Effects of Sodium Chloride in Soil Stabilization: Improving the Behavior of Clay Deposits in Northern Cartagena, Colombia
Abstract
1. Introduction
2. Materials and Methods
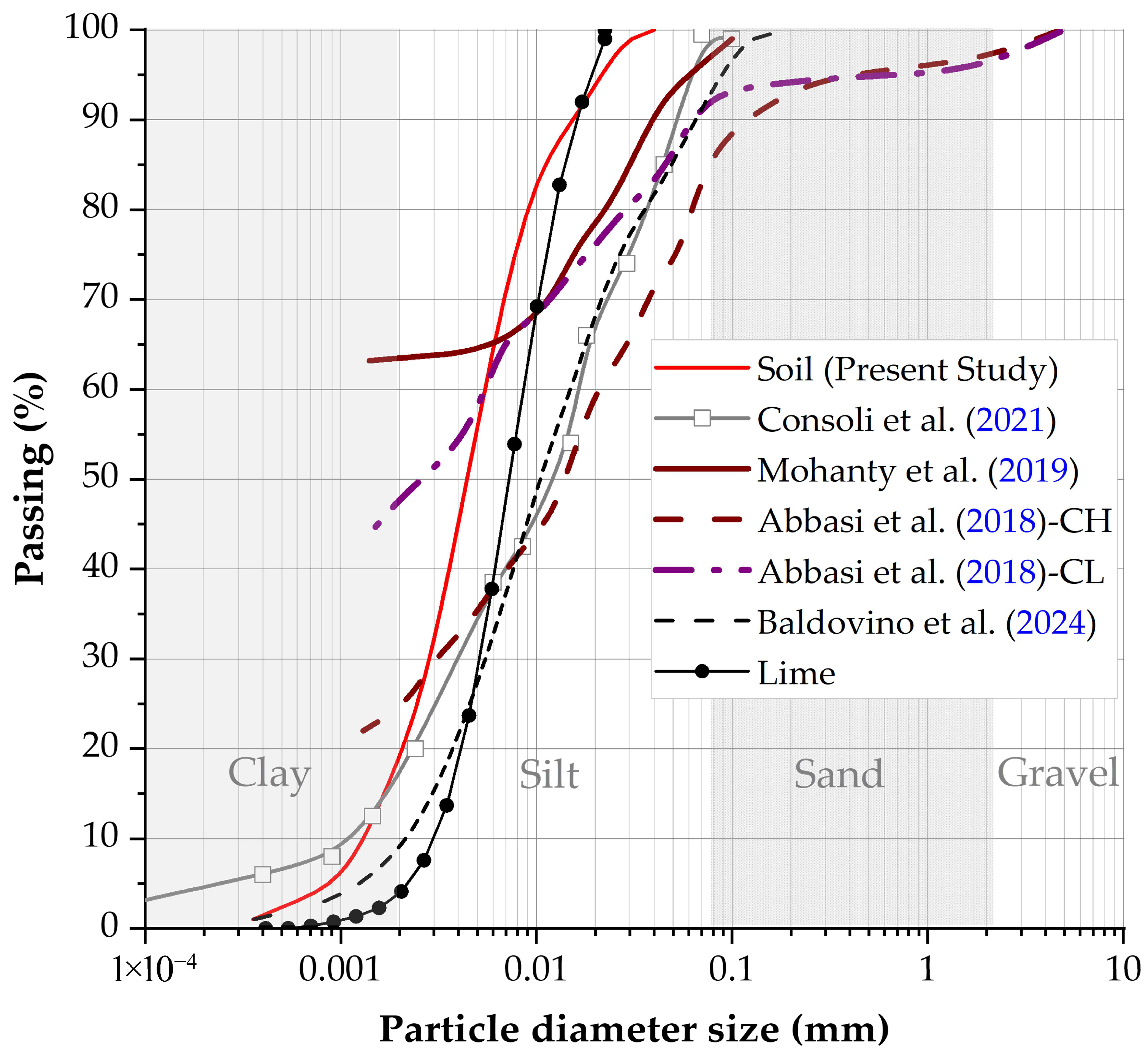
2.1. Clayey Soil Characteristics
2.2. Dispersity Tests
2.3. Fixation of Lime and NaCl Contents
2.4. Experimental Program
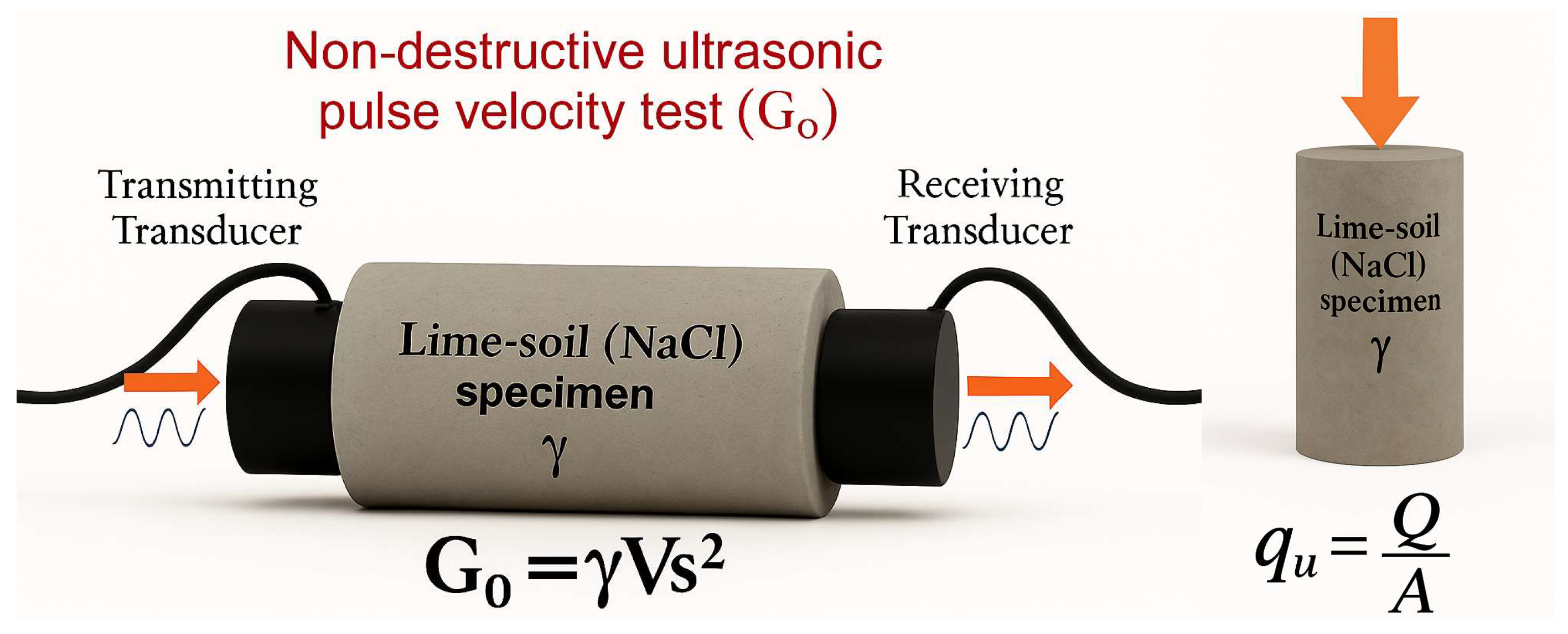
2.5. Preparing Specimens for qu and Go Tests
2.6. Unconfined Compressive and Stiffness
2.7. Microstructure Analysis
3. Results and Discussions
3.1. Chemical, Geotechnical, Mineralogy, and Microstructure Properties of Clay
3.2. Pinhole and Crumb Test Results of the Soil Sample
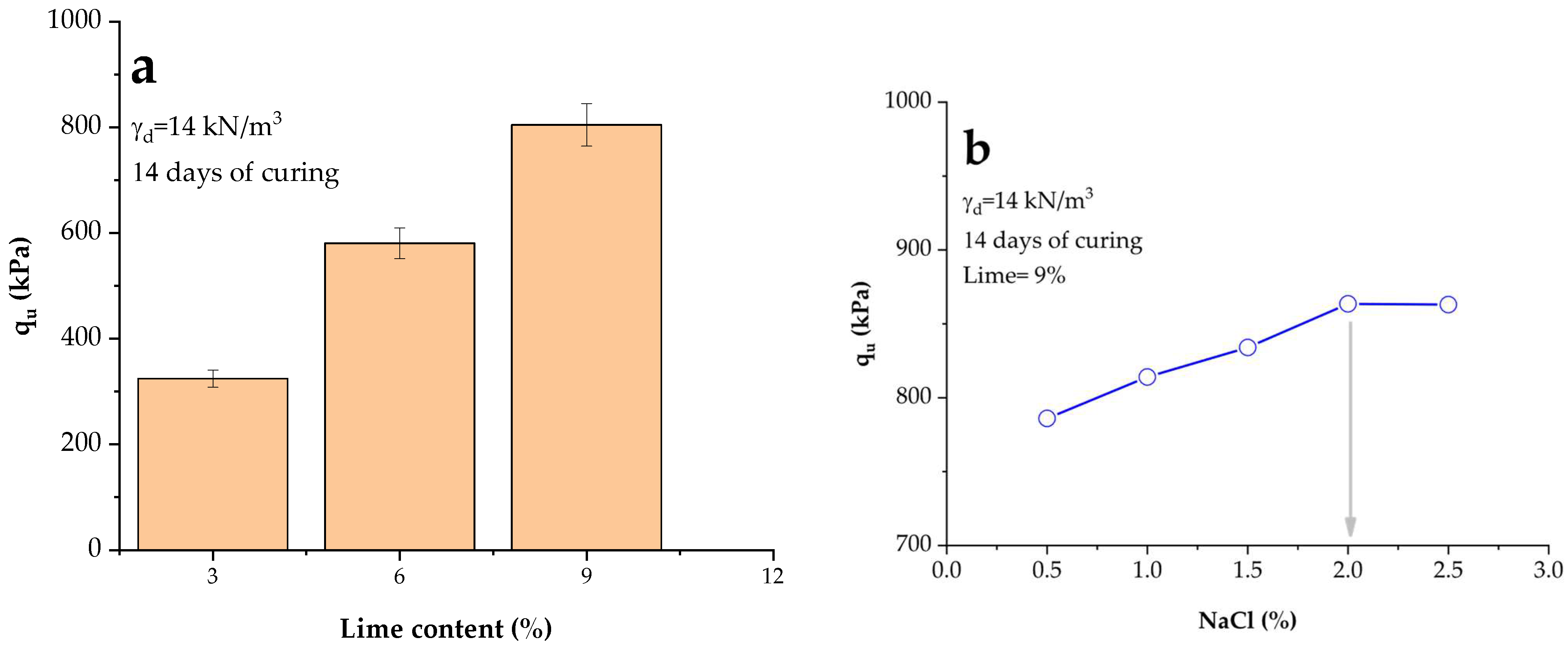
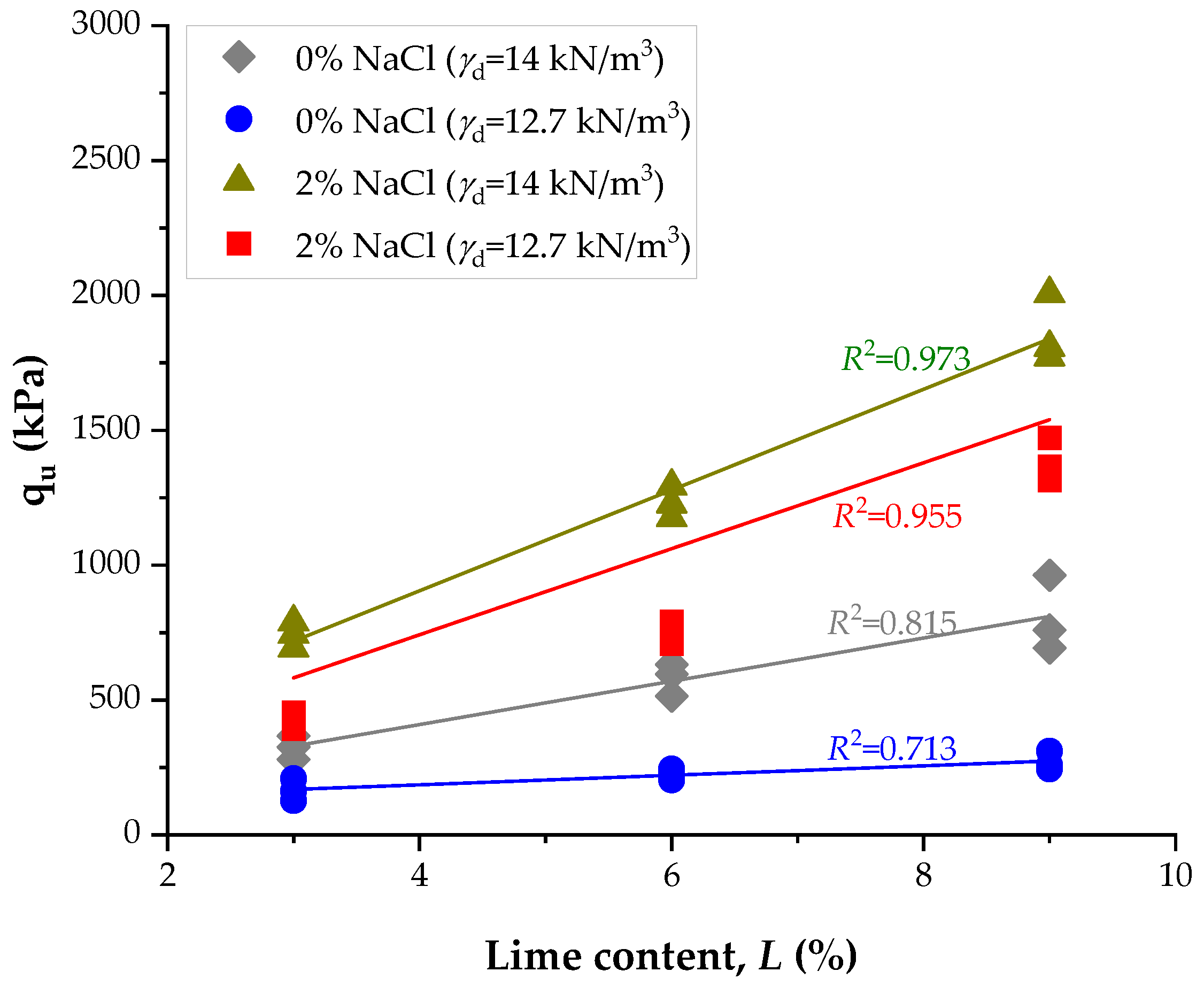
3.3. Influence of NaCl and Lime Content on Unconfined Compressive Strength
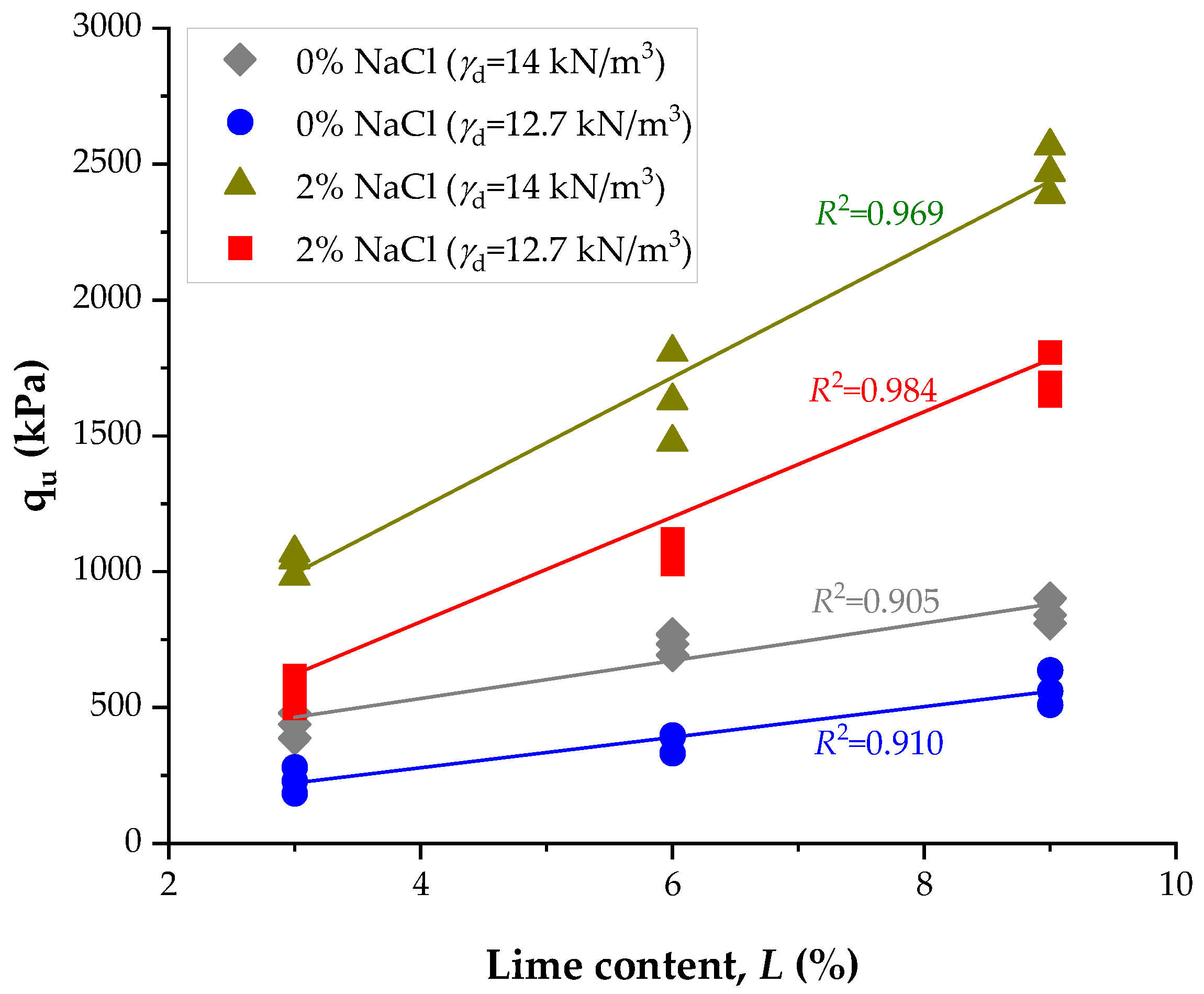
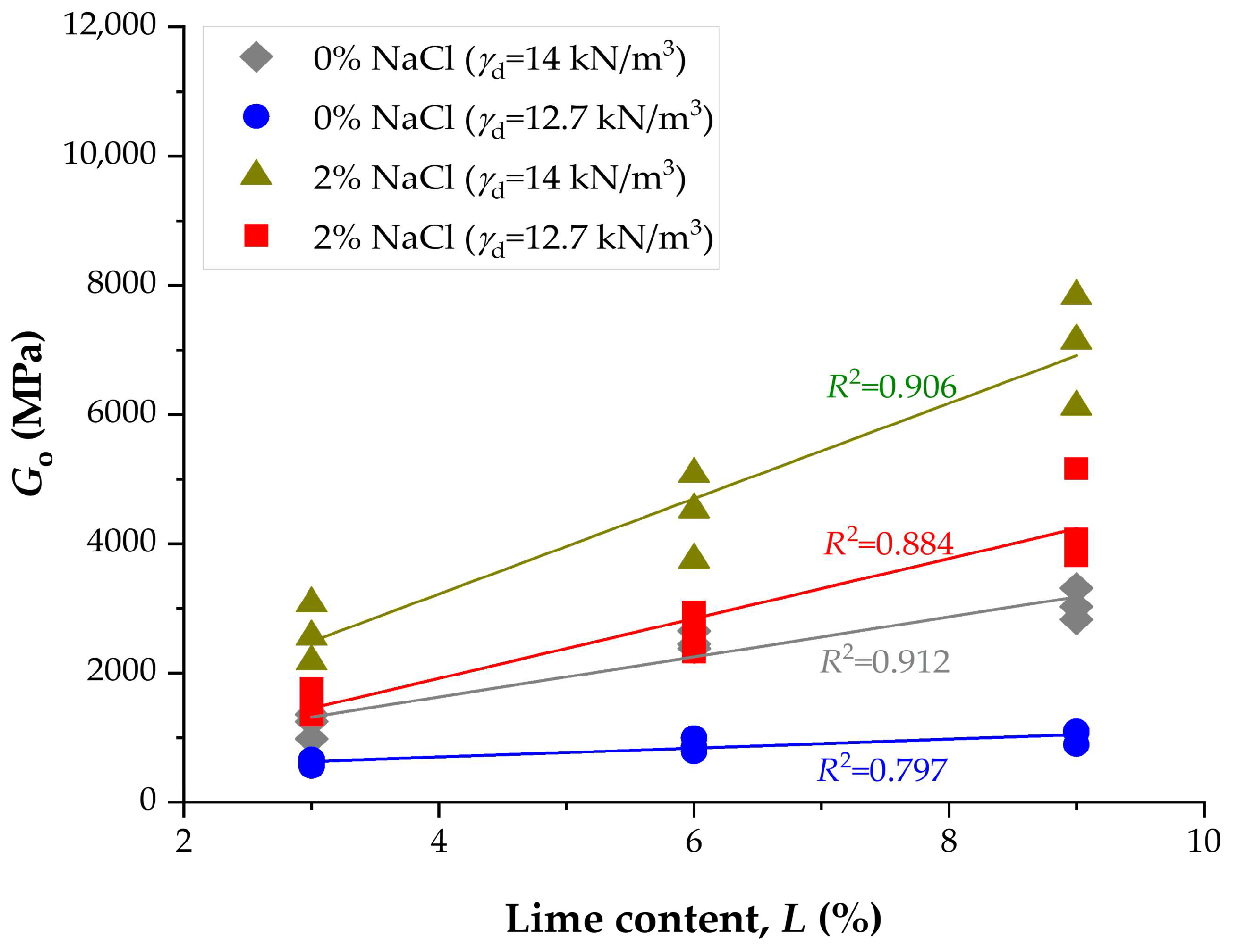
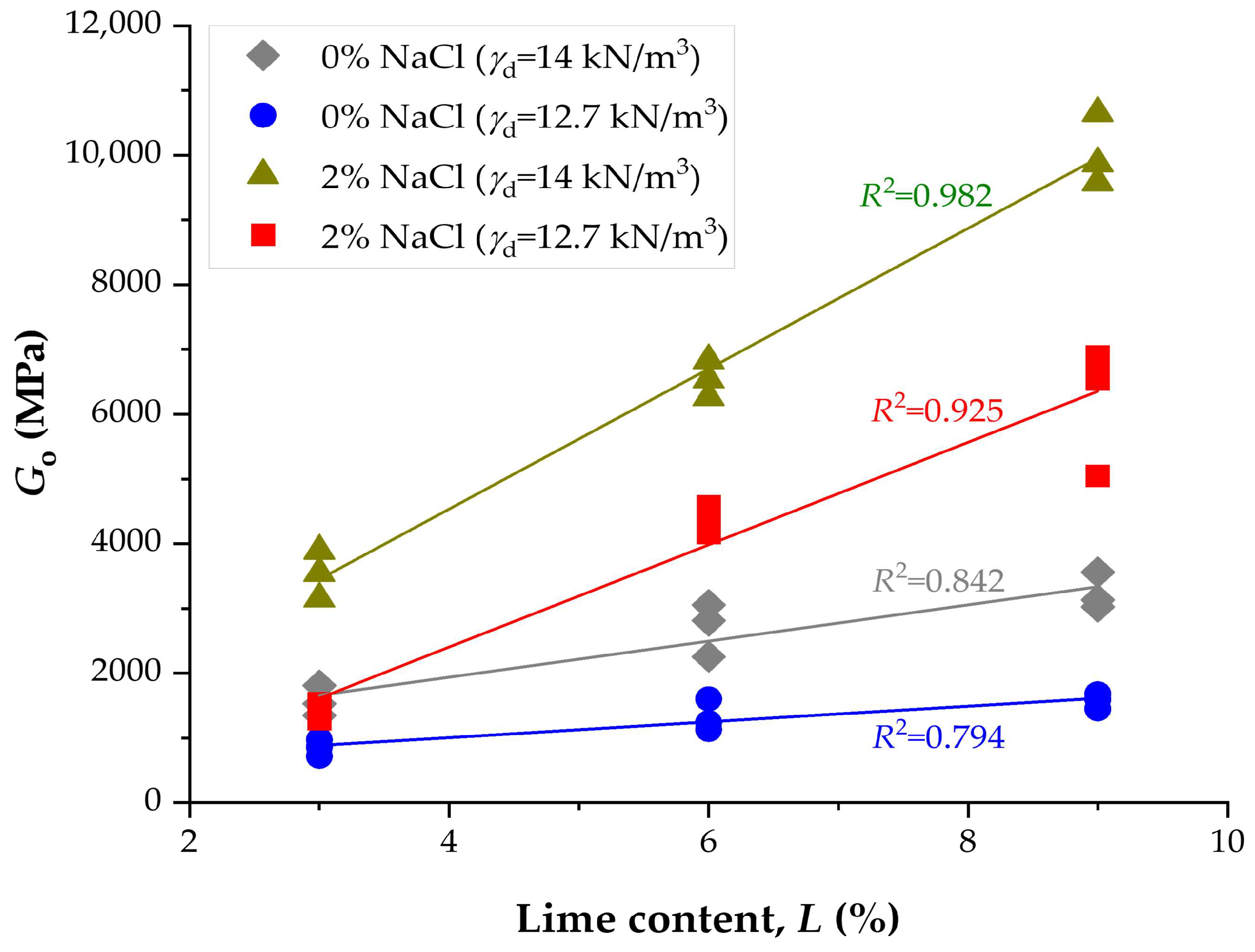
3.4. Influence of NaCl and Lime Content on Stiffness
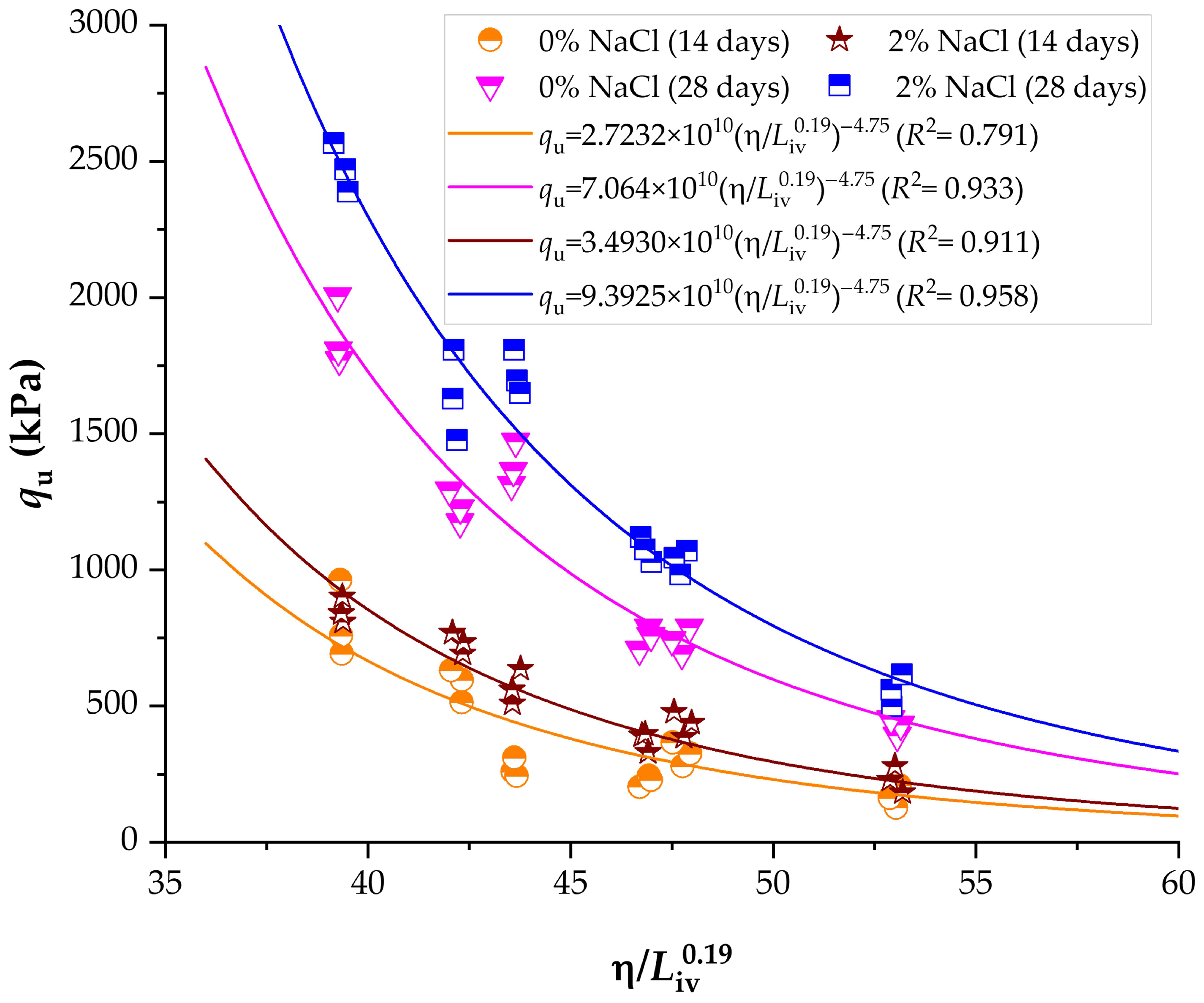
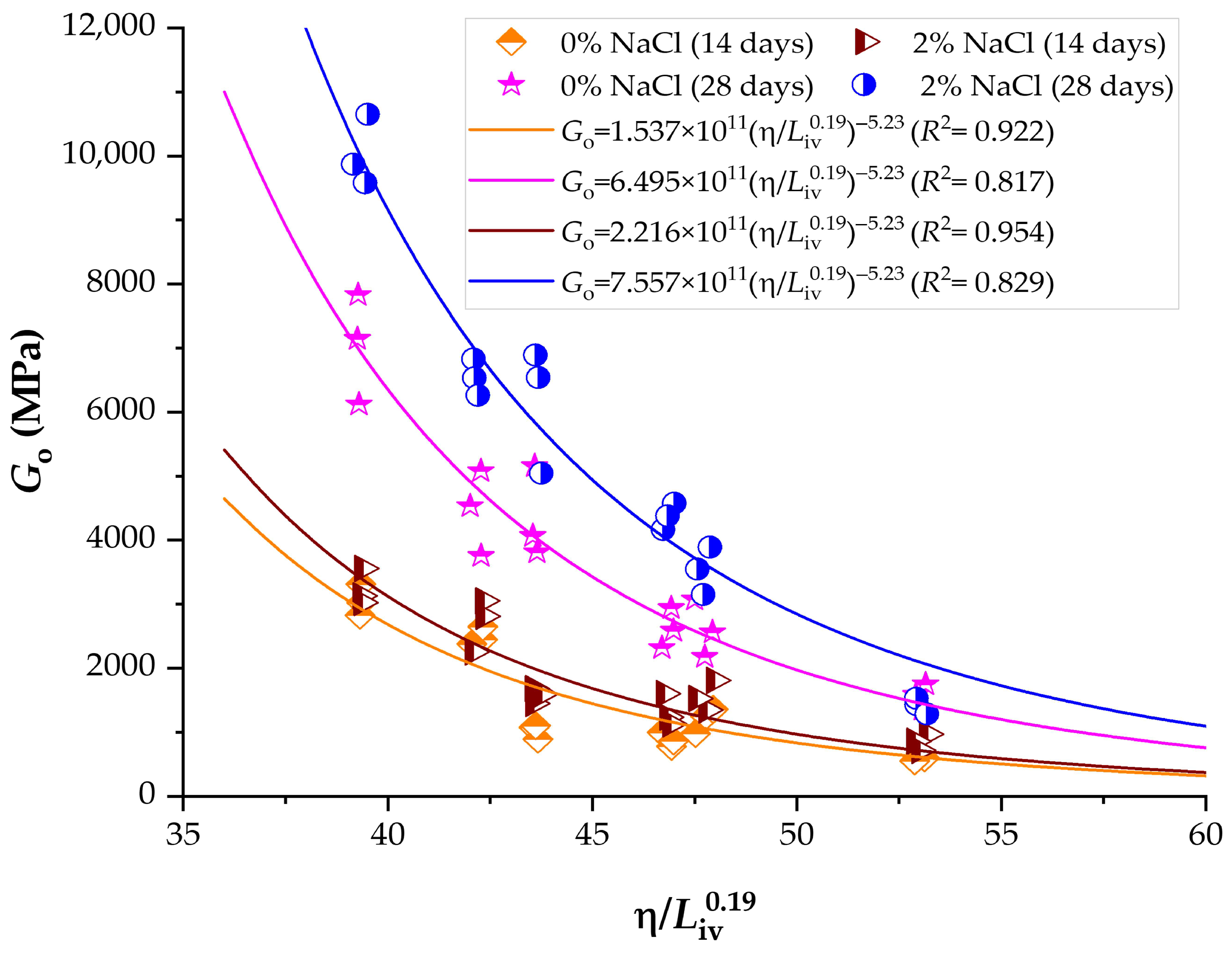
3.5. Influence of Porosity-to-Lime Index on Unconfined Compressive Strength and Stiffness of Compacted Blends
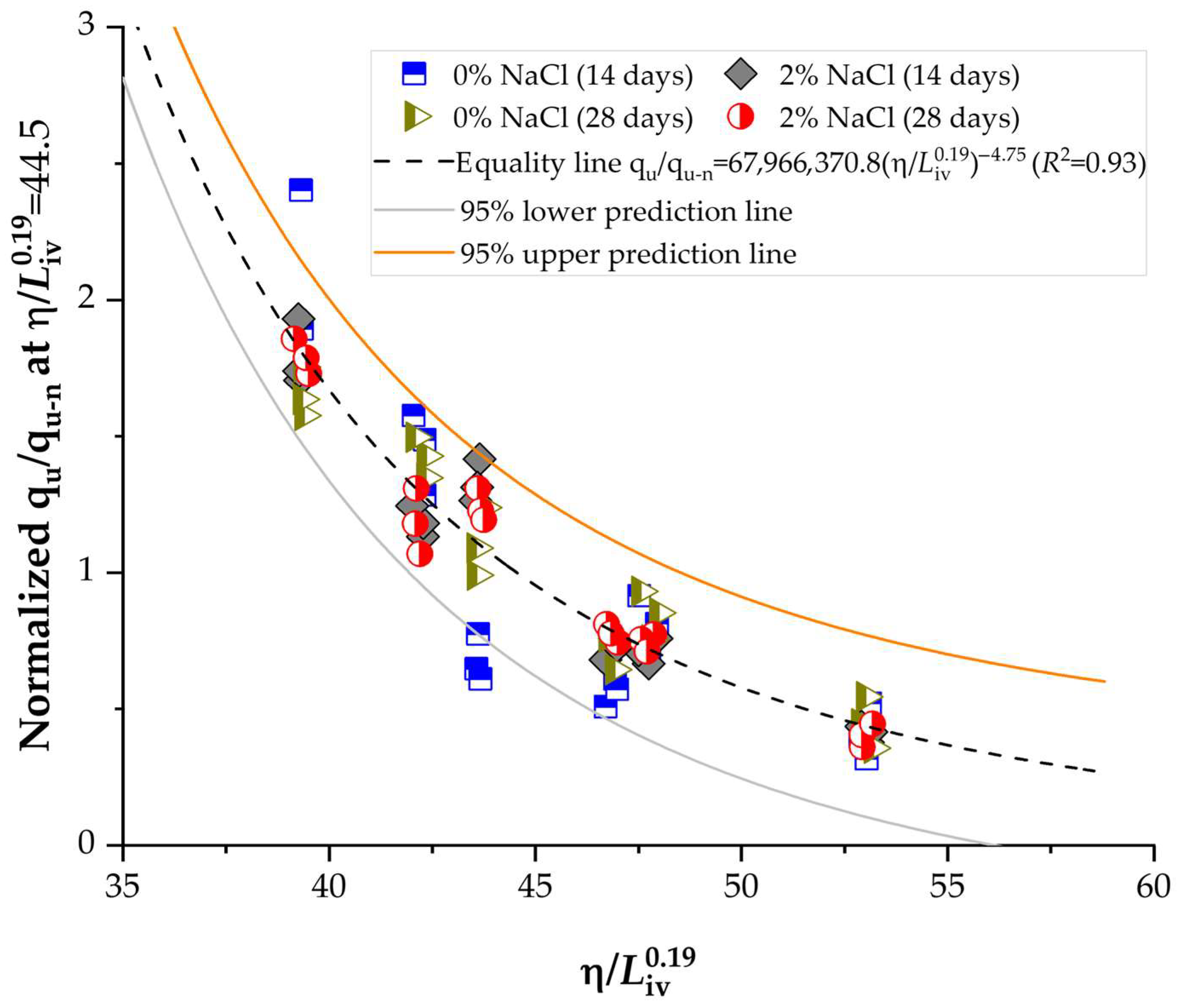
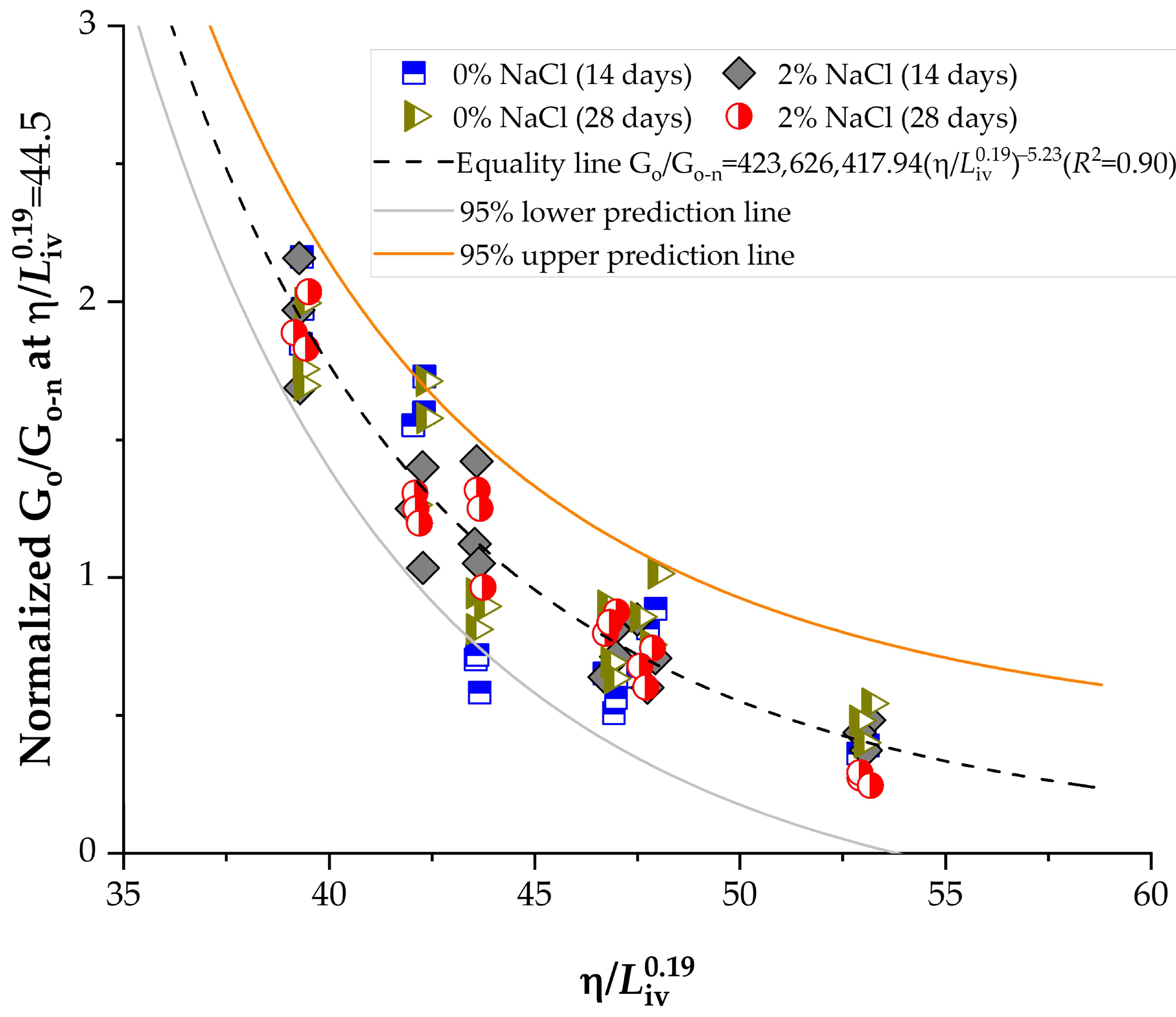
3.6. Normalization of Strength and Stiffness Using the Porosity-to-Lime Index
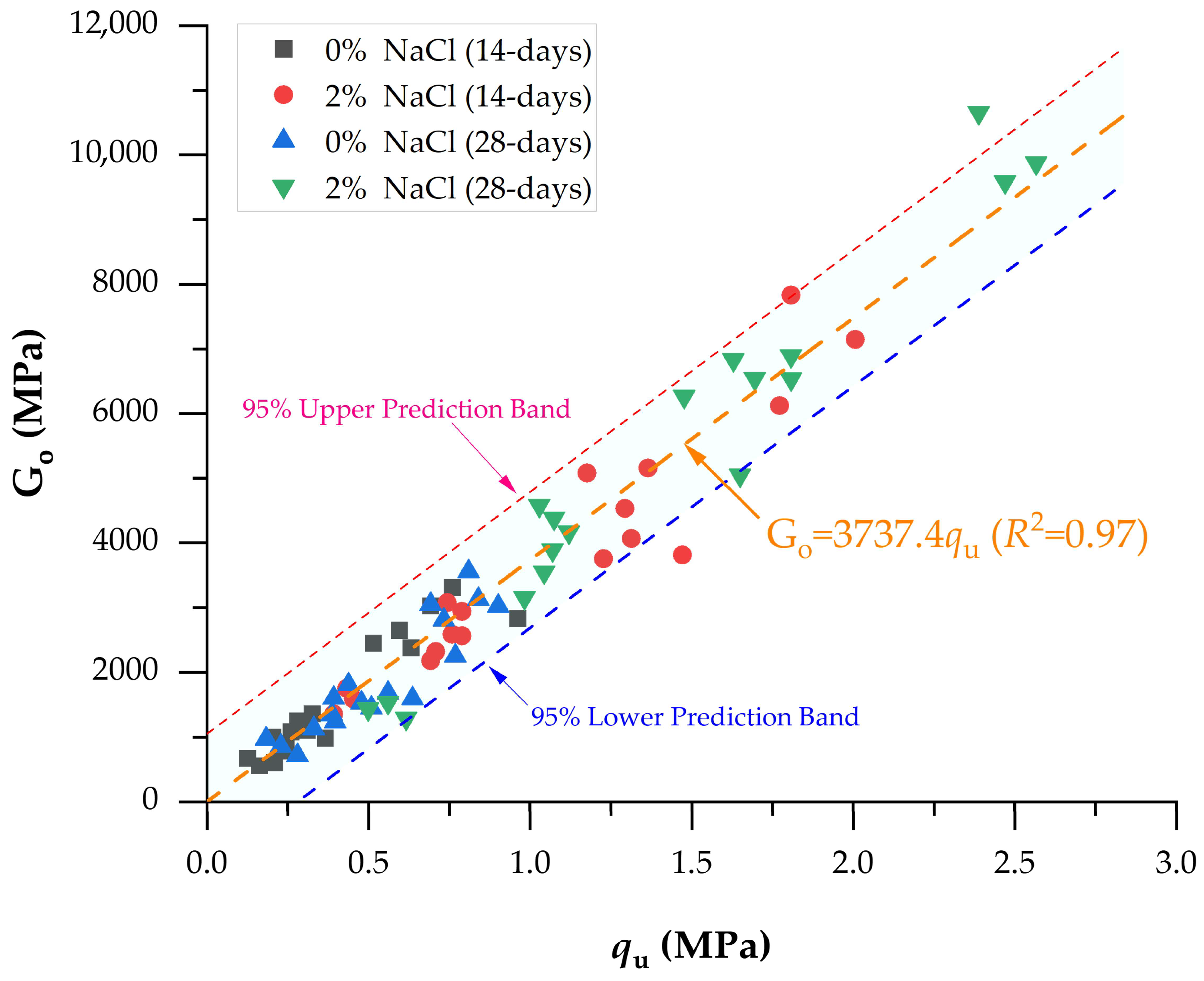
3.7. Go/qu Index
| Type of Compacted Mix | UCS Equation | R2 | Reference |
|---|---|---|---|
| Present Study | qu = 3737.4 Go | 0.970 | - |
| Soil–cement–natural rubber latex | qu = 3686.6 Go | 0.910 | [15] |
| Soil–RAP–xanthan gum–XG (0.5% XG) | qu = 2601Go | 0.981 | [17] |
| Soil–RAP–xanthan gum–XG (1.0% XG) | qu = 1958.1Go | 0.964 | [17] |
| Soil–RAP–xanthan gum–XG (1.5% XG) | qu = 1202.5Go | 0.923 | [17] |
| Soil–RAP–xanthan gum–XG (2.0% XG) | qu = 695.47Go | 0.812 | [17] |
| Clayey soil–cement–limestone waste | qu = 4828.8Go | 0.960 | [14] |
| Sand–cement | qu = 7465.9Go | 0.860 | [39] |
| Clayey soil–xanthan gum | qu = 1915.3Go | 0.980 | [17] |
| Clayey soil–glass powder–cement | qu = 2909.68Go | 0.970 | [31] |
| Sand–ground glass–carbide lime (7 days) | qu = 21,690Go | 0.990 | [42] |
| Sand–ground glass–carbide lime (180 days) | qu = 30,690Go | 0.980 | [42] |
| Osorio sand–glass powder–carbide lime | qu = 2169.49Go | 0.940 | [41] |
| Rio Pardo sand–glass powder–carbide lime | qu = 1785.74Go | 0.850 | [41] |
| Porto Alegre sand–glass powder–carbide lime | qu = 985.34Go | 0.820 | [41] |
| Clay–tire rubber fiber–cement | qu = 0.00124Go1.73 | 0.870 | [43] |
| Alluvial clay–marble dust–Portland cement | qu = 1 × 10−5Go2.3055 | 0.880 | [44] |
| Clay–sintered gypsum– glass powder | qu = 8.508Go0.78 | 0.900 | [45] |
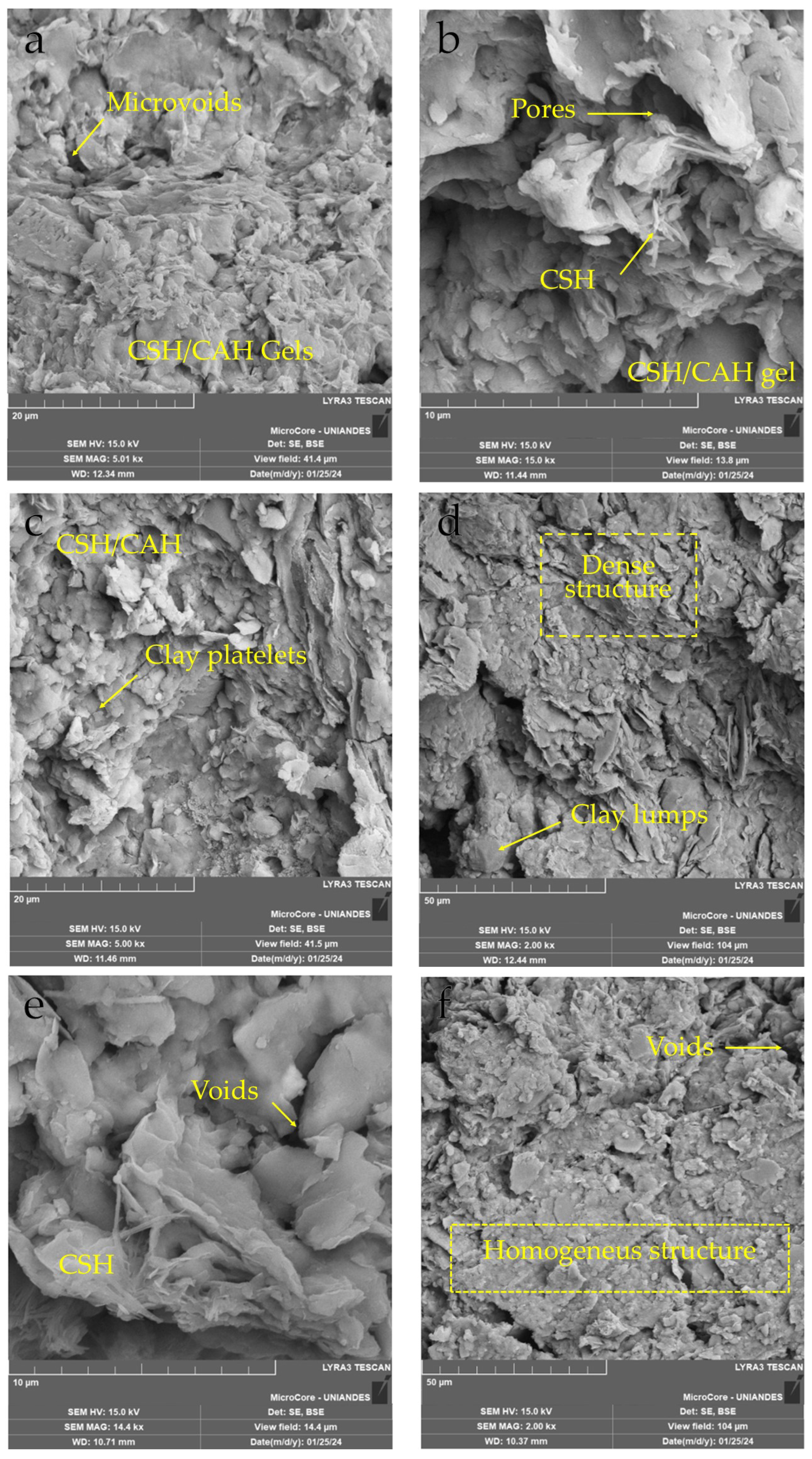
3.8. Microstructure of Lime–Soil-NaCl Compacted Blends
4. Conclusions
- −
- Lime treatment alone improved the unconfined compressive strength (qu) of the soil from 324 kPa at the early stage of 14 days (3% lime, 14 kN/m3) to 901 kPa for a longer term of 28 days (9% lime, 14 kN/m3, 28 d), confirming its effectiveness as a stabilizing agent.
- −
- The incorporation of 2% NaCl at 9% lime and high compaction density increased qu from 901 kPa (lime only, 28 d) to 2567 kPa, representing an enhancement of approximately 185%. At low density, qu rose from 637 kPa to 1808 kPa under the same conditions.
- −
- Initial stiffness (Go) exhibited a similar trend. At 9% lime and high density, values increased from 3315 MPa (0% NaCl, 14 d) to 10,652 MPa (2% NaCl, 28 d), corresponding to a nearly threefold improvement. At lower densities, Go increased from ~1103 MPa (0% NaCl, 14 d) to 6891 MPa (2% NaCl, 28 d).
- −
- The stiffness-to-strength ratio (Go/qu) obtained in this study was 3737.4, which is consistent with values reported for cemented geomaterials such as lime–limestone waste (4828.8) and soil–natural rubber latex blends (3686.6), and significantly higher than biopolymer-based systems such as xanthan gum (1915.3).
- −
- The porosity-to-lime index strongly governed unconfined compressive strength (qu) and initial stiffness (Go), following consistent power-type relationships with exponents of −4.75 and −5.23, respectively.
- −
- Normalization of qu and Go produced master curves with high determination coefficients (R2 > 0.90), confirming the robustness of the porosity-to-lime framework as a predictive design tool for lime–NaCl–soil systems.
- −
- The transition from a flocculated, porous system to a compact, gel-rich microstructure demonstrates a clear micro–macro correlation. As cementitious products such as C–S–H and C–A–H develop and interconnect the particles, the soil matrix becomes more deformation-resistant and can better distribute applied stresses. This is further enhanced by the role of NaCl, which likely accelerates lime dissolution and promotes early reaction kinetics.
- −
- The combined lime–NaCl treatment produced a denser, more cemented microstructure strongly associated with higher stiffness and strength. At the same time, this transformation suggests enhanced resistance to moisture-related degradation and potential reduction in dispersivity.
- −
- This study demonstrates that combined lime–NaCl stabilization converts moderately dispersive clay into a dense, durable, non-dispersive material, enabling local soils to be safely reused for foundations and embankments. This approach reduces the need for imported granular materials, lowers transportation emissions, and extends the service life of earth structures, aligning with sustainable construction practices
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fattah, M.Y.; Ismael, R.H.; Aswad, M.F. Dispersion Characteristics of MgO-Treated Dispersive Clay. Arab. J. Geosci. 2021, 14, 605. [Google Scholar] [CrossRef]
- Zhang, L.; Du, Y.H.; Yang, X.J.; Fan, H.H. Application of Artificial Neural Network in Predicting the Dispersibility of Soil. Iran. J. Sci. Technol. Trans. Civ. Eng. 2022, 46, 2315–2324. [Google Scholar] [CrossRef]
- Filho, H.C.S.; Saldanha, R.B.; da Rocha, C.G.; Consoli, N.C. Sustainable Binders Stabilizing Dispersive Clay. J. Mater. Civ. Eng. 2021, 33, 06020026. [Google Scholar] [CrossRef]
- Mohd Yunus, N.Z.; Wanatowski, D.; Marto, A.; Jusoh, S.N. Strength Improvement of Lime-Treated Clay with Sodium Chloride. Geotech. Res. 2017, 4, 192–202. [Google Scholar] [CrossRef]
- Saldanha, R.B.; Mallmann, J.E.C.; Consoli, N.C. Salts Accelerating Strength Increase of Coal Fly Ash-Carbide Lime Compacted Blends. Geotech. Lett. 2016, 6, 23–27. [Google Scholar] [CrossRef]
- Razeghi, H.R.; Ghadir, P.; Javadi, A.A. Mechanical Strength of Saline Sandy Soils Stabilized with Alkali-Activated Cements. Sustainability 2022, 14, 13669. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Liu, X.; Li, Y. Effect of Salt Content on Freezing Temperature and Unconfined Compression Strength of Lime-Treated Subgrade Clay. Appl. Clay Sci. 2018, 158, 65–71. [Google Scholar] [CrossRef]
- Saldanha, R.B.; Scheuermann Filho, H.C.; Ribeiro, J.L.D.; Consoli, N.C. Modelling the Influence of Density, Curing Time, Amounts of Lime and Sodium Chloride on the Durability of Compacted Geopolymers Monolithic Walls. Constr. Build. Mater. 2017, 136, 65–72. [Google Scholar] [CrossRef]
- Ramesh, H.N.; Rakesh, C. Influence of Lime Sludge and Sodium Salts on the Strength and Structural Behavior of Clayey Soils–Granite Stone Slurry Dust Composite with Curing. Indian Geotech. J. 2020, 50, 801–809. [Google Scholar] [CrossRef]
- Godoy, V.B.; Tomasi, L.F.; Benetti, M.; de Araújo, M.T.; Dalmolin, D.A.; Heineck, K.S. Effects of Curing Temperature on Sand-Ash-Lime Mixtures with Fibres and NaCl. Geotech. Geol. Eng. 2023, 41, 2221–2235. [Google Scholar] [CrossRef]
- Gravina da Rocha, C.; Marin, E.J.B.; Quiñónez Samaniego, R.A.; Consoli, N.C. Decision-Making Model for Soil Stabilization: Minimizing Cost and Environmental Impacts. J. Mater. Civ. Eng. 2021, 33, 06020024. [Google Scholar] [CrossRef]
- Saldanha, R.B.; Lotero, A.; Jaskulski, F.; Consoli, N.C.; da Rocha, C.G. A Decision-Making Flowchart for Tailings Stabilization: Assessing Environmental and Economic Impacts. J. Clean. Prod. 2025, 519, 145893. [Google Scholar] [CrossRef]
- Ferrazzo, S.T.; Tonini de Araújo, M.; Consoli, N.C.; da Rocha, C.G. Geotech Social Impacts: Development, Application, and Comparative Analysis. Env. Impact Assess. Rev. 2024, 108, 107577. [Google Scholar] [CrossRef]
- Román Martínez, C.; Nuñez de la Rosa, Y.E.; Estrada Luna, D.; Baldovino, J.A.; Jordi Bruschi, G. Strength, Stiffness, and Microstructure of Stabilized Marine Clay-Crushed Limestone Waste Blends: Insight on Characterization through Porosity-to-Cement Index. Materials 2023, 16, 4983. [Google Scholar] [CrossRef]
- Baldovino, J.A.; Diaz, K.C.; Royero, J.M.; Sierra, R.S.; Nuñez de la Rosa, Y.E. Developing New Geomaterials: The Case of the Natural Rubber Latex Polymers in Soil Stabilization. Materials 2025, 18, 1720. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Liu, X.; Gao, H.; Pan, Y. Stabilization of Micaceous Residual Soil with Industrial and Agricultural Byproducts: Perspectives from Hydrophobicity, Water Stability, and Durability Enhancement. Constr. Build. Mater. 2024, 430, 136450. [Google Scholar] [CrossRef]
- Baldovino, J.A.; Durán, A.P.; de la Rosa, Y.E.N. Sustainable Stabilization of Soil–RAP Mixtures Using Xanthan Gum Biopolymer. Sustainability 2025, 17, 4601. [Google Scholar] [CrossRef]
- López, L.C.S.; Ramos, J.C.L.; de la Rosa, Y.E.N.; Bruschi, G.J.; Baldovino, J.d.J.A. Stabilization of Clay Soils Using a Lime Derived from Seashell. Materials 2025, 18, 2723. [Google Scholar] [CrossRef] [PubMed]
- ASTM D854; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM 4318; Standard Test Methods for Liquid Limit, Plastic Limit and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D4647/D4647M-13; Standard Test Methods for Identification and Classification of Dispersive Clay Soils by the Pinhole Test. ASTM International: West Conshohocken, PA, USA, 2013.
- Consoli, N.C.; da Silva Lopes, L.; Foppa, D.; Heineck, K.S. Key Parameters Dictating Strength of Lime/Cement-Treated Soils. Proc. Inst. Civ. Eng.-Geotech. Eng. 2009, 162, 111–118. [Google Scholar] [CrossRef]
- Ciancio, D.; Beckett, C.T.S.; Carraro, J.A.H. Optimum Lime Content Identification for Lime-Stabilised Rammed Earth. Constr. Build. Mater. 2014, 53, 59–65. [Google Scholar] [CrossRef]
- ASTM D 2166-03; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil 1. ASTM International: West Conshohocken, PA, USA, 2003; p. 4.
- ASTM D 2487-11; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–5. [CrossRef]
- ASTM D7928; Standard Test Method for Particle-Size Distribution (Gradation) of Fine-Grained Soils Using the Sedimentation (Hydrometer) Analysis. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D698-12; Standard Test Method for Laboratory Compaction Characteristics of Soils Using Standard Effort (12.400 Ft-Lbf/Ft3 (600 KN-m/m3)). ASTM International: West Conshohocken, PA, USA, 2012.
- Consoli, N.C.; Festugato, L.; Miguel, G.D.; Moreira, E.B.; Scheuermann Filho, H.C. Fatigue Life of Green Stabilized Fiber-Reinforced Sulfate-Rich Dispersive Soil. J. Mater. Civ. Eng. 2021, 33, 04021249. [Google Scholar] [CrossRef]
- Mohanty, S.; Roy, N.; Singh, S.P.; Sihag, P. Estimating the Strength of Stabilized Dispersive Soil with Cement Clinker and Fly Ash. Geotech. Geol. Eng. 2019, 37, 2915–2926. [Google Scholar] [CrossRef]
- Abbasi, N.; Farjad, A.; Sepehri, S. The Use of Nanoclay Particles for Stabilization of Dispersive Clayey Soils. Geotech. Geol. Eng. 2018, 36, 327–335. [Google Scholar] [CrossRef]
- Baldovino, J.A.; Nuñez de la Rosa, Y.E.; Namdar, A. Sustainable Cement Stabilization of Plastic Clay Using Ground Municipal Solid Waste: Enhancing Soil Properties for Geotechnical Applications. Sustainability 2024, 16, 5195. [Google Scholar] [CrossRef]
- Ekinci, A.; Scheuermann Filho, H.C.; Consoli, N.C. Copper Slag–Hydrated Lime–Portland Cement Stabilised Marine-Deposited Clay. Proc. Inst. Civ. Eng.-Ground Improv. 2022, 175, 51–63. [Google Scholar] [CrossRef]
- Castellar Buelvas, G.; Perez Luna, J.; De Avila Florez, W.; Aldana Rivera, H.; Arrieta, J. Using Ground Glass Powder and Recycled Gypsum Powder in Clay Stabilization. Int. Rev. Civ. Eng. 2025, 16, 36. [Google Scholar] [CrossRef]
- Diambra, A.; Ibraim, E.; Peccin, A.; Consoli, N.C.; Festugato, L. Theoretical Derivation of Artificially Cemented Granular Soil Strength. J. Geotech. Geoenviron. Eng. 2017, 143, 04017003. [Google Scholar] [CrossRef]
- Diambra, A.; Festugato, L.; Ibraim, E.; Peccin da Silva, A.; Consoli, N.C. Modelling Tensile/Compressive Strength Ratio of Artificially Cemented Clean Sand. Soils Found. 2018, 58, 199–211. [Google Scholar] [CrossRef]
- ASTM D4609-08; Standard Guide for Evaluating Effectiveness of Admixtures for Soil Stabilization. ASTM International: West Conshohocken, PA, USA, 2008.
- Favretto, F.; Bruschi, G.J.; Secco, M.P.; Festugato, L. Strength and Stiffness of High-Moisture Content Kaolin–Bentonite Blends Cemented with an Alternative Lime. Indian Geotech. J. 2025. [Google Scholar] [CrossRef]
- Ekinci, A.; Hanafi, M.; Aydin, E. Strength, Stiffness, and Microstructure of Wood-Ash Stabilized Marine Clay. Minerals 2020, 10, 796. [Google Scholar] [CrossRef]
- López, L.C.S.; Vergara, J.A.A.; de la Rosa, Y.E.N.; Arrieta, A.; Baldovino, J.d.J.A. Effect of Grain Size and Porosity/Binder Index on the Unconfined Compressive Strength, Stiffness and Microstructure of Cemented Colombian Sands. Materials 2024, 17, 5193. [Google Scholar] [CrossRef]
- Consoli, N.C.; Winter, D.; Leon, H.B.; Scheuermann Filho, H.C. Durability, Strength, and Stiffness of Green Stabilized Sand. J. Geotech. Geoenviron. Eng. 2018, 144, 04018057. [Google Scholar] [CrossRef]
- Consoli, N.C.; da Silva Carretta, M.; Festugato, L.; Leon, H.B.; Tomasi, L.F.; Heineck, K.S. Ground Waste Glass–Carbide Lime as a Sustainable Binder Stabilising Three Different Silica Sands. Géotechnique 2021, 71, 480–493. [Google Scholar] [CrossRef]
- Al-Subari, L.; Ekinci, A. Evaluation of Mechanical and Microstructural Properties of Waste Tire Improved Cemented Clay. J. Nat. Fibers 2024, 21. [Google Scholar] [CrossRef]
- Hanafi, M.; Javed, I.; Ekinci, A. Evaluating the Strength, Durability and Porosity Characteristics of Alluvial Clay Stabilized with Marble Dust as a Sustainable Binder. Results Eng. 2025, 25, 103978. [Google Scholar] [CrossRef]
- Nategh, M.; Ekinci, A.; Iravanian, A.; Fahrioğlu, M. Enhancing Clay Soil’s Geotechnical Properties Utilizing Sintered Gypsum and Glass Powder. Appl. Sci. 2024, 14, 4961. [Google Scholar] [CrossRef]
- Yao, L.; Yang, J.; Ma, X.; Wei, P.; Wang, Y.; Wang, W.; Li, N. Short-Term Mechanical Properties and Microstructure of Coastal Cement Soil Modified by Sugarcane Bagasse Ash. Results Eng. 2024, 24, 103279. [Google Scholar] [CrossRef]
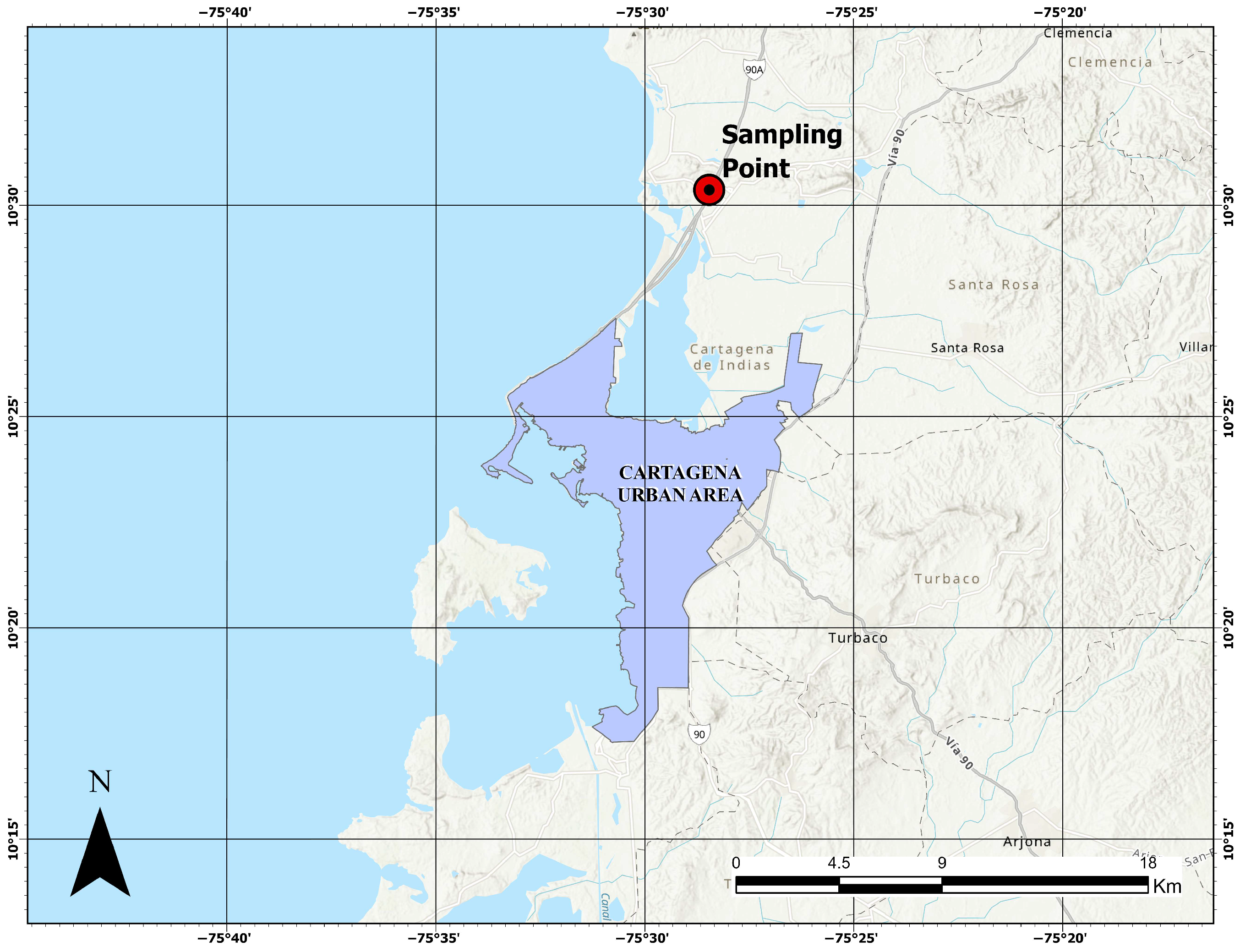

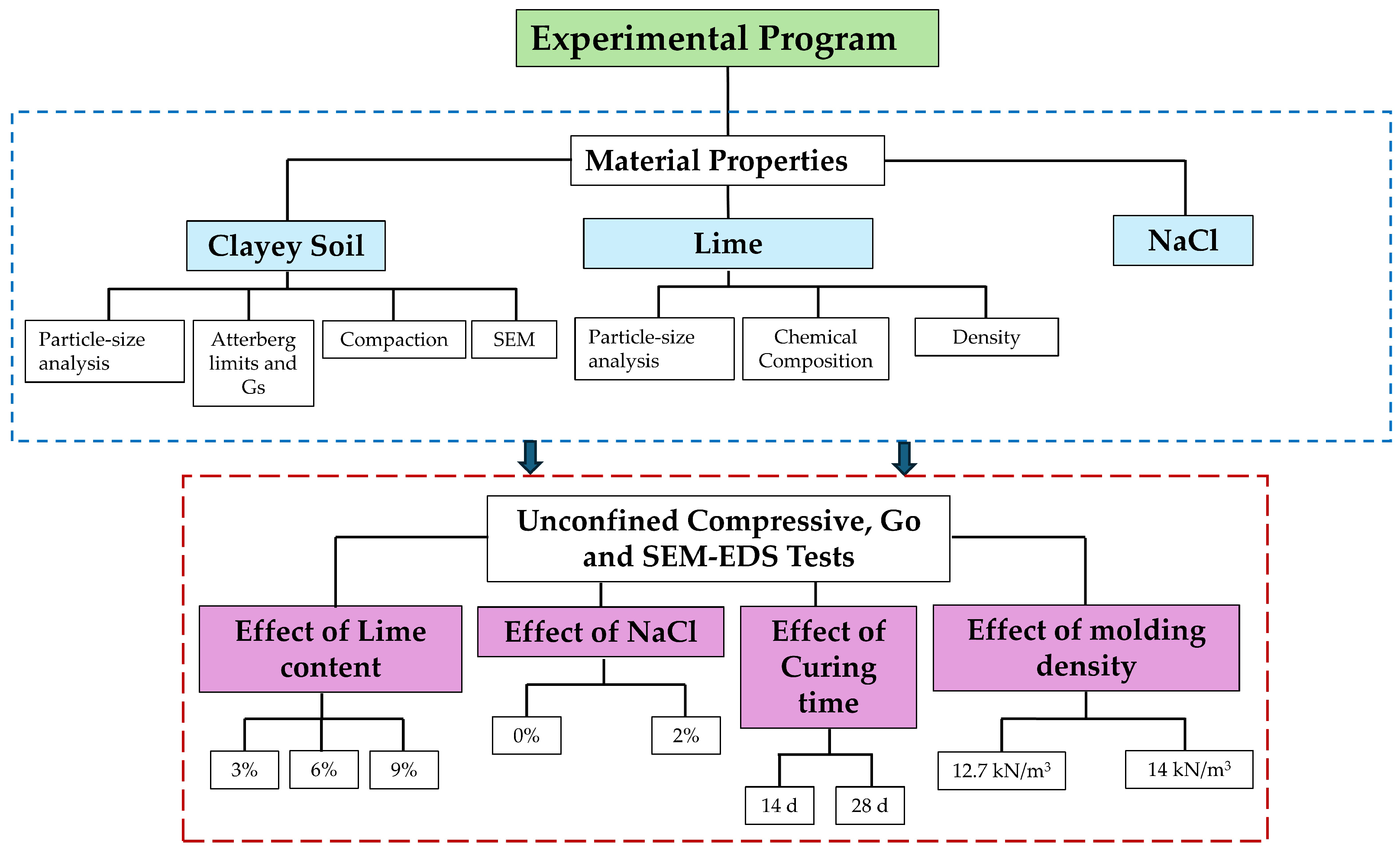
| Material | Moisture (%) | Lime (%) | NaCl (%) | Curing Time (Days) | qu (kPa) | Reference |
|---|---|---|---|---|---|---|
| Clay | 16 | 10 | 2 | 7 | 420 | [7] |
| Clay | 22 | 10 | 2 | 7 | 380 | [7] |
| Fly Ash | 31.5 | 8 | 1 | 28 | 8500 | [8] |
| Clay | 30.6 | 5 | 2 | 28 | 200 | [4] |
| Black cotton | 33 | 12 | 1 | 28 | 200 | [9] |
| Sand-Fly ash | 14 | 7 | 0.5 | 7 | 250 | [10] |
| Molding γd (kN/m3) | Soil (%) | Lime (%) | NaCl (%) | Curing Times (Days) | Specimens |
|---|---|---|---|---|---|
| 12.7 | 100 | 3 | 0 and 2 | 14 and 28 | 12 |
| 100 | 6 | 0 and 2 | 14 and 28 | 12 | |
| 100 | 9 | 0 and 2 | 14 and 28 | 12 | |
| 14.0 | 100 | 3 | 0 and 2 | 14 and 28 | 12 |
| 100 | 6 | 0 and 2 | 14 and 28 | 12 | |
| 100 | 9 | 0 and 2 | 14 and 28 | 12 |
| Element | Name | Content (%) |
|---|---|---|
| SiO2 | Silicon Dioxide | 62.6 |
| Al2O3 | Aluminum Oxide | 24.1 |
| SO3 | Sulfur Trioxide | 5.6 |
| K2O | Potassium Oxide | 4.4 |
| CaO | Calcium Oxide | 1.7 |
| Fe2O3 | Iron (III) Oxide | 1.2 |
| TiO2 | Titanium Dioxide | 0.5 |
| Physical Property of the Soil | Results | Standard |
|---|---|---|
| Liquid limit, % | 53.1 | ASTM 4318 [20] |
| Plastic limit, % | 29.0 | ASTM 4318 [20] |
| Plastic index, % | 24.1 | ASTM 4318 [20] |
| Specific gravity | 2.73 | ASTM D854 [19] |
| Fine gravel (4.75 mm–19 mm), % | - | ASTM D7928 [26] |
| Coarse sand (2.0 mm–4.75 mm), % | - | ASTM D7928 [26] |
| Medium sand (0.425 mm–2.0 mm), % | 7.5 | ASTM D7928 [26] |
| Fine sand (0.075 mm–0.425 mm), % | 25.9 | ASTM D7928 [26] |
| Silt (0.002 mm–0.075 mm), % | 57.6 | ASTM D7928 [26] |
| Clay (diameter < 0.002 mm), % | 9.0 | ASTM D7928 [26] |
| Medium diameter (D50), mm | 0.0245 | - |
| USCS Classification | CH | ASTM D2487 [25] |
| Maximum dry unit weight, standard Proctor, kN/m3 | 13.87 | ASTM D698 [27] |
| Optimum water content, Standard Proctor, % | 26.0 | ASTM D698 [27] |
| Blend | Normalization Index | For Normalization | ||
|---|---|---|---|---|
| Soil–L–0%NaCl (14 d) | 400.68 | 1533.17 | ||
| Soil–L–2%NaCl (14 d) | 1039.44 | 3628.24 | ||
| Soil–L–0%NaCl (28 d) | 513.92 | 1783.90 | ||
| Soil–L–2%NaCl (28 d) | 1381.93 | 5230.72 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldovino, J.A.; Parra, J.D.T.; Nuñez de la Rosa, Y.E. Effects of Sodium Chloride in Soil Stabilization: Improving the Behavior of Clay Deposits in Northern Cartagena, Colombia. Sustainability 2025, 17, 8715. https://doi.org/10.3390/su17198715
Baldovino JA, Parra JDT, Nuñez de la Rosa YE. Effects of Sodium Chloride in Soil Stabilization: Improving the Behavior of Clay Deposits in Northern Cartagena, Colombia. Sustainability. 2025; 17(19):8715. https://doi.org/10.3390/su17198715
Chicago/Turabian StyleBaldovino, Jair Arrieta, Jesús David Torres Parra, and Yamid E. Nuñez de la Rosa. 2025. "Effects of Sodium Chloride in Soil Stabilization: Improving the Behavior of Clay Deposits in Northern Cartagena, Colombia" Sustainability 17, no. 19: 8715. https://doi.org/10.3390/su17198715
APA StyleBaldovino, J. A., Parra, J. D. T., & Nuñez de la Rosa, Y. E. (2025). Effects of Sodium Chloride in Soil Stabilization: Improving the Behavior of Clay Deposits in Northern Cartagena, Colombia. Sustainability, 17(19), 8715. https://doi.org/10.3390/su17198715







