Biological Purification of Heterogenous Car Wash Effluents: Selection of Tolerant Bacteria and Development of Microbial Consortia for Pollutant Biodegradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Car Wash Wastewater
2.2. Bacterial Screening
2.3. Bacterial Identification
2.4. Bacterial Frequency Analyses
2.5. Car Wash Wastewater Utilization with Bacterial Monocultures
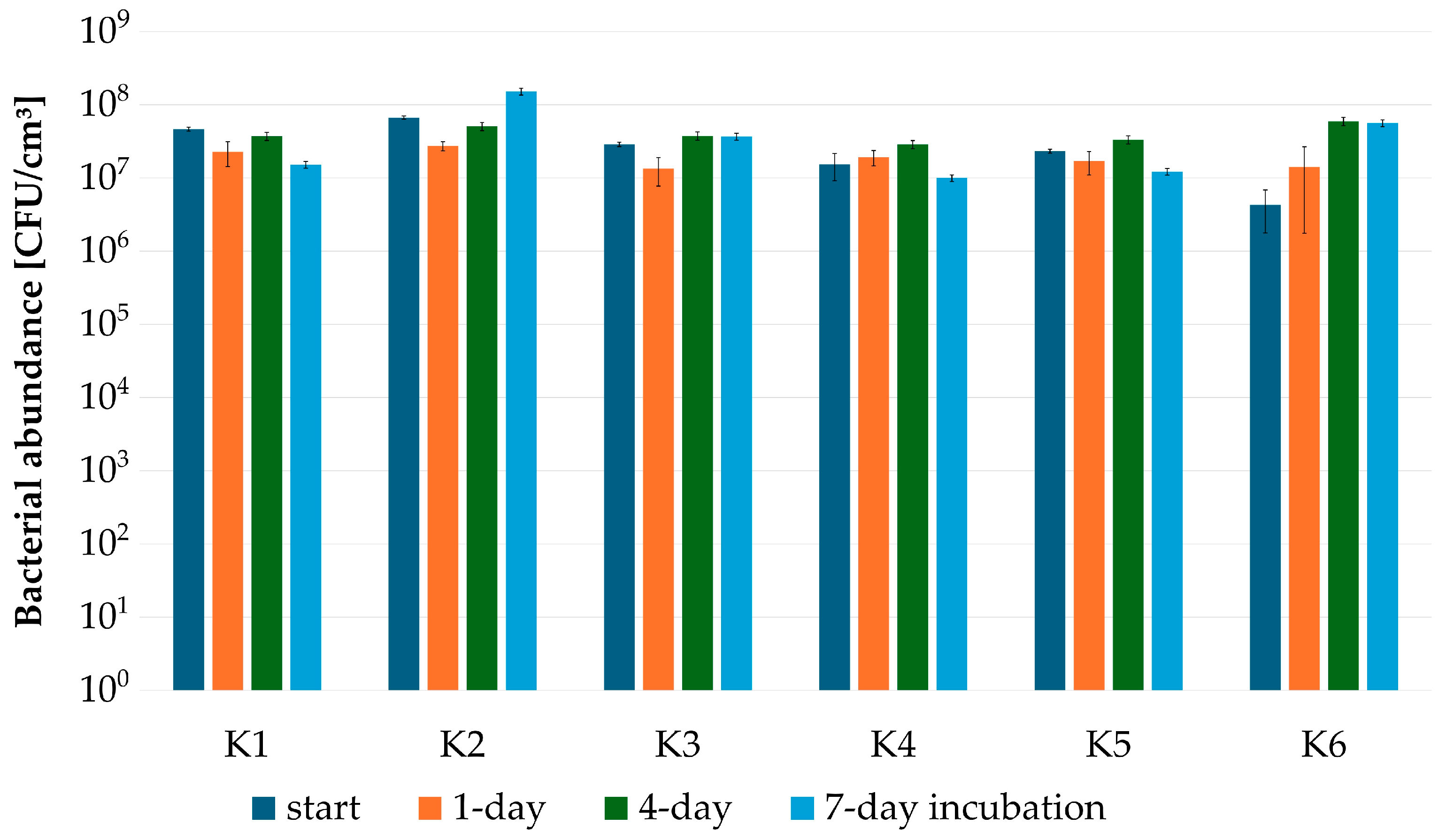
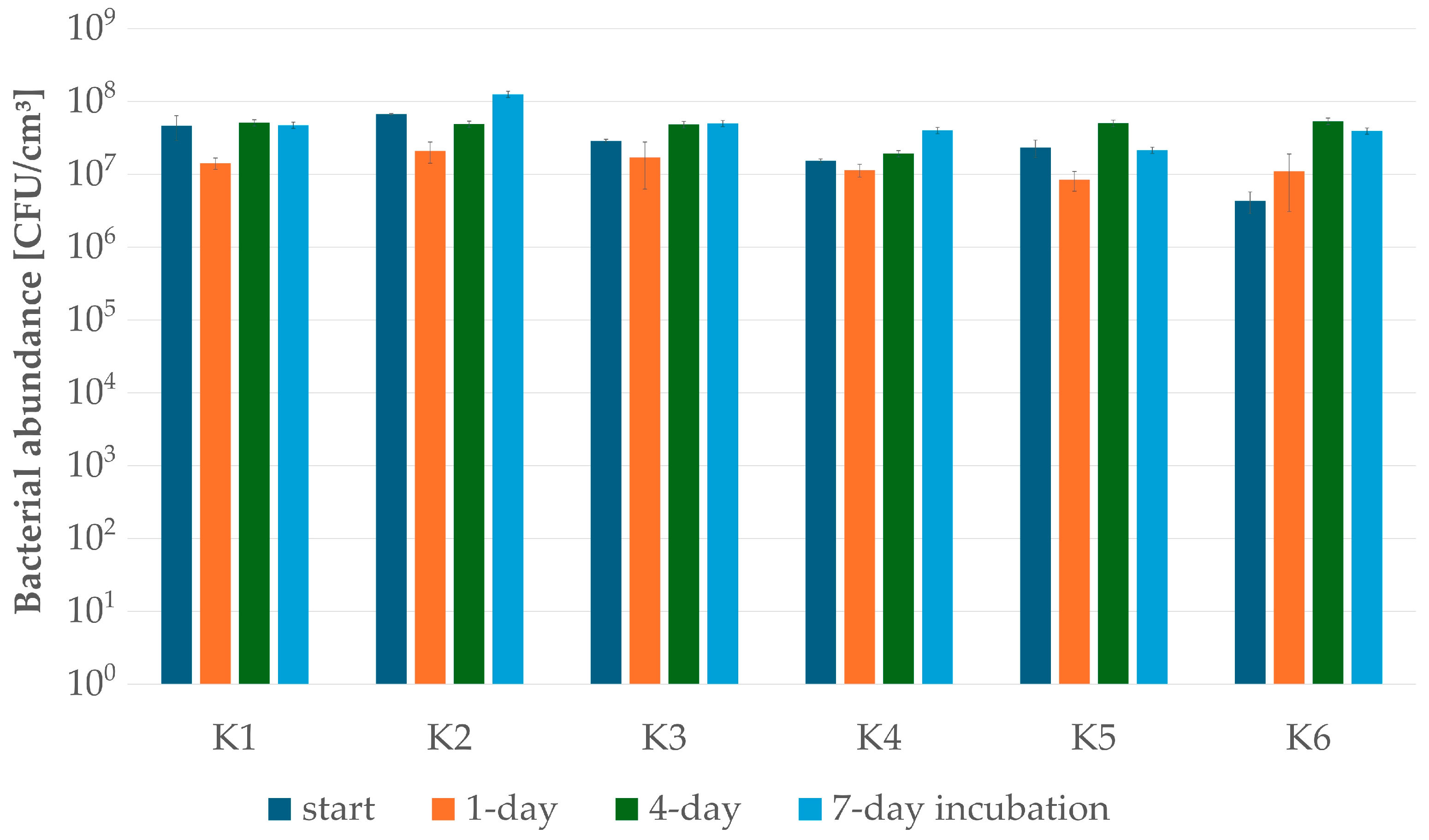
2.6. Construction of Bacterial Consortia for CWW Treatment and Biodegradation Tests
3. Results
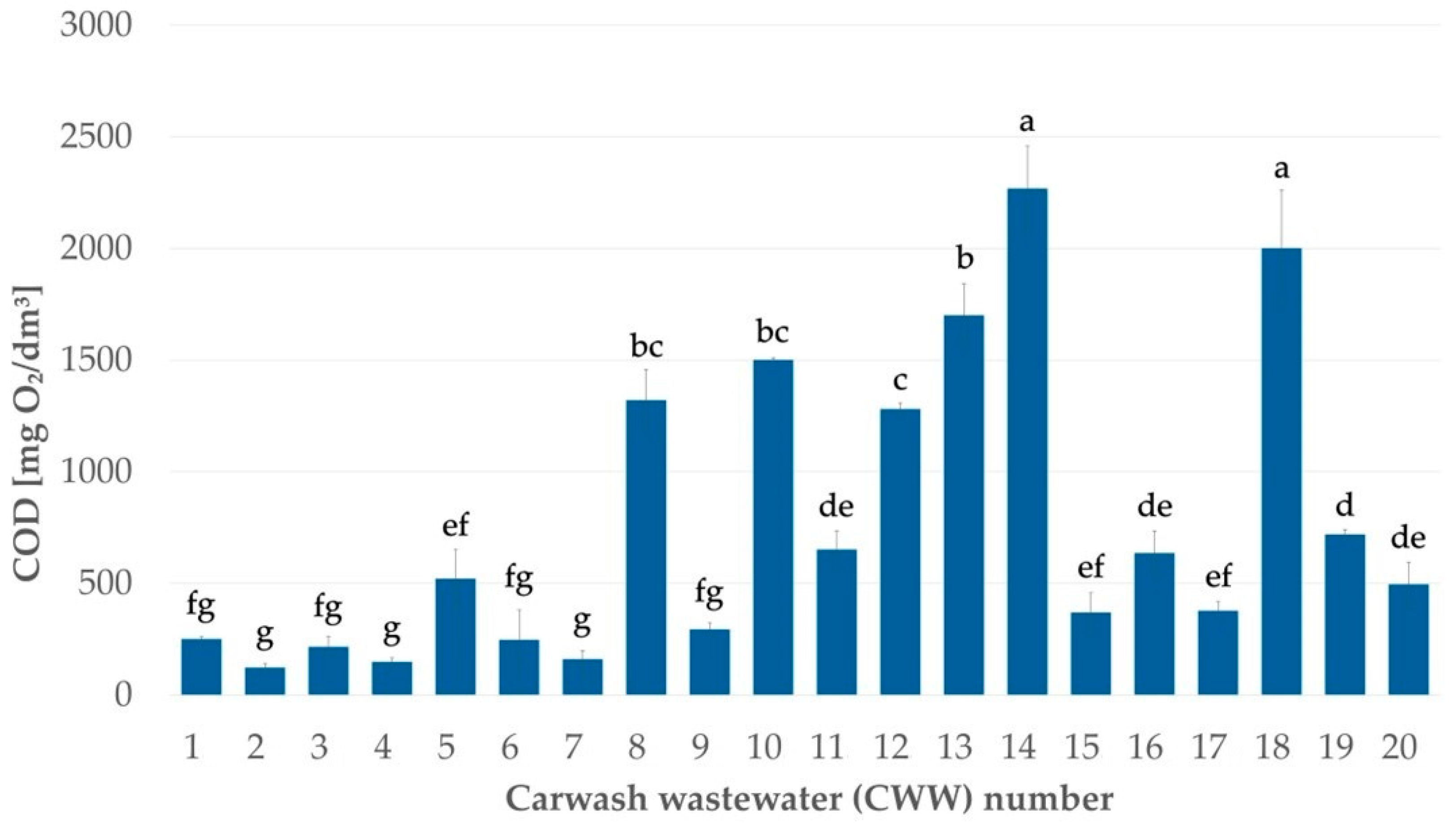
3.1. Heterogeneity of the Organic Load
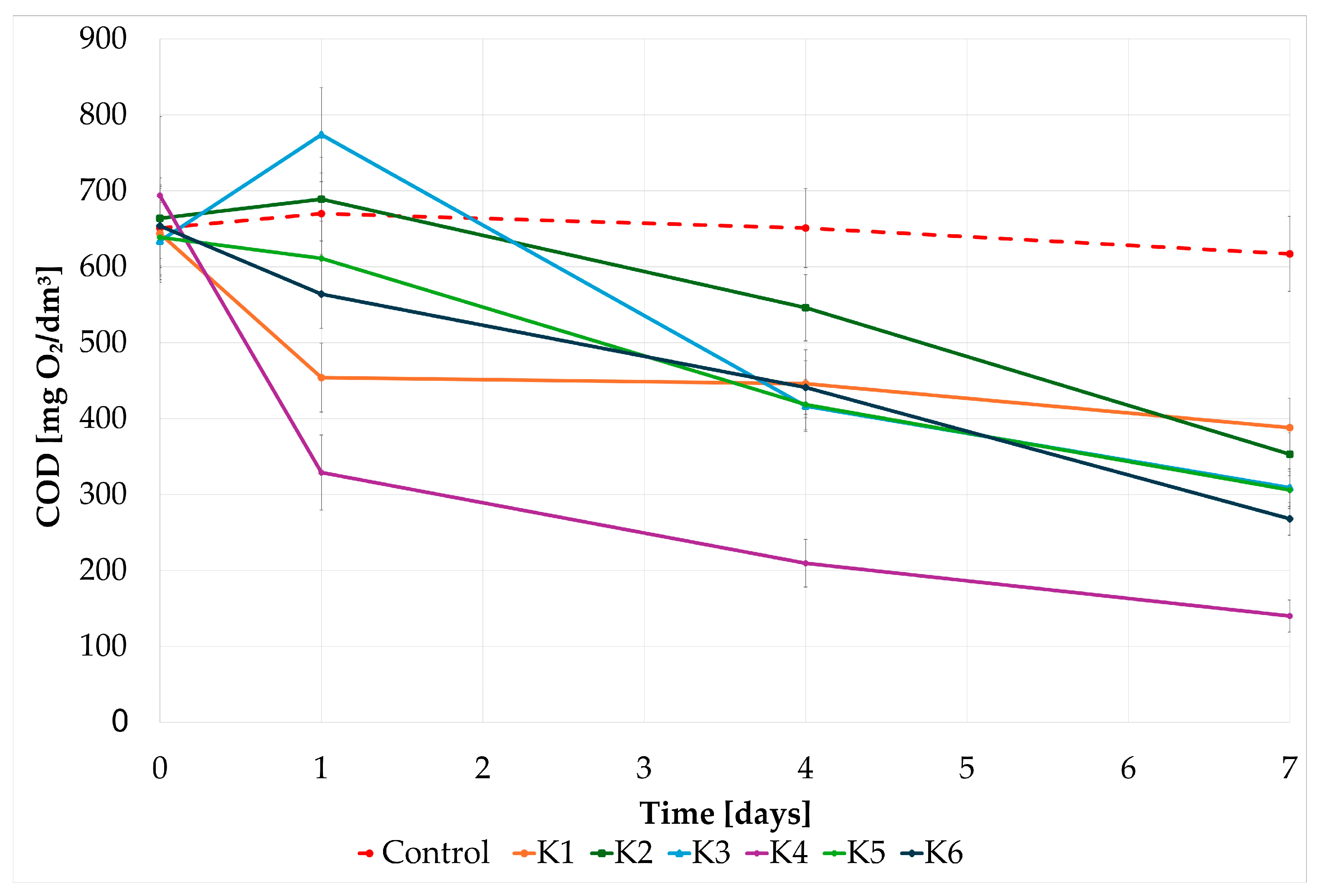
3.2. Testing CWW Self-Attenuation with Indigenous Microbiota
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEA | European Automobile Manufacturers’ Association |
| BH | Bushnell Haas medium |
| BOD | Biological oxygen demand |
| CFU | Colony-forming unit |
| COD | Chemical oxygen demand |
| CWW | Car wash wastewater |
| DOM | Dissolved organic matter |
| HRT | Hydraulic retention time |
| MBBR | Moving bed biofilm reactor |
| MBR | Membrane bioreactor |
| NTU | Nephelometric turbidity units |
| SEC | Specific electrical conductivity |
| SNB | Standard nutrient broth medium |
| TSS | Total suspended solids |
| WWTP | Wastewater treatment plant |
References
- ACEA European Automobile Manufacturers’ Association. The Automobile Industry. Pocket Guide 2024/2025. 2025. Available online: https://www.acea.auto/files/ACEA-Pocket-Guide-2024-2025.pdf (accessed on 5 March 2025).
- Berger, R. Automotive Outlook 2040. 2024. Available online: https://www.rolandberger.com/en/Media/Roland-Berger-Automotive-Outlook-2040.html (accessed on 17 July 2025).
- Smith, M.N. The Number of Cars Worldwide Is Set to Double by 2040. 2016. Available online: https://www.weforum.org/stories/2016/04/the-number-of-cars-worldwide-is-set-to-double-by-2040/ (accessed on 17 July 2025).
- Monney, I.; Donkor, E.A.; Buamah, R. Clean Vehicles, Polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 2020, 6, e03952. [Google Scholar] [CrossRef] [PubMed]
- Kazembeigi, F.; Bayad, S.; Nasab, A.Y.; Doraghi, M.; Parseh, I. Techno-environmental study on the consequences of carwash wastewater and its management methods. Heliyon 2023, 9, e19764. [Google Scholar] [CrossRef] [PubMed]
- Torkashvand, J.; Pasalari, H.; Gholami, M.; Younesi, S.; Oskoei, V.; Farzadkia, M. On-site carwash wastewater treatment and reuse: A systematic review. Int. J. Environ. Anal. Chem. 2020, 102, 3613–3627. [Google Scholar] [CrossRef]
- Phipps, D.; AlKhaddar, R.; Stiller, M. Water saving in domestic car washing. In Proceedings of the World Environmental and Water Resources Congress 2013: Showcasing the Future, Cincinnati, OH, USA, 19–23 May 2013. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Biotechnol. 2017, 16, 591–609. [Google Scholar] [CrossRef]
- Zhang, D.; Sial, M.S.; Ahmad, N.; Filipe, A.J.; Thu, P.A.; Zia-Ud-Din, M.; Caleiro, A.B. Water scarcity and sustainability in an emerging economy: A management perspective for future. Sustainability 2020, 13, 144. [Google Scholar] [CrossRef]
- Gleick, P.H.; Cooley, H. Freshwater scarcity. Annu. Rev. Environ. Resour. 2021, 46, 319–348. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Alzghoul, T.M.; Amr, S.A.; Ramu, M.B.; Nassani, D.E. Bibliometric insights into car wash wastewater treatment research: Trends and perspectives. Water 2024, 16, 2034. [Google Scholar] [CrossRef]
- Sarmadi, M.; Foroughi, M.; Saleh, H.N.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient technologies for carwash wastewater treatment: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 34823–34839. [Google Scholar] [CrossRef]
- Agyen, K.; Monney, I.; Antwi-Agyei, P. Contemporary carwash wastewater recycling technologies: A systematic literature review. World Environ. 2021, 11, 83–98. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Mahmoud, N.S.; Ibrahim, M.M.; Mostafa, E.A.; Abdelrahman, E.N.; Emam, R.H.; Kassem, M.A.; Hatem, M.H. Carwash wastewater treatment and reclamation technologies for water conservation and pollution load reduction: A review. Int. J. Biopro. Biotechnol. Adv. 2021, 7, 274–310. [Google Scholar]
- Kuan, W.; Hu, C.; Ke, L.; Wu, J. A Review of on-site carwash wastewater treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Espinoza-Montero, P.J.; Martínez-Huitle, C.A.; Loor-Urgilés, L.D. Technologies employed for carwash wastewater recovery. J. Clean. Prod. 2023, 11, 136722. [Google Scholar] [CrossRef]
- Subtil, E.L.; Rodrigues, R.; Hespanhol, I.; Mierzwa, J.C. Water reuse potential at heavy-duty vehicles washing facilities—The mass balance approach for conservative contaminants. J. Clean. Prod. 2017, 166, 1226–1234. [Google Scholar] [CrossRef]
- Kashi, G.; Younesi, S.; Heidary, A.; Akbarishahabi, Z.; Kavianpour, B.; Kalantary, R.R. Carwash wastewater treatment using the chemical processes. Water Sci. Technol. 2021, 84, 16–26. [Google Scholar] [CrossRef]
- Włodyka-Bergier, A.; Mazur, R.; Kowalewski, Z.; Bergier, T. Research on the possibility of using moving bed biofilm reactors for treating car wash wastewater. Desalin. Water Treat. 2023, 301, 150–158. [Google Scholar] [CrossRef]
- Sarmadi, M.; Zarei, A.A.; Ghahrchi, M.; Sepehrnia, B.; Meshkinian, A.; Moein, H.; Nakhaei, S.; Bazrafshan, E. Carwash wastewater characteristics—A systematic review study. Desalin. Water Treat. 2021, 225, 112–148. [Google Scholar] [CrossRef]
- Rai, R.; Sharma, S.; Gurung, D.; Sitaula, B.K.; Shah, R.D.T. Assessing the impacts of vehicle wash wastewater on surface water quality through physico-chemical and benthic macroinvertebrates analyses. Water Sci. 2020, 34, 39–49. [Google Scholar] [CrossRef]
- Efendi, N.M.; Murtius, N.W.S.; La Choviya Hawa, N. Car wash wastewater treatment combination: A systematic review. OA Res. J. Sci. Technol. 2023, 8, 045–057. [Google Scholar] [CrossRef]
- Tomczak, W.; Woźniak, P.; Gryta, M.; Grzechulska-Damszel, J.; Daniluk, M. Cleaning of ultrafiltration membranes: Long-term treatment of car wash wastewater as a case study. Membranes 2024, 14, 159. [Google Scholar] [CrossRef]
- Dadebo, D.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Transition towards sustainable carwash wastewater management: Trends and enabling technologies at global scale. Sustainability 2022, 14, 5652. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef] [PubMed]
- Bakacs, M.E.; Yergeau, S.E.; Obropta, C.C. Assessment of car wash runoff treatment using bioretention mesocosms. J. Environ. Eng. 2013, 139, 1132–1136. [Google Scholar] [CrossRef]
- Tamiazzo, J.; Breschigliaro, S.; Salvato, M.; Borin, M. Performance of a wall cascade constructed wetland treating surfactant-polluted water. Environ. Sci. Pollut. Res. 2015, 22, 12816–12828. [Google Scholar] [CrossRef] [PubMed]
- Torrens, A.; Sepúlveda-Ruiz, P.; Aulinas, M.; Folch, M. Innovative carwash wastewater treatment and reuse through nature-based solutions. Clean Technol. 2025, 7, 12. [Google Scholar] [CrossRef]
- Mazumder, D.; Mukherjee, S. Treatment of auto mobile service station wastewater by coagulation and activated sludge process. Int. J. Environ. Sci. Dev. 2011, 2, 64–69. [Google Scholar] [CrossRef]
- Do, K.; Kim, J.; Chu, X. Sludge characteristics and performance of a membrane bioreactor for treating oily wastewater from a car wash service station. Desalin. Water Treat. 2018, 120, 166–172. [Google Scholar] [CrossRef]
- Mallick, S.K.; Chakraborty, S. Bioremediation of wastewater from automobile service station in anoxic-aerobic sequential reactors and microbial analysis. Chem. Eng. J. 2018, 361, 982–989. [Google Scholar] [CrossRef]
- Khondee, N.; Tathong, S.; Pinyakong, O.; Powtongsook, S.; Chatchupong, T.; Ruangchainikom, C.; Luepromchai, E. Airlift bioreactor containing chitosan-immobilized Sphingobium sp. P2 for treatment of lubricants in wastewater. J. Hazard. Mater. 2012, 213–214, 466–473. [Google Scholar] [CrossRef]
- Hsu, S.; Chen, C.; Chang, W. Reclamation of car washing wastewater by a hybrid system combining bio-carriers and non-woven membranes filtration. Desalin. Water Treat. 2011, 34, 349–353. [Google Scholar] [CrossRef]
- Mazur, R.; Włodyka-Bergier, A.; Kowalewski, Z.; Kaszycki, P.; Starzec, K. The impact of microbiological consortia of autochthonous and allochthonous microorganisms on the parameters of wastewater treatment from car washes. Instal 2023, 12, 99–103. [Google Scholar] [CrossRef]
- Boluarte, I.A.R.; Andersen, M.; Pramanik, B.K.; Chang, C.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Moazzem, S.; Ravishankar, H.; Fan, L.; Roddick, F.; Jegatheesan, V. Application of enhanced membrane bioreactor (eMBR) for the reuse of carwash wastewater. J. Environ. Manag. 2019, 254, 109780. [Google Scholar] [CrossRef]
- DIN 38409-H41-H44; German Standard Methods for the Examination of Water, Waste Water and Sludge; Parameters Characterizing Effects and Substances (Group H); Determination of the Chemical Oxygen Demand (COD) in the Range Over 15 mg/L (H41) and Ranging from 5 to 50 mg/L (H44). German Institute for Standardisation: Berlin, Germany, 1980.
- PN-EN 872:2007+A1:2007; Water Quality—Determination of Suspended Solids—Method by Filtration Through Glass Fiber Filters. Polish Committee for Standardization: Warsaw, Poland, 2007.
- Supel, P.; Śliwa-Cebula, M.; Miszalski, Z.; Kaszycki, P. Cadmium-tolerant rhizospheric bacteria of the C3/CAM intermediate semi-halophytic common ice plant (Mesembryanthemum crystallinum L.) grown in contaminated soils. Front. Plant Sci. 2022, 13, 820097. [Google Scholar] [CrossRef] [PubMed]
- Starzec, K.; Stańkowska, E.; Supel, P.; Mazur, R.; Surma, P.; Kaszycki, P. Microbiological treatment of post-industrial water: Example of efficient bioremediation of the heavily polluted Kalina pond, Poland. J. Water Land Dev. 2024, 60, 236–245. [Google Scholar] [CrossRef]
- Kaszycki, P.; Supel, P.; Petryszak, P. Bacterial population dynamics in waste oily emulsions from the metal-processing industry. J. Ecol. Eng. 2014, 15, 14–22. [Google Scholar] [CrossRef]
- Włodyka-Bergier, A.; Kowalewski, Z.; Mazur, R. Effect of temperature and rigor of aeration on the efficiency of wastewater treatment from a car wash in bioreactors with a moving bed (MBBR). Instal 2023, 10, 33–37. [Google Scholar] [CrossRef]
- Sathya, R.; Arasu, M.V.; Al-Dhabi, N.A.; Vijayaraghavan, P.; Ilavenil, S.; Rejiniemon, T.S. Towards sustainable wastewater treatment by biological methods—A challenges and advantages of recent technologies. Urban Clim. 2022, 47, 101378. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2010, 409, 4141–4166. [Google Scholar] [CrossRef]
- Pu, S.; Ma, H.; Deng, D.; Xue, S.; Zhu, R.; Zhou, Y.; Xiong, X. Isolation, identification, and characterization of an Aspergillus niger bioflocculant-producing strain using potato starch wastewater as nutrilite and its application. PLoS ONE 2018, 13, e0190236. [Google Scholar] [CrossRef]
- Gzar, H.A.; Al-Rekabi, W.S.; Shuhaieb, Z.K. Application of moving bed biofilm reactor (MBBR) for treatment of industrial wastewater: A mini review. J. Phys. Conf. Ser. 2021, 1973, 012024. [Google Scholar] [CrossRef]
- Sabliy, L.; Kuzminskiy, Y.; Zhukova, V.; Kozar, M.; Sobczuk, H. New approaches in biological wastewater treatment aimed at removal of organic matter and nutrients. Ecol. Chem. Eng. S 2019, 26, 331–343. [Google Scholar] [CrossRef]
- Sanghamitra, P.; Mazumder, D.; Mukherjee, S. Treatment of wastewater containing oil and grease by biological method—A review. J. Environ. Sci. Health A 2021, 56, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Geerdink, R.B.; van den Hurk, R.S.; Epema, O.J. Chemical oxygen demand: Historical perspectives and future challenges. Anal. Chim. Acta 2017, 961, 1–11. [Google Scholar] [CrossRef]
- Dhanjai, A.S.; Zhao, H.; Chen, J.; Mugo, S.M. Water Analysis. Determination of Chemical Oxygen Demand. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Academic Press: Cambridge, MA, USA, 2019; Volume 10, pp. 258–270. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, R.; Bhattacharya, P. Mixed consortia in bioprocesses: Role of microbial interactions. Appl. Microbiol. Biotechnol. 2016, 100, 4283–4295. [Google Scholar] [CrossRef]
- Seo, H.; Kim, J.; Jung, J.; Jin, H.M.; Jeon, C.O.; Park, W. Complexity of cell–cell interactions between Pseudomonas sp. AS1 and Acinetobacter oleivorans DR1: Metabolic commensalism, biofilm formation and quorum quenching. Res. Microbiol. 2011, 163, 173–181. [Google Scholar] [CrossRef]
- Dejonghe, W.; Berteloot, E.; Goris, J.; Boon, N.; Crul, K.; Maertens, S.; Höfte, M.; De Vos, P.; Verstraete, W.; Top, E.M. Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading Variovorax Strain. Appl. Environ. Microbiol. 2003, 69, 1532–1541. [Google Scholar] [CrossRef]
- Mougi, A.; Kondoh, M. Stability of competition–antagonism–mutualism hybrid community and the role of community network structure. J. Theor. Biol. 2014, 360, 54–58. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, G.; Wang, D.; Zhu, X.; Xie, L.; Zuo, D.; Wang, L.; Tian, L.; Peng, F.; Xu, B.; et al. Water periods impact the structure and metabolic potential of the nitrogen-cycling microbial communities in rivers of arid and semi-arid regions. Water Res. 2024, 267, 122472. [Google Scholar] [CrossRef]
- Xie, Q.; Zhao, K.; Li, S.; Lian, Y.; Yang, M.; Liu, H.; Chen, C.; Fang, C. Degradation of typical tetracycline antibiotics in landfill leachate by three-dimensional aerated electrocatalytic reactor (3D-AER): Electrode properties, influencing factors and degradation mechanism. J. Environ. Manag. 2025, 386, 125787. [Google Scholar] [CrossRef] [PubMed]


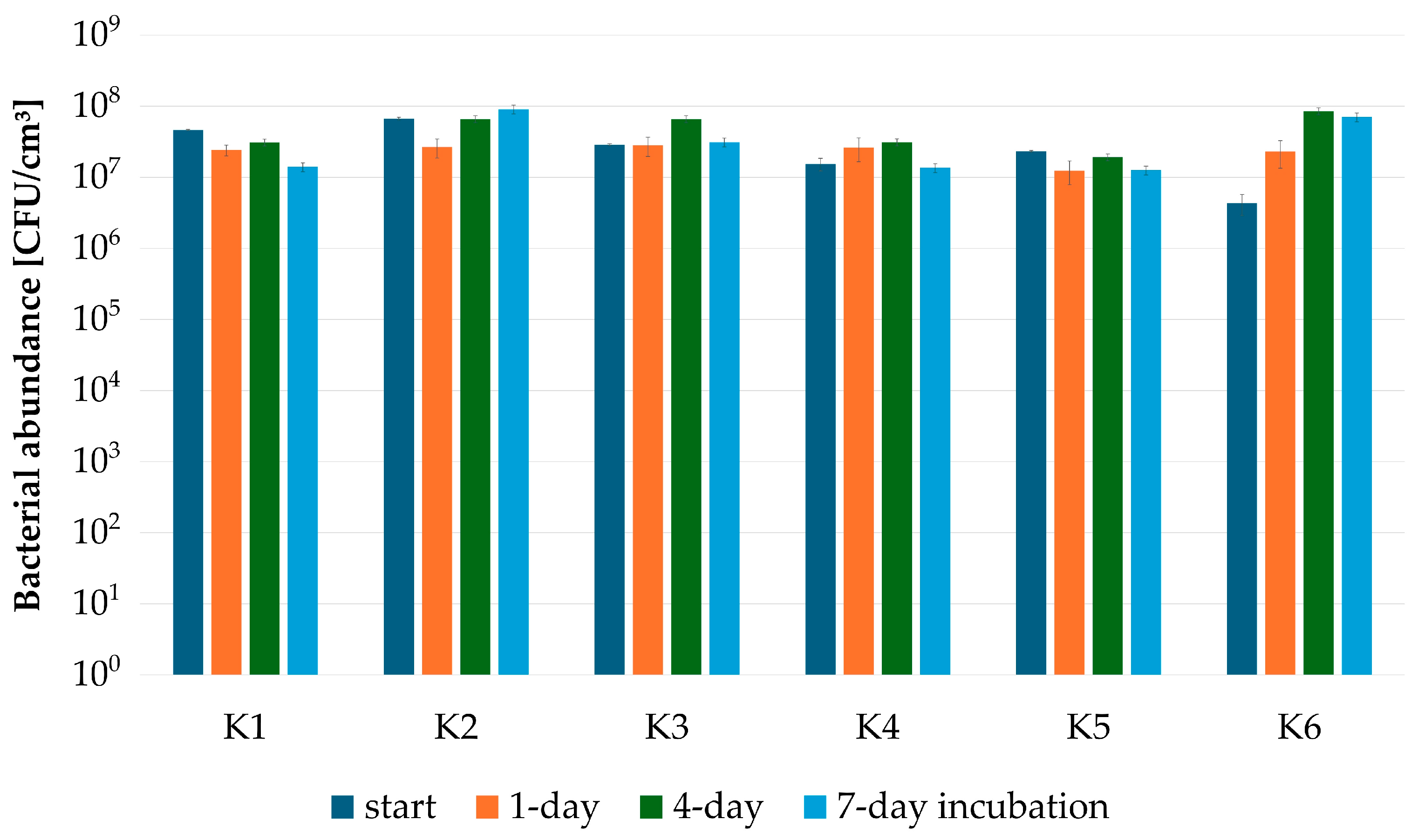
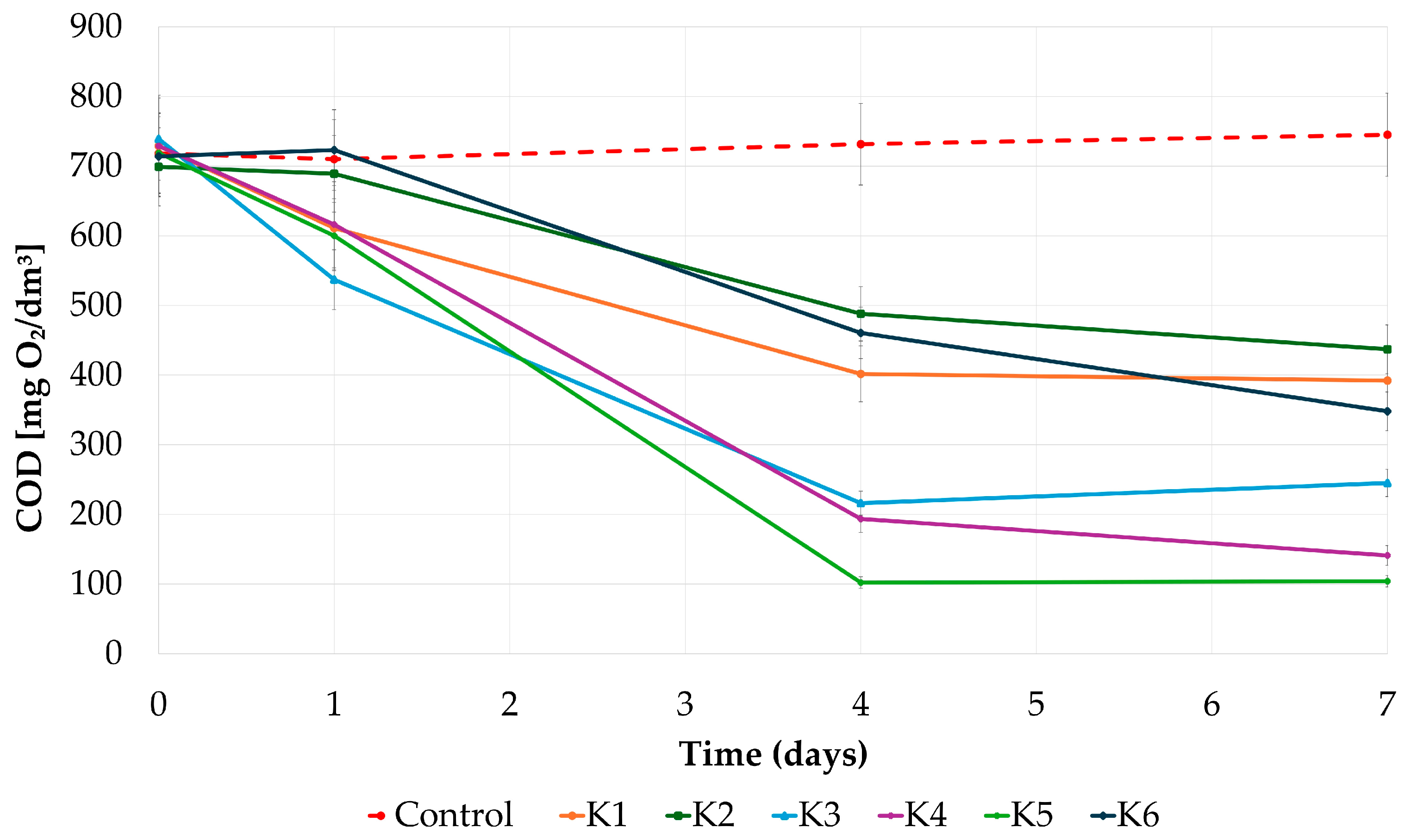
| Strain | Source of Isolated Strain(s) |
|---|---|
| Stenotrophomonas maltophilia SC9 Alcaligenes faecalis SC5 Alcaligenes faecalis SC7 | Indigenous strains obtained from car wash wastewater |
| Alcaligenes faecalis OC1 Lysinibacillus fusiformis OC2 | Activated sludges of municipal and industrial wastewater treatment plants |
| Cellulomonas pakistanensis OW1 | The soil polluted with a natural crude oil leachate |
| Ochrobactrum anthropi ZB1 Shewanella frigidimarina ZB2 | Polluted ground-water environments, the strains capable of biodegradation of selected petroleum substances |
| Bacillus pumilus R2 | Water contaminated with mono- and polyaromatic hydrocarbons |
| Rhodococcus opacus C11 | Lignite (brown coal) deposits colonized with bacteria |
| Kocuria rhizophila G3 Cupriavidus necator G4 Sphingomonas yabuuchiae G5 Pseudomonas koreensis G6 Priestia megaterium G7 Pseudomonas brassicacearum G8 | Soils anthropogenically contaminated with products of crude oil processing |
| Microbacterium aerolatum K18 | The soil contaminated with pesticides |
| CWW Sample Number | |||
|---|---|---|---|
| 3 | 8 | 18 | |
| COD | 215 mg O2/dm3 | 1320 mg O2/dm3 | 2000 mg O2/dm3 |
| Control (uninoculated, sterilized sample) | 0 | 1 | 25 |
| Stenotrophomonas maltophilia SC9 | 28 | 41 | 81 |
| Alcaligenes faecalis SC5 | 28 | 71 | 46 |
| Alcaligenes faecalis SC7 | 51 | 61 | 0 |
| Lysinibacillus fusiformis OC2 | 15 | 22 | 0 |
| Alcaligenes faecalis OC1 | 11 | 53 | 77 |
| Cellulomonas pakistanensis OW1 | 0 | 34 | 78 |
| Shewanella frigidimarina ZB2 | 16 | 38 | 79 |
| Ochrobactrum anthropi ZB1 | 0 | 36 | 71 |
| Bacillus pumilus R2 | 0 | 29 | 80 |
| Rhodococcus opacus C11 | 42 | 37 | 78 |
| Pseudomonas brassicacearum G8 | 95 | 18 | 74 |
| Priestia megaterium G7 | 0 | 29 | 74 |
| Pseudomonas koreensis G6 | 92 | 15 | 79 |
| Sphingomonas yabuuchiae G5 | 77 | 19 | 77 |
| Cupriavidus necator G4 | 69 | 44 | 77 |
| Kocuria rhizophila G3 | 69 | 44 | 77 |
| Microbacterium aerolatum K18 | 10 | 55 | 76 |
| Microbial Consortium | ||||||
|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | |
| Stenotrophomonas maltophilia SC9 | x | x | x | |||
| Alcaligenes faecalis SC5 | x | x | x | |||
| Alcaligenes faecalis SC7 | x | x | ||||
| Lysinibacillus fusiformis OC2 | x | x | x | |||
| Alcaligenes faecalis OC1 | x | x | ||||
| Cellulomonas pakistanensis OW1 | x | |||||
| Shewanella frigidimarina ZB2 | x | x | x | |||
| Ochrobactrum anthropi ZB1 | x | x | ||||
| Bacillus pumilus R2 | x | x | ||||
| Rhodococcus opacus C11 | x | x | ||||
| Pseudomonas brassicacearum G8 | x | x | ||||
| Priestia megaterium G7 | x | x | x | |||
| Pseudomonas koreensis G6 | x | x | x | |||
| Sphingomonas yabuuchiae G5 | x | x | ||||
| Cupriavidus necator G4 | x | x | x | |||
| Kocuria rhizophila G3 | x | x | x | |||
| Microbacterium aerolatum K18 | x | x | x | x | ||
| TOTAL NUMBER OF STRAINS | 6 | 5 | 11 | 5 | 7 | 9 |
| Indicator | Time (Days) | |
|---|---|---|
| 0 | 7 | |
| COD (mg O2/dm3) | 640.0 ± 249.5 | 138.7 ± 7.6 |
| BOD (mg O2/dm3) | 186.7 ± 41.6 | 31.7 ± 14.4 |
| Anionic surfactants (mg/dm3) | 4.91 ± 1.2 | 0.42 ± 0.02 |
| Turbidity (NTU) | 552.3 ± 174.3 | 44.0 ± 7.6 |
| Total suspended solids (TSS, mg/dm3) | 1166.7 ± 442.2 | 46.0 ± 28.5 |
| Specific electrical conductivity (SEC, µS/cm) | 2478.0 ± 614.4 | 2582.0 ± 275.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starzec, K.; Supel, P.; Kaszycki, P. Biological Purification of Heterogenous Car Wash Effluents: Selection of Tolerant Bacteria and Development of Microbial Consortia for Pollutant Biodegradation. Sustainability 2025, 17, 8414. https://doi.org/10.3390/su17188414
Starzec K, Supel P, Kaszycki P. Biological Purification of Heterogenous Car Wash Effluents: Selection of Tolerant Bacteria and Development of Microbial Consortia for Pollutant Biodegradation. Sustainability. 2025; 17(18):8414. https://doi.org/10.3390/su17188414
Chicago/Turabian StyleStarzec, Katarzyna, Paulina Supel, and Paweł Kaszycki. 2025. "Biological Purification of Heterogenous Car Wash Effluents: Selection of Tolerant Bacteria and Development of Microbial Consortia for Pollutant Biodegradation" Sustainability 17, no. 18: 8414. https://doi.org/10.3390/su17188414
APA StyleStarzec, K., Supel, P., & Kaszycki, P. (2025). Biological Purification of Heterogenous Car Wash Effluents: Selection of Tolerant Bacteria and Development of Microbial Consortia for Pollutant Biodegradation. Sustainability, 17(18), 8414. https://doi.org/10.3390/su17188414







