1. Introduction
The demand for agricultural products (food), energy, and the production of organic waste are increasing along with the global population. According to recent projections, the world population is expected to increase from an estimated 8.2 billion in 2024 to 10.3 billion in the mid-2080s, resulting in an increasing dependency between water, energy and food [
1]. This surge is further exacerbated by urgent challenges such as climate change and environmental degradation. Together, these factors will pose significant challenges to effective waste management and long-term resource sustainability [
2].
The exploitation of natural resources is also intensifying, and scarcity has become unavoidable, making overuse one of the most critical challenges of the modern era. Promoting the integrated management of water, energy, and food resources enhances sustainability and strengthens resilience [
3]. These resources are fundamental to achieving the United Nations Sustainable Development Goals (SDGs) [
4]. As a result, Water–energy–food (WEF) nexus-based frameworks have been explored and implemented at various scales [
5,
6].
Household-scale organic waste can be managed through a range of technologies that recover resources while minimizing environmental impacts. One such option is anaerobic digestion (AD). AD is a microbial process that operates without oxygen, yielding two main outputs: biogas and digestate. Primary advantage of this process is the capture of methane, a potent greenhouse gas that would otherwise be released into the atmosphere. In addition to AD, which yields biogas [
7], other widely adopted methods include composting and small-scale thermal conversion. Composting is the most accessible and widely used method that relies on aerobic microbial decomposition. Composting is a simple aerobic process that stabilizes food and garden waste into a soil amendment that improves soil fertility [
8]. This method offers less costly and simpler implementation but releases some greenhouse gases and lacks the energy capture potential of AD. Small-scale thermal conversion, such as pyrolysis, can transform organic waste into biochar and heat, providing both a renewable energy source and a stable carbon product that supports soil improvement [
8]. However, this technology is in a very early stage for household practical use. The main reasons are (1) the possibility of the production of flammable and poisonous gases and (2) the need for specialized equipment.
The most effective choice for household-scale systems depends on initial capital availability, desired output (e.g., energy or fertilizer), local climate, and the interest of end-users to engage in system maintenance. Anaerobic digestion produces both renewable energy and a nutrient-rich soil amendment (i.e., digestate), which provides a net global warming benefit. However, this is more capital-intensive compared to composting, which is the most practical and affordable technology without energy output. Additionally, environmental performance depends on proper site management to minimize greenhouse gas emissions. Small-scale thermal conversion has great potential for significant waste volume reduction but is expensive for household use and faces challenges with public perception and air pollution control.
This highlights the need for innovative solutions in household-scale WEF to achieve sustainability goals. Household-scale systems can offer a practical and efficient approach to integrating the WEF nexus by simultaneously addressing waste management, energy generation, and nutrient recycling. Organic waste, which includes food waste, agricultural by-products, and other biodegradable materials, can be used to produce clean energy and boost agricultural productivity in a WEF Nexus. Such organic waste can be utilized in anaerobic digestion processes to produce methane-rich biogas, which can then be used as a renewable energy source [
7]. Use of impure methane created using the bio-digestion process requires some consideration. Combustion engines and gas turbines are both viable options for small-scale biogas energy conversion, each with unique characteristics. Combustion engines stand out because of their higher efficiency, lower cost, minimal maintenance requirements, and ability to operate without biogas purification. Conversely, gas turbines have notable disadvantages, including their reliance on purified, compressed biogas, which makes them less attractive in comparison to combustion engines [
9]. For optimal performance, combustion engines require a biogas methane concentration of at least 45% [
10]. Methane-rich biogas can also be combusted for direct applications such as heating or grilling.
Municipal solid waste (MSW) represents a significant energy resource, with an average energy content of approximately 6.6 GJ/ton, although this varies based on waste composition [
9]. In 2018, the United States disposed of roughly 150 million tons of MSW in landfills [
11], highlighting the vast untapped potential for energy recovery. At the household level, food waste presents an opportunity for small-scale energy generation. For instance, the average annual food waste per person in Europe and North America is 105 kg (231.49 lb) [
12]. Using corn as a reference material with an energy content of 10.8 GJ/ton [
9] and assuming a combustion engine efficiency of 24%, the usable energy output per ton of corn would be approximately 2.59 GJ.
Closed-loop technological systems play a crucial role in maintaining sustainability by mimicking cycles where waste from one process becomes a resource for another [
13]. Self-sustaining closed-loop systems make them particularly effective in creating sustainable solutions across different sectors. Such systems not only eliminate waste but also generate usable energy and produce fertilizer, making them efficient in providing multiple benefits simultaneously [
8,
14]. Decentralized WEF systems have been recognized as an effective strategy to achieve significant environmental and social benefits, moving communities closer to a closed-loop or circular economy model. Within WEF, anaerobic digestion has been widely applied to reduce organic waste volumes and divert material from landfills, thereby minimizing leachate formation. Household systems also encourage active user engagement, integrating residents into circular economy practices. Real-world examples further demonstrate the feasibility of such systems; for instance, closed-loop organic waste management has been applied by family farmers in Brazil to recover energy and nutrients for crop cultivation [
14]. Small-scale systems have been designed to combine anaerobic digestion, pyrolysis for biochar, hydroponics, and vermifiltration, offering opportunities to increase resilience and income for small-scale farmers while supporting sustainable waste management practices.
Biogas is a mixture of methane, carbon dioxide, nitrogen, hydrogen sulfide, and small amounts of other trace gases [
15]. It is commonly created by the anaerobic breakdown of a substance with a high organic content such as food waste, agricultural waste, and other sources [
16].
Constant production and availability of organic waste make biogas an excellent, clean and safe source of renewable energy. Biogas can be filtered to maximize the ratio of methane to other gases and to remove impurities such as hydrogen sulfide, which can harm equipment and be hazardous to human health. Methane-containing biogas is combustible and can be used as fuel for cooking and producing heat or electricity. Use of created biogas is an ideal option for generating renewable energy, as it efficiently converts organic waste into a sustainable energy source, while contributing to environmental conservation and waste management [
17].
A biodigester system is used to break down organic waste for biogas production. A commonly used technology for industrial biogas production is the continuous stirred tank reactor (CSTR) [
18,
19]. This system has been extensively used for substrates with high moisture content which includes sewage sludge, municipal solid waste, manure, and agricultural and industrial wastewater [
19,
20]. The biodigester is filled with organic waste, and an inoculum of bacterial diversity is added. The inoculum is a biologically active liquid rich in microorganisms [
21]. These microorganisms begin to break down the waste in an anaerobic process called microbiological fermentation. The final output of this process is a biogas that includes a calorific value of 21–24 MJ/m
3 and a composition of 50–70% methane and 30–50% carbon dioxide [
22]. The biogas is stored in an attached gasbag or tank for later usage. Several parameters that affect the anaerobic system, including temperature, organic loading rate (OLR), pH, alkalinity, heavy metals, and substrate concentration, must be controlled to maximize biogas yield and keep the biodigester from failing [
23].
The biodigester effluent can be effectively utilized as a nutrient source to support agriculture [
24]. Wastewater from domestic sources, which share similarities with digested organic residues, contains essential nutrients such as nitrogen, phosphorus, potassium, and other micronutrients that plants can absorb [
25]. Similarly, Siddiqui et al. [
26] demonstrated that food waste-derived organic fertilizers, such as “FoodLift,” could serve as sustainable alternatives to synthetic fertilizers, effectively supplying key nutrients for crops like lettuce and cucumber. Therefore, residuals from biogas production can be used as sustainable fertilizers, reducing the need for synthetic fertilizers for promoting plant growth and soil health, particularly when properly monitored and managed.
Biodigestors support a closed-loop system compared to composting and small-scale thermal conversion. Anaerobic digestion generates both renewable energy and nutrient-rich digestate. Composting primarily focuses on soil improvement which enhances crop growth and water-use efficiency. The thermal conversion method is generally used for drier organic waste and yields highly stable products, but it is limited to large-scale applications rather than household scale. To date, there are also few integrated studies that simultaneously evaluate biogas production, water quality, and hydroponic plant performance from a household-scale sink waste stream. To address this, the study aims to develop and evaluate a household-scale, closed-loop WEF system that is directly connected to the kitchen sink, providing an innovative solution to address the increasing challenges of waste management, energy generation, and sustainable resource utilization for agricultural production. This study also demonstrates the feasibility and practical effectiveness of household-scale biodigester systems by identifying and establishing parameters necessary for their successful operation.
2. Materials and Methods
2.1. WEF System Setup
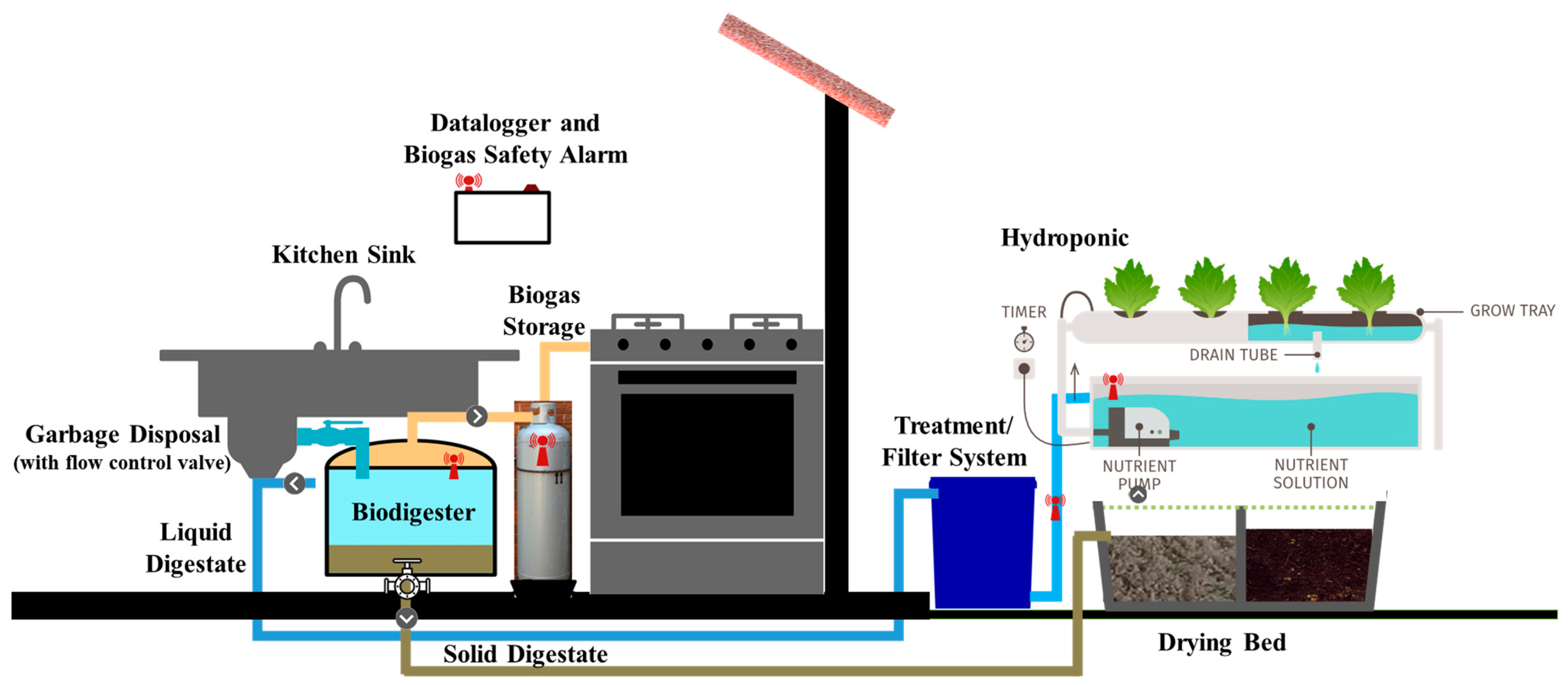
The system was designed and constructed at the Emergent Technologies Institute (ETI) Lab at Florida Gulf Coast University. The biodigester was designed to connect to a household kitchen sink and food disposal system to turn food waste into biogas for an energy source and nutrient-rich fertilizer that supports food crop production. This dual functionality underscores the system’s role in reducing waste while simultaneously generating valuable resources. The main components of the system include a custom-made anaerobic biodigester, kitchen sink garbage disposal, settling tank, slow sand filter, holding tanks, and hydroponic systems. The preliminary design of this comprehensive system is demonstrated in
Figure 1, which provides a conceptual overview of its layout and functionality.
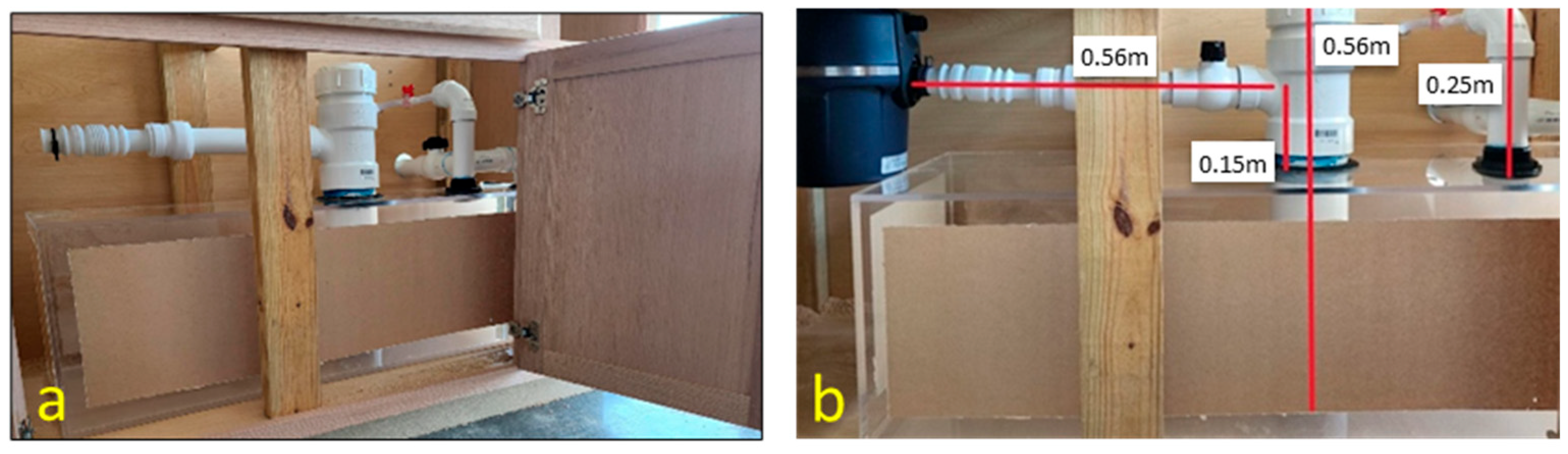
Specific dimensions of the biodigester are shown in
Figure 2, including the placement and size of its inlet and outlet openings. The design accounts for the flow dynamics of food waste and effluent, optimizing the placement and size of the openings to facilitate efficient input of waste materials and output of biogas and digestate. The size of the digester carefully considers practical constraints, such as the limited clearance height available beneath a residential kitchen sink cabinet. This ensures the system is compact and adaptable for household use.
The biodigester was constructed using acrylic sheets with a protective brown covering as presented in
Figure 3. Acrylic is a strong and flexible impact-resistant clear thermoplastic material that offers high resistance to variations in temperature. Acrylic also enables an option for visual inspection of the anaerobic digestion process and biogas production. Digesters were designed to run at different target temperature ranges. The temperature ranges are typically 30–38 °C for mesophilic organisms and 50–60 °C for thermophilic organisms [
27]. The system was designed to operate under mesophilic conditions. Heating pads were installed beneath the acrylic biodigester to maintain the target temperature, which was continuously monitored using the In-Situ Aqua Troll sonde (In-Situ Inc., Fort Collins, CO, USA).
The biodigester was mounted under the kitchen cabinet and connected to a common household garbage disposal unit. The garbage disposal grinds food waste into smaller particles and combines it with water, before rerouting it into the biodigester for anaerobic digestion. In this experiment, biogas produced from anaerobic digestion of food waste rises through the tube and is captured in an inflatable air mattress for storage. This air mattress, as shown in
Figure 4a, has a volume of approximately 119,298 cm
3 and the biogas is sampled once a week for eight weeks. In addition, the system produces a nutrient-rich effluent that can be repurposed for agricultural or landscaping use, providing an environmentally friendly alternative to synthetic fertilizers. By contrast, food waste typically disposed of in a landfill, sewage system, or septic tank often leads to methane emissions or complex waste-handling challenges. A broad range of PVC pipe sizes were utilized for the feedstock inflow and digestate outflow. This engineered design with automated flow control ensures smooth operation and optimal performance of the biodigester within the constraints of a residential kitchen setup.
The digestate was processed through a 121,000 cm
3 slow sand filter system, as shown in
Figure 4b, before being directed to the hydroponic planter. Digestate, an exceedingly nutrient-rich byproduct of anaerobic digestion, requires filtration to achieve acceptable levels of pH, total coliforms (TC), biological oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS), and total suspended solids (TSS) for agricultural irrigation [
28]. Untreated digestate can introduce high levels of ammonia and salt, potentially causing stress to plants [
29]. This indicates that filtration and treatment of digestate are essential to ensure it is safe for agricultural use. Slow sand filtration is a simple and inexpensive method for treating digestate. Although it is not as effective as more sophisticated filtration methods, slow sand filtration can nevertheless raise water quality to substantial standards for crop irrigation [
28]. It has been demonstrated that crops cultivated with filtered digestate grow just as well as crops grown with conventional fertilizers [
29].
A typical slow sand filter media can be developed with fine- and course-grained sand with an effective diameter of 0.15–0.35 mm, and a filter depth of 0.6–1.2 m [
30]. The effectiveness of slow sand filters lies in their combination of mechanical (absorption, diffusion, screening, and sedimentation) and biological (predation, natural death, and metabolic breakdown) processes to remove organic materials and microorganisms [
28,
31]. Moreover, slow sand filters are highly reliable and require minimal maintenance due to their lack of moving parts. Maintenance is limited to simple procedures like backwashing, making them an accessible and sustainable option for digestate treatment in agricultural systems.
After being processed through the slow sand filtration system, the filtered digestate becomes a valuable resource for plant growth due to its nutrient-rich composition. This filtered digestate is then utilized in two hydroponic systems residing within the Gorilla Grow Tent Lite Line 20.3 × 20.3 cm, as shown in
Figure 5. The Gorilla Grow Tent (The Original Gorilla Grow Tent
® 5 × 5, Gorilla Inc., Santa Rosa, CA, USA) is specifically designed to create a controlled environment for optimal plant development. The tent is equipped with sinching port ducts that allow for efficient management of environmental conditions. These ports enable the entry of electrical wiring, LED lights, and aerators while maintaining the tent’s sealed environment to optimize light exposure for the plants. The LED lights simulate natural sunlight, ensuring consistent photosynthesis, while the aerators promote oxygenation in the hydroponic nutrient solution, supporting healthy root development. This setup provides an ideal environment for hydroponic cultivation by combining advanced lighting, nutrient delivery, and controlled air circulation.
2.2. General Procedures
The food waste used in this study was collected from the South Village dining hall at Florida Gulf Coast University, and the waste was transported to the Emergent Technology Institute testing laboratory. The feedstock consisted mainly of vegetables, fruits, and staple foods. Vegetables, including leafy greens, carrots, corn, and broccoli, made up about 60% of the waste, while fruits such as pineapple and cantaloupe contributed around 20%, and staples like bread, rice, pasta, and eggs accounted for the remaining 20%. The waste was first homogenized and diluted with an equal amount of water before being added to the biodigester. The system was fed three times a week, with roughly 2.1 kg of food waste (wet mass), and no pH adjustment was applied during the study. The food waste was then directed into an anaerobic/biodigester, where bacteria grew and flourished, producing nutrients and biogas with a high methane content as a byproduct. The digestate trickled into a storage tank and was pumped into a slow sand filtration system, while the biogas was routed to an inflatable mattress that captured approximately 0.12 m3 of gas each week. The filtered effluent delivered nutrient-rich water to half of a hydroponic system, while nitrogen/phosphorus-rich water was delivered to the other half of the hydroponic system as an experimental control.
The hydroponic system, consisting of Rex lettuce (Lactuca sativa), was equally germinated in starter growth cubes for one week, and transplanted into two plastic bins constructed to hold twelve plants each, where the plant roots absorbed the nutrients. The water level was maintained immediately below the net pots to ensure the plant roots were in the water. The closed-loop system using the Nutrient Film Technique (NFT) of hydroponics, connected via flexible tubes with accessible valves at each station, was closely monitored by collecting water and biogas samples weekly. A multi-parameter water quality sonde was used to analyze the water samples, which were collected each week at all the system locations. Test strip kits were used to measure the concentrations of phosphorus and nitrite/nitrate in the water. The water quality monitoring data was used to ensure that the water quality remained suitable for plant growth and to demonstrate that the system was operating efficiently. Weekly 1-L gas samples were collected for on-site gas chromatography analysis to determine the type of compounds present, and their percent composition occurring in each sample. These analyses helped to provide insights into the efficiency of the anaerobic digestion process.
To develop a method enabling individuals to maximize energy output without depleting nonrenewable resources, various boundaries have been explored through trial and error. These boundaries tested include design optimization, water quality testing, gas tracing, and plant monitoring.
2.3. Gas Chromatography Testing
Biogas analysis was conducted using the Shimadzu GC-8A (Shimadzu Corporation, Kyoto, Japan), gas chromatography analyzer (GC) system equipped with a thermal conductivity detector (TCD) system utilizing Helium (He) as the carrier medium. GC-based techniques are widely used since the system can concurrently interpret the quantitative and qualitative traces in the gas samplings through various biotechnological procedures. According to the interaction of the targets with the stationary phase, the degree of the target dispersion in the mobile phase, and their boiling point, the GC machine can additionally distinguish compounds that arise in complex matrices [
32]. In general, the design of GC-based methods is based on optimizing key operating variables, including the kind of stationary and mobile phases, the column temperature and its corresponding heating rate, as well as the flow rate and composition of the carrier gas, to maximize effectiveness [
32]. By tailoring these variables, GC-based systems ensure accurate and reliable analyses, further strengthening their role as indispensable tools in gas analysis and related fields.
The inclusion of a thermal conductivity detector (TCD) in the GC approach is considered especially effective for tracing gases such as hydrogen (H
2), carbon dioxide (CO
2), nitrogen (N
2) and methane (CH
4) which are the primary components of biogas [
32]. Storing biogas reduces the amount of methane released into the atmosphere, thereby decreasing the overall levels of greenhouse gases that contribute to greenhouse warming. This reduction in methane emissions is estimated to be equivalent to 11 million vehicles annually [
33].
The versatility of biogas as a source of energy is worth noting from a technological standpoint. Heating buildings and even powering boilers can be accomplished by burning the biogas on-site. There are many ways biogas can be converted into electricity. A combustion engine or gas turbine can generate this electricity which can be distributed to other locations. Using biogas as a source of energy has a variety of advantages including improved quality of the environment, decreasing the amount of greenhouse gases emitted into the atmosphere, and limiting society’s oil and fossil fuel demand [
33]. The transformative potential of biogas cannot be overlooked, as it provides powerful solutions to many pressing environmental issues.
There are many microbes in the biogas fermenter community. The most common substrates found throughout the biogas production process are bacteria that are efficiently able to break down polysaccharides (e.g.,
Clostridium thermocellum,
Clostridium cellulolyticum and
Caldicellulosiruptor saccharolyticus) [
34]. Polysaccharides are carbohydrates found in sources like starch from vegetables and cellulose from fruits, which serve as sources for biogas production. The
Clostridia class of microbes dominates the biogas fermenting community. To generate methane, cellulose and other lignocellulosic substrates must be broken down by
Clostridia. All Clostridial hydrogenases are remarkably active. Furthermore,
Clostridia helps the hydrolysis of polymeric substrates, production of H
2, and decreases the growth rate of hydrogenotrophic methanogens within the biodigester [
34].
The process of converting food waste into biogas involves four biological stages [
34]. By hydrolyzing the lipids, proteins, carbohydrates, and cellulose from the food, facultative anaerobic bacteria produce fatty acids, amino acids, and sugars. The covalent bonds of food waste are disrupted by the water and facultative anerobic bacteria. Propionate, butyrate, alcohols, and lactate are produced by the product’s continued fermentation by acidogenic bacteria. Acetate, H
2, and CO
2 are further produced by acetogenic bacteria during acetogenesis via the acetyl-CoA enzyme route for synthesis. Finally, the end-product of methane and carbon dioxide biogas is produced by hydrogenotrophic methanogens using H
2 and CO
2 and acetotrophic methanogens using acetate [
35].
Samples with concentrations of 25%, 50%, 75%, and 100% were formulated to generate calibration curves for CO2 and CH4 from compressed pure gas cylinders. Each of these two calibration curves resulted in equations expressing y as a function of x. The measured area under each spike in terms of retention time recorded every two seconds from each sample is replaced with y and x corresponds to the concentration percentage. The calibration standards and retention times of each constituent were used to validate the identification of biogas components in the samples by comparing chromatographic patterns.
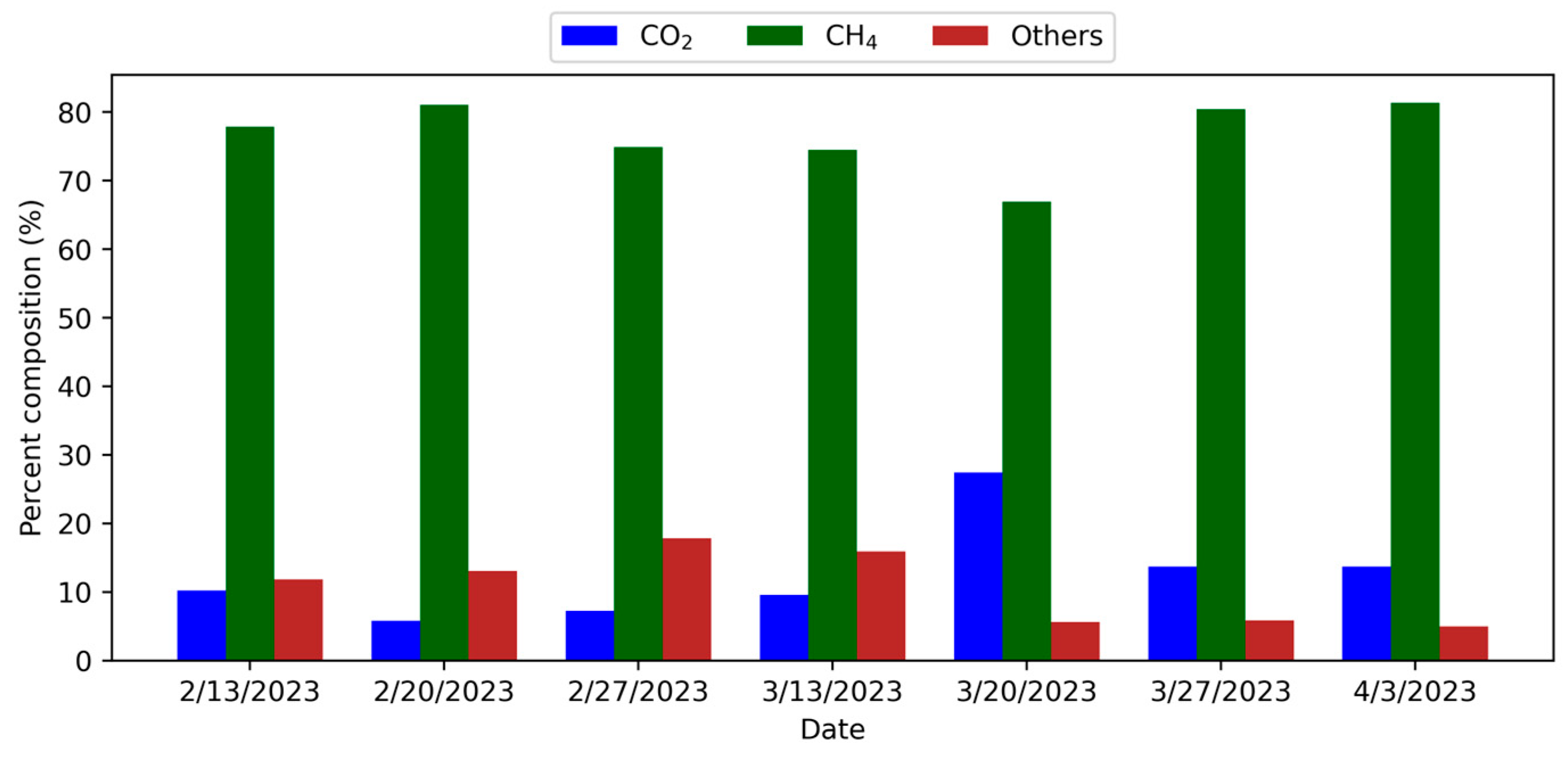
The equations for the methane and carbon dioxide calibration curves are shown in Equations (1) and (2), respectively. From 13 February through 3 April 2023, eight 1-L samples of biogas were collected weekly for testing. The GC-TCD system was used to identify the types of gases and determine their concentrations in each sample. Each biogas sample was loaded and injected via syringe through the flow control and injection port (a 1 mL loop) at 150 °C. Helium (He) carrier gas at 200 kPa and reference gas at 100 kPa (mobile phase) were used to facilitate the movement of gas to the column oven and further separation within the column (stationary phase). The 60/80 Carboxen-1000 column (38.1 cm × 0.175 cm SS) was calibrated to 140 °C and separated each compound detected within the biogas (Supelco, Bellefonte, PA, USA). The chromatographic areas of the target compounds measured in the samples were compared with areas derived from varying concentrations of the targets included in a typical mixture that imitated biogas to create the analytical curves [
32].
The area under the methane curve on 13 February 2023, was 982.6. The methane percentage was derived by substituting the y variable in Equation (1) with the area. Equation (3) was used to determine that 77.9% of the gas generated on 13 February 2023 was methane.
The peak retention time of each component, along with the coil material and dimensions, were carefully considered in this analysis. After putting the gas sample into the GC machine, the nitrogen component, which is represented by the first curve, peaks 2.26 min later. The methane content can be observed by the second curve, which peaks at 4.87 min. The third curve, which represents carbon dioxide, has a 9.23-min peak.
2.4. Water Quality Testing
The samples of the effluent generated by this engineered system were tested by environmental sensors manufactured by In-Situ. For this research, the In-Situ Aqua Troll 600 Multi-Parameter Water Quality Sonde (In-Situ Inc., Fort Collins, CO, USA) was used to conduct daily water quality monitoring at multiple locations within the system. During the initial phase of the study, a comprehensive dataset was collected, including measurements of RDO concentrations (mg/L), RDO saturation (%), oxygen partial pressure (Torr), actual conductivity (µS/cm), resistivity (Ω·cm), density (g/cm
3), total dissolved solids (g/L), pH (pH), oxidation-reduction potential (ORP) (mV), temperature (°C), barometric pressure (psi), pressure (psi), turbidity (NTU), external voltage (V), and barometric pressure (mbar). Among these parameters, the pH values were the focus of the research, as they play the critical role in determining the optimal conditions for hydroponic plant growth. According to Brechner and Both [
36], the optimal pH value for lettuce production in a hydroponic system is 5.8, with an acceptable range between 5.6 and 6.0. By aligning water quality parameters with this pH range, the system provided valuable insights into optimizing conditions for hydroponic agriculture, paving the way for improved plant or crop health and yield.
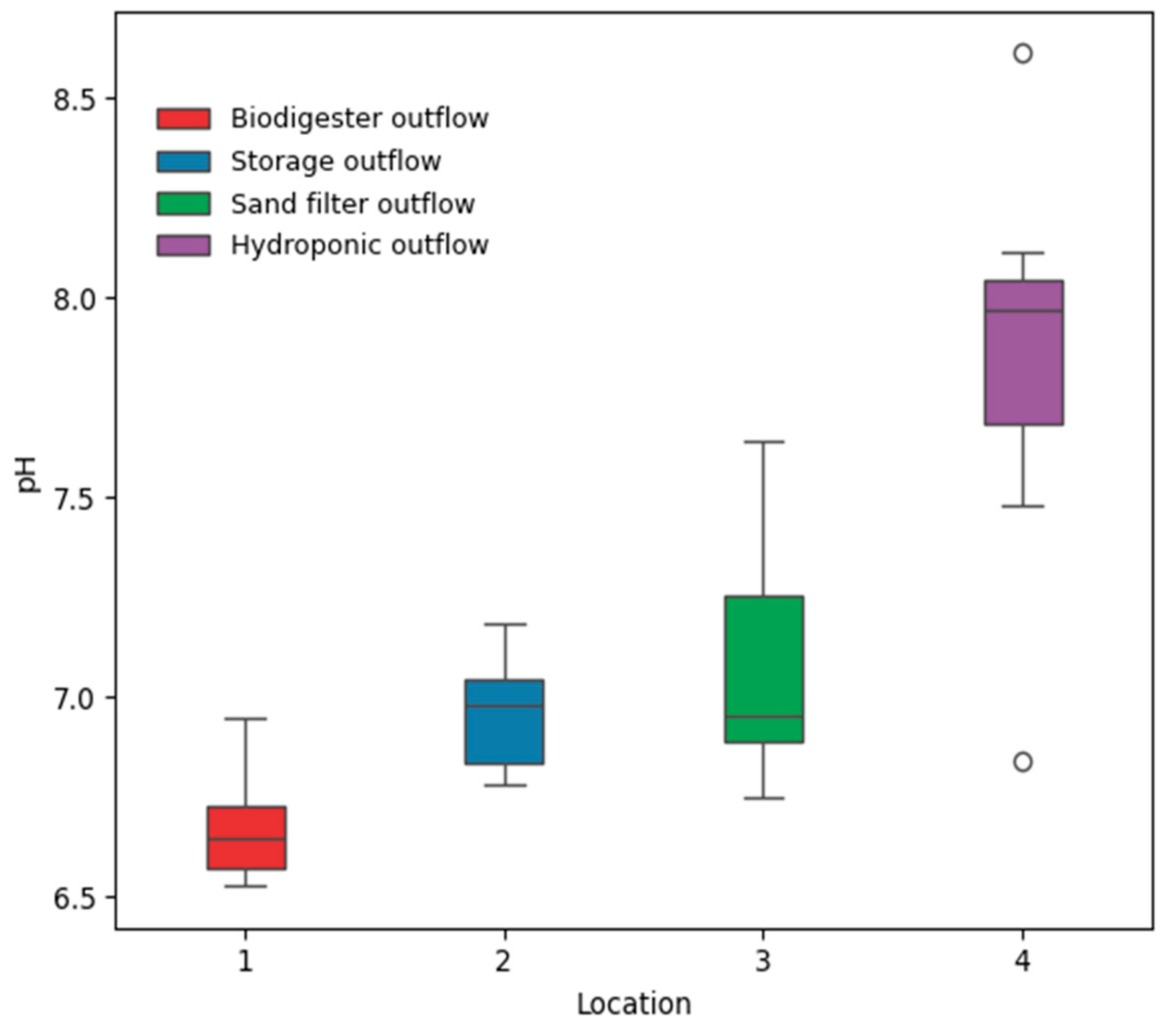
Tests were conducted weekly at four distinct locations within the experimental WEF-Nexus framework system to measure the nutrient contents, as illustrated in
Figure 6. The framework integrated a biodigester, storage for settling, filtration, and hydroponic cultivation. The biodigester outflow is transferred to a storage tank, which acts as a buffer, allowing large suspended solids to settle and ensuring a steady supply for the next stages. From storage, the water moves through a sand filter, which removes suspended solids, pathogens, and other impurities to enhance the water quality. The filtered water was then utilized in a hydroponic system, where it supported agriculture. The outflow from the hydroponic system is connected back to the biodigester, creating a continuous and iterative cycle within the system. This framework demonstrates the WEF-Nexus by recycling water, potentially recovering energy through the biodigester, and supporting sustainable food production through hydroponics.
The WEF system is coupled with a hydroponic unit in which crops are cultivated using only the recycled digestate from the digester. This linkage closes the loop by reusing nutrients and water that would otherwise be wasted, while also reducing the need for external fertilizers. At the same time, anaerobic digestion supplies biogas that can substitute for conventional energy sources. Taken together, these outcomes highlight key advantages of the system, reducing dependence on clean water and non-renewable energy inputs while supporting resilient food production.
4. Conclusions
A novel household-scale, closed-loop WEF system was designed, installed, and operated to manage food waste while recovering water for irrigation, nutrients for plant growth, and biogas for energy supply. The experimental analysis of water quality and gas chromatography samples from the biodigester and hydroponic system showed the system’s potential for household use, providing renewable energy and nutrient-rich effluent for plant growth. The research focused on biogas production and on ensuring a consistent wastewater byproduct with sufficient natural fertilizer value for agricultural irrigation use. The addition of the hydroponic system to food waste management demonstrated that reduced harmful levels of phosphate, nitrite, and nitrate released to the environment could be sustainably used to grow plants. Results from gas chromatography showed that methane levels were substantially above typical small biodigester systems for the eight weeks of sampling, averaging 76.7%, with a peak of 81.3%. Key indicators such as conductivity (mean: 3120.81 µS/cm), TDS (2.18 g/L), and substantial levels of phosphate (20 mg/L), nitrate (26 mg/L), and nitrite (5 mg/L) confirm its potential as a nutrient source. The pH was near neutral (7.01). While ORP values fluctuated, the overall water quality supports its reuse in agriculture or hydroponics with minimal treatment.
The integration of this WEF system into a household setting presents numerous benefits, including decentralized food waste management and a renewable biogas supply for cooking and potentially small-scale heating. By transforming food waste into both energy and nutrients, the system aligns with circular economic principles, reducing waste while promoting sustainable energy and agricultural productivity. This pilot study utilized typical kitchen sink space and common household food waste to demonstrate the system’s feasibility. However, further research is warranted to analyze nutrient composition across diverse food waste types, evaluate their impacts on various plants in household settings and explore pathways to scale up the household toward community or large-scale applications. Such studies will help optimize the system for broader applications in sustainable home food waste management and urban agriculture. Household adoption of such systems collectively advances SGDs by diverting organic waste from landfills, lowering carbon emissions through biogas substitution, and supporting food production. While this study focused on technical performance, future work should also incorporate techno-economic analysis (TEA) and life cycle assessment (LCA) to provide a comprehensive assessment of both economic and environmental performance. Additional focus should be on understanding how socio-cultural factors and human behavior influence the performance of household WEF systems. Such analyses are essential to quantify household-scale benefits, including potential cost-effectiveness, energy savings, waste reduction, and greenhouse gas mitigation, thereby strengthening the case for broader adoption of these systems. There is also potential for household-scale WEF systems to be applied in urban homes, integrated with smart home technologies, and leveraged as a disaster-resilience strategy by providing decentralized sources of renewable energy and food production. In addition, practical barriers such as space constraints, regulatory requirements, maintenance needs, seasonal variability, and feedstock management must be considered to provide a realistic assessment of implementation feasibility. Addressing these challenges alongside system performance and quantified benefits will be critical for advancing household-scale WEF systems from pilot demonstrations toward broader adoption.