1. Introduction
Fossil fuels have long been the cornerstone of global energy systems, serving as the primary driver of industrialization, economic growth, and modern living standards [
1,
2]. However, the rapid rise in global population, coupled with accelerated industrialization and urbanization, has dramatically intensified energy consumption. This surge in demand has been paralleled by fluctuating and steadily rising petroleum prices, which create instability in global markets. Beyond economic volatility, the extensive dependence on fossil fuels has also raised critical environmental concerns, including severe greenhouse gas emissions, particulate pollution, and ecological imbalance [
3,
4,
5,
6]. These converging issues, resource depletion, economic instability, and environmental degradation, have motivated researchers worldwide to intensify efforts toward developing renewable and sustainable energy alternatives. Addressing this challenge is not only a scientific necessity but also a practical imperative to ensure long-term energy security and environmental sustainability.
Among various renewable energy options, biodiesel has gained prominence due to its renewability, carbon-neutral cycle, and compatibility with compression-ignition engines. It can be produced from a wide range of feedstocks, including edible oils such as soybean, palm, sunflower, and rapeseed, as well as non-edible oils like jatropha, neem, mahua, cottonseed, and karanja [
7,
8]. This feedstock versatility supports biodiesel production in both developed and developing regions, offering a means to reduce dependence on petroleum imports. Biodiesel also provides additional benefits, including improved lubricity and lower emissions of carbon monoxide (CO), hydrocarbons (HC), and particulate matter compared to conventional diesel. However, reliance on edible oils raises concerns about competition with food crops, which can threaten food security in low-income nations, particularly in Africa and Asia. In contrast, major food-producing countries like China and Brazil may experience less impact on food availability, though biofuel production can still influence land use and market prices. To address these challenges, agricultural biomass residues such as corn stover, wheat straw, rice husk, coffee husk, and coffee pulp are increasingly being explored for their potential to simultaneously enable waste valorization and renewable energy generation [
9,
10].
The coffee industry represents one of the largest agro-industrial sectors globally, generating significant amounts of solid residues during processing. Key by-products include coffee husk (CH), coffee pulp (CP), coffee silverskin (CS), spent coffee grounds (SCG), mucilage (CM), and coffee cut-stems (CCS) [
6,
11,
12]. These wastes are generated at enormous scales, approximately 6 million tons of SCG and more than 6.08 tons of CH and CP annually worldwide [
13,
14]. Notably, almost 50% of a coffee cherry’s weight is lost as waste during processing, reflecting the magnitude of the challenge [
15,
16]. Coffee husk, in particular, constitutes the main solid by-product of dry coffee processing and contains high proportions of carbon-rich compounds such as carbohydrates (58–85%) and proteins (8–11%), along with minerals and other organic matter [
17,
18]. Despite this high bioenergy potential, CH also contains anti-nutritional factors and harmful compounds such as caffeine, tannins, and polyphenols, which render it unsuitable for animal feed and problematic for direct disposal. Consequently, improper management of CH not only represents an environmental liability but also a lost opportunity for sustainable energy generation. Thus, converting CH into biodiesel (CHOME) transforms an environmental challenge into an energy solution, positioning coffee residues as a promising feedstock for waste-to-energy pathways, particularly in coffee-producing nations like Ethiopia.
Several researchers have explored biochemical conversion techniques to convert lignocellulosic biomass into bioethanol and biodiesel, with results demonstrating the viability of such residues for renewable fuel production [
19,
20]. For instance, a researcher. reported the efficiency of cellulose hydrolysis for ethanol production from agricultural waste, while Sime et al. successfully produced bioethanol from Ethiopian coffee residues [
21]. These findings confirm the technical feasibility of harnessing coffee residues for biofuel production. However, despite this progress, biodiesel derived from such unconventional feedstocks faces critical operational challenges when used in diesel engines. Compared to petroleum diesel, biodiesel typically exhibits higher viscosity and lower calorific value, which adversely affect engine performance. Specifically, studies have consistently reported reductions in brake thermal efficiency (BTE), increases in brake-specific fuel consumption (BSFC), and lower cylinder pressures and heat release rates for biodiesel-fueled engines [
22,
23,
24]. These drawbacks highlight a key limitation in biodiesel utilization that demands targeted solutions to improve combustion quality and overall engine efficiency.
One practical strategy that has been widely proposed to mitigate the unfavorable properties of biodiesel is fuel preheating. Preheating reduces viscosity and density, improves atomization, and enhances mixing between fuel and air during injection [
14,
25,
26]. This results in better combustion efficiency and reduced emissions of incomplete combustion products. While fuel preheating has been studied for a variety of biodiesels, there remains a complete absence of research investigating this technique for coffee husk oil methyl ester (CHOME) under diesel engine operating conditions. This gap is scientifically significant because CHOME’s unique chemical composition, compared to conventional biodiesel feedstocks, may respond differently to preheating. Moreover, given Ethiopia’s status as a major coffee producer, establishing CHOME as a viable biodiesel source is of high practical relevance for local renewable energy strategies.
The present study directly addresses this gap by conducting, for the first time, a systematic investigation into the performance, combustion, and emission characteristics of a diesel engine fueled with both preheated and unheated CHOME biodiesel. Various blend ratios (B10, B30, B50, and B100) are tested and compared against baseline diesel (B0), allowing for a comprehensive evaluation of the effects of blending and preheating. The central research question guiding this work is whether preheating CHOME biodiesel can significantly enhance engine performance while simultaneously reducing harmful emissions. We hypothesize that preheating will improve atomization and ignition quality, leading to higher cylinder pressure, improved heat release rates, and reductions in CO, HC, and smoke opacity, with only a marginal increase in NOx emissions that can be controlled through exhaust gas recirculation [
27]. While preheating has been examined for other biodiesel feedstocks such as Dhupa seed oil [
28], fish oil [
29], and jatropha [
30], the unique composition of CHOME characterized by unusually high FFA, high viscosity, and bioactive compounds (caffeine, tannins) suggests that its combustion response under preheated conditions cannot be generalized from prior biodiesel studies. Thus, this work provides new insights into how a waste-derived, chemically distinct biodiesel behaves when subjected to preheating.
By addressing this unexplored research problem, the novelty and contribution of this study are twofold. First, it introduces CHOME biodiesel preheating as a practical intervention to overcome viscosity-related limitations, thereby enhancing engine efficiency and combustion characteristics. Second, it demonstrates a sustainable waste-to-energy pathway by valorizing coffee husk, one of the most abundant yet underutilized agricultural residues in coffee-producing countries. Scientifically, this research provides new insights into the combustion dynamics of waste-derived biodiesel under preheated conditions. At the same time, practically, it offers a scalable approach for renewable fuel utilization in regions where coffee processing waste is abundant. The findings are expected to open new avenues for sustainable energy research and provide policymakers with actionable strategies for integrating biofuels into national energy portfolios.
2. Materials and Methodology
2.1. Production of Biodiesel
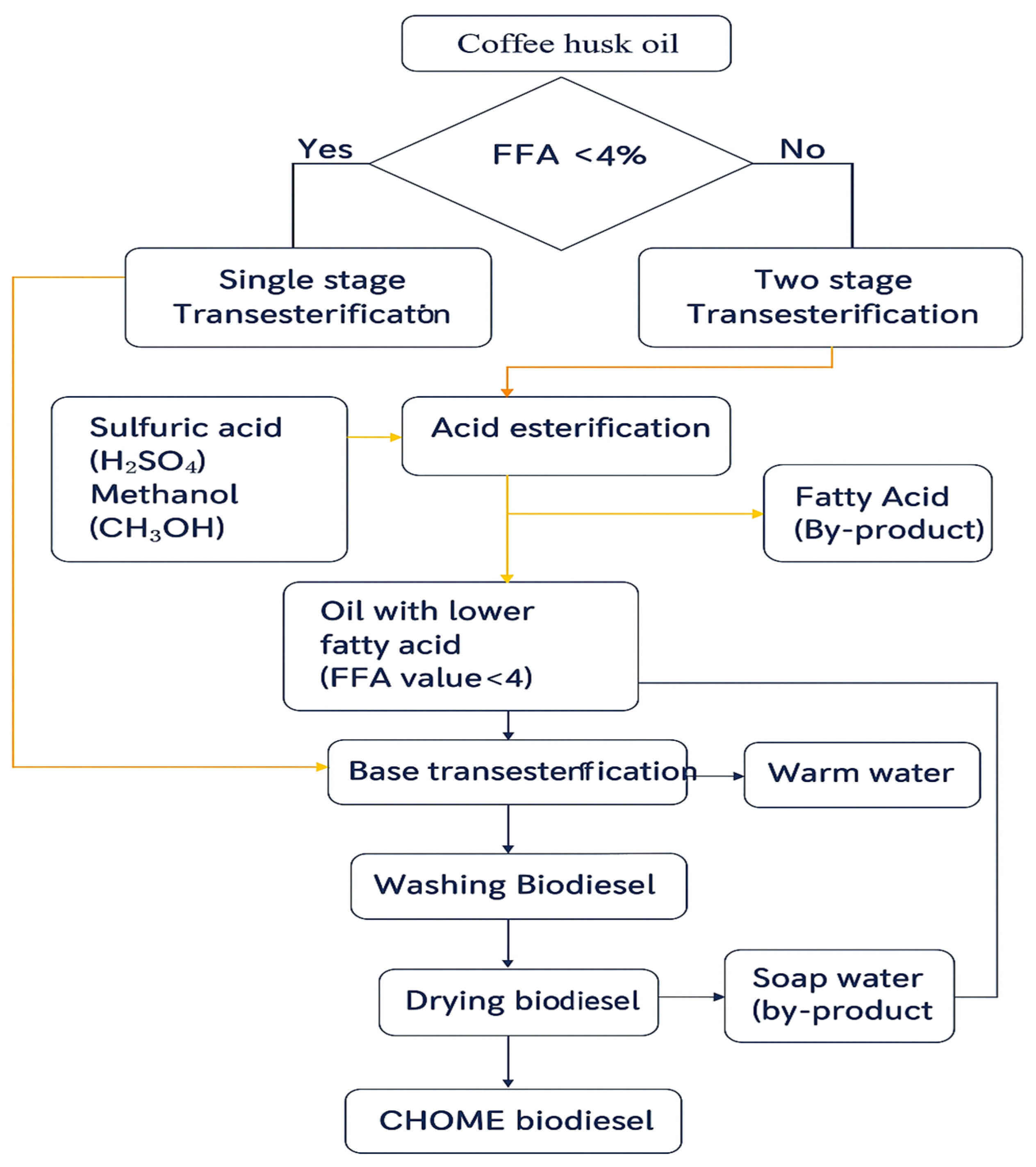
The production of biodiesel from coffee husk oil (CHO) was performed using a two-step transesterification process, a widely adopted method for converting triglycerides in oils into their corresponding methyl esters (biodiesel) and glycerol. In this process, methanol reacts with the oil in the presence of a catalyst, breaking ester bonds and forming methyl esters with enhanced fuel properties [
31,
32]. The effectiveness of transesterification depends strongly on the free fatty acid (FFA) content of the raw oil. When the FFA level exceeds 4%, a single-stage base-catalyzed reaction becomes unsuitable, as high FFA promotes soap formation and reduces ester yield. In such cases, a two-step approach—acid esterification followed by base transesterification—is necessary, while oils with FFA below 4% can be processed directly through a single base-catalyzed step.
For CHO, the FFA content was determined using ASTM D5555-95 [
33] titrimetric analysis, calculated as
where V is the titrant volume (mL), N is the normality of KOH, and W is the sample weight (g). The measured FFA was 21%, confirming the need for a two-stage process.
In the first stage (acid esterification), CHO was treated with methanol at a 6:1 molar ratio and 1 wt% H2SO4 (relative to oil) at 60 °C for 1 h under continuous stirring (600 rpm), reducing FFA to below 2%. In the second stage (base transesterification), the pretreated oil was reacted with methanol at a 6:1 molar ratio and 0.8 wt% KOH at 60 °C for 90 min. After settling for 12 h, two distinct layers formed: biodiesel (upper phase) and glycerol (lower phase). The biodiesel was then washed with warm deionized water and dried at 105 °C to remove residual methanol and moisture.
This sequential method effectively reduced the high FFA content and ensured complete conversion of CHO into coffee husk oil methyl ester (CHOME).
Figure 1 presents the adopted biodiesel production pathway, highlighting the two-stage strategy used to overcome the unusually high FFA content of CHO—a challenge rarely encountered in conventional feedstocks.
The contribution lies in the valorization of CHO, a waste biomass with an exceptionally high FFA content that has historically been considered unsuitable for direct biodiesel conversion. The successful implementation of a two-stage transesterification strategy demonstrates the feasibility of transforming this problematic residue into a viable renewable fuel.
2.2. Physicochemical Properties of Produced Biodiesel
The physicochemical characteristics of CHOME and its blends with petroleum diesel were analyzed in accordance with the ASTM D6751 [
34] specifications for biodiesel fuels. The key properties investigated included density, kinematic viscosity, flash point, fire point, and calorific value. These parameters were measured using a Redwood viscometer, bomb calorimeter, and Cleveland flash point tester.
Table 1 summarizes the measured values of CHOME blends (B10, B30, B50, and B100) alongside conventional diesel.
The results indicate that CHOME exhibits higher density, viscosity, flash point, and fire point compared to diesel, but a lower calorific value. These differences are crucial because they directly influence engine performance. For example, the elevated viscosity can lead to poor fuel atomization and delayed combustion, while the lower calorific value reduces the energy available per unit mass of fuel. However, the higher flash and fire points improve fuel safety during storage and handling, a desirable feature in biodiesel applications.
These findings are consistent with trends observed in biodiesels from other unconventional feedstocks, but the notably high viscosity and low heating value of neat CHOME reinforce the necessity of performance optimization strategies such as fuel preheating, which constitutes a central innovation of this study.
2.3. Structural Characterization by FTIR
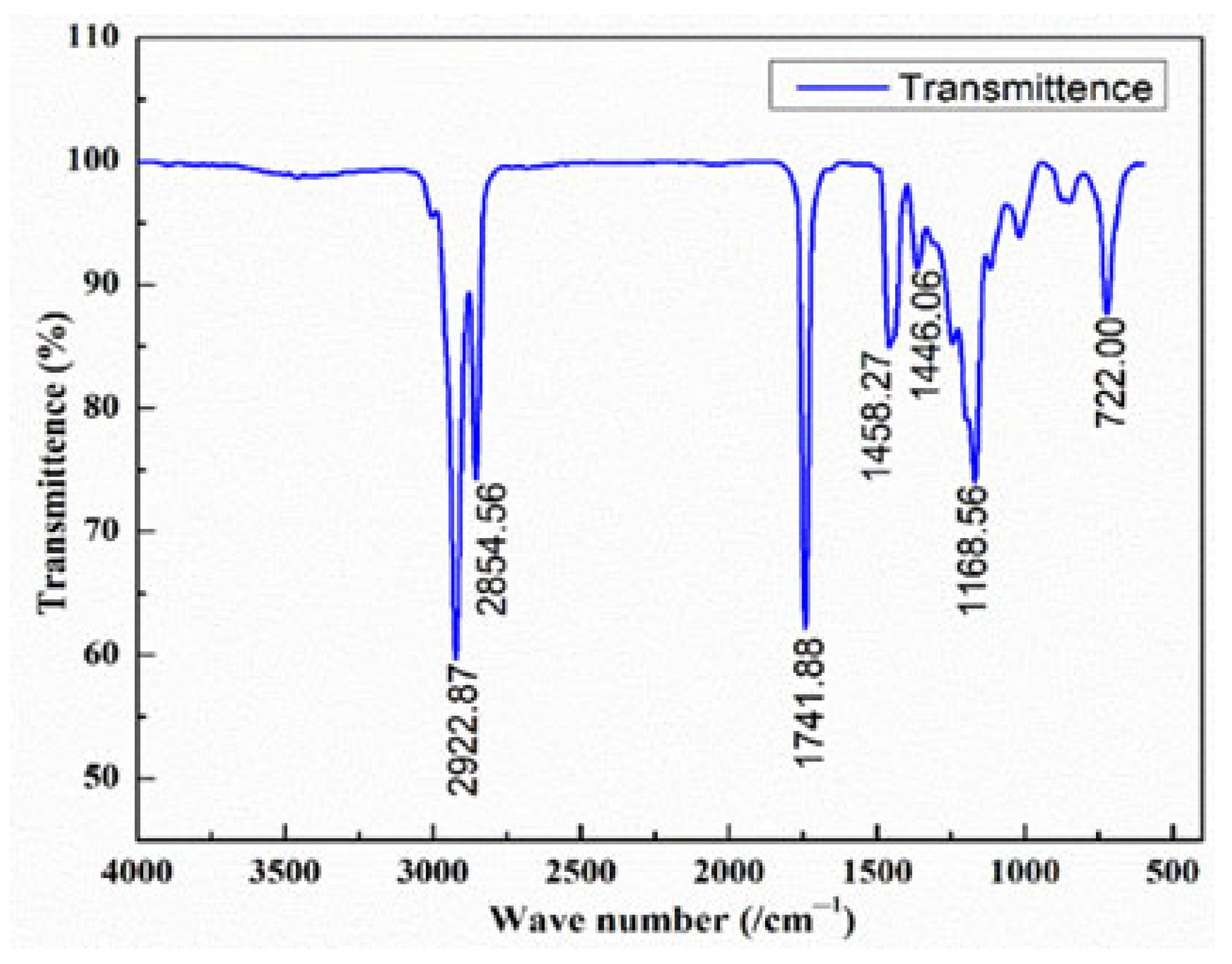
To confirm the successful conversion of CHO into methyl esters, Fourier Transform Infrared (FTIR) spectroscopy was employed using a Bruker Series FTIR spectrometer (Bruker Corporation, Billerica, MA, USA). FTIR is a powerful diagnostic tool that identifies functional groups based on their characteristic infrared absorption frequencies, thereby providing a molecular fingerprint of the fuel. Spectra were recorded in the range of 4000–500 cm
−1 for both CHOME and regular diesel (
Figure 2 and
Figure 3).
The FTIR spectrum of CHOME displayed prominent absorption bands at 2922.87 cm
−1 and 2854.56 cm
−1, corresponding to C–H stretching vibrations of aliphatic CH
2 and CH
3 groups. A sharp peak at 1741.88 cm
−1 confirmed the presence of ester carbonyl (C=O) groups, while the band at 1168.56 cm
−1 was assigned to C–O stretching vibrations of esters. Additional peaks at 1458.27 cm
−1 and 1446.06 cm
−1 indicated bending vibrations of methyl and alkene groups, respectively, while the rocking vibration of -(CH
2) n chains appeared at 722 cm
−1. These spectral features (
Table 2) unambiguously confirm the transesterification of CHO to CHOME, distinguishing it from unreacted triglycerides.
Additional peaks unique to CHOME were observed in the 500–1500 cm−1 region, corresponding to C–O stretching (esters), C–H bending of alkanes, and other fingerprint absorptions absent in conventional diesel. These features confirm the formation of ester functional groups and explain the differences in combustion reactivity between diesel (hydrocarbon-only) and biodiesel (oxygenated functional groups). The FTIR analysis thus provides molecular-level evidence of successful CHOME production, verifying its chemical identity as a biodiesel. Notably, these findings validate the two-step transesterification process as an effective approach for converting high-FFA oils into usable biodiesel.
2.4. Stability Testing of CHOME Biodiesel
Figure 4 (Day 0) and
Figure 5 (Day 30) show visual comparisons of diesel–CHOME blends, revealing no noticeable crystallization, phase separation, or discoloration during storage. Long-term stability is a crucial factor for assessing the practical suitability of biodiesel, as exposure to air, light, and heat can trigger oxidative degradation, polymerization, and phase separation [
29,
39,
40]. To evaluate the storage stability of CHOME, blended fuels were stored under ambient laboratory conditions for 30 days following ASTM D6751 guidelines, with periodic visual inspections to detect any signs of crystallization, gel formation, separation, or color changes. As shown in
Figure 4 and
Figure 5, no signs of degradation were observed, indicating that CHOME blends maintained excellent oxidative and physical stability throughout the storage period. The absence of gelling, separation, or discoloration confirms that CHOME biodiesel resists short-term oxidative deterioration—a notable outcome considering the high FFA content of the original feedstock, which could have otherwise increased its susceptibility to instability.
The demonstrated stability of CHOME biodiesel reinforces its potential as a viable alternative fuel. This finding highlights a key novelty of the study: despite being derived from a high-FFA feedstock, CHOME not only complies with ASTM specifications but also maintains satisfactory storage stability, supporting its suitability for large-scale use in diesel engines. Moreover, alongside visual assessments, kinematic viscosity and density were measured before (Day 0) and after 30 days of storage, showing variations within ±2%. This confirms that the observed stability was not only visual but also substantiated by consistent fuel property measurements.
2.5. Engine Setup
In this experimental investigation, a Kirloskar TV1 diesel engine was employed, which is widely recognized for research and testing purposes due to its robust design, reliability, and adaptability for alternative fuel evaluations. The engine is a single-cylinder, four-stroke, constant-speed, naturally aspirated, water-cooled, vertical cylinder, and direct injection (DI) compression ignition engine. Such an engine configuration was deliberately selected because its simple geometry facilitates accurate measurements of in-cylinder parameters, while its single-cylinder nature minimizes experimental uncertainties and ensures repeatability. Moreover, the DI configuration closely resembles practical diesel engine applications, thereby improving the real-world relevance of the results.
For load variation and control, the experimental engine was coupled to an eddy current dynamometer, which allowed precise adjustment of engine load and provided rapid response under transient conditions an essential capability when evaluating biodiesel blends with combustion and ignition characteristics distinct from conventional diesel. The complete experimental setup is shown photographically in
Figure 6. The test bed included a Kirloskar TV1 engine, a fuel line equipped with a heating system, a computer-based data acquisition unit, the eddy current dynamometer, and a control panel. Additional instrumentation consisted of a fuel consumption indicator, engine speed display, temperature sensors, and a load control unit. Emission data were collected using an INDUS 5 gas analyzer and an AVL smoke analyzer, enabling comprehensive assessment of engine performance and exhaust characteristics.
A preheating temperature of 95 °C was selected based on preliminary trials and literature reports [
41,
42] which indicate that the viscosity of biodiesel approaches that of diesel at temperatures between 90 and 100 °C. Operating above this range risks thermal degradation and excessive energy consumption, whereas lower temperatures provide insufficient viscosity reduction. Engine tests were carried out at five steady-state load conditions—0%, 25%, 50%, 75%, and 100% of rated load with data recorded after 10 min of stable operation to ensure measurement repeatability.
The presence of a fuel line heater, a distinctive feature of this experimental setup. The heating system consisted of a 500 W electric resistance heater with an adjustable thermostat (ambient to 120 °C, ±1 °C stability). Fuel temperature was monitored using a K-type thermocouple and controlled with a PID controller, ensuring a stable heating rate of approximately 2 °C/min and maintaining a constant temperature of 95 °C before injection. The heating time from ambient to 95 °C was approximately 20 min. Because biodiesel blends generally possess higher viscosity than diesel, this controlled preheating improved fuel atomization, enhanced mixture formation, and promoted stable combustion. Incorporating this heating arrangement represents a key distinguishing feature of the present study, as it directly addresses the viscosity-related limitations of biodiesel-based fuels—an aspect often overlooked in similar investigations A Data Acquisition System (DAS) integrated with Engine Soft software (Version 2.4, Apex Innovations Pvt. Ltd., Nagpur, India) was used to archive all real-time data. High-frequency sampling ensured accurate resolution of combustion characteristics. The in-cylinder pressure and Heat Release Rate (HRR) profiles were obtained by averaging over 100 consecutive engine cycles, minimizing cycle-to-cycle variation and noise. This methodological rigor provides high fidelity to the combustion data and strengthens the reliability of comparative performance evaluation. Furthermore, the system continuously logged brake power, fuel consumption, exhaust gas temperature, and loading percentage, enabling a comprehensive thermodynamic and emissions assessment.
To ensure a robust emissions analysis, the experimental setup was equipped with an INDUS 5 exhaust gas analyzer and an AVL smoke meter. The INDUS 5 analyzer was employed to quantify major exhaust species, including unburned hydrocarbons (HC), carbon monoxide (CO), carbon dioxide (CO
2), and oxides of nitrogen (NOₓ), with its precision levels provided in
Table 3. The AVL smoke analyzer was used for particulate matter assessment in terms of smoke opacity, thereby providing a holistic view of gaseous as well as particulate emissions. The combination of these instruments ensures the validity of emissions data, which is crucial in evaluating the environmental viability of biodiesel diesel blends.
As presents in
Table 4, the experimental engine specifications, highlighting the metrological rigor of the study and ensuring that all measurements adhere to internationally recognized standards of accuracy and reproducibility. The selected instrumentation system significantly strengthens biodiesel research in multiple ways. The inclusion of a fuel preheating system, capable of raising the temperature from ambient up to 95 °C, addresses the viscosity-related challenges of biodiesel blends and improves their adaptability in conventional engines. In-cylinder pressure data were averaged over 100 consecutive cycles, providing a statistically robust representation of combustion characteristics—a methodological rigor often absent in similar studies that typically consider fewer cycles. Additionally, simultaneous monitoring of both gaseous and particulate emissions allows the creation of a comprehensive emissions database, filling a notable gap in biodiesel experimental literature. The experiments were conducted at a constant engine speed of 1500 rpm under varying electrical loads (0, 25, 50, 75, and 100%), with cooling water maintained at 27 ± 2 °C and ambient laboratory conditions carefully controlled. Collectively, these deliberate design choices enhance the accuracy, reliability, and novelty of this study, positioning it beyond conventional biodiesel engine performance analyses.
The engine used in this investigation was a Kirloskar TV1, a single-cylinder, water-cooled, four-stroke, variable compression ratio diesel engine. The engine specifications are as follows: a displacement of 661 cc, bore and stroke dimensions of 87.5 mm × 110 mm, and a connecting rod length of 234 mm. The compression ratio can be varied between 12:1 and 18:1, with the manufacturer’s default setting at 17.5:1. The maximum rated power output is 3.5 kW at 1500 rpm, while the fuel injection timing is fixed at 23° before top dead center (bTDC) for diesel operation. These characteristics make the Kirloskar TV1 engine an ideal platform for investigating the influence of biodiesel blends on engine performance, combustion, and emission behavior.
2.6. Performance and Combustion Equations
Brake thermal efficiency (BTE), brake specific fuel consumption (BSFC), brake specific energy consumption (BSEC), and heat release rate (HRR) were calculated as follows:
where
BP is brake power,
ṁf fuel mass flow rate,
CV calorific value,
P pressure,
V cylinder volume,
γ ratio of specific heats, and
θ crank angle.
3. Results and Discussion
The findings of this experimental study are presented and interpreted in terms of combustion characteristics, engine performance, and emission behavior. A major focus of the present investigation is the effect of fuel preheating on reducing viscosity, enhancing spray atomization, and ultimately improving combustion when Coffee Husk Oil Methyl Ester (CHOME) biodiesel and its blends are utilized in a compression ignition engine. By systematically analyzing these aspects, this work highlights preheating as a simple yet highly effective strategy for optimizing biodiesel performance.
It is well recognized that one of the most critical limitations of biodiesel fuels lies in their relatively high kinematic viscosity compared to conventional petroleum diesel. Elevated viscosity leads to poor atomization of the fuel spray, formation of larger droplets, extended ignition delay, and incomplete combustion. These factors collectively diminish thermal efficiency and contribute to higher emissions [
43]. Conventionally, two strategies have been adopted to mitigate this issue. The first is
transesterification, a chemical process in which triglycerides are converted into methyl esters, thereby reducing viscosity. The second is
thermal preheating, a physical approach in which the biodiesel is heated before injection, reducing viscosity directly and improving spray characteristics under real operating conditions. While transesterification modifies the molecular structure of biodiesel permanently, preheating enables real-time control and adaptability of fuel properties.
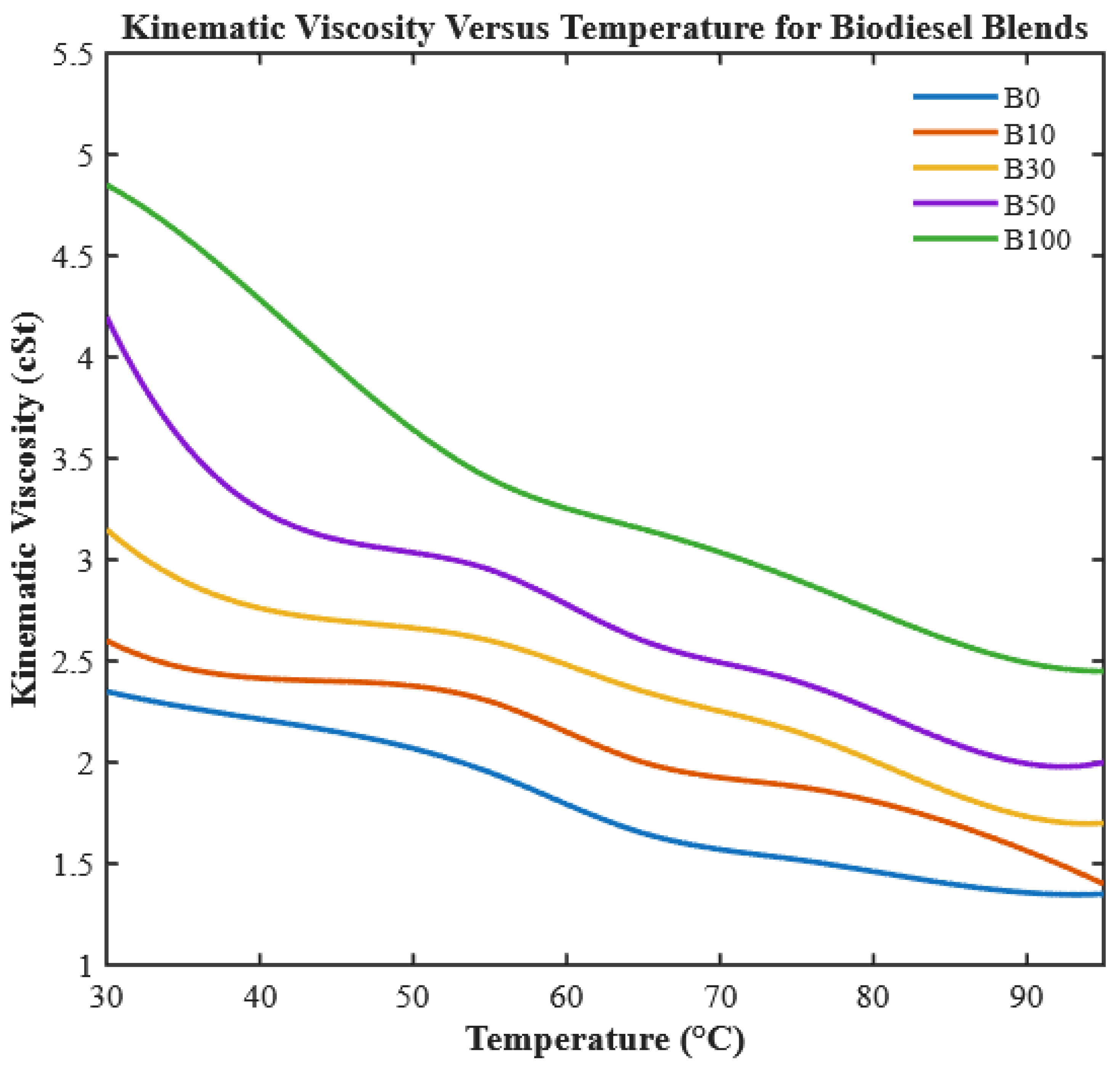
In this study, neat CHOME biodiesel and CHOME-diesel blends were preheated to 95 °C before injection.
Figure 7 shows the effect of temperature on kinematic viscosity, measured using a Redwood viscometer according to ASTM D445. Preheating reduced the viscosity of neat CHOME by ~49.5%, narrowing the gap with conventional diesel and improving atomization, ignition, and combustion efficiency. Unlike previous studies that evaluated biodiesel at ambient conditions or focused solely on blending ratios, this work highlights controlled preheating as an operational optimization strategy.
The contribution of this research lies in demonstrating that preheating biodiesel represents a sustainable and adaptable solution to overcome viscosity-related challenges. Unlike transesterification or the use of chemical additives, preheating does not require additional processing steps or infrastructure, and it can be implemented in real time within the engine system. This approach not only improves in-cylinder combustion dynamics and brake thermal efficiency (BTE) but also reduces emissions without relying on costly after-treatment technologies. Furthermore, the adaptability of this technique allows the same fuel to be optimized under varying operating conditions, making it a highly practical option for real-world diesel engine applications. This is particularly relevant in regions where biodiesel, such as cottonseed oil-based fuel, is produced locally and used directly, reinforcing the sustainability and feasibility of the approach.
To systematically examine the impact of this preheating strategy, the subsequent analysis is divided into three key subsections. First, combustion characteristics are evaluated, focusing on in-cylinder pressure variation, net heat release rate (HRR), and ignition delay. Next, performance characteristics are analyzed through brake thermal efficiency (BTE), brake specific fuel consumption (BSFC), and brake specific energy consumption (BSEC). Finally, emission characteristics are assessed by measuring variations in carbon monoxide (CO), carbon dioxide (CO2), hydrocarbons (HC), nitrogen oxides (NOₓ), and smoke opacity under biodiesel–diesel blends with preheating.
Through this structured investigation, the study establishes a direct correlation between the modified fuel properties achieved via preheating and the resulting improvements in combustion efficiency, performance outcomes, and emission reduction. The results underscore the central claim of this work: that preheating is not merely a supplementary step but a transformative operational strategy for enhancing biodiesel’s competitiveness as a diesel substitute.
3.1. Engine Performance Test
The performance behavior of the engine was evaluated in terms of brake thermal efficiency (BTE), brake specific fuel consumption (BSFC), and brake specific energy consumption (BSEC). These parameters were analyzed for CHOME biodiesel–diesel blends at varying mixing ratios (B0, B10, B30, B50, and B100) under different engine loads. Both preheated and unheated fuels were examined to assess the influence of viscosity reduction through preheating. Standard diesel (B0) was used as a baseline reference for direct comparison. This dual approach provides unique insights into how fuel preheating alters the combustion performance relationship of biodiesel, thereby differentiating this study from prior works.
3.1.1. Brake Thermal Efficiency (BTE)
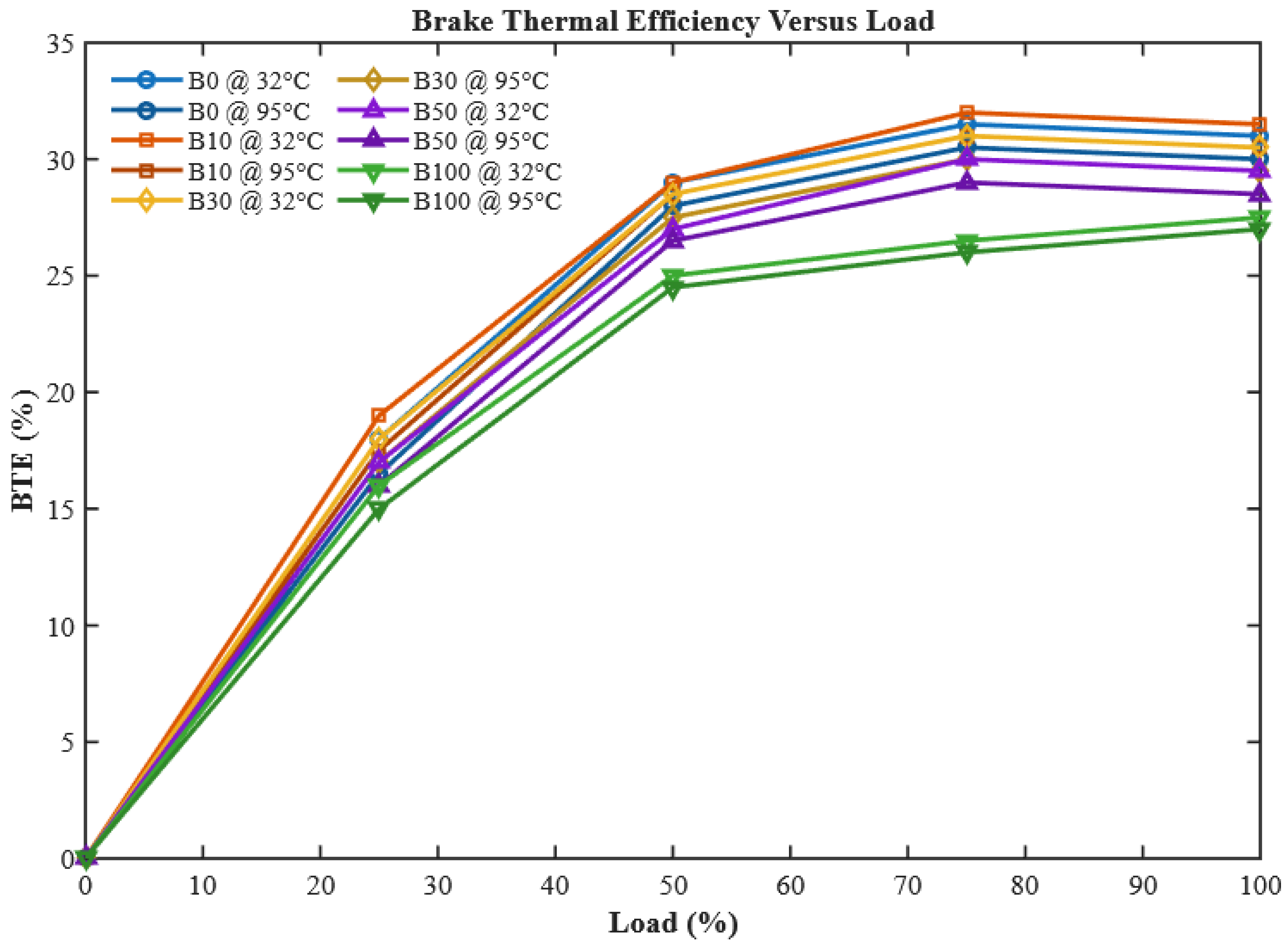
Brake thermal efficiency represents the ability of the engine to convert the chemical energy of fuel into useful brake power, thereby serving as a crucial performance indicator.
Figure 8 illustrates the BTE variation in neat diesel and CHOME biodiesel blends across different engine loads (0–100%).
The trend observed indicates that BTE increases with engine load up to a certain point, after which it begins to decline. This can be attributed to the rise in in-cylinder temperature with higher loads, which enhances combustion initially, but at extreme loads leads to reduced efficiency due to incomplete combustion and heat losses. Importantly, the results confirm that CHOME biodiesel blends consistently show lower BTE than neat diesel across all loading conditions. At full load, B100 exhibits an 11.8% lower BTE than diesel, largely due to its higher viscosity, poor atomization, and lower calorific value. These findings align with the observations of researchers reported in [
39,
44]. However, the novelty of this work emerges clearly when considering the preheated biodiesel blends. Preheating the fuel to 95 °C significantly improved atomization and spray quality, thereby raising BTE across all blending ratios. For instance, preheated B100 demonstrated a 5% increase in BTE compared to its unheated counterpart. Notably, preheated B10 surpassed neat diesel by 0.5%, while preheated B30 closely approached B0 performance levels. These results establish preheating as a simple yet effective strategy to compensate for biodiesel’s inherent drawbacks, thus enhancing engine efficiency without the need for additives or engine modifications.
Although preheated B10 exhibited 0.5% higher BTE than diesel, this difference lies within the ±2.3% measurement uncertainty (
Section 3.5). A two-tailed t-test indicated
p > 0.05, suggesting the difference is not statistically significant. Thus, the improvement should be interpreted as a positive trend rather than a conclusive superiority.
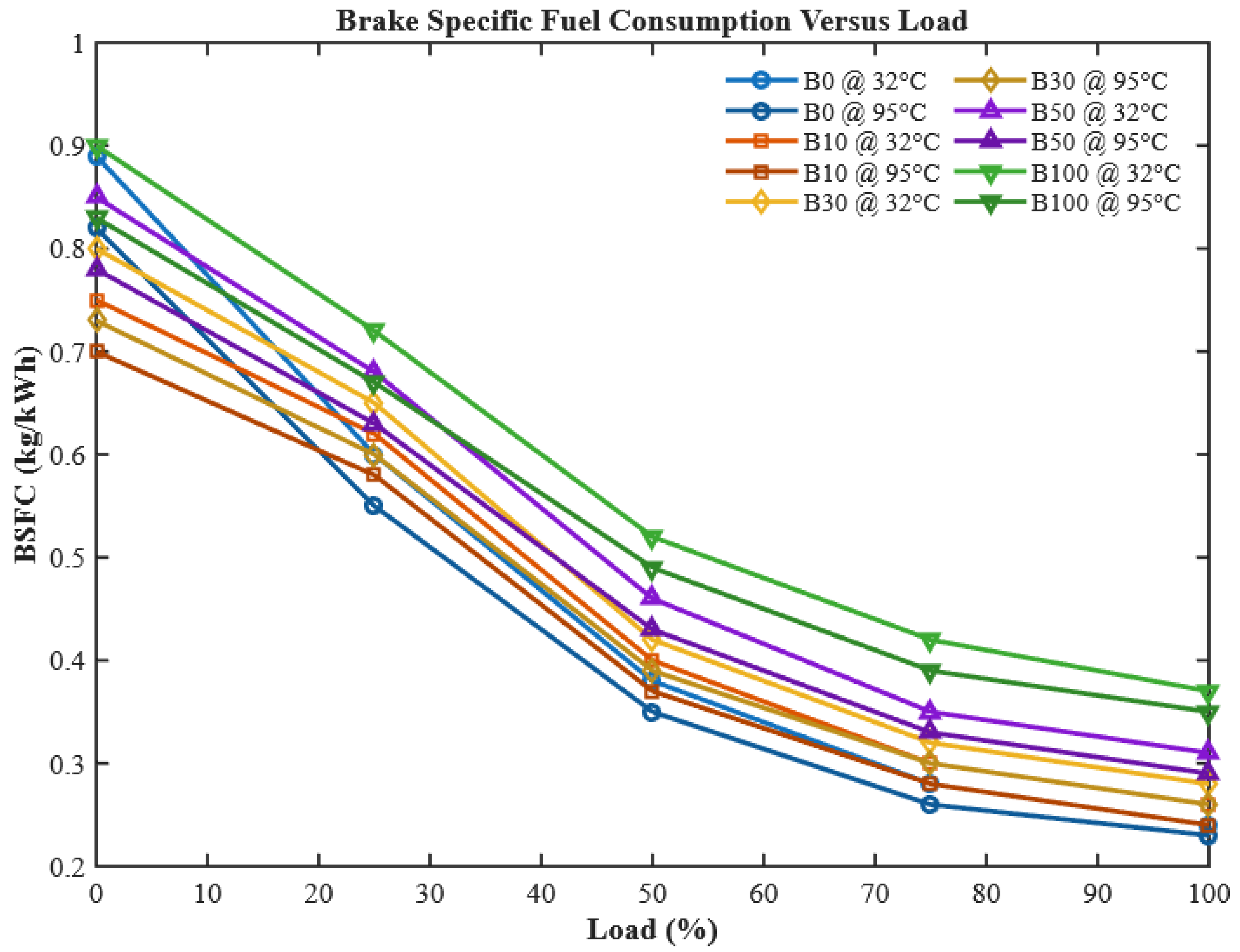
3.1.2. Brake Specific Fuel Consumption (BSFC)
Brake-specific fuel consumption, defined as the mass of fuel consumed per unit brake power, is an essential metric to gauge fuel efficiency.
Figure 9 shows the BSFC trends of CHOME biodiesel–diesel blends compared to neat diesel under varying load conditions.
As expected, BSFC decreased with rising engine load across all fuels. This reduction can be explained by the increased proportion of fuel energy converted into useful work at higher loads, as well as relatively smaller heat losses. However, blends with higher CHOME content consistently exhibited higher BSFC values than diesel. This is attributed to biodiesel’s higher viscosity, greater density, and lower calorific value, which result in inferior atomization and fuel–air mixing. These findings are consistent with prior studies [
40,
45]. The influence of preheating becomes particularly evident when comparing unheated and heated fuels. Preheating CHOME biodiesel to 95 °C lowered viscosity by nearly 49.5%, thereby improving atomization and reducing BSFC. Specifically, BSFC was reduced by 7.75% for B100, 6.5% for B50, 5.3% for B30, 4.6% for B10, and 4% for B0. In certain cases, such as preheated B10, the BSFC was even lower than diesel. These outcomes demonstrate that viscosity control via preheating plays a decisive role in improving the fuel economy of biodiesel-fueled engines. Comparable results were reported in studies involving preheated cashew nut shell liquid biodiesel, spirulina microalgae biodiesel, and palm oil methyl ester [
41,
46].
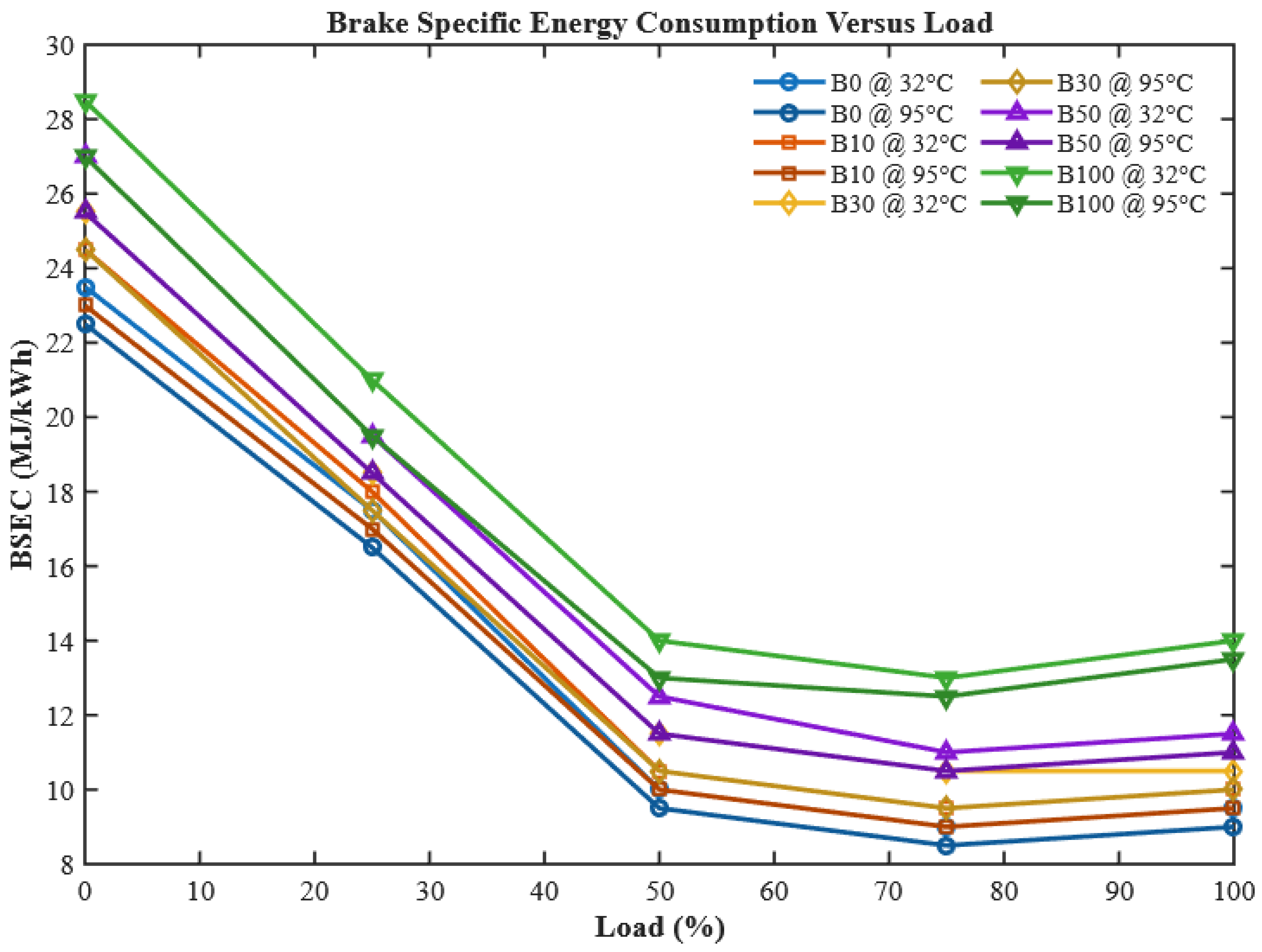
3.1.3. Brake Specific Energy Consumption (BSEC)
While BSFC provides an important measure of efficiency, it does not account for differences in fuel heating values. Brake specific energy consumption (BSEC), which normalizes fuel mass flow against its calorific value, therefore provides a more reliable parameter for comparing fuels with varying energy contents.
Figure 10 presents the BSEC results for diesel and CHOME biodiesel blends under both preheated and unheated conditions.
The results demonstrate a clear decreasing trend in BSEC with rising engine load for all tested fuels, primarily due to the more efficient utilization of injected energy at higher loads. However, similar to BSFC, blends with higher CHOME content recorded higher BSEC values than neat diesel. This is explained by biodiesel’s relatively lower calorific value and higher viscosity, leading to incomplete combustion and greater energy demand per unit brake power [
47]. Once again, preheating proved to be a highly effective intervention. By lowering viscosity and improving spray characteristics, preheating reduced BSEC by 2.04%, 2.6%, 3.21%, 4.29%, and 4.09% for B0, B10, B30, B50, and B100, respectively. These results are in strong agreement with previous findings on preheated corn biodiesel, Pongamia oil, and other non-edible biodiesel sources [
48,
49,
50].
This study demonstrates for the first time that controlled preheating of CHOME biodiesel up to 95 °C can offset performance penalties traditionally associated with biodiesel use, particularly in terms of BTE, BSFC, and BSEC. Unlike chemical modification routes such as transesterification or blending with costly additives, preheating offers a real-time, low-cost, and scalable strategy to enhance biodiesel usability in conventional diesel engines. Moreover, the adaptability of this method makes it suitable for field applications, especially in regions where cottonseed oil biodiesel is locally available. Thus, the experimental results not only validate preheating as a performance-boosting technique but also position it as a practical pathway toward sustainable diesel substitution, aligning with both economic and environmental goals.
3.2. Combustion Characteristics
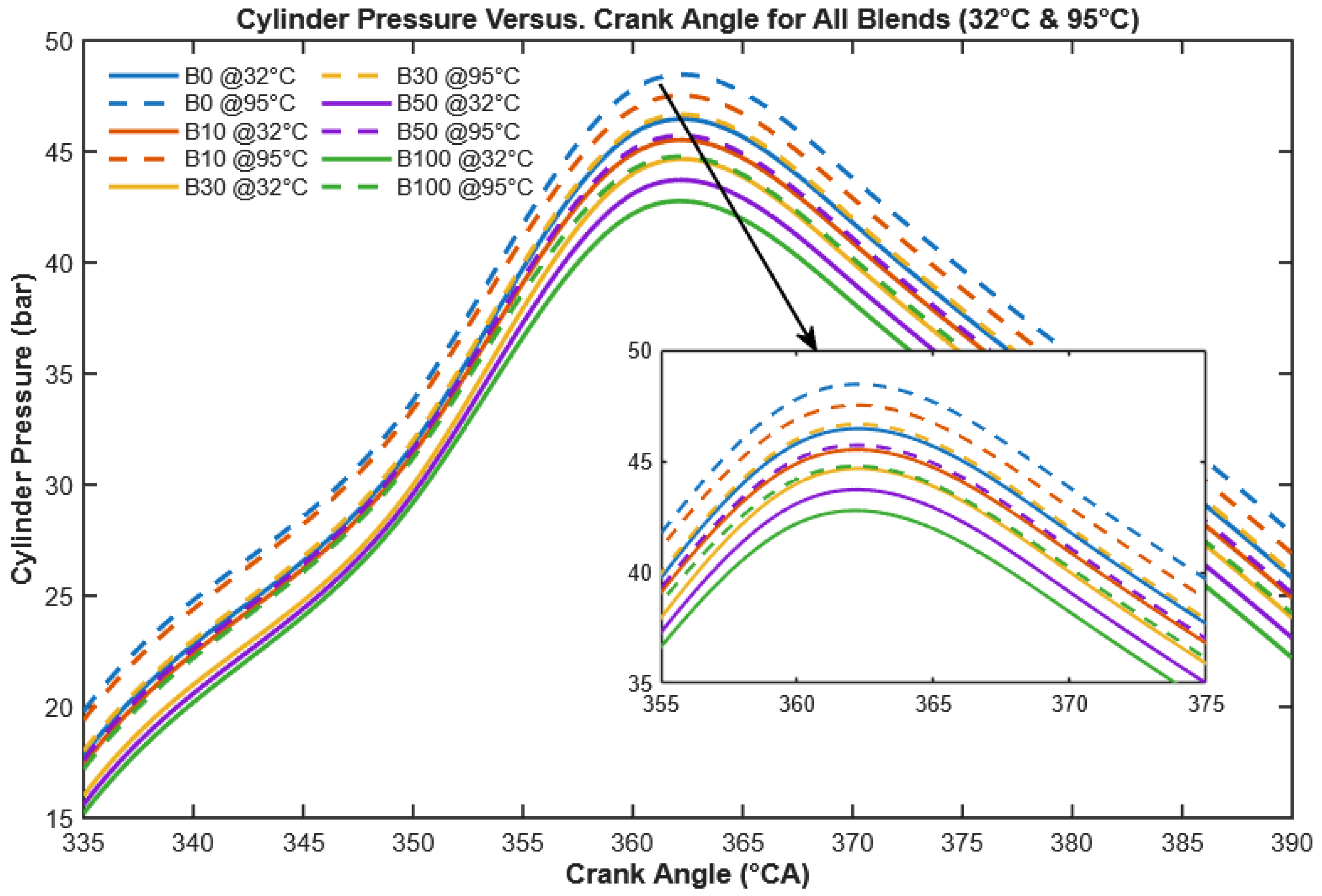
3.2.1. Cylinder Pressure
Cylinder pressure is a key combustion parameter that reflects fuel burning efficiency and energy conversion within the engine.
Figure 11 shows the in-cylinder pressure profiles of unheated and preheated CHOME biodiesel and its blends compared to conventional diesel at full load. While all fuels exhibit trends similar to diesel, notable differences appear in peak pressures. For B100 at 32 °C, slight fluctuations near the peak are attributed to cycle-to-cycle variations caused by poor atomization and delayed ignition. Preheating reduces fuel viscosity, promoting smaller droplets and more uniform premixed combustion, which smooths the pressure profile.
As seen in
Figure 11, neat diesel exhibits the highest peak cylinder pressure (45.25 bar), which is slightly greater than that of neat CHOME biodiesel (41.31 bar). The lower peak pressure of biodiesel is attributed to its higher viscosity, lower calorific value, and higher density relative to diesel [
13,
17]. These properties hinder atomization and slow down combustion, thereby reducing cylinder pressure. However, the unique contribution of this study lies in demonstrating that preheating biodiesel to 95 °C effectively reduces viscosity (down to 2.45 cSt), which enhances atomization, promotes more homogeneous fuel–air mixing, and subsequently improves in-cylinder pressure development.
Quantitatively, preheating the test fuels increases cylinder pressure by 2.81%, 2.97%, 3.11%, 3.4%, and 3.95% for B0, B10, B30, B50, and B100, respectively, compared to their unheated counterparts. This enhancement highlights the novel advantage of preheating as a practical alternative to transesterification for viscosity reduction, making biodiesel combustion more competitive with conventional diesel. Similar observations were also reported by [
28] for Dhupa seed oil biodiesel, but the present study provides a systematic quantification of improvements across multiple biodiesel blend ratios, which strengthens the case for preheating as a robust strategy for biodiesel utilization.
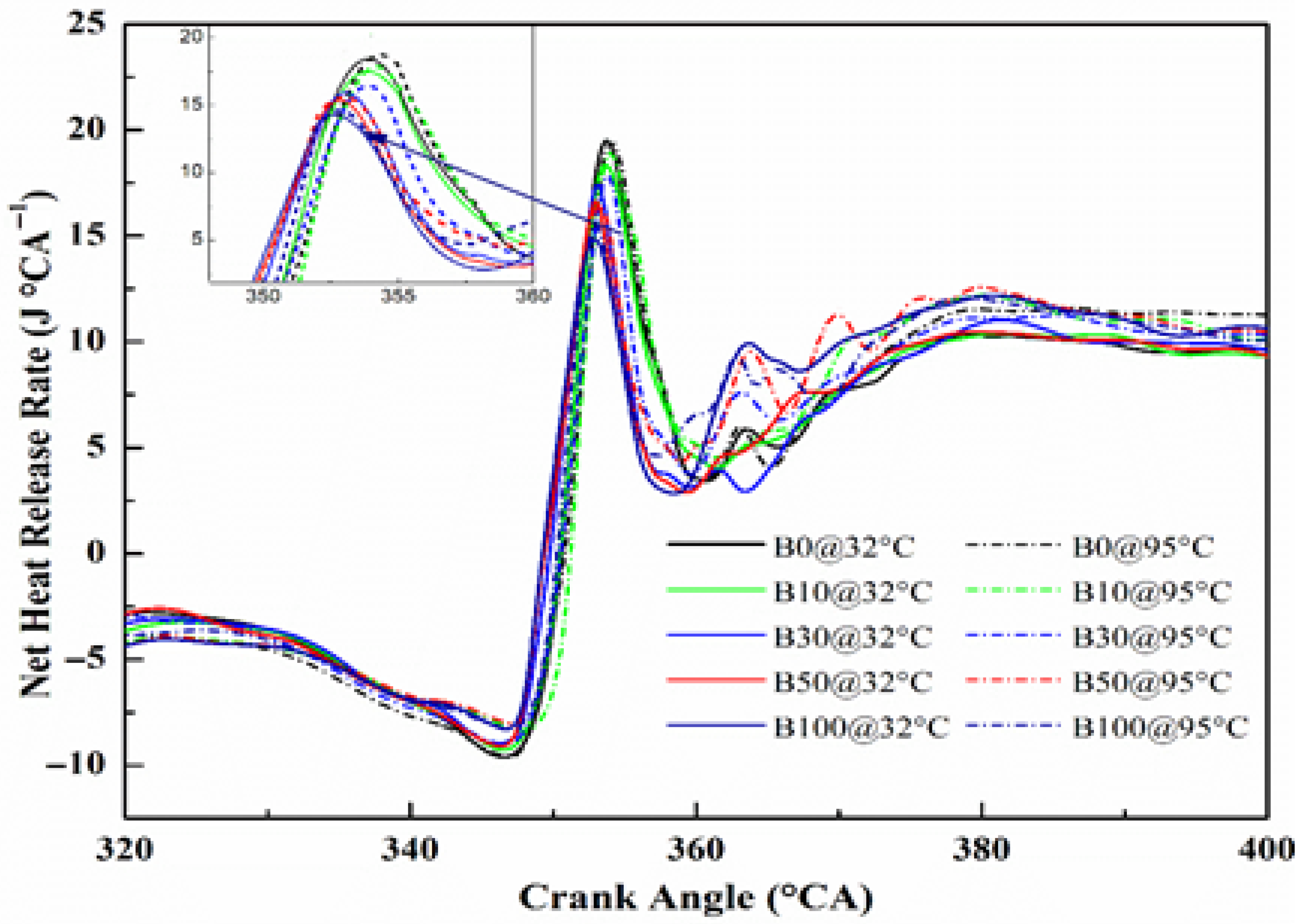
3.2.2. Net Heat Release Rate (NHRR)
The Net Heat Release Rate (NHRR) is a fundamental indicator of the combustion process, revealing how efficiently the chemical energy of the fuel is released as heat inside the combustion chamber.
Figure 12 compares the NHRR of diesel, unheated CHOME biodiesel, and preheated CHOME biodiesel blends at 100% engine load with respect to crank angle. A negative NHRR is observed during the initial phase of injection due to the ignition delay period, where energy is absorbed for fuel atomization and vaporization before combustion begins [
51,
52].
Biodiesel blends exhibit lower maximum NHRR compared to diesel fuel. Specifically, the peak NHRR values for diesel, B10, B30, B50, and B100 are 20.14, 18.62, 16.87, 15.00, and 14.66 J °CA−1, respectively. This reduction is again linked to the higher viscosity and lower calorific value of biodiesel, which limits the premixed combustion phase and reduces heat liberation. Despite this limitation, the novelty of this work is in demonstrating that preheating the biodiesel blends markedly improves their NHRR performance. For example, the NHRR of neat diesel increases slightly from 20.35 to 20.56 J °CA−1, while neat CHOME biodiesel shows a more substantial increase from 14.66 to 16.21 J °CA−1 after preheating.
This improvement can be attributed to enhanced spray atomization, reduced ignition delay, and better premixed combustion facilitated by the lowered viscosity at elevated temperatures. Such findings indicate that preheating narrows the combustion performance gap between biodiesel and diesel, reinforcing its role as an effective strategy for overcoming biodiesel’s inherent drawbacks. Comparable improvements have been observed by Viswanathan et al. [
31] when using preheated fish oil ethyl ester, but the present study goes further by systematically analyzing the progressive effect across different blend ratios and quantifying the improvements, thereby contributing new insights into optimizing biodiesel utilization in compression ignition engines.
3.3. Engine Emission Analysis
In modern diesel engine research, emission behavior is as critical as performance and combustion characteristics, especially with alternative fuels such as biodiesel. The present study evaluates the emission profile of Coffee Husk Oil Methyl Ester (CHOME) biodiesel and its blends with regular diesel under both preheated and unheated conditions. The experimental investigation spans varying engine loads (0–100%) and biodiesel blending ratios (B10, B30, B50, B100), providing a comprehensive assessment of how preheating influences the interaction between fuel properties, combustion dynamics, and exhaust emissions. This approach is crucial because biodiesel’s higher viscosity and oxygen content significantly influence both performance and emission characteristics.
3.3.1. Oxides of Nitrogen (NOx)
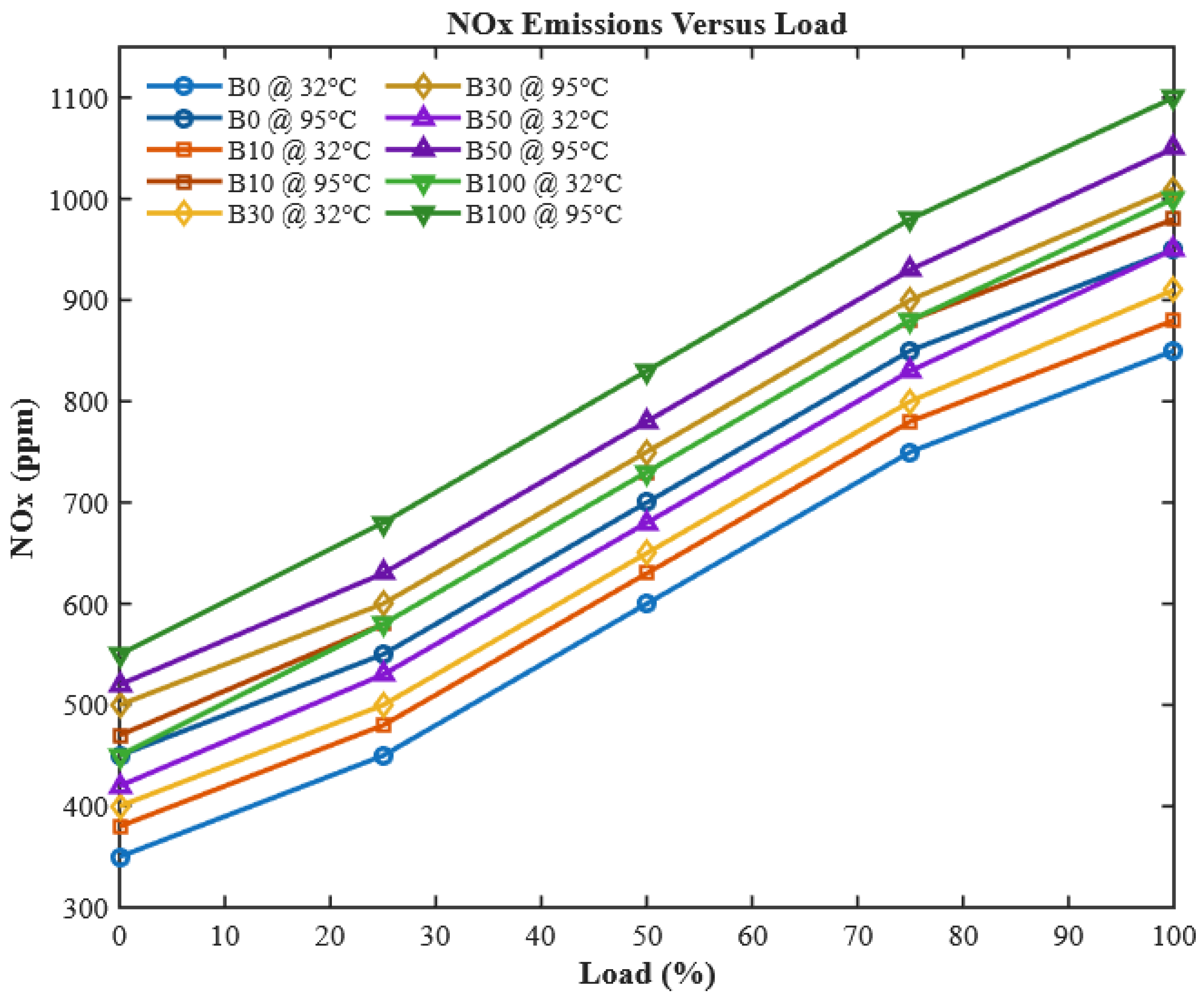
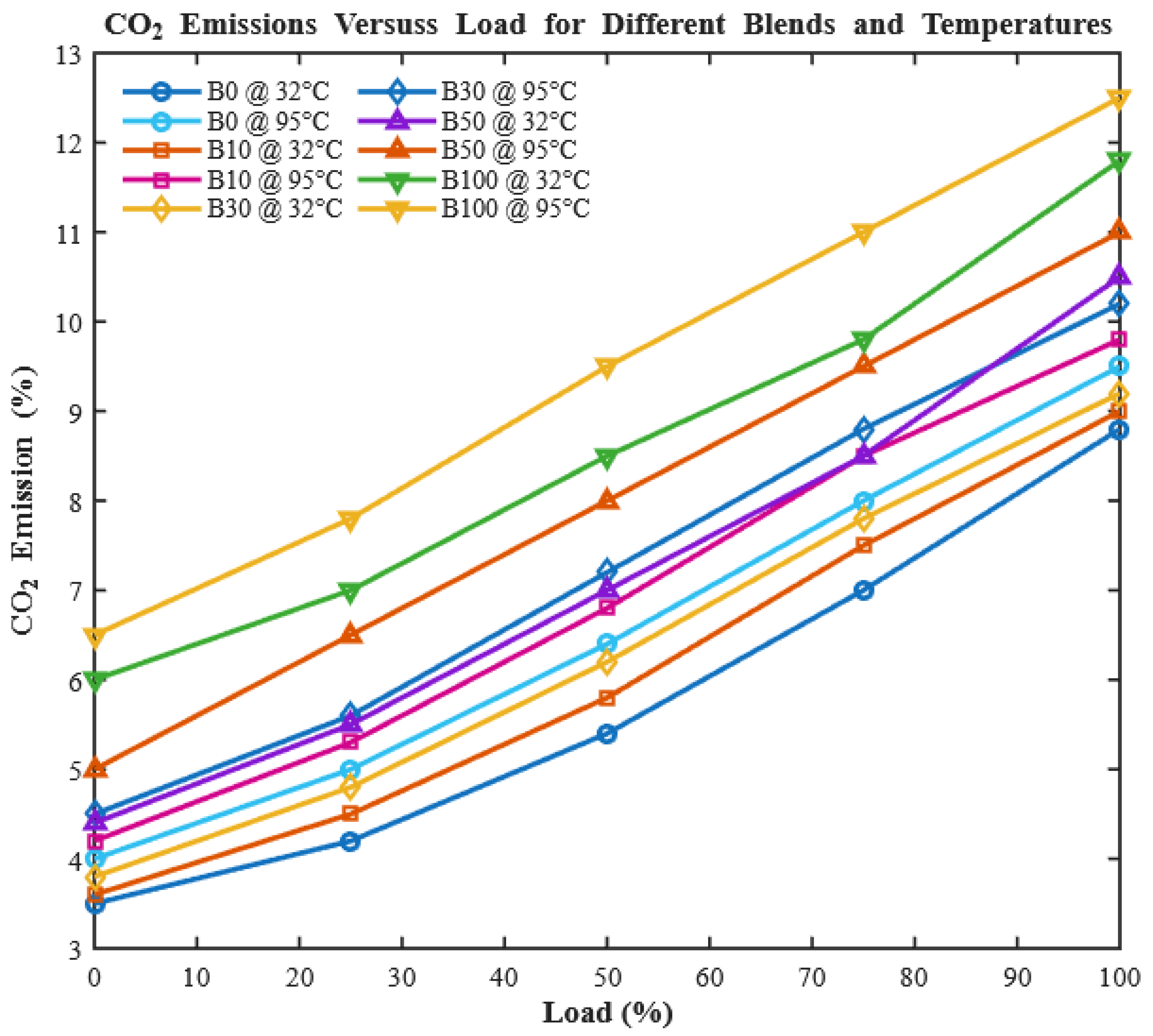
Nitrogen oxides (NOx) are key pollutants from diesel engines, formed at high combustion temperatures and contributing to smog, acid rain, and health hazards. As shown in
Figure 13, NOx emissions increase with engine load for all fuels due to elevated in-cylinder temperatures that accelerate the reaction between atmospheric nitrogen and oxygen. For CHOME biodiesel, the oxygenated molecular structure promotes more complete combustion, enhancing peak flame temperatures and providing additional reactive oxygen species that accelerate thermal NO formation. Preheating further reduces fuel viscosity, improves atomization, and increases flame temperature, intensifying NOx generation.
For B100, these combined chemical and physical effects result in a 2.86% increase in NOx compared to diesel. While preheating and oxygen content improve combustion efficiency, reducing unburned hydrocarbons and particulate emissions, they also exacerbate thermal NOx formation. This trade-off highlights the need for mitigation strategies, such as exhaust gas recirculation (EGR), selective catalytic reduction (SCR), or optimized injection timing, to balance improved combustion with NOx control in biodiesel-fueled engines.
Interestingly, the oxygen content of CHOME biodiesel contributes to higher NOx emissions compared to conventional diesel, with neat B100 producing 34.3% more NOx at full load. This demonstrates a fundamental trade-off: oxygenated biodiesel improves combustion efficiency but increases NOx formation. Preheating the fuel further enhances combustion, slightly increasing NOx emissions, with increments of 1.69%, 2.67%, 2.86%, 2.38%, and 2.34% for B0, B10, B30, B50, and B100, respectively. While this could appear detrimental, it actually confirms the efficacy of preheating in improving fuel atomization and combustion completeness, a key contribution of this work. These observations align with [
28], but contrast with [
49], highlighting the sensitivity of NOx emissions to fuel conditioning methods such as preheating.
3.3.2. Unburned Hydrocarbons (HC)
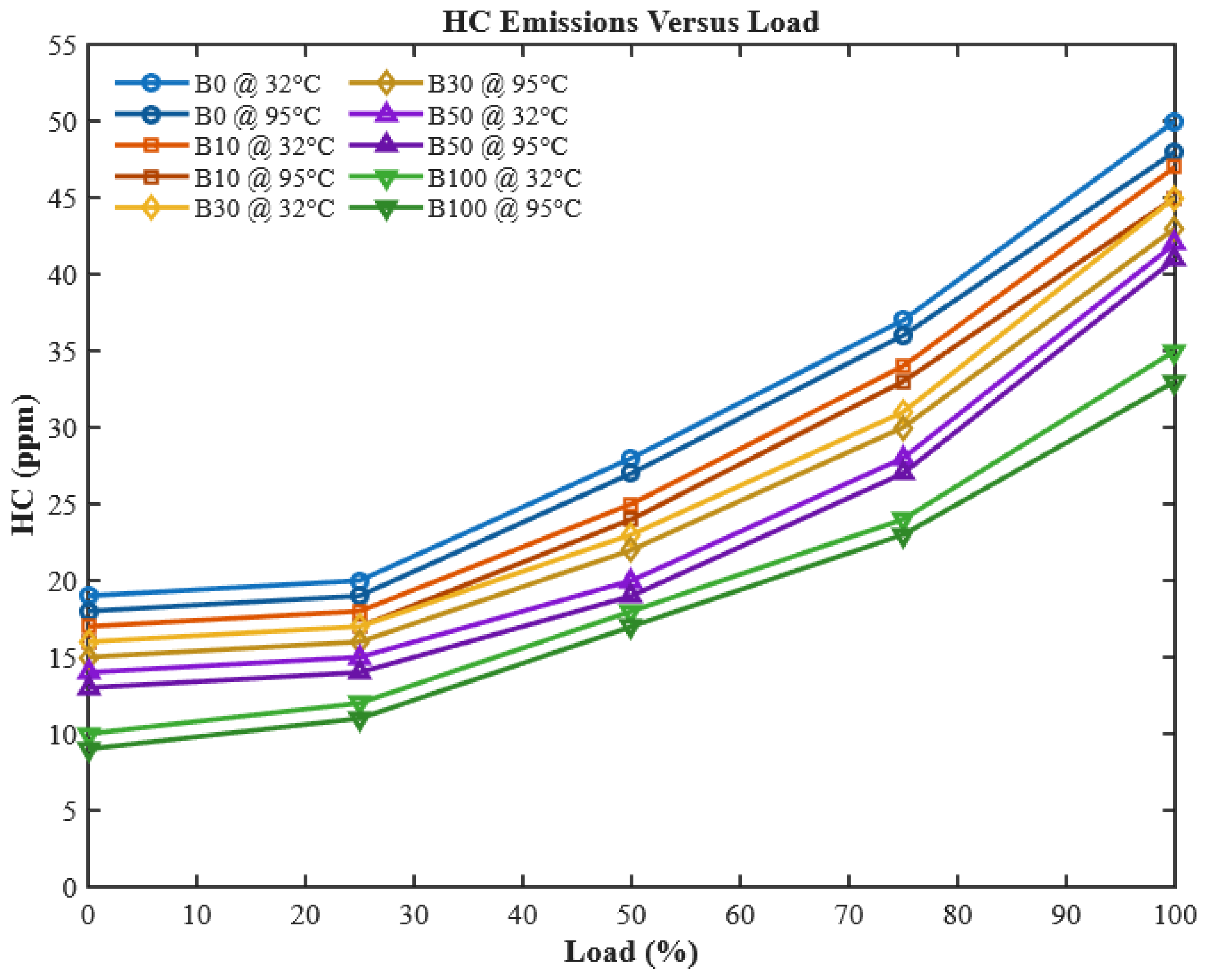
Unburned hydrocarbon (HC) emissions are direct indicators of incomplete combustion, often caused by insufficient atomization, poor mixing, or low combustion chamber temperatures. As illustrated in
Figure 14, HC emissions generally rise with increasing engine load because higher loads reduce the effective oxygen-to-fuel ratio in localized regions of the cylinder, leading to incomplete oxidation.
However, an increase in CHOME blending ratio markedly reduces HC emissions. This reduction is attributed to the intrinsic oxygen content of biodiesel, which promotes a more complete combustion reaction even in fuel-rich zones [
48]. The effect is further amplified when fuels are preheated. Preheating reduces viscosity, enhancing atomization and vaporization, leading to a more homogeneous fuel–air mixture. Consequently, HC emissions decreased by 4%, 4.08%, 5.2%, 6.5%, and 8.33% for B0, B10, B30, B50, and B100, respectively. This significant reduction highlights preheating as a practical, low-cost strategy to mitigate one of the key emission challenges of biodiesel fuels, supporting findings by [
28,
52].
3.3.3. Carbon Monoxide (CO)
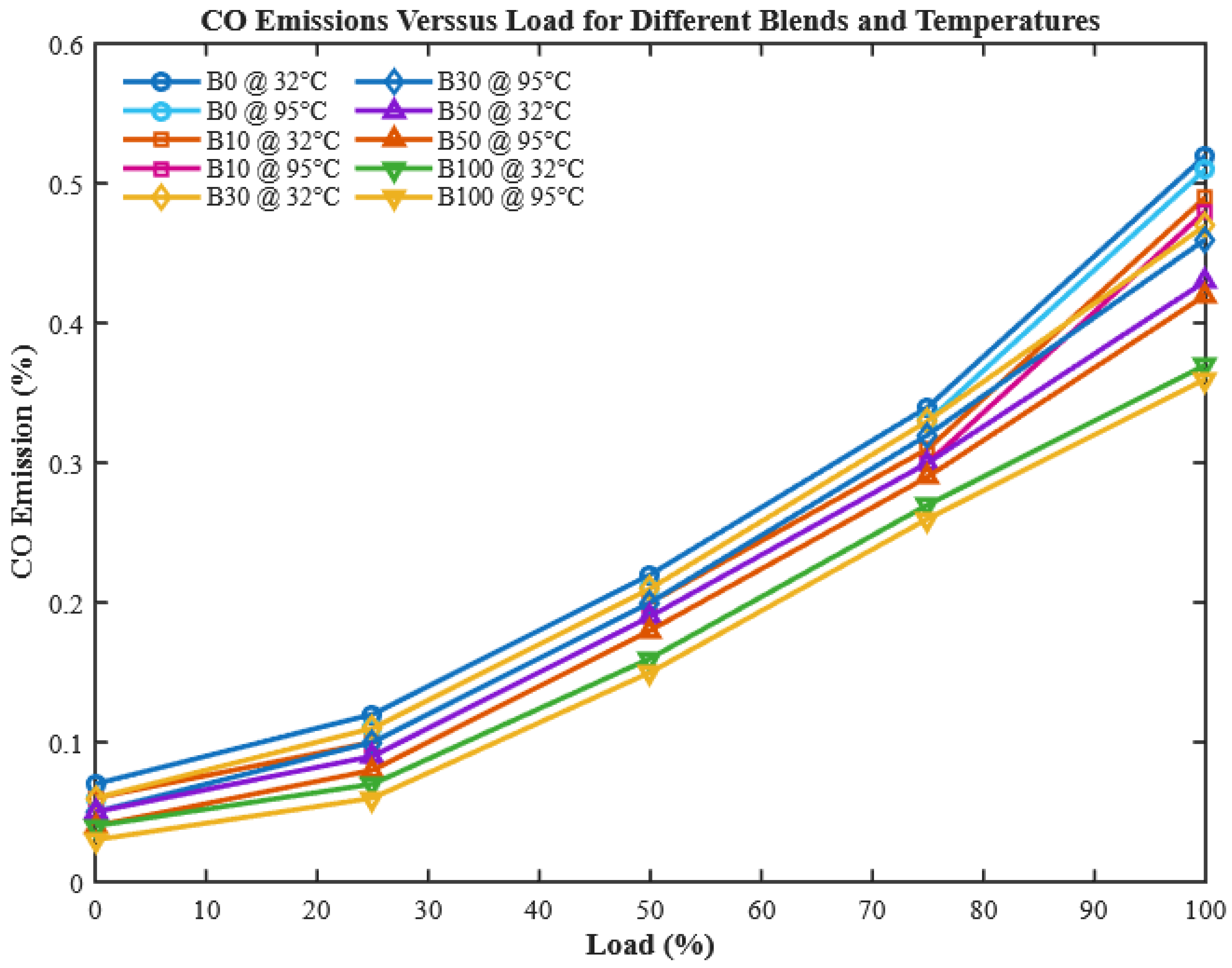
CO is generated due to incomplete oxidation of carbon in regions of local oxygen deficiency or poor combustion. As shown in
Figure 15, CO emissions increase with engine load for all tested fuels due to higher fuel injection and a temporarily rich mixture in some combustion zones. Notably, increasing CHOME content in the blend reduces CO formation, which can be explained by the higher oxygen content of biodiesel, facilitating CO to CO
2 conversion [
49,
53].
Preheating the fuels further reduces CO emissions by 3.8%, 4.6%, 4.84%, 5.14%, and 6.4% for B0, B10, B30, B50, and B100, respectively. This improvement is due to the enhanced spray atomization and rapid vaporization of preheated biodiesel, resulting in more complete combustion. This finding underscores the novelty of this work: preheating enables biodiesel blends to achieve combustion efficiency comparable to diesel, without chemical additives. Similar trends were reported by [
10,
11,
18], and other studies on preheated alternative fuels, confirming the broader applicability of this approach.
3.3.4. Carbon Dioxide (CO2)
CO
2 emissions serve as a proxy for combustion completeness and are correlated with fuel energy conversion efficiency.
Figure 16 illustrates that CO
2 emissions increase with engine load for all tested fuels. Biodiesel blends generally exhibit slightly higher CO
2 emissions than diesel due to their higher oxygen content, which promotes more complete oxidation [
50].
Preheating further increases CO2 emissions, indicating enhanced fuel utilization. For instance, unheated B100 produces a CO2 concentration of 12.02%, while preheating raises it to 12.74% at full load. Across blends, preheating enhances CO2 by 1.6%, 1.67%, 2.48%, 2.64%, and 3.37% for B0, B10, B30, B50, and B100, respectively. This clearly demonstrates that preheating improves energy extraction from fuel, a key advantage in optimizing biodiesel performance without changing the chemical composition.
3.3.5. Smoke Opacity
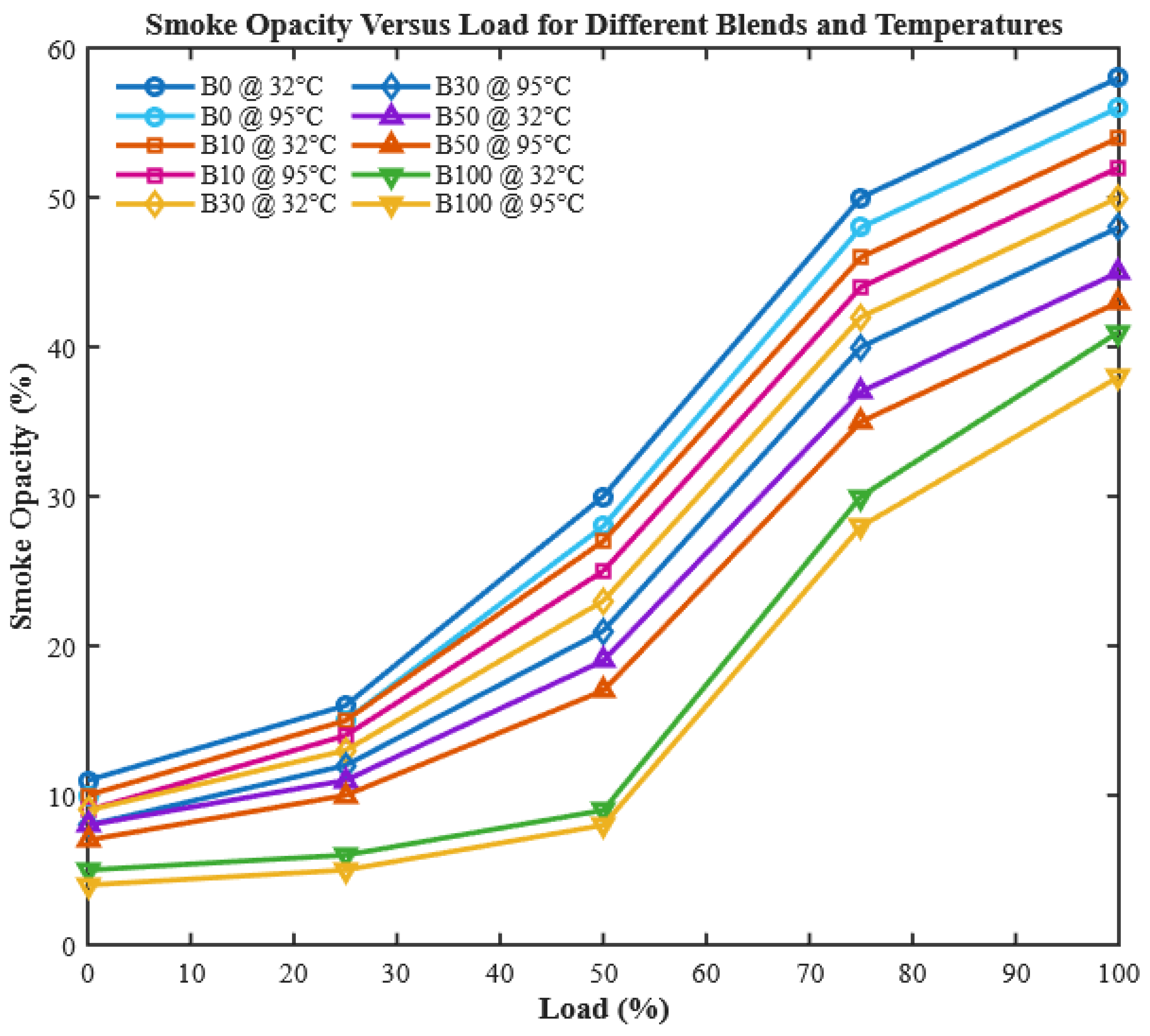
Smoke opacity results from incomplete combustion, local oxygen deficiency, and poor fuel atomization.
Figure 17 shows that smoke opacity rises with increasing engine load, particularly for unheated fuels, due to richer mixtures in the cylinder at high loads [
54].
CHOME biodiesel blends exhibit lower smoke emissions than diesel due to their oxygen content, promoting cleaner combustion. Preheating further reduces smoke opacity; for example, opacity drops from 57% to 55% for B0 and 41% to 37% for B100. These results highlight a novel dual benefit of preheating: improving atomization and combustion efficiency while simultaneously reducing particulate emissions [
55]. Such reductions are critical for meeting stringent emission regulations in diesel engines.
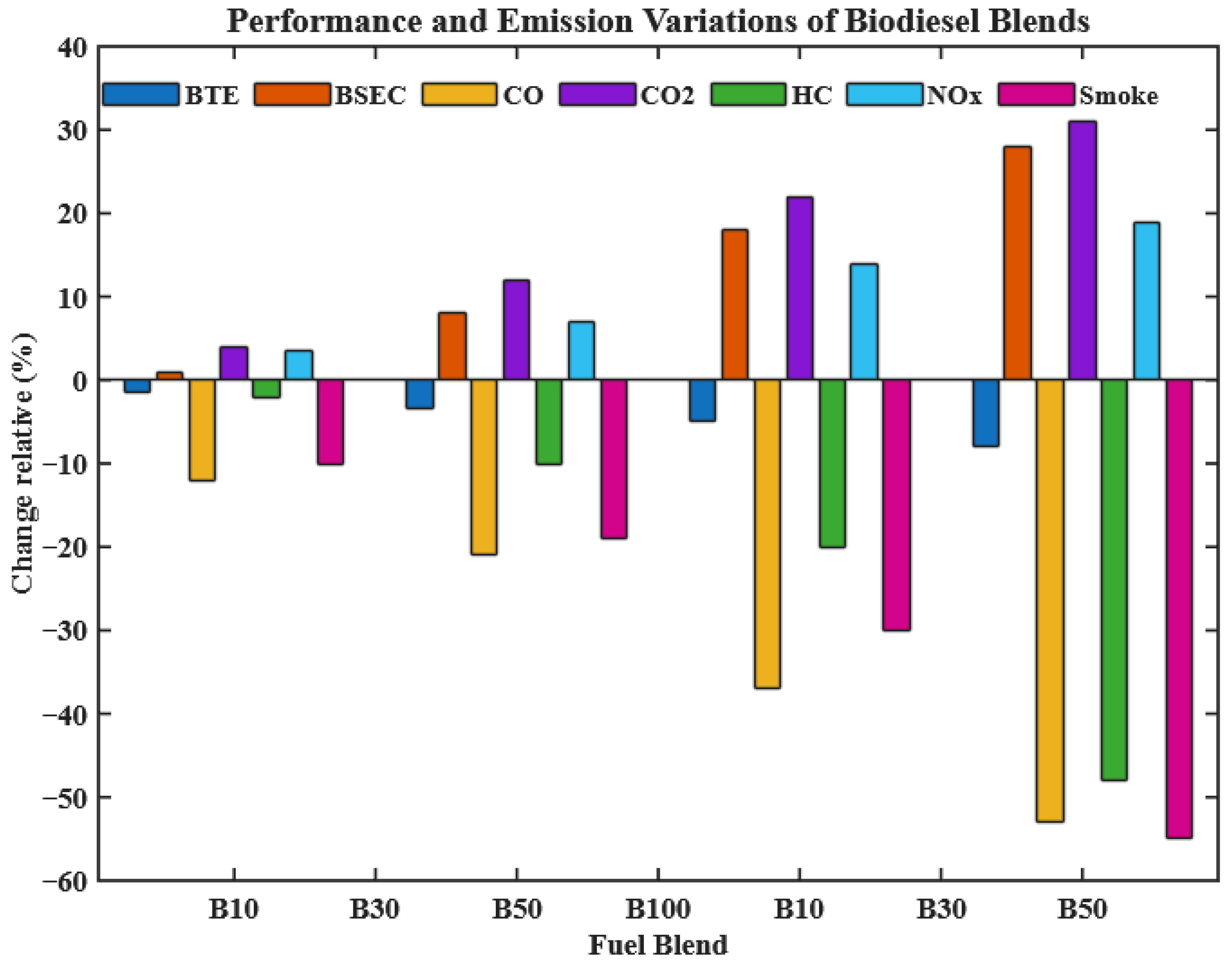
3.3.6. Rate of Change in Performance and Emission Parameters
To quantify the effect of preheating, the percentage change in performance and emission parameters was calculated using unheated diesel as a baseline:
Percentage change for performance/emission parameters was calculated as
where
X is the respective parameter (BTE, BSFC, NOx, CO, HC, etc.). For example, preheated B100 reduced HC emissions by 8.33% compared to unheated B100.
As illustrated in
Figure 18, preheated CHOME blends show significant improvements in engine performance, with B10 achieving higher BTE than unheated diesel, while B30 approaches the performance of unheated B0. The BSEC also shows proportional improvement due to reduced viscosity and enhanced fuel–air mixing.
Emissions are substantially improved: CO decreases by 51.43%, HC by 51.52%, and smoke opacity by 54.05%, while CO2 rises by 31.27%, reflecting more complete and efficient combustion. These results strongly demonstrate the novelty of preheating CHOME biodiesel as a practical, low-cost strategy to enhance combustion and reduce emissions, bridging the performance gap between biodiesel and conventional diesel.
3.4. Result Outcomes
The experimental investigation of preheated CHOME biodiesel and its blends demonstrates significant improvements in both engine performance and combustion characteristics compared to unheated fuels. Preheating to 95 °C effectively reduces kinematic viscosity by nearly 50%, which enhances fuel atomization, spray penetration, and vaporization inside the combustion chamber. As a result, in-cylinder pressure and net heat release rates (NHRR) increase across all blends, with peak cylinder pressure improvements of up to ~3.95% for B100. These observations confirm that preheating mitigates one of the primary limitations of biodiesel, its high viscosity, without altering its chemical composition, providing a practical method to optimize combustion dynamics in real-time under variable engine loads.
In terms of engine performance, the study highlights that preheating significantly enhances brake thermal efficiency (BTE) and reduces both brake specific fuel consumption (BSFC) and brake specific energy consumption (BSEC) for all blends. The preheated B10 blend even exceeds the performance of unheated regular diesel, while B100 demonstrates a notable 5% BTE improvement over its unheated counterpart. These outcomes underscore the adaptive advantage of preheating, allowing biodiesel blends to approach or surpass conventional diesel performance without additional chemical modification. The results establish that fuel preheating can be a low-cost, field-applicable strategy for improving energy conversion efficiency in engines running on locally produced biodiesel feedstocks.
The emission analysis reveals that preheated CHOME biodiesel blends markedly reduce harmful pollutants while slightly increasing CO2 due to more complete combustion. Specifically, CO, HC, and smoke opacity are reduced by over 50% in some blends, while NOx shows only marginal increases attributable to enhanced combustion temperatures and oxygen content. These findings demonstrate that preheating not only enhances fuel utilization and engine efficiency but also contributes to cleaner exhaust emissions, balancing performance and environmental objectives. Collectively, the results of this study establish preheating as a novel, practical approach for optimizing biodiesel utilization, offering significant implications for sustainable diesel engine operation and emission mitigation strategies.
From an economic perspective, preheating requires additional energy input, which may affect the practicality of large-scale implementation. In stationary power generation or hybrid systems, waste heat recovery from the exhaust could be used to maintain fuel preheating with minimal additional cost. For mobile applications, such as vehicles, an auxiliary heating unit would be required, which may marginally increase travel costs. However, given the significant performance and emission benefits observed, further techno-economic studies are needed to evaluate cost–benefit trade-offs and the feasibility of integrating preheating systems in real-world transportation fleets.
3.5. Uncertainty Analysis
Experimental measurements are always subject to uncertainties arising from instrument precision, calibration errors, environmental conditions, and human factors. In this study, the uncertainty analysis was performed by considering both systematic and random errors associated with each measuring instrument. The overall uncertainty for a derived parameter was calculated using the root-sum-square (RSS) method:
where UR is the resultant uncertainty of the calculated parameter, and Ui represents the uncertainty of each measured variable contributing to that parameter.
The accuracy specifications of the major instruments were as follows: the fuel flow meter (±0.5%), the dynamometer for brake power measurement (±0.2%), the AVL smoke analyzer (±1%), and the INDUS gas analyzer (±0.06% for CO, ±12 ppm for HC, ±0.5% for CO2, and ±1 ppm for NOₓ). In-cylinder pressure data were obtained using a piezoelectric pressure transducer with an accuracy of ±0.3%, while crank angle measurements using an encoder had an uncertainty of ±0.1°.
Based on these values, the uncertainties of the calculated performance parameters were determined. The brake thermal efficiency exhibited an overall uncertainty of ±2.3%, brake-specific fuel consumption ±1.9%, and brake-specific energy consumption ±2.5%. Combustion parameters, including peak cylinder pressure and heat release rate, showed uncertainties of ±1.8% and ±2.1%, respectively. For emission parameters, the maximum uncertainties were ±3.2% for CO, ±2.8% for HC, ±2.6% for NOₓ, ±2.4% for CO2, and ±2.7% for smoke opacity.
These values indicate that the uncertainties remained within acceptable limits for experimental engine research. Therefore, the observed improvements in efficiency (up to 5%), reductions in brake-specific fuel consumption (up to 7.75%), and emission reductions (>50% in CO, HC, and smoke opacity) significantly exceed the calculated uncertainty margins. This confirms that the trends and conclusions reported in this work are both reliable and statistically meaningful.
4. Conclusions
The present study confirmed that coffee husk oil methyl ester (CHOME) can be effectively produced through a two-step transesterification process despite the high free fatty acid (FFA) content of the raw oil. The successful conversion of this abundant agro-industrial residue into biodiesel demonstrates the technical feasibility of valorizing a waste stream that is otherwise underutilized or disposed of in environmentally harmful ways, positioning coffee husk as a promising feedstock for renewable energy production, particularly in coffee-producing regions with large biomass availability.
Engine performance evaluation revealed that unheated CHOME biodiesel exhibited lower brake thermal efficiency (BTE) and higher fuel consumption than conventional diesel, primarily due to its higher viscosity and lower calorific value. To address these drawbacks, controlled fuel preheating was introduced as a practical intervention. Preheating to 95 °C reduced viscosity by nearly 50%, which enhanced atomization, improved in-cylinder combustion, and increased BTE by up to 5% for neat biodiesel compared to its unheated form. In certain cases, such as the B10 blend, preheated biodiesel even surpassed the efficiency of neat diesel, highlighting preheating as a low-cost and field-adaptable solution to biodiesel performance limitations.
Emission analysis further demonstrated the environmental benefits of fuel preheating. Carbon monoxide (CO), unburned hydrocarbons (HC), and smoke opacity were reduced by over 50% across blends, indicating cleaner combustion and more complete fuel oxidation. Although nitrogen oxide (NOx) emissions increased slightly (up to 2.86%), these increments were relatively small and could be mitigated through exhaust gas recirculation (EGR), water or steam injection, selective catalytic reduction (SCR), or fuel additives such as cetane improvers and metal-based catalysts. The FTIR analysis highlighted functional groups in CHOME (C=O, C–O, CH2 bending) absent in diesel, which facilitate more complete combustion and lower CO/HC emissions, while contributing to the modest increase in NOx under elevated temperatures. This functional distinction is central to understanding CHOME’s combustion behavior.
Beyond experimental outcomes, the study underscores the broader significance of converting coffee husk waste into a viable fuel, providing both environmental and economic benefits. This waste-to-energy pathway aligns with circular economy principles by turning an abundant residue into a valuable resource, supporting energy diversification, reducing reliance on fossil fuels, and contributing to Sustainable Development Goals (SDG 7: Affordable and Clean Energy, and SDG 13: Climate Action). The simplicity and scalability of fuel preheating ensure practical adoption in regions with limited access to advanced fuel processing infrastructure.
Future research should investigate the long-term durability of engines operating with preheated CHOME, evaluating potential impacts on injector wear, lubrication, and material compatibility. Additionally, integrating preheating with emission control strategies could mitigate NOx formation while maintaining efficiency gains. Techno-economic and life-cycle assessments are also recommended to assess the feasibility of large-scale CHOME biodiesel deployment, particularly in coffee-producing nations where this technology could simultaneously support rural development, energy security, and environmental sustainability.