Abstract
This study investigates the effects of NaOH pretreatment on the microstructural distribution and biomethane released from Xyris capensis. Xyris capensis was pretreated with NaOH using 1, 2, 3, 4, and 5% w/w concentrations for 60, 45, 30, 20, and 15 min of exposure time, respectively, at a 90 °C autoclave temperature. The impacts of the pretreatment technique on microstructural arrangement, crystallinity, and functional groups were examined with a scanning electron microscope (SEM), X-ray diffraction, and Fourier transform infrared (FTIR), respectively. NaOH-pretreated and untreated feedstocks were digested at the laboratory scale at a mesophilic temperature (37 ± 2 °C) for 35 days for their biomethane potential. It was discovered from the SEM analysis that NaOH pretreatment affects the microstructural arrangement of Xyris capensis, and the sample with the longer exposure time is the most affected. The results of XRD and FTIR also indicated that NaOH pretreatment lowered the crystallinity of the feedstock and significantly influenced the functional groups at varying degrees. Biomethane yield was recorded to be 258.68, 287.80, 304.02, 328.20, 310.20, and 135.06 mL CH4/gVSadded, representing 91.53, 113.09, 125.10, 143.00, and 129.68% more increases than the untreated feedstock. It was discovered that the optimum biomethane generation was achieved when 4% w/w of NaOH concentration was utilized for 20 min. This study shows that a higher NaOH concentration with a shorter retention time is more suitable for Xyris capensis. This pretreatment method can improve the biomethane yield of Xyris capensis and can be investigated for industrial applications and its use on other lignocellulose feedstocks, especially energy grasses.
1. Introduction
There is a need for an increased search for renewable and sustainable energy sources globally to replace conventional energy production from fossil fuels because of the increase in demand in developing and developed countries [1]. Energy from fossil fuel origins harms the environment and causes environmental challenges like climate change and global warming. The past few decades have witnessed an exponential increase in greenhouse gas emissions through fossil fuel combustion to generate power [2]. Therefore, renewable energy sources like bioenergy, hydro, geothermal, wind, and solar power must substitute fossil fuels in energy production to overcome the present environmental challenges [3]. Renewable energy is eco-friendly and can produce energy with little or no emissions, and it is becoming more acceptable because of the intense awareness of the need to clean the environment globally [4]. It is a critical element for environmentally friendly, economical, and sustainable sources of energy production. Energy from renewable sources can also be a secondary source of income since the producer can sell the energy generated to other consumers or the power grid [2]. This search for the origins of renewable and sustainable energy with which to substitute fossil fuels has turned attention to the waste and residue attained from various activities. Compiling waste poses a danger to the environment and the sustainability of the production chain. Most of this waste and residue is lignocellulose in nature. It is a biodegradable material that can degrade, release methane into the environment when piled up, and grow pathogens. Most importantly, energy crops and agricultural residues are gaining more attention as a potential feedstock for bioenergy generation and biobased products [5]. Using energy crops and agricultural residues as energy sources can enhance the agricultural sustainability and forestry system, minimize the total reliance on fossil fuels, and lower greenhouse gas release [6]. Bioenergy, such as biogas, bioethanol, biohydrogen, briquette, pyrolysis oil, syngas, and biodiesel, are some energy sources that can be produced from lignocellulose biomass [7,8].
Biogas is a biofuel generated from organic feedstocks with the potential to replace conventional energy sources like natural gas. It is produced through anaerobic digestion and the composition of methane, carbon dioxide, and other gasses like hydrogen sulfide, nitrogen, hydrogen, ammonia, siloxane, and water vapor in traces [9]. Biogas can be used as fuel to produce electricity with less air pollution, and its application in thermal power plants releases a lower quantity of greenhouse gasses to the environment. When biogas is used as an energy source, the quantity of carbon dioxide released into the atmosphere is equivalent to the carbon required for photosynthesis by plants [10]. Utilizing organic waste/residues to generate eco-friendly energy is one of the significant benefits of biogas. It can also assist in reducing the odor and pathogens that could be generated during decomposition since the waste can be converted to biogas. It is a bright, renewable, and sustainable energy that can be injected into the public grid for electricity and heat production [11]. The primary sources of feedstock for biogas production are agricultural and forest residues, which are abundant in nature [12,13]. Recently, more attention has been paid to energy grass as a biogas feedstock because of its lower water requirements than other crops, its non-competition with food supply, and its ability to grow on non-fertile lands [13]. The biogas potential of some energy grass has been investigated and reported as a promising biogas feedstock [14,15]. Energy grasses are lignocellulose in nature and have a high energy content. They are regarded as waste-polluting in the environment because of their low utilization, burn-off, and loss of inherent energy value. Lignocellulose feedstock is a composition of phenol aldehyde polymer lignin, polysaccharide cellulose, hemicellulose, and some percentage soluble and non-polar constituents strongly intertwined to form a strong material [16]. The morphological arrangement of these feedstocks hinders the hydrolysis stage of the anaerobic digestion process [17]. Anaerobic digestion is a biochemical process where organic materials like lignocellulose are digested without oxygen to generate biogas [18]. Anaerobic digestion has four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [19]. The hydrolysis stage is regarded as the rate-limiting step, especially during the digestion of lignocellulose materials due to the resistance of the feedstock to the microorganism’s activities. These recalcitrant properties of lignocellulose feedstock make the digestion process inefficient because only a percentage of the potential feedstock is digested [20]. Pretreatment techniques are required before anaerobic digestion to improve lignocellulose feedstocks’ efficiency. This is required to rupture the strong arrangement and make cellulose and hemicellulose contents accessible for microorganisms’ activities during digestion [21]. This stage is costly in biogas production; therefore, selecting appropriate techniques is of interest to minimize the cost input, improve efficiency, and increase the recovery potential of pretreated feedstock [22].
Biomass pretreatment techniques include biological, chemical, mechanical/physical, thermal, and combined techniques [23]. The efficiency of the pretreatment technique is determined by different factors like the age of the feedstock, its type, characteristics, etc.; therefore, every feedstock has varying pretreatment conditions for effective treatment. Irrespective of the pretreatment method selected, some important factors like the potential for commercial-scale processing, recording high yields regardless of the biomass type and source, a reduction in the quantity of waste and harmful chemicals, the adaptability of the pretreated substrate during anaerobic digestion, lignin removal efficiency, and the minimal cost of energy and equipment required are top priorities [24]. The biomethane yield of corn stover was improved by 48% when it was pretreated with deep eutectic solvents with a 1:2 ratio of the solid-to-liquid for 60 min at 100 °C [25]. Arachis hypogea shells were pretreated under different conditions of thermal pretreatment, and it was reported that the biomethane generated was enhanced by 23.96% at 100 °C for 30 min, but after this condition, the yield declined [16]. Chemical pretreatment is one of the most acceptable techniques that alter biomass’s recalcitrant structure. It involves using alkalis, acids, oxidizing agents, deep eutectic solvents, organic solvents, ionic liquids, etc., to pretreat the feedstock before anaerobic digestion [23]. The degree of depolymerization of lignocellulose feedstocks during chemical pretreatment depends on the type of chemical used and the exposure time [20]. Alkali pretreatment is an essential chemical technique with two crucial actions resulting in the decrystallization and swelling of the feedstock’s lignin–carbohydrate linkages. The solvation properties of the alkali disintegrate the lignin arrangement and significantly remove/redistribute the lignin from hemicellulose, acetyl groups, and uronic acid. The efficiency of alkali pretreatment is induced by the percentage of the lignin content present in lignocellulose [26]. This technique breaks the hydrolyzable bonds and enhances saccharification, which improves solubilization and reduces polymerization and crystallinity. The major limitations of this technique are its high operational cost, the production of inhibitory products, and longer processing time [27]. The methane yield of corn stover was improved by 56.40% when pretreated with 0.5% potassium hydroxide for 12 h at 60 °C [28]. Sodium hydroxide is one of the alkali pretreatments that has been investigated on some lignocellulose feedstock, and it was observed that its influence varies on different feedstocks. NaOH pretreatment can assist in removing lignin, hemicellulose, extractive materials, and others like oil and wax that protect the cell walls of the feedstock at particular concentrations. This technique can convert cellulose I to cellulose III groups through depolymerization to alter the microstructural arrangement [29]. This pretreatment method can extract organic and inorganic compounds like low-molecular-weight carbohydrates, sugars, kinomes, tannins, and dyes in the substrate. It alters the lignocellulose feedstocks’ morphological structure and functional groups [30]. When X-ray diffraction (XRD) and Fourier transform infrared (FTIR) were used to characterize the influence of NaOH on lignocellulose feedstock, it was observed that at 4% w/v, there was optimal removal of extractive materials, lignin, and hemicelluloses. It was also noticed that at this concentration, the amorphous content of the cellulose was altered, as recorded by the highest absorption ratio of FTIR compared to the untreated feedstock [31]. The highest methane released was recorded when 18% w/w of NaOH was used for the pretreatment of date palm [32], but 6% w/w produced the optimum methane yield when rice straw was pretreated with the same NaOH [33]. In a related study, when NaOH was applied for the treatment of Arachis hypogea shells, optimum methane generation was noticed when a 3% w/w concentration was used [34], whereas 4% w/w released the optimum methane yield for rose stalk [35]. It can be observed that NaOH does not have the same influence on the biomethane yield, but the impact depends on the morphological arrangement of the feedstock.
NaOH pretreatment was investigated on herbaceous and woody lignocellulose materials with 2% concentration at 80 °C for 2 h using a solid–liquid ratio of 1:10 (m/v). It was observed that the fascicular arrangement in corn straw, wheat straw, and sugarcane bagasse was loosened, indicating lignin elimination. However, for the cypress and Pterocarpus soyauxii, the microstructure was slightly altered. The influence of pretreatment on the functional group was higher in herbaceous materials than in woody biomass. The peaks noticed at 1730 cm−1 and 1732 cm−1 were linked to the C=C valence vibration of acetyl or carboxyl groups of hemicellulose for the herbaceous feedstock, with 1738 cm−1 traced to C-O stretching in unconjugated ketones, carbonyls, and ester groups for hard and softwood. The 833 cm−1 wavelength was assigned to the G unit for sugarcane bagasse, corn straw, and Pterocarpus soyauxii, which were observed to be eliminated after NaOH pretreatment. The enzymatic hydrolysis of NaOH-pretreated biomass showed that pretreatment improved the hydrolysis rate. Compared with untreated feedstock, it was observed that saccharification increased by 32.4, 14.8, and 61.6% for wheat straw, corn straw, and sugarcane bagasse, respectively. In contrast, cypress and Pterocarpus soyauxii improved by only 5.1% and 2.5%, respectively. It was concluded that NaOH pretreatment improved the microstructural and enzymatic saccharification of the selected biomass but at varying degrees [36]. Several microstructural modifications that can enhance enzymatic hydrolysis were reported when NaOH was applied to pretreat the bagasse seeds of Ricinus communis. The bonds that intertwined the shielded lignin hindrance with hemicellulose were degraded. The treatment conditions determine the lignin solubilization level and the hemicellulose portion’s destabilization. The pretreatment was observed to increase the surface area and availability of the cellulose portion. The morphological image showed remarkable degradation compared to the untreated substrate, with microspheres noticed on the feedstock. The anaerobic digestion process was also noticed to improve, and the biogas yield increased by about 56% [37]. The results of this study indicated that NaOH pretreatment significantly influenced the microstructural, digestion process, and biogas yield. In another study, Arachis hypogea shells were pretreated with NaOH at different treatment conditions. The morphological analysis of the pretreated samples indicated that NaOH treatment reduced cellulose crystallinity and impacted the functional compositions of the feedstock. It was noticed that the SEM images showed an alteration in the recalcitrant properties of Arachis hypogea shells. The activities of microorganisms were reported to have been improved during anaerobic digestion, and biomethane yield improved by about 94% [34]. This pretreatment technique can improve the morphological and biogas yield of lignocellulose feedstocks. However, comparing the results shows that the degree of severity depends on the microstructural arrangement of the lignocellulose materials. It was inferred that most of the available literature did not focus on energy grass, which has different morphological arrangements from agricultural residues that are primarily in consideration.
Xyris capensis is an example of energy grass that does not compete with the food supply and is the feedstock with the lowest concern with the potential for biogas production [38]. Xyris capensis is available in the Southwestern Cape and tropical Africa, South America, Malaysia, India, and China. It can be found in almost all the Provinces of South Africa with little or no usefulness [39]. Morphological analysis of Xyris capensis showed that it is a lignocellulose in nature, and its anaerobic digestion is inefficient due to its complicated structural arrangement [38]. Considering its availability and capacity as a biogas feedstock, it is necessary to investigate the most efficient pretreatment techniques to enhance the biomethane release from this bright feedstock. Therefore, this study examines the effect of different concentrations of NaOH and exposure times on the morphological and biomethane generated from Xyris capensis. The feedstock was pretreated under different conditions, and the impacts of pretreatment on morphological, crystallinity, and functional groups were studied using scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR), respectively. Pretreated and untreated feedstocks were digested under anaerobic conditions to study the impact of NaOH pretreatment on biomethane yield. The results from this research are expected to serve as a baseline for subsequent studies on lignocellulose feedstocks in the search for alternative energy sources to fossil fuels and can be replicated at the commercial scale.
2. Materials and Methods
2.1. Feedstock Collection
The feedstock used for this study was sourced locally in the Limpopo Province of South Africa (24°40′ S 30°20′ E), reduced to smaller sizes between 2 and 4 cm, and dried to reduce the moisture content to 25%. The dried sample was kept in a well-ventilated, controlled environment with a temperature of about 4 °C. The substrate was then subjected to NaOH pretreatment, and both pretreated and untreated feedstocks were analyzed for proximate and ultimate characteristics. The proximate and ultimate characterization of the feedstock was carried out according to the Association of Official Analytical Chemists (AOAC) standard guidelines [40].
2.2. Inoculum Sourcing
Inoculum, which serves as seeding material for the anaerobic digestion process, was sourced from a recently concluded anaerobic digestion process. The material was derived from the anaerobic digestion process of wastewater sludge, and lignocellulose feedstock was digested at a mesophilic temperature in a batch digester for 40 days. This stable inoculum was characterized in the laboratory for its proximate composition in line with the AOAC guidelines [40]. It was then stored in the laboratory at room temperature until the experiment was set up.
2.3. Alkali Pretreatment
The focus of this study was to investigate the influence of sodium hydroxide pretreatment on the morphological arrangement and biomethane yield of Xyris capensis. Therefore, the chopped feedstock was pretreated with a NaOH alkaline solution. Sodium hydroxide pellets (SLCF8143, Bioxtra ≥ 98%, Sigma-Aldrich, Stockholm, Sweden) were procured locally in Johannesburg, South Africa, from Sigma-Aldrich (Pty), Ltd. NaOH pretreatment was carried out as reported in previous studies with slight adjustments [34]. Different NaOH concentrations were prepared, as illustrated in Table 1, and the feedstock was soaked in the solution for a specified exposure time. The feedstock was dipped into the solution using 1:10 of w:v, heated at a specified temperature, and stirred at 200 rpm using a magnetic stirrer. The solution containing NaOH concentrations and feedstock was allowed to cool down for 10 min before the solid content was separated from the filtrate at room temperature using quantitative analysis filter papers (Grade 1, 55 mm diameter). The solid portion was then washed with running water to remove the NaOH solution, and it was checked with a pH meter until a neutral pH was recorded. NaOH-pretreated Xyris capensis was then dried in an oven at 100 °C for 4 h to reduce the moisture content. After drying, the feedstocks were stored in zip-lock plastic bags and kept in the fridge at 4 °C for characterization and anaerobic digestion.

Table 1.
NaOH pretreatment conditions.
2.4. Morphological Analysis
The impacts of NaOH pretreatment were investigated on the structural compositions of Xyris capensis with appropriate equipment. Scanning electron microscopy (SEM) (VEGA 3 TESCAN X-Max, Brno-Kohoutovice, Czech Republic) was utilized to examine the influence of different NaOH conditions and their pretreatment on the microstructural arrangement of the feedstock [41]. The samples were coated with gold before being loaded into the machine; gold coating was used over carbon coating to not compromise the samples’ carbon content. Different magnification was considered, and the most precise image was selected. X-ray diffraction (XRD) (D-8 Advance, Bruker, Canton, MA, USA) was utilized to study the cellulose crystallinity of NaOH-pretreated and untreated substrates [42]. The machine was set at 5–35 °C with a scanning speed of 5 °C/min and a diffraction angle of 2Ө. The crystallinity index of both NaOH-pretreated and untreated feedstock was determined with Equation (1) using the numerical data generated from XRD analysis [43]. The influence of NaOH pretreatment at different conditions and when untreated on the surface functional groups of the feedstocks was studied using Fourier transform infrared (FTIR spectroscopy) (SHIMADZU—IRAfinity-1, Kyoto, Japan) [44]. This was carried out between 500 and 4000 cm−1, and the data from the analysis were used to calculate the percentage relative variation in the functional groups using Equation (2) [45].
where Ic = crystallinity index; Imax = maximum diffraction at the peak position at 2Ө = 22.23°; and Ix = the intensity at 2Ө = 18°
where Bx = the absorbance of the untreated substrate; By = the absorbance of the pretreated substrate.
2.5. Theoretical Methane Yield and Biodegradability Rate
The biomethane potential of the feedstock was estimated using the theoretical methane yield (TMY) with the Buswell equation. The stoichiometry value of the substrate was calculated from the elemental analysis result and the values are used in Equations (3) and (4) to determine the TMY [46]. The biodegradation rate (Bd) of the pretreated and untreated Xyris capensis was calculated using the result of the calculated TMY and experimental yield (EMY). The Elbeshbishy expression presented in Equation (5) was used to calculate the Bd [47].
2.6. Anaerobic Digestion
As shown in Figure 1, the anaerobic digestion of NaOH-pretreated and untreated Xyris capensis was performed in the Automatic Methane Potential Testing System II (AMPTS II) at a mesophilic temperature following the VDI 4630 standard [48]. Reactor bottles of 500 mL were loaded with 400 g of the inoculum, which is 80% of the digester as recommended [48], and the weight of feedstocks loaded into each digester was determined with Equation (6). This calculation was based on the samples’ volatile solids (VSs), and a ratio of 2:1 of the substrate and inoculum was used. The water bath where the reactors were arranged was set at mesophilic temperature (37 ± 2 °C) and maintained throughout the retention period. As illustrated in Table 1, the digesters were loaded, the set up was replicated twice, and the average values were reported. Two reactors containing only inoculum were operated in parallel to measure the quantity of the gas produced by the inoculum to adjust the yield. To ascertain the precise amount of gas generated, the gas generated from the parallel reactors was removed from the reactors containing both substrates and inoculum. The software of the AMPTS II (2015 version) was programmed such that carbon dioxide flush gas was set at 10%, with a mixing time of 60 s, and off-time of 60 s, with a stirrer speed of 80%, consistent throughout the retention period. Methane release was assumed to be 60% [49], with a reactor head space of 100 mL, and the reactors were purged with nitrogen gas to remove trapped oxygen gas and set anaerobic conditions before connecting to the gas purification unit. Carbon dioxide and other impurities in the gas produced were purified with 75 mL of sodium hydroxide (3M NaOH) solution inside 100 mL screw bottles. The gas released was sent directly to the purification unit using silicon tubes, and another silicon tube transported the purified gas to the next unit, where the quantity of the biomethane yield generated was noted. When it became apparent that the daily gas output was lower than 1% of the accumulated gas, the digestion process was stopped at the 35-day retention period.
where Mi = the mass of inoculums (g); Ci = the concentration of inoculum (%); Ms = the mass of the substrate (g); and Cs = the concentration of substrate (%) [48].

Figure 1.
AMPTS II batch experiment set up for anaerobic digestion of NaOH-pretreated Xyris capensis.
3. Results and Discussions
3.1. Physicochemical Characterization
The results of the proximate and ultimate analysis of the NaOH-pretreated and untreated Xyris capensis are shown in Table 2. It was noticed that pretreatment conditions influenced the TS of the substrate. The results indicate that treatments P, R, and T had the same TS values, while treatments Q and S also had the same values. However, treatment U, the untreated feedstock, had a value different from all the NaOH-pretreated feedstocks. The values of TS observed in this study were higher than what was reported for similar lignocellulose feedstocks like corn stovers and fruit and vegetable waste [28,50]. A higher percentage of TS can affect the compaction rate of the feedstock in the digester and can result in digestion at the reactor’s outlet [51]. The recommended TS for the efficient biodigestion of lignocellulose feedstocks is between 28 and 40% [48]. Since the TS recorded in this study was higher than the acceptable range, the calculated amount of water was added to lower the TS to an acceptable level. The percentage of VSs in the feedstock was the quantity of organic matter available for the biogas released in the substrate. The VS values recorded for both feedstocks indicate that the pretreatment method influenced the feedstock’s VS. It was noticed that all the VS values were higher (>90%), indicating that feedstock has a higher potential for biomethane release. Compared to other similar lignocellulose feedstocks like corn straw, groundnut shells, and rice straw, the values recorded were higher [20,52]. This higher value of VSs implies better buffering strength for microbes during anaerobic digestion. Biomethane yield depends on the available VS, and with these percentages of VSs, higher percentages of biomethane are expected to be released [53]. The carbon–nitrogen ratio (C/N) is an important parameter influencing the potential of biomethane released. Carbon is the food source that microorganisms feed on to liberate gas, and some percentage of nitrogen is also required for their growth. An imbalanced C/N ratio can cause a digester failure due to the underproduction or overaccumulation of volatile fatty acids [54]. When the value of the C/N ratio is higher, it indicates sufficient carbon for the microorganisms to convert to biomethane, and a lower percentage does not support the growth of the microorganisms since it is needed for growth. The literature has it that for optimum process performance, a C/N ratio of 20 to 30 is needed [55]. As presented in Table 2, it was observed that all treatments have higher C/N ratios, indicating a higher degree of carbon for microbe feeding and the release of biomethane. It was also noticed that pretreatment impacts the C/N ratio when the value of the untreated feedstock is compared to the NaOH-pretreated feedstocks. This result aligns with what was recorded when another chemical pretreatment technique was examined on lignocellulose feedstock [25].

Table 2.
The physicochemical characteristics of NaOH-pretreated and untreated Xyris capensis.
Data from the elemental investigation of untreated Xyris capensis were utilized to determine the stoichiometry values of the element and organic contents as C38.89H61.44O32.11N; this was used to determine the theoretical biomethane yield (TMY) of the feedstock using Equation (4).
where a = 38.89; x = 61.44; y = 32.11; and z = 1
TMY = 397.14 mLCH4/gVSadded
3.2. Impacts of NaOH Pretreatment on Microstructural Arrangement of Xyris capensis
The impacts of different conditions of NaOH pretreatment on the microstructural arrangement of the Xyris capensis were studied with SEM. The SEM images showing the surface morphology of the feedstock picked from pretreated and untreated Xyris capensis are shown in Figure 2. It was observed that NaOH pretreatment conditions had significant impacts on microstructural arrangement when treatments P to T were compared to treatment U. It was noticed that the microstructural structure has a smooth and more intact arrangement with a compact and unaltered microstructure, as presented in Figure 2U. It was confirmed from the image that the original microstructure has a regular, complicated, and stronger microstructural arrangement than the pretreated feedstock (Figure 2P–T). This is aligned with earlier studies that alkali pretreatment methods significantly impact the microstructural arrangement of lignocellulose feedstocks but at varying degrees [34,56]. Images from the pretreated feedstocks show evident transformation after NaOH pretreatment at different conditions but at various degrees. Some level of rupturing and fragmentation with the fickle traces was noticed in the SEM images of the pretreated feedstock. Also, it was observed from the images of the pretreated feedstock that there was an agglomeration of flat and rounded microstructures, which is an indication that the lignin was broken down to make hemicellulose and cellulose available for microorganisms during anaerobic digestion [57]. It can be noticed from Figure 2P that there is a broken and amorphous arrangement, and Figure 2Q shows more broken and chiseled arrangements of Xyris capensis, which increased the crystallinity of the feedstock and was expected to reduce the microorganism accessibility, hydrolysis rate, and biomethane yield.
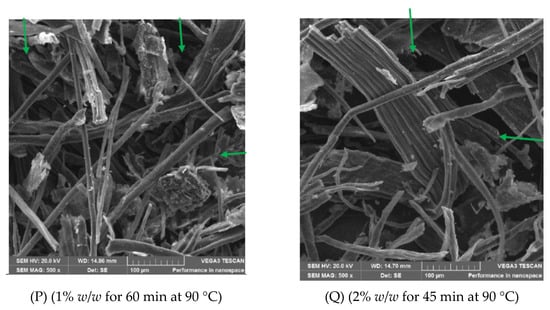

Figure 2.
SEM images of NaOH-pretreated and untreated Xyris capensis.
Further microstructure alterations and the rupture of Xyris capensis can be observed in Figure 2R, which shows that an increase in NaOH concentrations improves the degree of feedstock rupturing, which creates more space for microbial activities. Figure 2S shows the lower rupturing of the structure compared to the one noticed in Figure 2R. This indicates that increased NaOH concentrations with lesser exposure time have a lesser impact on the microstructural structure. This was further established in Figure 2T, where a lesser impact could be noticed. This result shows that a longer exposure time has more influence on the microstructural arrangement of Xyris capensis than higher concentrations of NaOH. During NaOH pretreatment, the feedstock fiber arrangement was altered and deconstructed due to the strength of the NaOH, which broke the covalent and hydrogen bonds that bind the feedstock. The images also show that the alkali led to some degree of lignin expansion irrespective of the NaOH concentration and exposure time [26]. The SEM images showed that different treatment conditions had varying levels of degradation on the surface and inner part. NaOH pretreatment could be noticed to create spaces within the feedstock, which could be due to the removal of hemicellulose. Still, the degradation was more pronounced in the external cellulose and lignin arrangement than in the internal parts. This improved polymerization and increased the surface area, enhancing the microbial accessibility and hydrolysis rate. Similar results were observed when Arachis hypogea shells were pretreated under different conditions of NaOH pretreatment [34]. A longer pretreatment time with NaOH on Napier grass was reported to show a significant influence on the microstructural arrangement [58]. It has been noticed that the alkali pretreatment of the lignocellulose substrate breaks down the microstructural structure of the substrate by degrading the inter-unit bonds that make them rough, porous, and irregular [59]. This indicates that the lignin content was altered and removed from hemicellulose and cellulose in the Xyris capensis cell wall arrangements. The SEM images from NaOH-pretreated Xyris capensis show that higher exposure times with lower alkali concentrations produce higher lignin and hemicellulose removal and enhanced cellulose accessibility when digested. This contradicts the report when the same method was applied to Arachis hypogea shells [34]. In this study, higher concentrations with shorter exposure times were reported to have a higher influence on the microstructural arrangement. This could be linked to variations in the feedstocks’ lignin content percentage, and the feedstock’s ages could also be a factor. This investigation has established that higher exposure times enhance the delignification properties, which assist in removing lignin, as reported in previous studies [60]. NaOH pretreatment has been noticed to react with aliphatic and aromatic lignin structures to alter the link between the substrate and cell wall components, enhance the inner surface area, and reduce the polymerization level [61].
3.3. Influence of NaOH Pretreatment on Crystalline Characteristics of Xyris capensis
Xyris capensis is a lignocellulose material with crystalline and non-crystalline arrangements, where cellulose falls within the crystalline region while lignin and hemicellulose are in the amorphous region [62]. The crystalline content hinders microbial activities during digestion compared to the amorphous content. The efficiency of the pretreatment technique can be verified from the crystallinity arrangement of the material. The impact of the NaOH pretreatment on Xyris capensis was investigated using XRD, and the numerical was used to plot the spectra image presented in Figure 3. XRD spectra diffract the crystallinity of the samples and form an explicit pattern, which is utilized to study the crystallinity of the feedstock. All the spectra images followed similar paths when the variation of 2Ө was considered between 22 and 18° for the changes in the crystalline. It was observed that NaOH pretreatment produced a sharp peak, whereas there was no sharp peak when Arachis hypogea shells were pretreated under the same conditions [34]. The result from Figure 3 indicates that NaOH at different treatment conditions influences the crystallinity of feedstock. It can be noticed from the figure that all the pretreated samples show higher picks than the untreated sample (treatment U). The higher peak recorded from the pretreated feedstock is a sign of high crystalline arrangement, while the second peak represents the amorphous structure. It could be observed that treatment Q has the most prominent second peak, which indicates the ability of higher concentrations of NaOH (compared to treatment P) and higher exposure times (compared to treatments R–T) to eliminate the lignin better and improve the crystallinity of the feedstock. It was noticed from the peaks that exposure time plays a major role in improving the crystallinity of the feedstock compared to acid concentrations. Generally, the figure shows that NaOH pretreatment affects the crystallinity of the feedstock and improves the crystallinity of Xyris capensis compared to the untreated material. This can be linked to the larger hydroxylation of the crystalline portion. NaOH pretreatment hydrolyzed lignin and hemicellulose and increased cellulose exposure. The longer exposure time increases the level of hydrogen bond alteration within the lattice of Xyris capensis, and it is expected that this will result in an improvement in biomethane yield compared to the untreated feedstock during anaerobic digestion. This finding supports previous investigations on the influence of pretreatment and on the crystallinity of lignocellulose feedstocks [16,25].
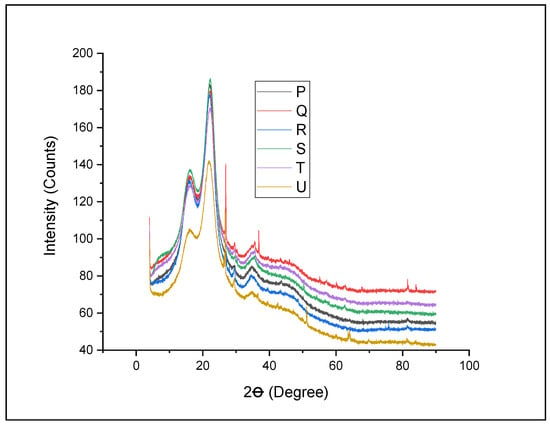
Figure 3.
XRD spectra image for NaOH-pretreated and untreated Xyris capensis. P—1% w/w NaOH for 60 min at 90 °C; Q—2% w/w NaOH for 45 min at 90 °C; R—3% w/w NaOH for 30 min at 90 °C; S—4% w/w NaOH for 20 min at 90 °C; T—5% w/w NaOH for 15 min at 90 °C; and U—control.
The percentage of crystalline cellulose in lignocellulose feedstocks can be ascertained by calculating the crystallinity index (Ic). The Ic of NaOH-pretreated and untreated Xyris capensis was determined using the numerical data from XRD analysis, and the result is illustrated in Table 3. It was observed that NaOH pretreatment influences the Ic of Xyris capensis; biodegradability is directly related to the exposure time. The table shows that the Ic for treatments P, Q, R, S, T, and U are 32.12, 31.88, 31.58, 31.21, 29.42, and 26.75%, respectively. The results followed the same pattern as the treatment with the highest exposure time, which produced a higher Ic and which also agreed with what was observed in microstructural analysis. It was noticed that the untreated feedstock has the lowest value of Ic, which indicates the strong bonding between the lignin, hemicellulose, and cellulose. The improvement in the Ic of the pretreated feedstocks could be linked to the ability of the NaOH to penetrate and remove/redistribute lignin during a longer exposure time than higher NaOH concentration within a short period. A higher concentration of NaOH could increase the melting rate of amorphous content and compromise the crystallinity. This finding aligns with earlier studies focusing on the influence of the pretreatment technique on the crystallinity of lignocellulose materials [25,28]. However, this result opposes what was recorded when the same pretreatment method was examined on Arachis hypogea shells. It was reported that higher Ic was achieved when the NaOH was the highest, with the shortest exposure time [34]. This difference in findings could be because of the different microstructural arrangements of the two feedstocks and some other environmental conditions that influence the growth of both plants. Compared with the values of Ic in previous studies, the values achieved in this study were lower. When corn stover was pretreated with the thermal–KOH process, an Ic value of 58.07% was recorded [28], and 62.10% was recorded when corn stover was pretreated with the deep eutectic solvent [25]. It was inferred that NaOH pretreatment with a longer time improves the Ic more than the alkali concentration, and treatment time could be more critical than the NaOH concentration. It is expected that this improvement will translate into biomethane yield during anaerobic digestion.

Table 3.
The crystallinity index of alkali-pretreated Xyris capensis.
3.4. Influence of NaOH Pretreatment on the Functional Groups of Xyris capensis
The impact of NaOH pretreatment on Xyris capensis for the functional group of the feedstock was examined with FTIR spectra. The spectrum peak assignment of pretreated and untreated Xyris capensis is shown in Figure 4 and Table 4, as observed in the previous literature [63]. It can be inferred from the figure that the feedstock comprises polymers (lignin, hemicellulose, and cellulose), which are the main components of lignocellulose feedstocks [64]. The figure showed a similar path with the untreated Xyris capensis, indicating no severe damage to the feedstock during pretreatment. The spectra result indicates that the material is a cellulose feedstock since bounds were noticed between 3350 and 2900 cm−1, primarily cellulose regions [65]. It was noticed that the absorbance ratio of the feedstock was enhanced after pretreatment and varied with the treatment conditions, as shown in Figure 4 and Table 4b. There was cellulose reduction in treatments P, Q, and S, with treatment Q having the highest reduction (Table 4b). This implies a reduction in the cellulosic O-H bonds, resulting in lower P, Q, and S absorbance at 3350 cm−1. It was observed that the O-H bonds of treatments P and T were not reduced with pretreatment techniques. It was noticed that there was an improvement in the absorbance ratio of the cellulosic O-H bond with increased NaOH concentrations. Still, the improvement started declining at a particular point (treatment R). This shows that NaOH concentrations play a more significant role in cellulose O-H bond reduction than the exposure time. It can be assumed that treatment with higher relative variation will release a higher biomethane yield because the cellulose bond has been reduced or broken, increasing microbial accessibility. This finding agrees with similar studies on the impact of pretreatment methods on cellulose O-H bonding [66].
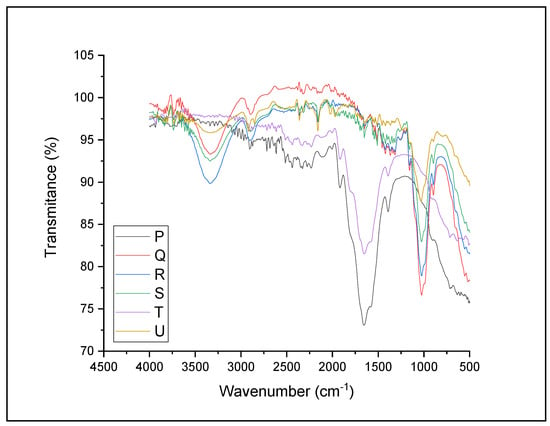
Figure 4.
FTIR spectra image for NaOH-pretreated and untreated Xyris capensis. P—1% w/w NaOH for 60 min at 90 °C; Q—2% w/w NaOH for 45 min at 90 °C; R—3% w/w NaOH for 30 min at 90 °C; S—4% w/w NaOH for 20 min at 90 °C; T—5% w/w NaOH for 15 min at 90 °C; and U—control.

Table 4.
(a): Wavelengths correlating to a selected functional group and their response to FTIR. (b): Wavelengths correlate to a particular functional group and their percentage relative variation to infrared spectroscopy.
Lignin components at around bands 1732, 1715, 1635, 1606, and 1515 cm−1 that are primarily linked to lignin content functional groups were noticed to have been altered after pretreatment but at a varying rate. It was noticed that NaOH pretreatment significantly affected all their peaks because they were noted to have either been decimated or redistributed due to the concentration of NaOH and the heat applied during pretreatment [30]. The carbonyl bond associated with lignin side chain removal could be affected mainly by treatment P followed by treatment T, which indicates that the lignin was reduced/removed better with longer exposure time and higher NaOH concentrations. This could be due to the strength of chemicals needed to break the Van der Waals bond that bonded the lignin with cellulose and hemicellulose [67]. The mild impacts of NaOH pretreatment on lignin components in some treatment conditions resulted in pseudo lignin, as observed in previous studies when other lignocellulose feedstocks were examined [28,68]. It is expected that this influences the cumulative biomethane yield during anaerobic digestion. For example, the aromatic ring stretch at 1635 and 1606 cm−1 peaks were reduced significantly at all the treatment conditions but at different degrees. On the contrary, the aromatic ring (1606 cm−1) in treatment Q was not reduced like other treatment conditions, as shown in Table 4b. The generic lignin found at 1515 cm−1 was significantly influenced by treatment P compared to other treatment conditions, indicating that the extended exposure time can remove the generic lignin better. All the treatments influenced every other lignin functional composition of Xyris capensis at varying degrees. This aligned with previous investigations when other lignocellulose feedstocks were pretreated with other methods [56,60]. A related pattern of influence was noticed from another type of lignin (syringyl lignin) at the 1320 cm−1 region [45]. Acetyl lignin was another functional group observed at the 1252 cm−1 region. The contents were noticed to have been reduced/redistributed for all the treatment conditions, with treatment P having the highest degree of alteration, followed by treatment T. It can be inferred from this that both the alkali concentrations and exposure time influence this functional group, and it is expected that this will enhance the biomethane release [34].
The influence of NaOH pretreatment on the hemicellulose portion of Xyris capensis was examined at the 1057 cm−1 band. A similar trend of results was noticed for the C-O-C stretch, which showed a reduction in the group, except for treatment T, which did not experience a reduction. In this case, treatment Q produced the highest reduction, followed by treatment R, indicating that alkali concentrations and exposure time play a major role in removing the C-O-C stretch. Amorphous cellulose recorded at 897 cm−1 was also reduced significantly with a very close value for all the treatments. This amorphous cellulose reduction indicates that there is higher crystalline cellulose for anaerobic digestion since the amorphous portion, which contributes to the feedstock’s rigidity, is removed/redistributed. The crystalline/amorphous cellulose ratio recorded at 1109/897 cm−1 increased with all the pretreatment conditions. This indicates a higher availability of readily available cellulose for microorganisms during anaerobic digestion [69]. It was observed from the FTIR findings that the NaOH pretreatment method influences the functional groups of Xyris capensis, and the degree of influence differs with alkali concentration; some were influenced by longer exposure, while NaOH concentrations also had influence. It was in only a few cases that both input parameters had a significant influence on functional groups. A general comparison of all the functional groups showed that treatment P significantly influenced the functional groups. This implies that the longer retention time for lower alkali concentration is the most suitable for improving the functional composition of Xyris capensis.
% Relative variation = 100 × (absorbance of untreated substrate—absorbance of NaOH pretreated substrate) ÷ absorbance of untreated substrate. All positive values imply a decrease.
3.5. Cumulative Biomethane Yield and Biodegradability of Xyris capensis
Both NaOH-pretreated and untreated Xyris capensis were digested for 35 days, and the cumulative biomethane released is illustrated in Figure 5. A thirty-five (35) day retention time is sufficient for methane generation, as it was noticed that biomethane generation became very low around day 20. It can be inferred from Figure 5 that NaOH pretreatment influences the biomethane yield from Xyris capensis. Biomethane yield was noted to be 258.68, 287.80, 304.02, 328.20, 310.20, and 135.06 mL CH4/gVSadded for treatments P, Q, R, S, T, and U, respectively. The expected electrical potential capacity of the biomethane yield was estimated, assuming that 1 m3 of methane is equivalent to 36 MJ with an electrical conversion efficiency of 35%. Therefore, 1 m3 of methane released generates 10 kWh of electricity [70]. It was observed that 0.0026, 0.0029, 0.0030, 0.0033, 0.0031, and 0.0014 kWh of electricity could be generated from treatments P, Q, R, S, T, and U, respectively. It was also observed that the untreated substrate releases the least biomethane yield, indicating the impact of the pretreatment technique on biomethane generation. This agrees with a previous study which reported that the oxidative pretreatment method increased the biomethane generated from [38]. Compared to the untreated feedstock, biomethane yield was enhanced by 91.53, 113.09, 125.10, 143.00, and 129.68% for treatments P, Q, R, S, and T, respectively. Compared with the TMY of Xyris capensis (397.14 mL CH4/gVSadded), it was noticed that about 34.86, 27.53, 23.45, 17.36, 21.89, and 65.99% of treatments P, Q, R, S, T, and U, respectively, of the feedstock potential were not released during anaerobic digestion. This shows that despite the pretreatment application, the full biomethane potential of the substrate was not released. The biodegradability rate (Bd) of the digestion process was calculated using Equation (5), and it was observed that treatments P, Q, R, S, T, and U have Bd rates 65.14, 72.47, 76.55, 82.64, 78.11, and 34.01%, respectively. It was observed that the cumulative biomethane yield agreed with Bd and, to some extent, with the morphological analysis. Figure 5 shows that all the pretreated feedstocks have a similar pathway, except the untreated feedstock, which differs. In terms of the retention period, pretreated feedstock appears to digest faster than untreated feedstock, which implies that the NaOH pretreatment shortens the retention time of the feedstock. This is aligned with the previous study, which reported that pretreatment of lignocellulose feedstock reduces the process’s retention time [71].
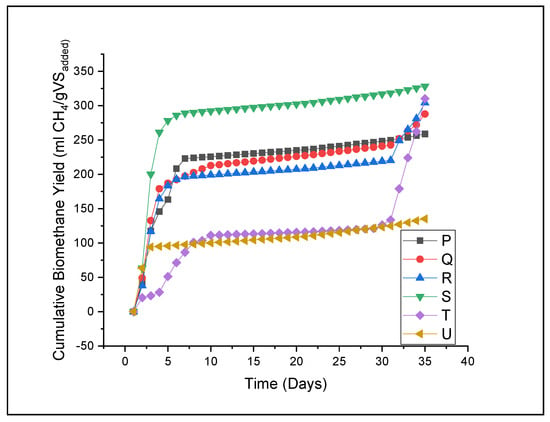
Figure 5.
Cumulative biomethane yield of NaOH pretreated and untreated Xyris capensis. P—1% w/w NaOH for 60 min at 90 °C; Q—2% w/w NaOH for 45 min at 90 °C; R—3% w/w NaOH for 30 min at 90 °C; S—4% w/w NaOH for 20 min at 90 °C; T—5% w/w NaOH for 15 min at 90 °C; and U—control.
It is noted that NaOH pretreatment breaks down the morphological arrangement of Xyris capensis significantly by partially altering hemicellulose and lignin, resulting in an improved surface area, fiber swelling, and cell wall disruption, making it more accessible for enzymatic hydrolysis. The feedstock was loosened because the NaOH concentration removed the binding agents and exposed the cellulose portion for biomethane release [72]. The linkage between lignin and hemicellulose, especially the ether and ester bonds, and carbon–carbon and ester linkages in lignin were affected by NaOH. The chemical bonds of lignin and the bonds between lignin and hemicellulose in the Xyris capensis are noted to be more vulnerable to NaOH [73]. The NaOH hydrolysis of Xyris capensis eliminates the acetyl groups and uronic replacement from hemicelluloses; therefore, the hemicelluloses surface becomes more available during the digestion process. Additionally, NaOH pretreatment improves the polyionic properties of Xyris capensis, which is connected to basic ion diffusion into the feedstock. These ions are kept in feedstock and serve as a countercharge to carboxylate ions. These polyionic properties of NaOH pretreatment encourage swelling, which supports the fast hydrolysis of Xyris capensis compared to the untreated substrate [74].
It was noticed that cumulative biomethane increased with the increase in NaOH concentrations and lower exposure time until the optimum biomethane yield was recorded at treatment S. An optimum biomethane yield of 328.20, which represents a 143% increase was observed when the feedstock was pretreated with 4% w/w for 20 min at 90 °C. This is contrary to what was reported when NaOH pretreatment was used to pretreat Arachis hypogea shells. The optimum biomethane yield with a 69.79% increase was recorded when 3% NaOH was applied for 15 min [34]. The variation in the performance of the same chemical on two different feedstocks could be linked to variations in the microstructural arrangement of the materials, as observed from the morphological analysis. Xyris capensis was pretreated with different oxidative conditions, and the highest improvement was 48.52% [38], which is lower than the observed value for all the NaOH conditions considered here. This difference in the performance of two different alkali pretreatments can be linked to the strength of NaOH to remove or reduce the lignin content and expose the cellulose to microbial activities. Another possibility could be due to the properties of the chemical used to generate inhibitory substances that can impede the release of biomethane [23]. The difference in biomethane yield among the NaOH-pretreated feedstock can be traced to the ability of each treatment condition to eliminate or rearrange the lignin contents or release inhibitory compounds. When discussing the morphological analysis, treatment P was observed to be more affected by pretreatment, which was linked to its longer exposure to the NaOH reaction. However, this does not translate to an increase in biomethane release, which can be a result of the production of inhibitory substances like furfural, 5-hydroxymethylfurfural (HMF), and acetic acid that hinders biomethane release [75]. Another possibility of producing a low biomethane yield at a longer exposure time could be the higher degradation of hemicellulose in the feedstock during pretreatment, reducing the available materials for gas production. Xyris capensis has high crystalline and amorphous cellulose, which is reduced significantly during pretreatment (treatment P), as reported in the FTIR report. When the exposure time increases, the amount of hydronium ions rises and can act as an acid that supports hemicellulose hydrolysis [76]. A longer exposure time can result in significant lignin removal, which makes organic matter accessible to microorganisms and encourages fast hydrolysis. Faster hydrolysis can produce the over-accumulation of volatile fatty acids (VFAs), which alters the pH of the process and reduces the biomethane yield or results in digester failure [54].
The biomethane released from the untreated substrate was small because hemicellulose was partially degraded due to the strong intertwining between the lignin, cellulose, and hemicellulose, as observed in the morphological and crystallinity analysis (Figure 2U). The lignin shielded the organic matter, resulting in low polymerization and partial decomposition, and limited the biomethane released. NaOH pretreatment on Xyris capensis produced varying structural alterations that supported enzymatic hydrolysis. The protective lignin bonds that linked the lignin barrier with hemicellulose were degraded, and the ester bonds were irreversibly hydrolyzed. The lignin was totally or partially solubilized based on the treatment conditions, and hemicellulose portion degradation was observed. The Xyris capensis particles also swelled during NaOH pretreatment, resulting in an improvement in the surface area and better availability of the cellulose portion. Furthermore, a reduction in the crystallinity and cellulose level and cellulose polymerization was noticed, with an increase in the enzymatic digestion of the polysaccharide [36]. Therefore, NaOH hydroxide pretreatment increased the efficiency of anaerobic digestion of Xyris capensis and agreed with previous investigations that considered NaOH pretreatment on lignocellulose feedstocks [58,77,78]. The optimum conditions and the degree of improvement differ from those of these studies, which can be due to the variation in the microstructural arrangement of the substrates. NaOH pretreatment can remove lignin effectively but is mild on cellulose content. This technique causes fiber swelling, improves the substrate’s surface area, and supports microbial accessibility [23]. As observed in the crystalline analysis, there was an improvement in the feedstock’s crystallinity, and the link between lignin and carbohydrates was altered, leading to lignin arrangement alteration, which encouraged an increase in the biomethane released. Findings from this study show that NaOH pretreatment improved the biomethane produced, and the optimum improvement was noticed when 4% w/w of NaOH was utilized for 20 min at 90 °C.
It can be inferred that treatment exposure time and NaOH concentrations are crucial to the pretreatment performance of lignocellulose feedstocks. The influence of this method also depends on the feedstock’s structural arrangement and functional groups. For example, the optimum increase in biogas and methane produced was observed at a 4% NaOH concentration when Napier grass, rice straw, and hazelnut were used, but at different exposure times [58,79,80]. It was noticed that the strength of NaOH above 4% w/w during a shorter period released inhibitory compounds that hindered the release of biomethane from the feedstock [81]. Pretreatment with NaOH for an extended time was observed not to have a significant impact on lignin elimination and cellulose recovery. Cellulose and lignin had a stronger resistance to NaOH treatment than hemicellulose. More prolonged feedstock exposure to NaOH pretreatment primarily influences hemicellulose because of its higher amorphous content and unstable arrangement compared to cellulose [82]. Therefore, a longer NaOH pretreatment time resulted in a reduction in the hemicellulose portion, which reduced the accessible materials during the methanogenic stage. Compared to related studies, findings from this investigation followed the same trend [34,58,77]. All the treatment conditions significantly influenced the morphological arrangement of Xyris capensis and biomethane yield. This pretreatment method is easy and does not need a unique design, equipment, or higher energy. However, NaOH pretreatment needs equipment that does not react with NaOH during pretreatment to obtain accurate results. This method can be observed to be economical at the laboratory scale, and further study is required at the prototype stage to establish it before it can be recommended for commercial usage. Also, there is a need to investigate the detailed kinetic parameters of the chemical concentration, treatment time, temperature, and retention time of microstructural alterations and the release of biomethane.
4. Conclusions
The strength of NaOH in enhancing the morphological and biomethane yield of Xyris capensis was investigated in this study. All the pretreatment conditions considered improved the morphological arrangement and biomethane yield of Xyris capensis. The performance of NaOH was discovered to be influenced by the alkali concentrations and pretreatment time. Pretreatment conditions that produced the most significant influence on morphological arrangements do not translate into biomethane yield, which could be due to some reasons, like the production of inhibitory compounds. The optimum biomethane yield improved by 143% when 4% w/w of NaOH was used for 20 min. The findings from this study have established conditions that can be adopted for other energy grasses and lignocellulose materials to produce improved renewable and sustainable energy. Since this study was conducted on a laboratory scale, further investigation is required to ascertain its viability on an industrial scale. The major limitation of this process is the need for special equipment for pretreatment due to the properties of the alkali.
Author Contributions
Conceptualization—K.O.O. and D.M.M.; methodology—K.O.O. and D.M.M.; investigation—K.O.O.; formal analysis—D.M.M. and K.O.O., resources—D.M.M. and K.O.O.; Original draft—K.O.O.; First draft review and editing—D.M.M.; Supervision—D.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data were presented in figures and tables in the manuscript and will be made available upon a reasonable request.
Acknowledgments
The authors would like to thank the Process, Energy and Environmental Technology Station, Faculty of Engineering and the Built Environment, University of Johannesburg, South Africa, for releasing the Automatic Methane Potential Testing System II (AMPTS II) used for this research.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Santika, W.G.; Anisuzzaman, M.; Bahri, P.A.; Shafiullah, G.M.; Rupf, G.V.; Urmee, T. From goals to joules: A quantitative approach of interlinkages between energy and the Sustainable Development Goals. Energy Res. Soc. Sci. 2019, 50, 201–214. [Google Scholar] [CrossRef]
- Ang, T.-Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Energy Strat. Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable Energy in the Sustainable Development of Electrical Power Sector: A Review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Qazi, A.; Hussain, F.; Rahim, N.A.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards Sustainable Energy: A Systematic Review of Renewable Energy Sources, Technologies, and Public Opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Rizzi, F.; van Eck, N.J.; Frey, M. The production of scientific knowledge on renewable energies: Worldwide trends, dynamics and challenges and implications for management. Renew. Energy 2014, 62, 657–671. [Google Scholar] [CrossRef]
- Mustafa, S.; Long, Y.; Rana, S. Role of domestic renewable energy plants in combating energy deficiency in developing countries. End-user perspective. Energy Rep. 2023, 11, 692–705. [Google Scholar] [CrossRef]
- Sudhakar, M.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Bijarchiyan, M.; Sahebi, H.; Mirzamohammadi, S. A sustainable biomass network design model for bioenergy production by anaerobic digestion technology: Using agricultural residues and livestock manure. Energy Sustain. Soc. 2020, 10, 19. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Ciotola, R.J.; Lansing, S.; Martin, J.F. Emergy analysis of biogas production and electricity generation from small-scale agricultural digesters. Ecol. Eng. 2011, 37, 1681–1691. [Google Scholar] [CrossRef]
- Eswari, A.P.; Ravi, Y.K.; Kavitha, S.; Banu, J.R. Recent insight into anaerobic digestion of lignocellulosic biomass for cost effective bioenergy generation. e-Prime 2023, 3, 100119. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M. Effect of acidic pretreatment on the microstructural arrangement and anaerobic digestion of Arachis hypogea shells; and process parameters optimization using response surface methodology. Heliyon 2023, 9, e15145. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.; Olabi, A. Pretreatment techniques used in biogas production from grass. Renew. Sustain. Energy Rev. 2016, 68, 1193–1204. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Sittijunda, S.; Reungsang, A. Influences of size reduction, hydration, and thermal-assisted hydration pretreatment to increase the biogas production from Napier grass and Napier silage. Bioresour. Technol. 2021, 331, 125034. [Google Scholar] [CrossRef]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Predicting methane yield by linear regression models: A validation study for grassland biomass. Bioresour. Technol. 2018, 265, 372–379. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M.; Ahmed, N.A.; Ogunkunle, O. Biomethane production from Arachis hypogea shells: Effect of thermal pretreatment on substrate structure and yield. Biomass Convers. Biorefinery 2022, 14, 6925–6938. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Antonopoulou, G.; Lyberatos, G. Production of biogas via anaerobic digestion. In Handbook of Biofuels Production: Processes and Technologies, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 266–304. [Google Scholar] [CrossRef]
- A Raja, I.; Wazir, S. Biogas Production: The Fundamental Processes. Univers. J. Eng. Sci. 2017, 5, 29–37. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M. Comparative Analysis of the Effects of Five Pretreatment Methods on Morphological and Methane Yield of Groundnut Shells. Waste Biomass Valorization 2023, 15, 469–486. [Google Scholar] [CrossRef]
- Olugbemide, A.D.; Lajide, L.; Adebayo, A.; Owolabi, B.J. Kinetic Study of Biogas Production from Raw and Solid-State Organosolv Pretreated Rice Husk. J. Biofuels 2016, 7, 110–118. [Google Scholar] [CrossRef]
- Deivayanai, V.; Yaashikaa, P.; Kumar, P.S.; Rangasamy, G. A comprehensive review on the biological conversion of lignocellulosic biomass into hydrogen: Pretreatment strategy, technology advances and perspectives. Bioresour. Technol. 2022, 365, 128166. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, L.; Deng, B.; Huang, C.; Zhu, J.; Liang, L.; He, X.; Wei, Y.; Qin, C.; Liang, C.; et al. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Olugbemide, A.D.; Oberlintner, A.; Novak, U.; Likozar, B. Lignocellulosic Corn Stover Biomass Pre-Treatment by Deep Eutectic Solvents (DES) for Biomethane Production Process by Bioresource Anaerobic Digestion. Sustainability 2021, 13, 10504. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Zou, H.; Sun, T.; Li, M.; Zhai, J.; He, Q.; Gu, L.; Tang, W.Z. Effects of acid/alkali pretreatments on lignocellulosic biomass mono-digestion and its co-digestion with waste activated sludge. J. Clean. Prod. 2020, 277, 123998. [Google Scholar] [CrossRef]
- Saravanan, A.; Yaashikaa, P.; Kumar, P.S.; Thamarai, P.; Deivayanai, V.; Rangasamy, G. A comprehensive review on techno-economic analysis of biomass valorization and conversional technologies of lignocellulosic residues. Ind. Crops Prod. 2023, 200, 116822. [Google Scholar] [CrossRef]
- Siddhu, M.A.H.; Li, J.; Zhang, J.; Huang, Y.; Wang, W.; Chen, C.; Liu, G. Improve the Anaerobic Biodegradability by Copretreatment of Thermal Alkali and Steam Explosion of Lignocellulosic Waste. BioMed Res. Int. 2016, 2016, 2786598. [Google Scholar] [CrossRef]
- Li, C.; Fan, M.; Xie, J.; Zhang, H. Effect of NaOH-catalyzed organosolv pretreatment on the co-production of ethanol and xylose from poplar. Ind. Crops Prod. 2023, 200, 116774. [Google Scholar] [CrossRef]
- Madyira, D.; Olatunji, K. Investigating the influence of different pretreatment methods on the morphological structure of Arachis hypogea shells. Mater. Today Proc. 2023, 105, 72–77. [Google Scholar] [CrossRef]
- Mustikaningrum, M.; Cahyono, R.B.; Yuliansyah, A.T. Effect of NaOH Concentration in Alkaline Treatment Process for Producing Nano Crystal Cellulose-Based Biosorbent for Methylene Blue. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012005. [Google Scholar] [CrossRef]
- Lahboubi, N.; Karouach, F.; Bakraoui, M.; El Gnaoui, Y.; Essamri, A.; El Bari, H. Effect of Alkali-NaOH Pretreatment on Methane Production from Anaerobic Digestion of Date Palm Waste. Ecol. Eng. Environ. Technol. 2022, 23, 78–89. [Google Scholar] [CrossRef]
- He, Y.; Pang, Y.; Liu, Y.; Li, X.; Wang, K. Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 2008, 22, 2775–2781. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M.; Ahmed, N.A.; Ogunkunle, O. Influence of alkali pretreatment on morphological structure and methane yield of Arachis hypogea shells. Biomass Convers. Biorefinery 2022, 14, 12143–12154. [Google Scholar] [CrossRef]
- Liang, Y.-G.; Cheng, B.; Si, Y.-B.; Cao, D.-J.; Li, D.-L.; Chen, J.-F. Effect of solid-state NaOH pretreatment on methane production from thermophilic semi-dry anaerobic digestion of rose stalk. Water Sci. Technol. 2016, 73, 2913–2920. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, Y.; Yu, Q.; Tan, X.; Zhuang, X.; Yuan, Z. Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Ind. Crops Prod. 2020, 145, 112145. [Google Scholar] [CrossRef]
- Quezada-Morales, D.L.; Campos-Guillén, J.; De Moure-Flores, F.J.; Amaro-Reyes, A.; Martínez-Martínez, J.H.; Chaparro-Sánchez, R.; Zavala-Gómez, C.E.; Flores-Macías, A.; Figueroa-Brito, R.; Rodríguez-Morales, J.A.; et al. Effect of Pretreatments on the Production of Biogas from Castor Waste by Anaerobic Digestion. Fermentation 2023, 9, 399. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M. Optimization of Biomethane Yield of Xyris capensis Grass Using Oxidative Pretreatment. Energies 2023, 16, 3977. [Google Scholar] [CrossRef]
- Pooley, E.; Herbarium, N. A Field Guide to Wild Flowers: KwaZulu-Natal and the Eastern Region; Natal Flora Publication Trust: Cape Town, South Africa, 1998; p. 630. [Google Scholar]
- Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MD, USA, 2019.
- Statnik, E.S.; Ignatyev, S.D.; Stepashkin, A.A.; Salimon, A.I.; Chukov, D.; Kaloshkin, S.D.; Korsunsky, A.M. The Analysis of Micro-Scale Deformation and Fracture of Carbonized Elastomer-Based Composites by In Situ SEM. Molecules 2021, 26, 587. [Google Scholar] [CrossRef]
- Bruker d8 XRD Procedures. Available online: https://xraysrv.wustl.edu/web/xrd/brukerd8.html (accessed on 6 December 2024).
- Atalla, R.H.; VanderHart, D.L. The role of solid state 13C NMR spectroscopy in studies of the nature of native celluloses. Solid State Nucl. Magn. Reson. 1999, 15, 1–19. [Google Scholar] [CrossRef]
- Barbes, L.; Radulescu, C.; Stihi, C. ATR-FTIR spectrometry characterisation of polymeric materials. Rom. Rep. Phys. 2014, 66, 765–777. [Google Scholar]
- Dahunsi, S.; Adesulu-Dahunsi, A.; Osueke, C.; Lawal, A.; Olayanju, T.; Ojediran, J.; Izebere, J. Biogas generation from Sorghum bicolor stalk: Effect of pretreatment methods and economic feasibility. Energy Rep. 2019, 5, 584–593. [Google Scholar] [CrossRef]
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical methane potential (BMP) of food waste and primary sludge: Influence of inoculum pre-incubation and inoculum source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Vergärung Organischer Stoffe Substratcharakterisierung. VEREIN DEUTSCHER INGENIEURE Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests VDI 4630 VDI-RICHTLINIEN. 2016. Available online: www.vdi.de/richtlinien (accessed on 17 June 2023).
- Olatunji, K.O.; Madyira, D.M.; A Ahmed, N.; Ogunkunle, O. Experimental evaluation of the influence of combined particle size pretreatment and Fe3O4 additive on fuel yields of Arachis hypogea shells. Waste Manag. Res. J. Sustain. Circ. Econ. 2022, 41, 467–476. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, X.; Ye, J.; Sun, Y.; Wang, W.; Zhang, Z. Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renew. Energy 2011, 36, 3313–3318. [Google Scholar] [CrossRef]
- Webster, J. The Biochemistry of Silage (Second Edition). By P. McDonald, A.R. Henderson and S. J. E. Heron. Marlow, Bucks, UK: Chalcombe Publications, (1991), pp. 340, £49.50, ISBN 0-948617-225. Exp. Agric. 1992, 28, 125. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.; Rahman, S.; Sunoj, S.; Igathinathane, C. Impact of corn stover particle size and C/N ratio on reactor performance in solid-state anaerobic co-digestion with dairy manure. J. Air Waste Manag. Assoc. 2020, 70, 436–454. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Marcus, A.K.; Kang, D.-W.; Rittmann, B.E.; Krajmalnik-Brown, R. pH-Mediated Microbial and Metabolic Interactions in Fecal Enrichment Cultures. mSphere 2017, 2, e00047-17. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Optimization of process parameters for accelerated methane yield from anaerobic co-digestion of rice straw and food waste. Renew. Energy 2019, 149, 1352–1359. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H. Improving biogas production from wheat plant using alkaline pretreatment. Fuel 2014, 115, 714–719. [Google Scholar] [CrossRef]
- Wang, C.; Shao, Z.; Qiu, L.; Hao, W.; Qu, Q.; Sun, G. The solid-state physicochemical properties and biogas production of the anaerobic digestion of corn straw pretreated by microwave irradiation. RSC Adv. 2021, 11, 3575–3584. [Google Scholar] [CrossRef] [PubMed]
- Pinpatthanapong, K.; Boonnorat, J.; Glanpracha, N.; Rangseesuriyachai, T. Biogas production by co-digestion of sodium hydroxide pretreated Napier grass and food waste for community sustainability. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 1678–1692. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Wunna, K.; Nakasaki, K.; Auresenia, J.L.; Abella, L.C.; Gaspillo, P.D. Effect of Alkali Pretreatment on Removal of Lignin from Sugarcane Bagasse. Chem. Eng. Trans. 2017, 56, 1831–1836. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Alkali lignin depolymerization under eco-friendly and cost-effective NaOH/urea aqueous solution for fast curing bio-based phenolic resin. Ind. Crops Prod. 2018, 120, 25–33. [Google Scholar] [CrossRef]
- Kim, S.; Holtzapple, M.T. Effect of structural features on enzyme digestibility of corn stover. Bioresour. Technol. 2006, 97, 583–591. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Rout, P.R.; Aslam, M.; Fuwad, A.; Choi, Y.; Park, J.H.; Kumar, G. A brief review of anaerobic membrane bioreactors emphasizing recent advancements, fouling issues and future perspectives. J. Environ. Manag. 2020, 270, 110909. [Google Scholar] [CrossRef]
- Pereira, S.C.; Maehara, L.; Machado, C.M.; Farinas, C.S. Physical–chemical–morphological characterization of the whole sugarcane lignocellulosic biomass used for 2G ethanol production by spectroscopy and microscopy techniques. Renew. Energy 2016, 87, 607–617. [Google Scholar] [CrossRef]
- Drygaś, B.; Depciuch, J.; Puchalski, C.; Zaguła, G. The impact of heat treatment on the components of plant biomass as exemplified by Junniperus sabina and Picea abies. ECONTECHMOD 2016, 5, 41–50. [Google Scholar]
- Awoyale, A.A.; Lokhat, D. Experimental determination of the effects of pretreatment on selected Nigerian lignocellulosic biomass in bioethanol production. Sci. Rep. 2021, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, K.O.; Madyira, D.M. Enhancing the biomethane yield of groundnut shells using deep eutectic solvents for sustainable energy production. Front. Energy Res. 2024, 12, 1346764. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem. 2019, 84, 81–90. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, E.-J.; Ban, S.-E.; Lee, J.-W. Structural characterization of the lignin-carbohydrate complex in biomass pretreated with Fenton oxidation and hydrothermal treatment and consequences on enzymatic hydrolysis efficiency. Carbohydr. Polym. 2021, 270, 118375. [Google Scholar] [CrossRef]
- Suhartini, S.; Lestari, Y.P.; Nurika, I. Estimation of methane and electricity potential from canteen food waste. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012075. [Google Scholar] [CrossRef]
- Dahunsi, S.; Oranusi, S.; Efeovbokhan, V. Optimization of pretreatment, process performance, mass and energy balance in the anaerobic digestion of Arachis hypogaea (Peanut) hull. Energy Convers. Manag. 2017, 139, 260–275. [Google Scholar] [CrossRef]
- Rai, P.S.; Unnikrishnan, S.; Chandrashekar, A. Influence of alkali treatment on physiochemical and morphological properties of palmyra fibers. Ind. Crops Prod. 2024, 224, 120298. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, R.C. Chapter 19—Recent Advances in Alkaline Pretreatment of Lignocellulosic Biomass. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–459. [Google Scholar] [CrossRef]
- Shah, T.A.; Khalid, S.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M. Sodium Hydroxide Hydrothermal Extraction of Lignin from Rice Straw Residue and Fermentation to Biomethane. Sustainability 2023, 15, 8755. [Google Scholar] [CrossRef]
- Moniz, P.; Pereira, H.; Quilhó, T.; Carvalheiro, F. Characterisation and hydrothermal processing of corn straw towards the selective fractionation of hemicelluloses. Ind. Crops Prod. 2013, 50, 145–153. [Google Scholar] [CrossRef]
- Suryawati, L.; Wilkins, M.R.; Bellmer, D.D.; Huhnke, R.L.; Maness, N.O.; Banat, I.M. Effect of hydrothermolysis process conditions on pretreated switchgrass composition and ethanol yield by SSF with Kluyveromyces marxianus IMB4. Process Biochem. 2009, 44, 540–545. [Google Scholar] [CrossRef]
- Şenol, H. Effects of NaOH, thermal, and combined NaOH-thermal pretreatments on the biomethane yields from the anaerobic digestion of walnut shells. Environ. Sci. Pollut. Res. 2021, 28, 21661–21673. [Google Scholar] [CrossRef] [PubMed]
- Salehian, P.; Karimi, K.; Zilouei, H.; Jeihanipour, A. Improvement of biogas production from pine wood by alkali pretreatment. Fuel 2013, 106, 484–489. [Google Scholar] [CrossRef]
- Şenol, H. Anaerobic digestion of hazelnut (Corylus colurna) husks after alkaline pretreatment and determination of new important points in Logistic model curves. Bioresour. Technol. 2019, 300, 122660. [Google Scholar] [CrossRef] [PubMed]
- Budiyono; Wicaksono, A.; Rahmawan, A.; Matin, H.H.A.; Wardani, L.G.K.; Kusworo, T.D.; Sumardiono, S. The effect of pretreatment using sodium hydroxide and acetic acid to biogas production from rice straw waste. MATEC Web Conf. 2017, 101, 02011. [Google Scholar] [CrossRef]
- Aklilu, E.G.; Waday, Y.A. Optimizing the process parameters to maximize biogas yield from anaerobic co-digestion of alkali-treated corn stover and poultry manure using artificial neural network and response surface methodology. Biomass Convers. Biorefinery 2021, 13, 12527–12540. [Google Scholar] [CrossRef]
- Thota, S.P.; Badiya, P.K.; Yerram, S.; Vadlani, P.V.; Pandey, M.; Golakoti, N.R.; Belliraj, S.K.; Dandamudi, R.B.; Ramamurthy, S.S. Macro-micro fungal cultures synergy for innovative cellulase enzymes production and biomass structural analyses. Renew. Energy 2017, 103, 766–773. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).