Nitrogen Transformation Mechanisms and Compost Quality Assessment in Sustainable Mesophilic Aerobic Composting of Agricultural Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Design
2.2. Sample Collection and Processing
2.3. Determination of Basic Index of Compost Sample
2.4. High-Throughput Sequencing
2.5. Statistical Analysis
3. Results and Discussion
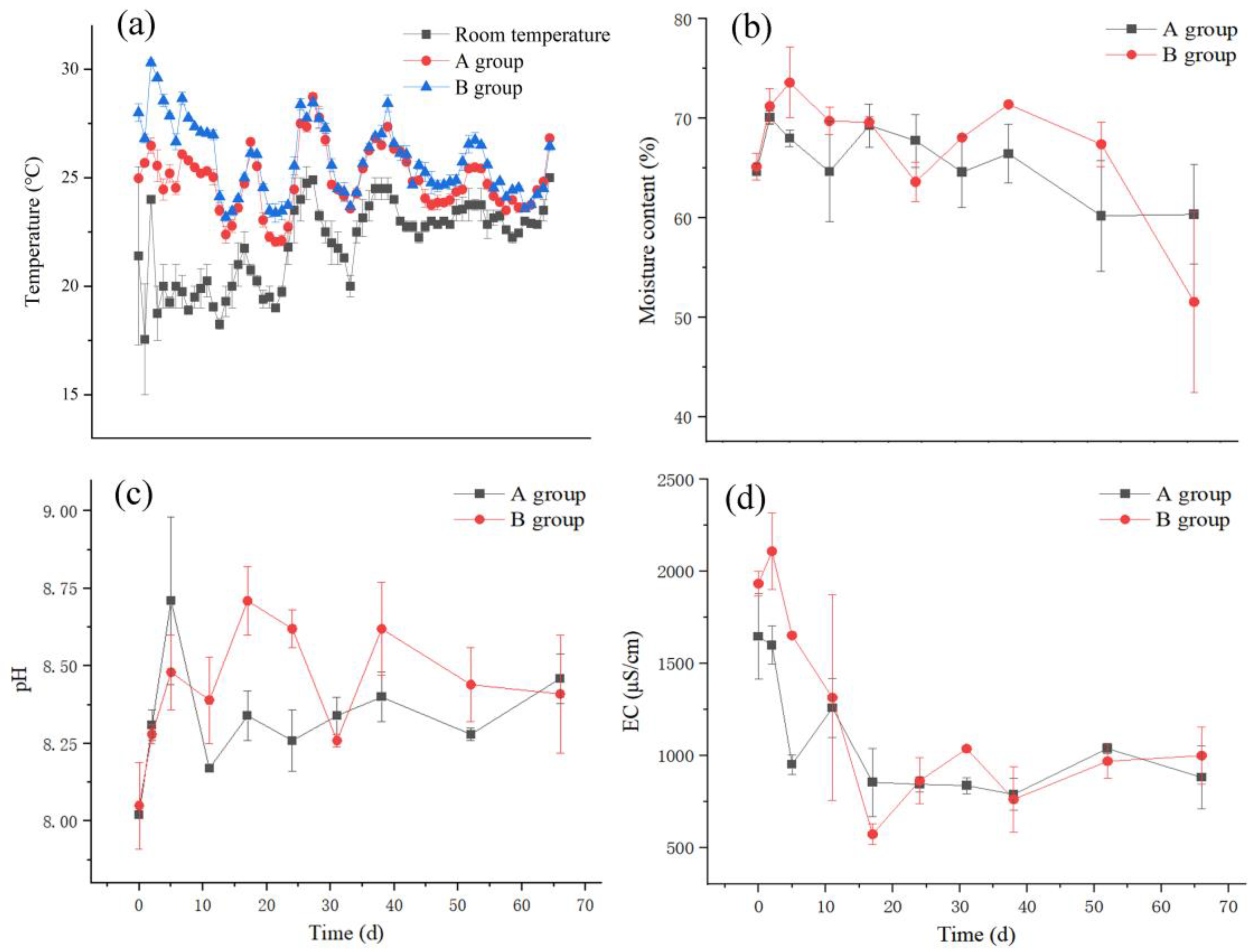
3.1. Physicochemical Properties of Compost
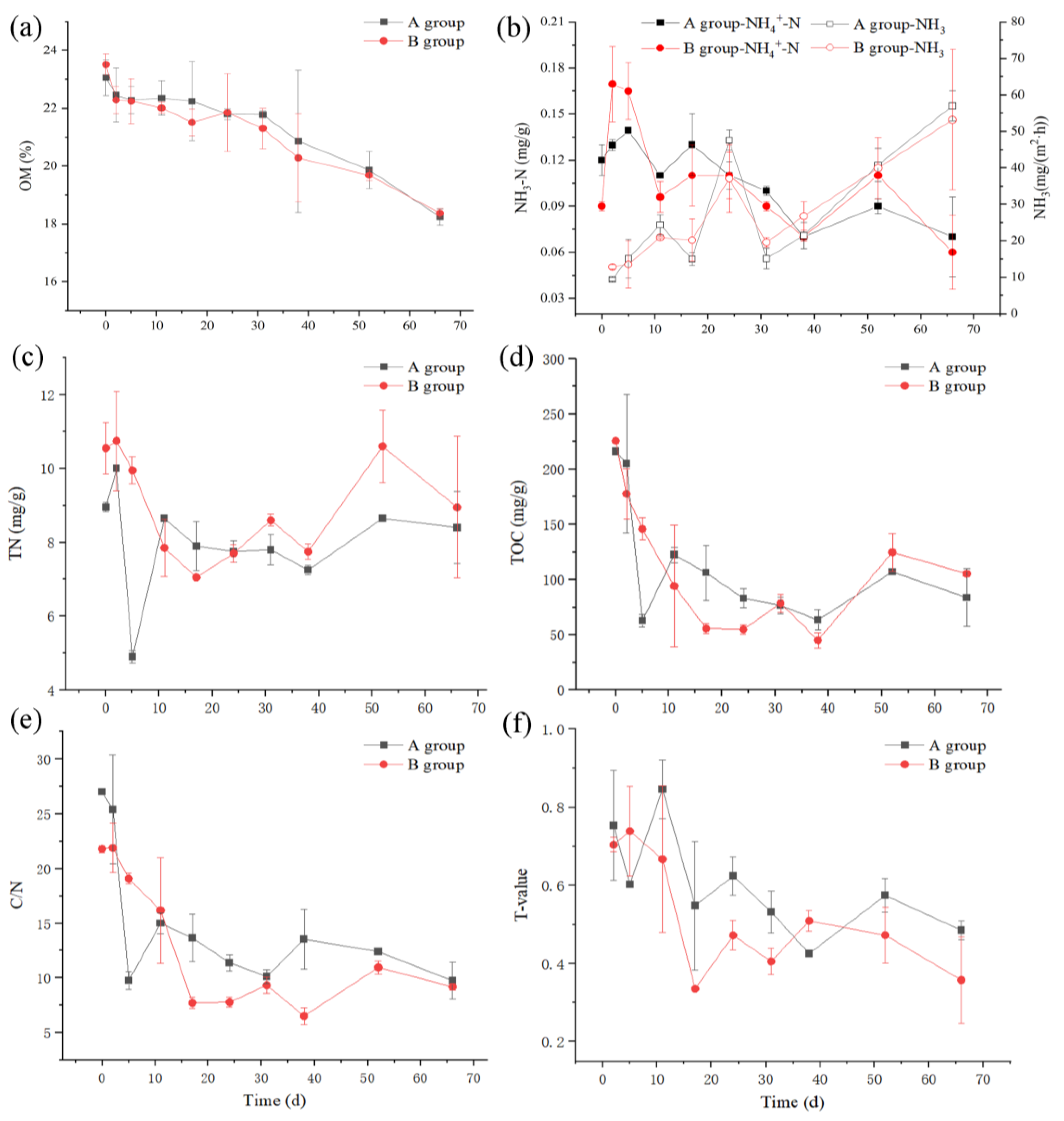
3.2. Changes in Representative Carbon and Nitrogen Compounds
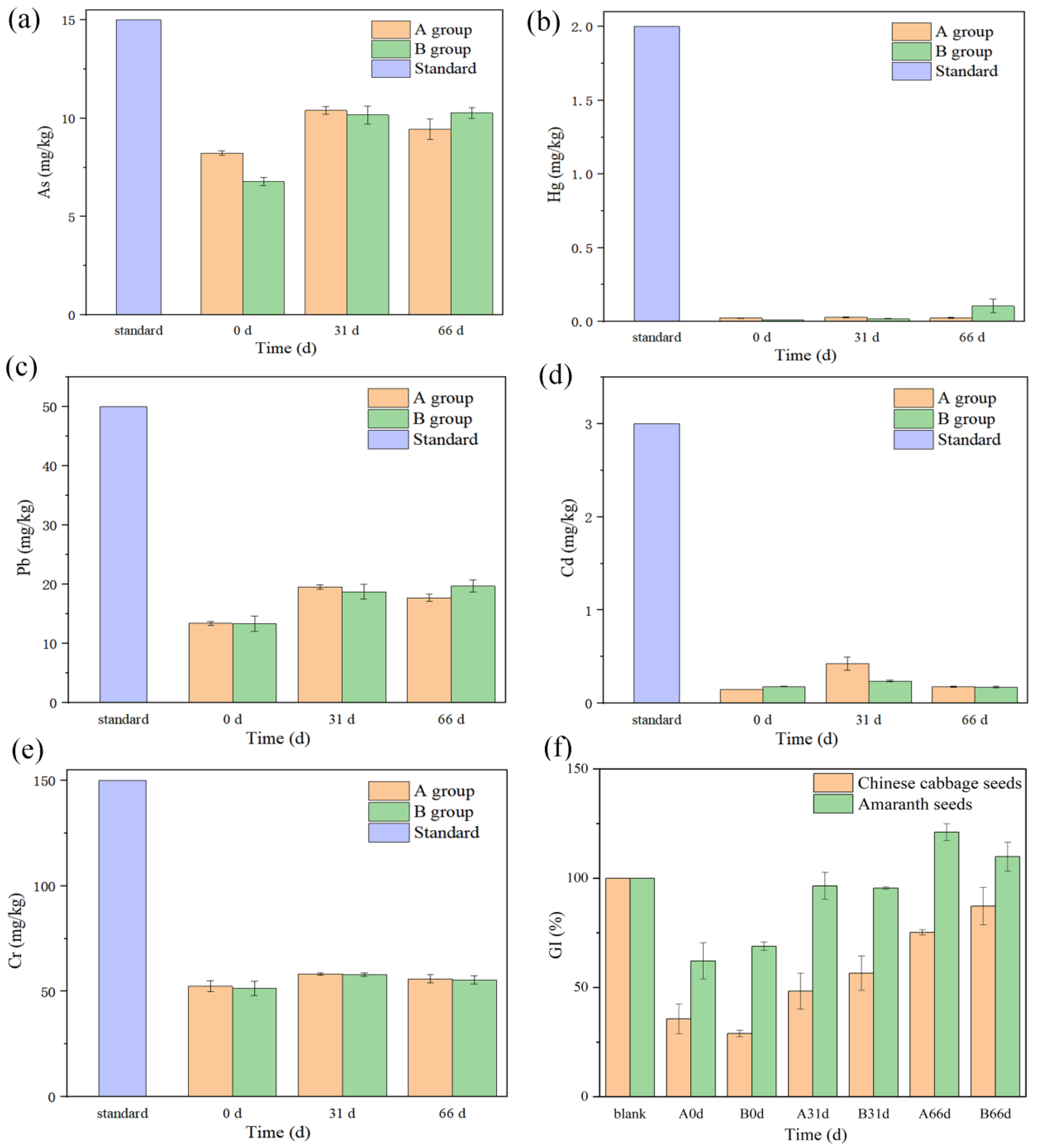
3.3. Heavy Metal Content and Seed Germination Index
3.4. Microbial Community
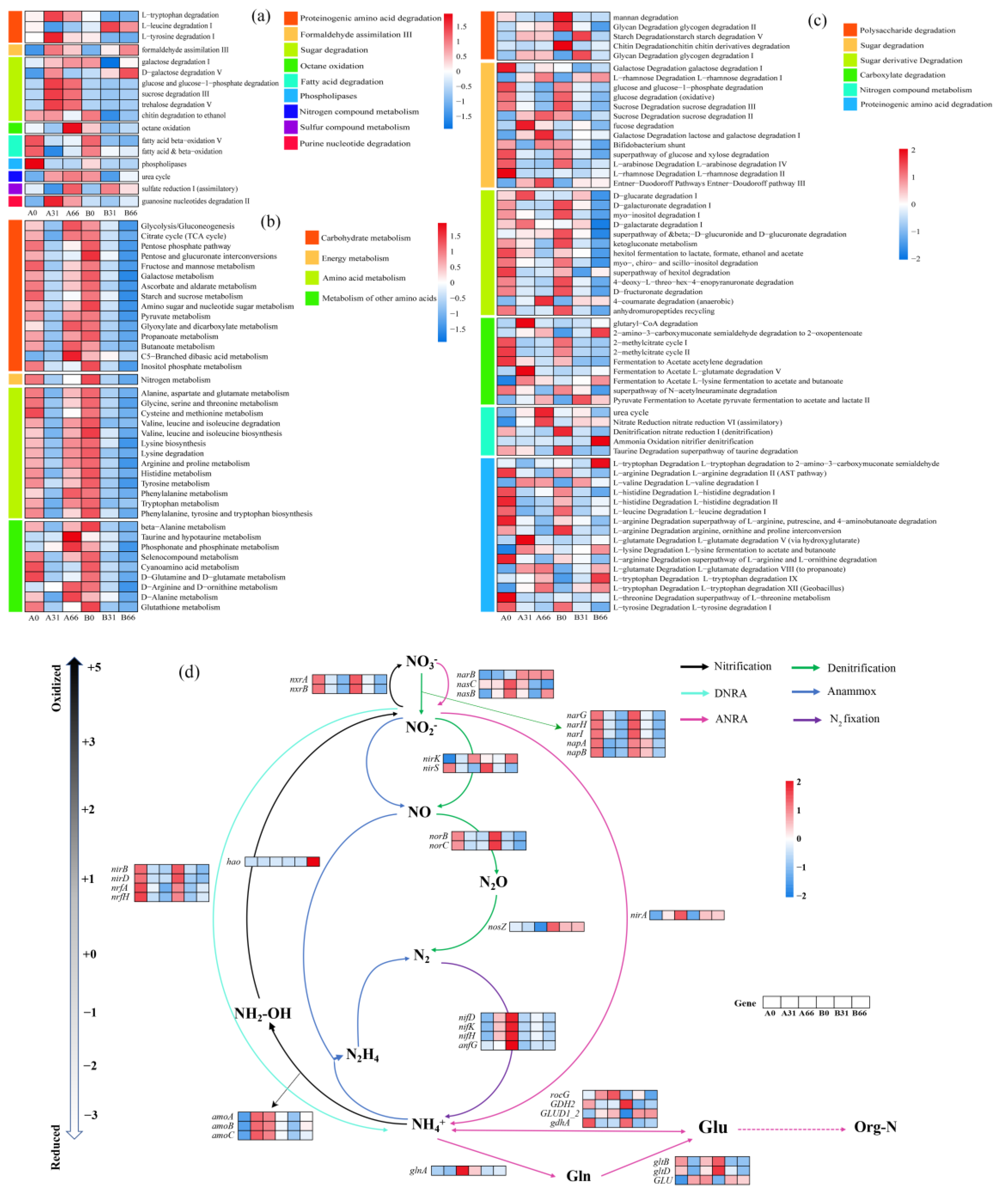
3.5. Metabolism of Microorganisms
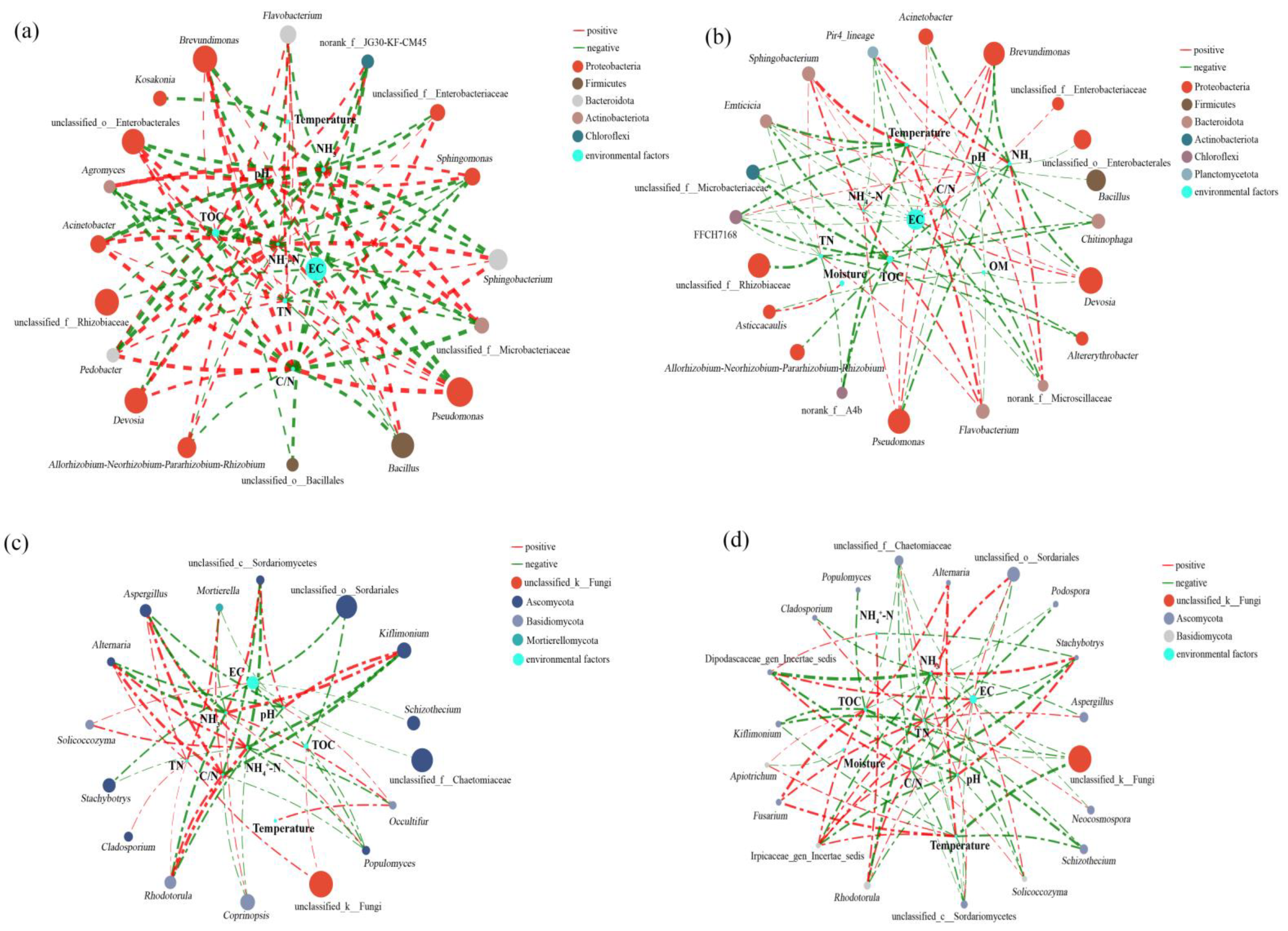
3.6. Correlation Analysis of Environmental Factors, and Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.-S.; Cai, R.-R. Preparation of solid organic fertilizer by co-hydrothermal carbonization of peanut residue and corn cob: A study on nutrient conversion. Sci. Total Environ. 2022, 838, 155867. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, Y. Ammonia emission mitigation in food waste composting: A review. Bioresour. Technol. 2018, 248, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Mazarji, M.; Li, M.; Li, A.; Li, R.; Zhang, Z.; Pan, J. Mechanism of magnetite-assisted aerobic composting on the nitrogen cycle in pig manure. Bioresour. Technol. 2024, 391, 129985. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, C.; Wang, B.; Bai, X.; Gao, H.; Huang, Y. Enzymatic mechanism of organic nitrogen conversion and ammonia formation during vegetable waste composting using two amendments. Waste Manag. 2019, 95, 306–315. [Google Scholar] [CrossRef]
- Martins, O.; Dewes, T. Loss of nitrogenous compounds during composting of animal wastes. Bioresour. Technol. 1992, 42, 103–111. [Google Scholar] [CrossRef]
- Szanto, G.L.; Hamelers, H.V.M.; Rulkens, W.H.; Veeken, A.H.M. NH3, N2O and CH4 emissions during passively aerated composting of straw-rich pig manure. Bioresour. Technol. 2007, 98, 2659–2670. [Google Scholar] [CrossRef]
- Nigussie, A.; Kuyper, T.W.; Bruun, S.; de Neergaard, A. Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J. Clean. Prod. 2016, 139, 429–439. [Google Scholar] [CrossRef]
- Liu, T.; Kumar Awasthi, M.; Verma, S.; Qin, S.; Awasthi, S.K.; Liu, H.; Zhou, Y.; Zhang, Z. Evaluation of cornstalk as bulking agent on greenhouse gases emission and bacterial community during further composting. Bioresour. Technol. 2021, 340, 125713. [Google Scholar] [CrossRef]
- Mo, J.; Xin, L.; Zhao, C.; Qin, Y.; Nan, Q.; Mei, Q.; Wu, W. Reducing nitrogen loss during kitchen waste composting using a bioaugmented mechanical process with low pH and enhanced ammonia assimilation. Bioresour. Technol. 2023, 372, 128664. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wei, Y.; Chen, W.; Ding, G.; Zhan, Y.; Liu, Y.; Xu, T.; Xiao, J.; Li, J. Impact of inoculation and turning for full-scale composting on core bacterial community and their co-occurrence compared by network analysis. Bioresour. Technol. 2022, 345, 126417. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, P.; Guo, X.; Bao, X.; Tian, J.; Li, G.; Zhang, J. Contribution of zeolite to nitrogen retention in chicken manure and straw compost: Reduction of NH(3) and N(2)O emissions and increase of nitrate. Bioresour. Technol. 2024, 391, 129981. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Chen, A.; Wang, C.; Wei, Z.; Zhao, Y.; Zhao, R. Nitrogen retention driven by cooperative succession of bacterial communities through promoting nitrogen fixation and inhibiting denitrification during straw composting with amino acids addition. Environ. Technol. Innov. 2024, 34, 103584. [Google Scholar] [CrossRef]
- Yan, B.; Lan, T.; Lv, Y.; Xing, C.; Liang, Y.; Wang, H.; Wu, Q.; Guo, L.; Guo, W.Q. Enhancing simultaneous nitrogen and phosphorus availability through biochar addition during Chinese medicinal herbal residues composting: Synergism of microbes and humus. Sci. Total Environ. 2024, 930, 172515. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hou, T.; Yin, H.; Han, L.; Huang, G. Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J. Hazard. Mater. 2019, 362, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhou, K.; Yang, T.; Zhao, X.; Li, R.; Li, J.; Xu, S.; Feng, Z.; Ding, X.; Zhang, L.; et al. Bacillus licheniformis inoculation promoted humification process for kitchen waste composting: Organic components transformation and bacterial metabolic mechanism. Environ. Res. 2023, 237, 117016. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Chen, L.; Zhou, Y.; Meng, L.; Zhang, S. Isolation and application of a thermotolerant nitrifying bacterium Gordonia paraffinivorans N52 in sewage sludge composting for reducing nitrogen loss. Bioresour. Technol. 2022, 363, 127959. [Google Scholar] [CrossRef]
- Tian, X.; Gao, R.; Li, Y.; Liu, Y.; Zhang, X.; Pan, J.; Tang, K.H.D.; Scriber Ii, K.E.; Amoah, I.D.; Zhang, Z.; et al. Enhancing nitrogen conversion and microbial dynamics in swine manure composting process through inoculation with a microbial consortium. J. Clean. Prod. 2023, 423, 138819. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Liu, F.; Han, K.; Ma, Q.; Wu, L. Membrane-covered systems improve compost quality and alter microbial communities during composting with microbial inoculation. J. Clean. Prod. 2024, 447, 141501. [Google Scholar] [CrossRef]
- Wang, F.; Xie, L.; Gao, W.; Wu, D.; Chen, X.; Wei, Z. The role of microbiota during chicken manure and pig manure co-composting. Bioresour. Technol. 2023, 384, 129360. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Env. Manag. 2013, 116, 18–26. [Google Scholar] [CrossRef]
- Tang, J.-C.; Shibata, A.; Zhou, Q.; Katayama, A. Effect of temperature on reaction rate and microbial community in composting of cattle manure with rice straw. J. Biosci. Bioeng. 2007, 104, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Semitela, S.; Pirra, A.; Braga, F.G. Impact of mesophilic co-composting conditions on the quality of substrates produced from winery waste activated sludge and grape stalks: Lab-scale and pilot-scale studies. Bioresour. Technol. 2019, 289, 121622. [Google Scholar] [CrossRef] [PubMed]
- Simujide, H.; Aorigele, C.; Wang, C.-J.; Lina, M.; Manda, B. Microbial activities during mesophilic composting of manure and effect of calcium cyanamide addition. Int. Biodeterior. Biodegrad. 2013, 83, 139–144. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Li, G.; Wang, K.; Gong, X. Performance of phosphogypsum and calcium magnesium phosphate fertilizer for nitrogen conservation in pig manure composting. Bioresour. Technol. 2018, 250, 53–59. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, X.-J.; Ju, X.-T.; Zhang, F.-S. Field in situ determination of ammonia volatilization from soil: Venting method. Plant Nutr. Fertil. Sci. 2002, 8, 205–209. [Google Scholar]
- Yang, X.; Li, R.; Wang, J.; Xu, W.; Wang, Y.; Yi, G.; Zhang, X.; Zhu, J.; Mazarji, M.; Syed, A.; et al. Exploring carbon conversion and balance with magnetite-amended during pig manure composting. Bioresour. Technol. 2023, 388, 129707. [Google Scholar] [CrossRef]
- Wen, Y.C.; Li, H.Y.; Lin, Z.A.; Zhao, B.Q.; Sun, Z.B.; Yuan, L.; Xu, J.K.; Li, Y.Q. Long-term fertilization alters soil properties and fungal community composition in fluvo-aquic soil of the North China Plain. Sci. Rep. 2020, 10, 7198. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, M.; Shi, X.; Wang, X.; Zhang, X.; Chai, L.; Liu, D.; Shen, Q. The functions of potential intermediates and fungal communities involved in the humus formation of different materials at the thermophilic phase. Bioresour. Technol. 2022, 354, 127216. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Abbasi, S.A. Solid Waste Management by Composting: State of the Art. Crit. Rev. Environ. Sci. Technol. 2008, 38, 311–400. [Google Scholar] [CrossRef]
- Zeng, G.; Huang, D.; Huang, G.; Hu, T.; Jiang, X.; Feng, C.; Chen, Y.; Tang, L.; Liu, H. Composting of lead-contaminated solid waste with inocula of white-rot fungus. Bioresour. Technol. 2007, 98, 320–326. [Google Scholar] [CrossRef]
- Chen, W.; Zhan, Y.; Zhang, X.; Shi, X.; Wang, Z.; Xu, S.; Chang, Y.; Ding, G.; Li, J.; Wei, Y. Influence of carbon-to-phosphorus ratios on phosphorus fractions transformation and bacterial community succession in phosphorus-enriched composting. Bioresour. Technol. 2022, 362, 127786. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, M.R.; Jacobsen, S.E.; Akhtar, S.S.; Muscolo, A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants 2014, 6, plu047. [Google Scholar] [CrossRef] [PubMed]
- Chenyin, P.; Yu, W.; Fenghou, S.; Yongbao, S. Review of the Current Research Progress of Seed Germination Inhibitors. Horticulturae 2023, 9, 462. [Google Scholar] [CrossRef]
- Xiong, J.; Zhuo, Q.; Su, Y.; Qu, H.; He, X.; Han, L.; Huang, G. Nitrogen evolution during membrane-covered aerobic composting: Interconversion between nitrogen forms and migration pathways. J. Environ. Manag. 2023, 345, 118727. [Google Scholar] [CrossRef]
- Hoang, H.G.; Thuy, B.T.P.; Lin, C.; Vo, D.N.; Tran, H.T.; Bahari, M.B.; Le, V.G.; Vu, C.T. The nitrogen cycle and mitigation strategies for nitrogen loss during organic waste composting: A review. Chemosphere 2022, 300, 134514. [Google Scholar] [CrossRef]
- Maeda, K.; Hanajima, D.; Toyoda, S.; Yoshida, N.; Morioka, R.; Osada, T. Microbiology of nitrogen cycle in animal manure compost. Microb. Biotechnol. 2011, 4, 700–709. [Google Scholar] [CrossRef]
- Wang, P.; Fu, G.; Guo, Z.; Zhao, L.; Pang, W.; Pan, C.; Wang, K.; Wu, Q.; Chen, Y. Composting of invasive plants in urban watercourses and its application in riverbanks: Mechanisms and compost quality assessment. J. Soils Sediments 2024, 24, 2695–2712. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Chen, L.; Meng, L.; Zheng, Z. Effect of enriched thermotolerant nitrifying bacteria inoculation on reducing nitrogen loss during sewage sludge composting. Bioresour. Technol. 2020, 311, 123461. [Google Scholar] [CrossRef]
- NY525-2012; Standards on Organic Fertilizer in Agriculture of People’s Republic of China. The Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- Sharma, M.; Sharma, S.; Paavan; Gupta, M.; Goyal, S.; Talukder, D.; Akhtar, M.S.; Kumar, R.; Umar, A.; Alkhanjaf, A.A.M.; et al. Mechanisms of microbial resistance against cadmium—A review. J. Environ. Health Sci. Eng. 2024, 22, 13–30. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef]
- Guo, H.-N.; Wang, L.-X.; Liu, H.-T. Potential mechanisms involving the immobilization of Cd, As and Cr during swine manure composting. Sci. Rep. 2020, 10, 16632. [Google Scholar] [CrossRef] [PubMed]
- El Mezouari El Glauoi, G.; El Hayany, B.; El Fels, L.; El Faiz, A.; Ezzariai, A.; Rihani, M.; Lebrihi, A.; Bekkaoui, F.; Hafidi, M. Physico-chemical and Spectroscopy Assessment of Sludge Biodegradation During Semi-industrial Composting Under Semi-arid Climate. Waste Biomass Valorization 2020, 11, 1217–1228. [Google Scholar] [CrossRef]
- Tian, X.; Qin, W.; Zhang, Y.; Liu, Y.; Lyu, Q.; Chen, G.; Feng, Z.; Ji, G.; Yan, Z. The inoculation of thermophilic heterotrophic nitrifiers improved the efficiency and reduced ammonia emission during sewage sludge composting. Chem. Eng. J. 2024, 479, 147237. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, L.; Zhong, M.; Kumar Awasthi, M.; Mao, H. Bacterial agents affected bacterial community structure to mitigate greenhouse gas emissions during sewage sludge composting. Bioresour. Technol. 2021, 337, 125397. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Yu, D.; Yao, X.; Han, J.; Cheng, R.; Cui, H.; Yan, G.; Zhang, X.; Zhu, G. Garbage enzymes effectively regulated the succession of enzymatic activities and the bacterial community during sewage sludge composting. Bioresour. Technol. 2021, 327, 124792. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, G.; Tang, J.; Wu, J.; Pang, W.; Guo, Z. Efficient nitrogen removal of mangrove constructed wetlands: Enhancing heterotrophic nitrification-aerobic denitrification microflora through quorum sensing. Chem. Eng. J. 2022, 430, 133048. [Google Scholar] [CrossRef]
- Sun, Y.; Men, M.; Xu, B.; Meng, Q.; Bello, A.; Xu, X.; Huang, X. Assessing key microbial communities determining nitrogen transformation in composting of cow manure using illumina high-throughput sequencing. Waste Manag. 2019, 92, 59–67. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, X.; Zhang, K.; Zhang, J.; Gao, N.; Min, J.; He, Y.; Wu, Z.; Chang, X. Enterobacter cloacae Rs-2 inoculum replaces fertiliser application by half in the field and modifies microbial community structure. Rhizosphere 2024, 31, 100942. [Google Scholar] [CrossRef]
- Yoo, S.H.; Weon, H.Y.; Jang, H.B.; Kim, B.Y.; Stackebrandt, E. Sphingobacterium composti sp. nov., isolated from cotton-waste composts. Int. J. Syst. Evol. Microbiol. 2007, 57, 1590–1593. [Google Scholar] [CrossRef][Green Version]
- Tudi, Y.; Pan, L.; Du, X.; Liu, B.; Li, X.; Zheng, F.; Zhang, Q. Combined Aerobic Fermentation of Maricultural and Agricultural Solid Waste: Physicochemical Property and Bacterial Community Structure. Sustainability 2024, 16, 4306. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Xia, J.; Chen, Y. Effect of microbial inoculation on physicochemical properties and bacterial community structure of citrus peel composting. Bioresour. Technol. 2019, 291, 121843. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, M.; Song, L.; Wang, C.; Yang, S.; Yan, Z.; Wang, Y. Study on nitrogen-retaining microbial agent to reduce nitrogen loss during chicken manure composting and nitrogen transformation mechanism. J. Clean. Prod. 2021, 285, 124813. [Google Scholar] [CrossRef]
- Guo, T.; Yao, X.; Wu, K.; Guo, A.; Yao, Y. Response of the rhizosphere soil microbial diversity to different nitrogen and phosphorus application rates in a hulless barley and pea mixed-cropping system. Appl. Soil. Ecol. 2024, 195, 105262. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Lopes, A.R.; Falsen, E.; Schumann, P.; Nunes, O.C.; Manaia, C.M. Microbacterium luticocti sp. nov., isolated from sewage sludge compost. Int. J. Syst. Evol. Microbiol. 2008, 58, 1700–1704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, T.; Murai, Y.; Tatsumi, K.; Iimura, Y. Biodegradation of polycyclic aromatic hydrocarbons by Sphingomonas sp. enhanced by water-extractable organic matter from manure compost. Sci. Total Environ. 2009, 407, 5805–5810. [Google Scholar] [CrossRef]
- Alessi, A.M.; Bird, S.M.; Oates, N.C.; Li, Y.; Dowle, A.A.; Novotny, E.H.; deAzevedo, E.R.; Bennett, J.P.; Polikarpov, I.; Young, J.P.W.; et al. Defining functional diversity for lignocellulose degradation in a microbial community using multi-omics studies. Biotechnol. Biofuels 2018, 11, 166. [Google Scholar] [CrossRef]
- Mistry, A.N.; Kachenchart, B.; Pinyakong, O.; Assavalapsakul, W.; Jitpraphai, S.M.; Somwangthanaroj, A.; Luepromchai, E. Bioaugmentation with a defined bacterial consortium: A key to degrade high molecular weight polylactic acid during traditional composting. Bioresour. Technol. 2023, 367, 128237. [Google Scholar] [CrossRef]
- Robledo, M.; Gomez-Silvan, C.; Andersen, G.L.; Calvo, C.; Aranda, E. Assessment of bacterial and fungal communities in a full-scale thermophilic sewage sludge composting pile under a semipermeable cover. Bioresour. Technol. 2020, 298, 122550. [Google Scholar]
- Wang, K.; Mao, H.; Li, X. Functional characteristics and influence factors of microbial community in sewage sludge composting with inorganic bulking agent. Bioresour. Technol. 2018, 249, 527–535. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.-P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef]
- Sáenz, V.; Lizcano Salas, A.F.; Gené, J.; Celis Ramírez, A.M. Fusarium and Neocosmospora: Fungal priority pathogens in laboratory diagnosis. Crit. Rev. Microbiol. 2024, 1–14. [Google Scholar] [CrossRef]
- Jiang, X.; Deng, L.; Meng, Q.; Sun, Y.; Han, Y.; Wu, X.; Sheng, S.; Zhu, H.; Ayodeji, B.; Egbeagu, U.U.; et al. Fungal community succession under influence of biochar in cow manure composting. Environ. Sci. Pollut. Res. 2020, 27, 9658–9668. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure-straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef] [PubMed]
- Sloma, A.; Rufo, G.A.; Theriault, K.A.; Dwyer, M.; Wilson, S.W.; Pero, J. Cloning and characterization of the gene for an additional extracellular serine protease of Bacillus subtilis. J. Bacteriol. 1991, 173, 6889–6895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, M.; Zhao, Y.; Zhu, L.; Song, X.; Tang, Y.; Qi, H.; Cao, H.; Wei, Z. Denitrification during composting: Biochemistry, implication and perspective. Int. Biodeterior. Biodegrad. 2020, 153, 105043. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Huang, Y.; Ran, X.; Xu, Y.; Chen, Y.; Wu, C.; Tang, J. Nitrogen Transformation Mechanisms and Compost Quality Assessment in Sustainable Mesophilic Aerobic Composting of Agricultural Waste. Sustainability 2025, 17, 575. https://doi.org/10.3390/su17020575
Zhao L, Huang Y, Ran X, Xu Y, Chen Y, Wu C, Tang J. Nitrogen Transformation Mechanisms and Compost Quality Assessment in Sustainable Mesophilic Aerobic Composting of Agricultural Waste. Sustainability. 2025; 17(2):575. https://doi.org/10.3390/su17020575
Chicago/Turabian StyleZhao, Lin, Yuhan Huang, Xue Ran, Yuwei Xu, Yuanyuan Chen, Chuansheng Wu, and Jun Tang. 2025. "Nitrogen Transformation Mechanisms and Compost Quality Assessment in Sustainable Mesophilic Aerobic Composting of Agricultural Waste" Sustainability 17, no. 2: 575. https://doi.org/10.3390/su17020575
APA StyleZhao, L., Huang, Y., Ran, X., Xu, Y., Chen, Y., Wu, C., & Tang, J. (2025). Nitrogen Transformation Mechanisms and Compost Quality Assessment in Sustainable Mesophilic Aerobic Composting of Agricultural Waste. Sustainability, 17(2), 575. https://doi.org/10.3390/su17020575





