut (Arachis hypogaea L.) cultivars grown on Cd-contaminated soils. Ecotox. Environ. Saf. 2014, 110, 174–181. [Google Scholar] [CrossRef]
- Su, G.Q.; Li, F.; Lin, J.S.; Liu, C.F.; Shi, G.R. Peanut as a potential crop for bioenergy production via Cd-phytoextraction: A life-cycle pot experiment. Plant Soil 2013, 365, 337–345. [Google Scholar] [CrossRef]
- Lin, L.H.; Wu, X.Y.; Deng, X.Y.; Lin, Z.; Liu, C.G.; Zhang, J.X.; He, T.; Yi, Y.Q.; Liu, H.; Wang, Y.F.; et al. Mechanisms of low cadmium accumulation in crops: A comprehensive overview from rhizosphere soil to edible parts. Environ. Res. 2024, 245, 118054. [Google Scholar] [CrossRef]
- Wang, L.Y.; Rengel, Z.; Zhang, K.; Jin, K.M.; Lyu, Y.; Zhang, L.; Cheng, L.Y.; Zhang, F.S.; Shen, J.B. Ensuring future food security and resource sustainability: Insights into the rhizosphere. iScience 2022, 25, 104168. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, Y.R.; Ding, C.F.; Yin, Y.P.; Zhou, Z.G.; Zhang, T.L.; Wang, X.X. Cadmium found in peanut (Arachis hypogaea L.) kernels mainly originates from root uptake rather than shell absorption from soil. Pedosphere 2024, 34, 726–735. [Google Scholar] [CrossRef]
- Jiang, H.F.; Okoye, C.O.; Chen, X.F.; Zhang, F.S.; Jiang, J.X. High-throughput 16S rRNA gene-based amplicon sequencing reveals the functional divergence of halophilic bacterial communities in the Suaeda salsa root compartments on the eastern coast of China. Sci. Total Environ. 2024, 942, 173775. [Google Scholar] [CrossRef]
- Wang, G.B.; Zhang, Q.Q.; Du, W.C.; Ai, F.X.; Yin, Y.; Ji, R.; Guo, H.Y. Microbial communities in the rhizosphere of different willow genotypes affect phytoremediation potential in Cd contaminated soil. Sci. Total Environ. 2021, 769, 145224. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Wu, K.J.; Shi, L.B.; Sun, X.C.; Tan, Q.L.; Hu, C.X. Recruitment of specific microbes through exudates affects cadmium activation and accumulation in Brassica napus. J. Hazard. Mater. 2023, 442, 130066. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality-Risk Control Standard for Soil Contamination of Agricultural Land. Standards Press of China: Beijing, China, 2018.
- NY/T 1121.6-2006; Technical Rules for Monitroing of Environmental Quality of Farmland Soil. China Agriculture Press: Beijing, China, 2006.
- Mo, X.H.; Wang, M.K.; Zeng, H.; Wang, J.J. Rhizosheath: Distinct features and environmental functions. Geoderma 2023, 435, 116500. [Google Scholar] [CrossRef]
- GB 5009.15-2014; National Food Safety Standard-Determination of Cadmium in Foods. Standards Press of China: Beijing, China, 2014.
- HJ 962-2018; Soil-Determination of pH-Potentiometry. National Environmental Protection Standard of the People’s Republic: Beijing, China, 2018.
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000.
- NY/T 1121.6-2006; Soil Testing Part 6: Method for Determination of Soil Organic Matter. China Agriculture Press: Beijing, China, 2006.
- Bai, L.Y.; Ding, S.; Huang, X.M.; Chen, X.G.; Chen, Y.H.; Cao, X.Y.; Wang, X.R.; Yu, X.J.; Dai, J.L. Prediction of the cadmium content in grains of low-accumulating wheat cultivars and soil cadmium threshold for safe production. J. Clean. Prod. 2023, 417, 138081. [Google Scholar] [CrossRef]
- GB 17141-1997; Soil Quality-Determination of Lead, Cadmium-Graphitefurnace Atomic Absorption Spectrophotometry. Standards Press of China: Beijing, China, 1997.
- Cheng, Z.Y.; Zheng, Q.; Shi, J.C.; He, Y.; Yang, X.L.; Huang, X.W.; Wu, L.S.; Xu, J.M. Metagenomic and machine learning-aided identification of biomarkers driving distinctive Cd accumulation features in the root-associated microbiome of two rice cultivars. ISME Communications 2023, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Chen, L.; Ma, T.F.; Li, X.F.; Zheng, M.S.; Zhou, X.; Chen, L.; Qian, X.B.; Xi, J.; Lu, H.Y.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta 2023, 2, e83. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.X.; Huang, L.Q. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Zhang, G.X.; Jiang, S.Y.; Liu, Y.X. Wekemo Bioincloud: A user-friendly platform for meta-omics data analyses. iMeta 2024, 3, e175. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mai, J.H.; Cao, X.; Burberry, A.; Cominelli, F.; Zhang, L.L.; Elofsson, A. ggpicrust2: An R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics 2023, 39, btad470. [Google Scholar] [CrossRef] [PubMed]
- GB 2762-2022; National Standards for Food Safety-Limits of Contaminants in Food. Standards Press of China: Beijing, China, 2022.
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Q.; Ren, B.Z.; Xie, Q.; Deng, X.P.; Yin, W.; Chen, L.Y. Assessment of health risks posed by toxicological elements of the food chain in a typical high geologic background. Ecol. Indic. 2024, 161, 111981. [Google Scholar] [CrossRef]
- Bai, L.Y.; Huang, X.M.; Li, Z.L.; Li, S.S.; Lv, C.; Zhang, K.; Dai, J.L. Stability and adaptability of wheat cultivars with low cadmium accumulation based on farmland trials. Eur. J. Agron. 2023, 144, 126764. [Google Scholar] [CrossRef]
- Li, S.S.; Huang, X.M.; Liu, N.; Chen, Y.H.; He, H.; Cao, X.Y.; Dai, J.L. Selection of low-cadmium and high-micronutrient wheat cultivars and exploration of the relationship between agronomic traits and grain cadmium. Environ. Sci. Pollut. Res. 2022, 29, 42884–42898. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Huang, X.M.; Sun, L.M.; Li, S.S.; Chen, Y.H.; Cao, X.Y.; Wang, W.X.; Dai, J.L.; Rinnan, R. Screening stably low cadmium and moderately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere 2020, 241, 125065. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Zhang, Z.; Shi, G.R. Genome-wide identification and expression profiling of heavy metal ATPase (HMA) genes in peanut: Potential roles in heavy metal transport. Int. J. Mol. Sci. 2024, 25, 613. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.G.; Ma, Y.Y.; Li, Y.; Li, X.; Liu, C.F.; Du, X.L.; Shi, G.R. Comparative transcriptome analysis revealed key factors for differential cadmium transport and retention in roots of two contrasting peanut cultivars. BMC Genom. 2018, 19, 938. [Google Scholar] [CrossRef]
- Yu, R.G.; Jiang, Q.; Xv, C.; Li, L.E.; Bu, S.J.; Shi, G.R. Comparative proteomics analysis of peanut roots reveals differential mechanisms of cadmium detoxification and translocation between two cultivars differing in cadmium accumulation. BMC Plant Biol. 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lv, J.L.; He, W.X.; Zhang, H.; Cao, Y.F.; Dai, Y.C. Major factors influencing cadmium uptake from the soil into wheat plants. Ecotox. Environ. Saf. 2015, 113, 207–213. [Google Scholar] [CrossRef]
- Bali, A.S.; Sidhu, G.P.S.; Kumar, V. Root exudates ameliorate cadmium tolerance in plants: A review. Environ. Chem. Lett. 2020, 18, 1243–1275. [Google Scholar] [CrossRef]
- Lin, K.N.; Williams, D.V.; Zeng, M.; Ahmed, I.M.; Dai, H.X.; Cao, F.B.; Wu, F.B. Identification of low grain cadmium accumulation genotypes and its physiological mechanism in maize (Zea mays L.). Environ. Sci. Pollut. Res. 2021, 29, 20721–20730. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.W.; Zhang, Z.; Su, Y.; Liu, C.F.; Shi, G.R. Cultivar variation in morphological response of peanut roots to cadmium stress and its relation to cadmium accumulation. Ecotox. Environ. Saf. 2013, 91, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, J.L.; Lu, Z.W.; Wang, X.M.; Zhang, Z.; Shi, G.R. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- Liu, C.F.; Yu, R.G.; Shi, G.R. Effects of drought on the accumulation and redistribution of cadmium in peanuts at different developmental stages. Arch. Agron. Soil Sci. 2016, 63, 1049–1057. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Swapnil, P. Plant growth-promoting rhizobacteria as a green alternative for sustainable agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Lu, M.; Huang, L.K.; Wang, Q.; Cao, X.R.; Lin, Q.; He, Z.L.; Feng, Y.; Yang, X.E. Soil properties drive the bacterial community to cadmium contamination in the rhizosphere of two contrasting wheat (Triticum aestivum L.) genotypes. J. Environ. Sci. 2023, 128, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Zhou, R.X.; Teng, L.D.; Chen, H.B.; Li, M.; Wang, L.; Zhran, M.; Cao, F.B. Genotypic variation in grain cadmium concentration in wheat: Insights into soil pollution, agronomic characteristics, and rhizosphere microbial communities. Environ. Pollut. 2024, 340, 122792. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liu, Q.; Min, J.M.; Zhang, S.J.; Li, S.S.; Chen, Y.H.; Dai, J.L. Specific bacterial communities in the rhizosphere of low-cadmium and high-zinc wheat (Triticum aestivum L.). Sci. Total Environ. 2022, 838, 156484. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.M.; Zhang, Y.X.; Wang, L.; Liu, C.G.; Sun, W.M.; Wang, Y.F.; Long, S.X.; He, X.T.; Lin, Z.; Liang, J.L.; et al. Rhizobacteria communities reshaped by red mud based passivators is vital for reducing soil Cd accumulation in edible amaranth. Sci. Total Environ. 2022, 826, 154002. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.D.; Wang, R.Z.; Gao, X.Y.; Wang, K.; Lin, Z.; Ge, J.; Liu, T.; Wei, S.; Chen, W.K.; Xie, R.H.; et al. Cultivar-specific response of bacterial community to cadmium contamination in the rhizosphere of rice (Oryza sativa L.). Environ. Pollut. 2018, 241, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kou, B.; He, Y.; Wang, Y.; Qu, C.T.; Tang, J.; Wu, Y.M.; Tan, W.B.; Yuan, Y.; Yu, T.Q. The relationships between heavy metals and bacterial communities in a coal gangue site. Environ. Pollut. 2023, 322, 121136. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chi, Y.; Su, X.Y.; Ye, Z.H.; Ren, X.X. Rhizobium soaking promoted maize growth by altering rhizosphere microbiomes and associated functional genes. Microorganisms 2023, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, C.; Lin, Y.; Chen, Y.J.; Zhang, Z.J.; Wei, K.H.; Lei, M. Biochar and organic fertilizer drive the bacterial community to improve the productivity and quality of Sophora tonkinensis in cadmium-contaminated soil. Front. Microbiol. 2024, 14, 1334338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Y.L.; Lin, D.S.; Yao, Y.P.; Song, N.N.; Wang, F.L. Co-application of biochar and nitrogen fertilizer promotes rice performance, decreases cadmium availability, and shapes rhizosphere bacterial community in paddy soil. Environ. Pollut. 2022, 308, 119624. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.F.; Li, Y.Y.; Dai, X.Z.; Zhang, Q.Z.; Zhang, M.; Zhang, Z.Q.; Tao, Y.; Chen, W.C.; Zhang, M.X.; et al. Improved immobilization of soil cadmium by regulating soil characteristics and microbial community through reductive soil disinfestation. Sci. Total Environ. 2021, 778, 146222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Xiong, Y.; Zhang, J.E.; Lu, X.N.; Wei, G.C. Naturally selected dominant weeds as heavy metal accumulators and excluders assisted by rhizosphere bacteria in a mining area. Chemosphere 2020, 243, 125365. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Song, K.; Shi, L.Z.; Duan, D.C.; Zhang, H.; Sun, Y.F.; Qin, Q.; Xue, Y. Influence of elemental sulfur on cadmium bioavailability, microbial community in paddy soil and Cd accumulation in rice plants. Sci. Rep. 2021, 11, 11468. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Huang, Y.C.; Yang, X.R.; Xue, W.J.; Zhang, X.; Zhang, Y.H.; Pang, J.; Liu, Y.M.; Liu, Z.Q. Burkholderia sp. Y4 inhibits cadmium accumulation in rice by increasing essential nutrient uptake and preferentially absorbing cadmium. Chemosphere 2020, 252, 126603. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, X.M.; Liu, C.F.; Shi, G.R. Variation in cadmium accumulation and translocation among peanut cultivars as affected by iron deficiency. Plant Soil 2013, 363, 201–213. [Google Scholar] [CrossRef]
- Hu, L.F.; Tan, X.Y.; Lu, L.L.; Meng, X.T.; Li, Y.Y.; Yao, H.Y. DNA-SIP delineates unique microbial communities in the rhizosphere of the hyperaccumulator Sedum alfredii which are beneficial to Cd phytoextraction. Ecotox. Environ. Saf. 2024, 272, 116016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Sheng, X.F.; He, L.Y.; Huang, Z.; Zhang, W.H. Effects of root inoculation with bacteria on the growth, Cd uptake and bacterial communities associated with rape grown in Cd-contaminated soil. J. Hazard. Mater. 2013, 244–245, 709–717. [Google Scholar] [CrossRef]
- Liu, H.K.; Huang, H.Y.; Xie, Y.L.; Liu, Y.K.; Shangguan, Y.X.; Xu, H. Integrated biochemical and transcriptomic analysis reveals the effects of Burkholderia sp. SRB-1 on cadmium accumulating in Chrysopogon zizanioides L. under Cd stress. J. Environ. Manag. 2023, 337, 117723. [Google Scholar] [CrossRef]
- Fan, W.J.; Deng, J.M.; Shao, L.; Jiang, S.M.; Xiao, T.F.; Sun, W.M.; Xiao, E.Z. The rhizosphere microbiome improves the adaptive capabilities of plants under high soil cadmium conditions. Front. Plant Sci. 2022, 13, 914103. [Google Scholar] [CrossRef]
- Li, Q.; Xing, Y.N.; Huang, B.; Chen, X.; Ji, L.; Fu, X.W.; Li, T.Y.; Wang, J.N.; Chen, G.H.; Zhang, Q. Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci. Total Environ. 2022, 825, 154136. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Jiang, L.H.; Guo, Z.W.; Sarkodie, E.K.; Li, K.W.; Shi, J.X.; Peng, Y.L.; Liu, H.W.; Liu, X.D. The Cd immobilization mechanisms in paddy soil through ureolysis-based microbial induced carbonate precipitation: Emphasis on the coexisting cations and metatranscriptome analysis. J. Hazard. Mater. 2024, 465, 133174. [Google Scholar] [CrossRef]
- Gu, T.Y.; Lu, Y.Q.; Li, F.; Zeng, W.M.; Shen, L.; Yu, R.L.; Li, J.K. Microbial extracellular polymeric substances alleviate cadmium toxicity in rice (Oryza sativa L.) by regulating cadmium uptake, subcellular distribution and triggering the expression of stress-related genes. Ecotox. Environ. Saf. 2023, 257, 114958. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L.F.; Zhao, S.W.; Li, S.S.; Lei, X.Q.; Qin, L.Y.; Sun, X.Y.; Chen, S.B. Manganese facilitates cadmium stabilization through physicochemical dynamics and amino acid accumulation in rice rhizosphere under flood-associated low pe+pH. J. Hazard. Mater. 2021, 416, 126079. [Google Scholar] [CrossRef]
- Wang, H.M.; Wu, P.X.; Liu, J.; Yang, S.S.; Ruan, B.; Rehman, S.; Liu, L.T.; Zhu, N.W. The regulatory mechanism of Chryseobacterium sp. resistance mediated by montmorillonite upon cadmium stress. Chemosphere 2020, 240, 124851. [Google Scholar] [CrossRef]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van Der Straeten, D. Folates in plants: Research advances and progress in crop biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.; Erdal, S.; Karayel, U.; Dumlupinar, R. Attenuation of lead toxicity by promotion of tolerance mechanism in wheat roots by lipoic acid. Cereal Res. Commun. 2018, 46, 424–435. [Google Scholar] [CrossRef]
- Hou, D.D.; Wang, K.; Liu, T.; Wang, H.X.; Lin, Z.; Qian, J.; Lu, L.L.; Tian, S.K. Unique rhizosphere micro-characteristics facilitate phytoextraction of multiple metals in soil by the hyperaccumulating plant Sedum alfredii. Environ. Sci. Technol. 2017, 51, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ahmad, N.; Ilyas, M.; Batool, T.S.; Gul, A. The role of ABC transporters in metal transport in plants. In Plant Metal and Metalloid Transporters; Kumar, K., Srivastava, S., Eds.; Springer: Singapore, 2022; pp. 55–71. [Google Scholar]
- Fajardo, C.; Costa, G.; Nande, M.; Botías, P.; García-Cantalejo, J.; Martín, M. Pb, Cd, and Zn soil contamination: Monitoring functional and structural impacts on the microbiome. Appl. Soil Ecol. 2019, 135, 56–64. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Li, B.Q.; Yang, Y.P. Cd accumulation characteristics of Salvia tiliifolia and changes of rhizospheric soil enzyme activities and bacterial communities under a Cd concentration gradient. Plant Soil 2021, 463, 225–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).