Sustainable Solid-Phase Extractant Based on Spent Coffee Waste-Derived Activated Carbon Functionalized with 1,10-Phenanthroline-5-Amine for Trace Metals from Groundwater Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Apparatuses
2.3. Preparation of AC from Spent Coffee Wastes
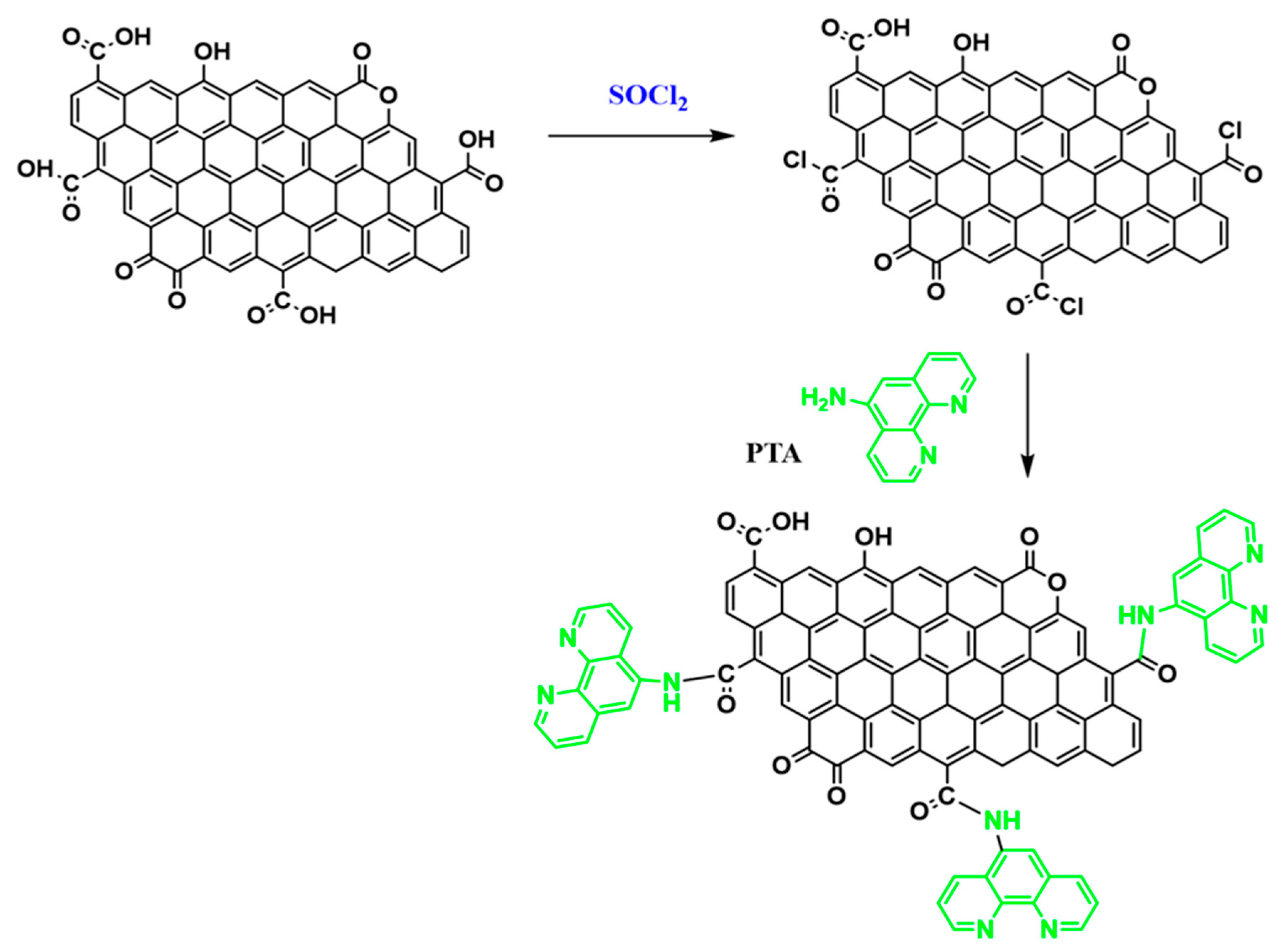
2.4. Preparation of AC-PTA Chelator
Sorption and Solid-Phase Extraction (SPE) Procedure
3. Results and Discussion
3.1. Preparation of AC Substrate and Its Modification with PTA Chelator
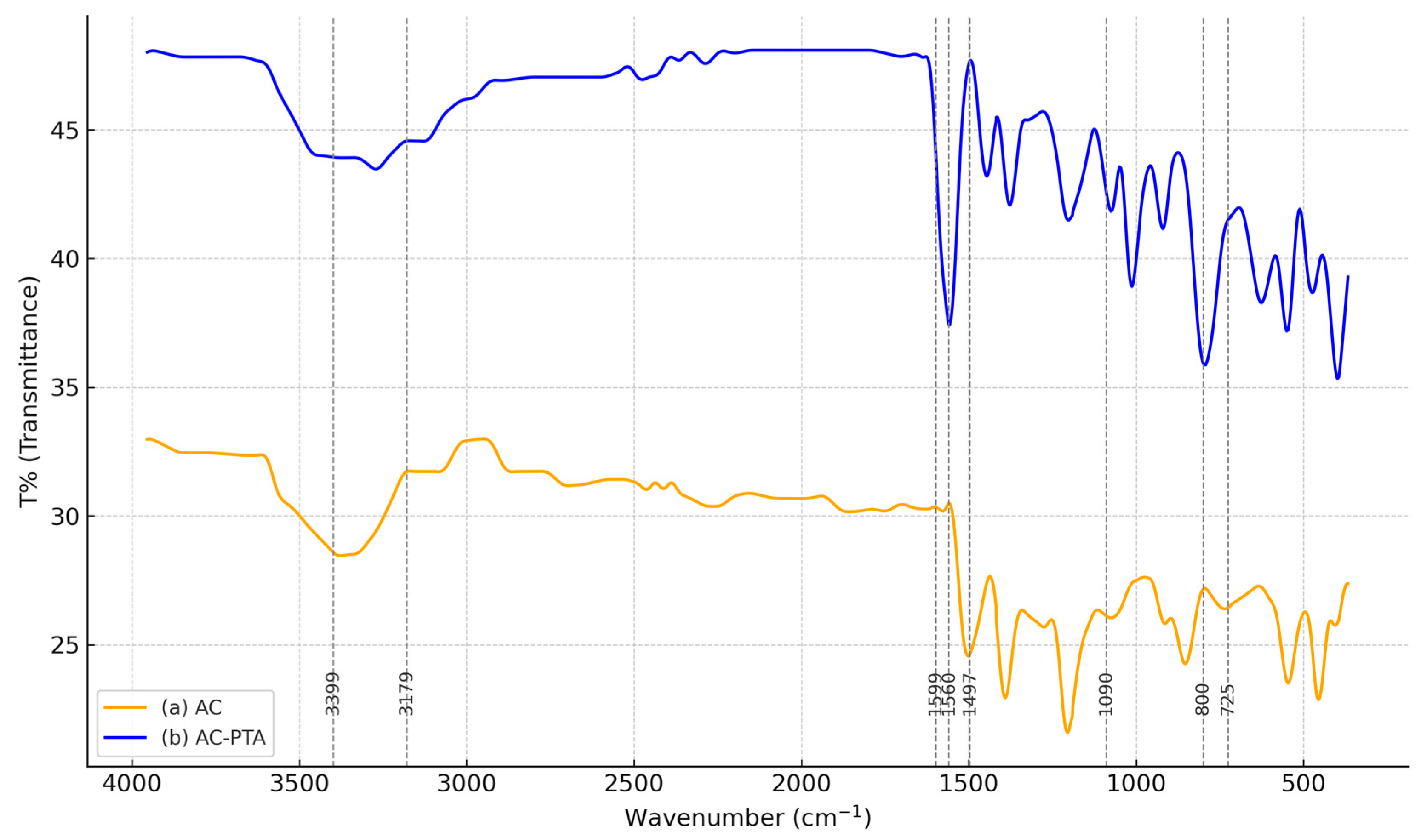
3.2. ATR–FTIR
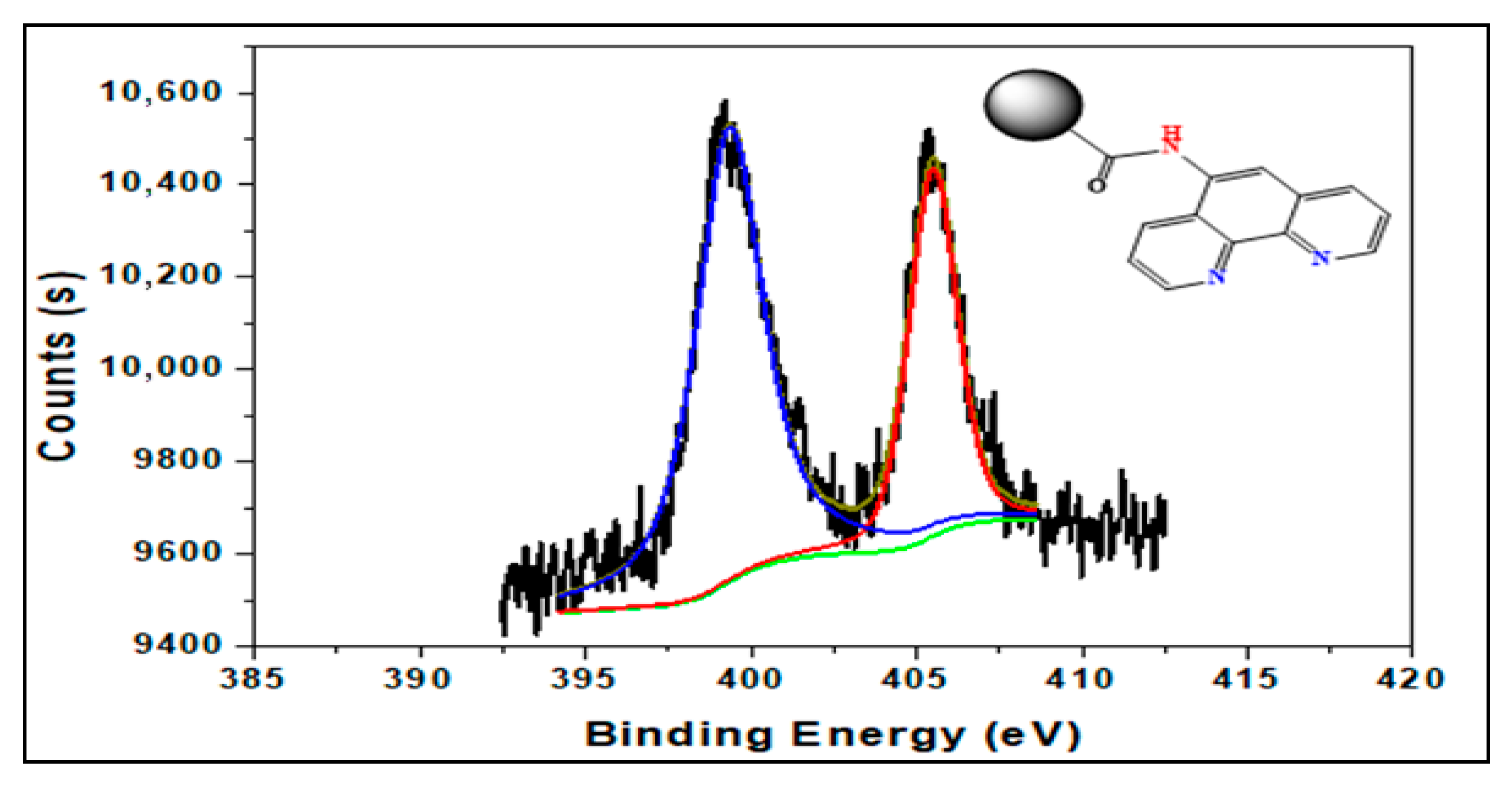
3.3. XPS
3.4. TGA
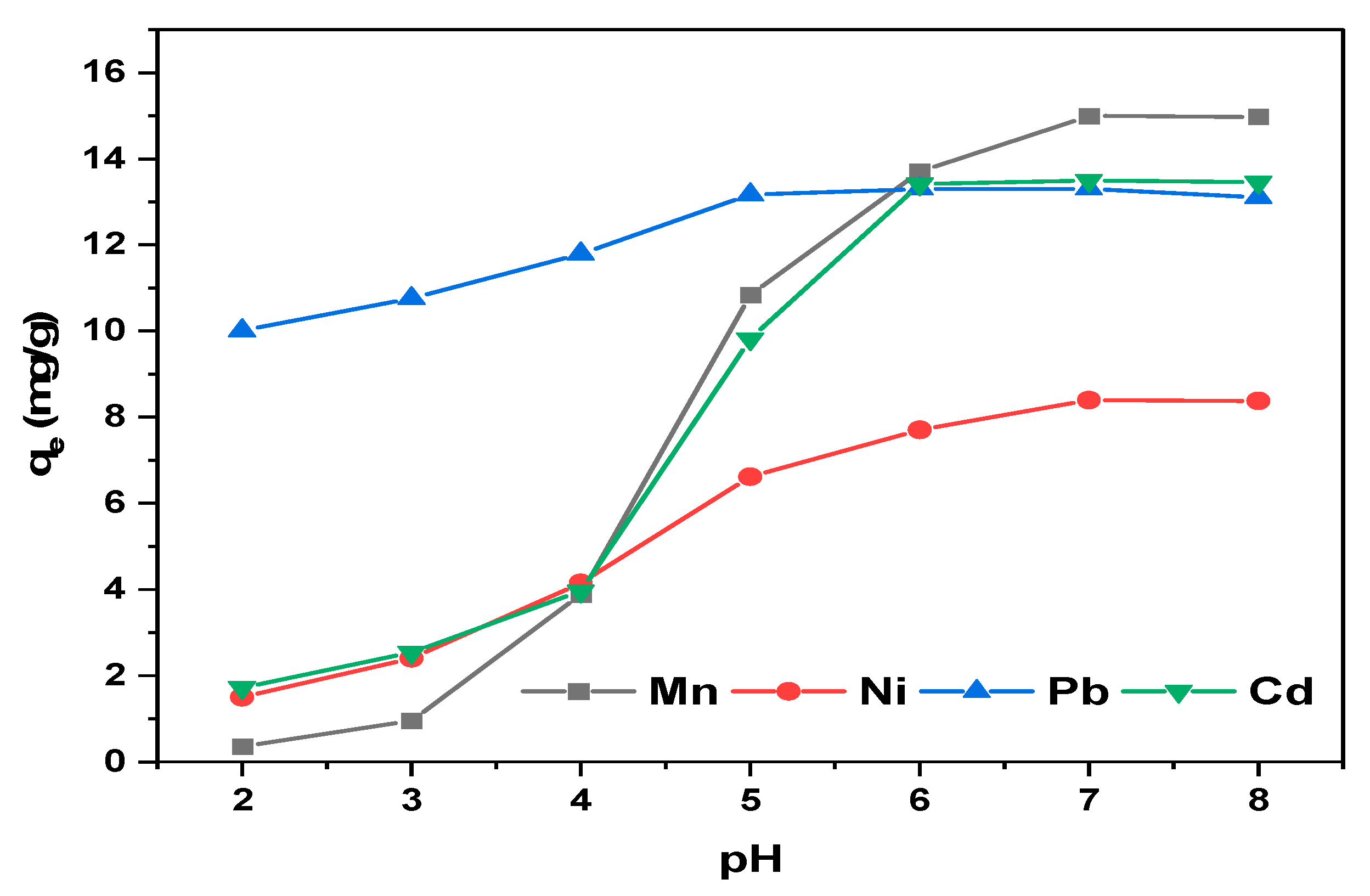
3.5. Influence of pH on Metals Sorption
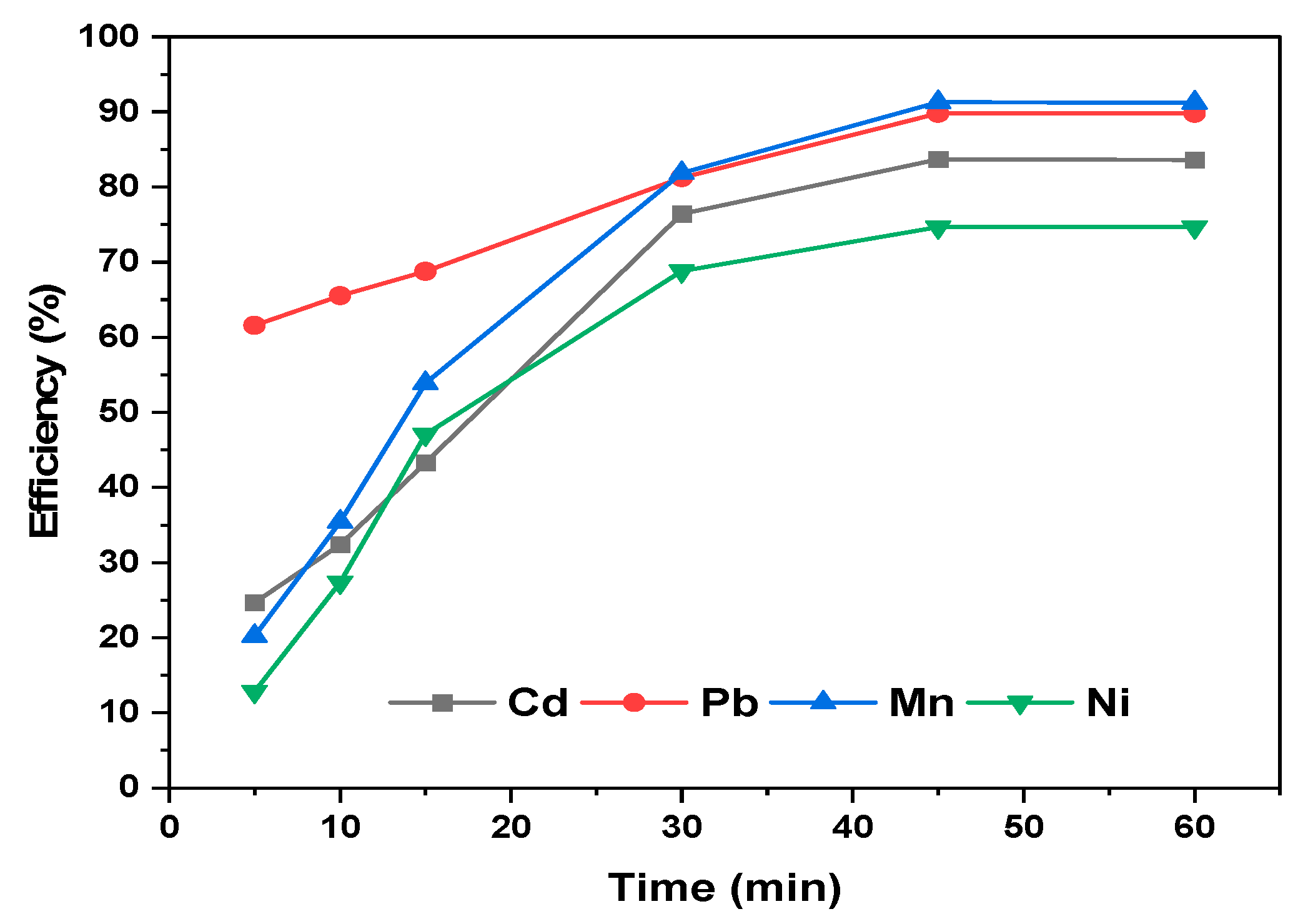
3.6. Effect of Contact Time on Sorption
3.7. Sorption Capacity
3.8. Method Validation: Analysis of Groundwater Reference Materials
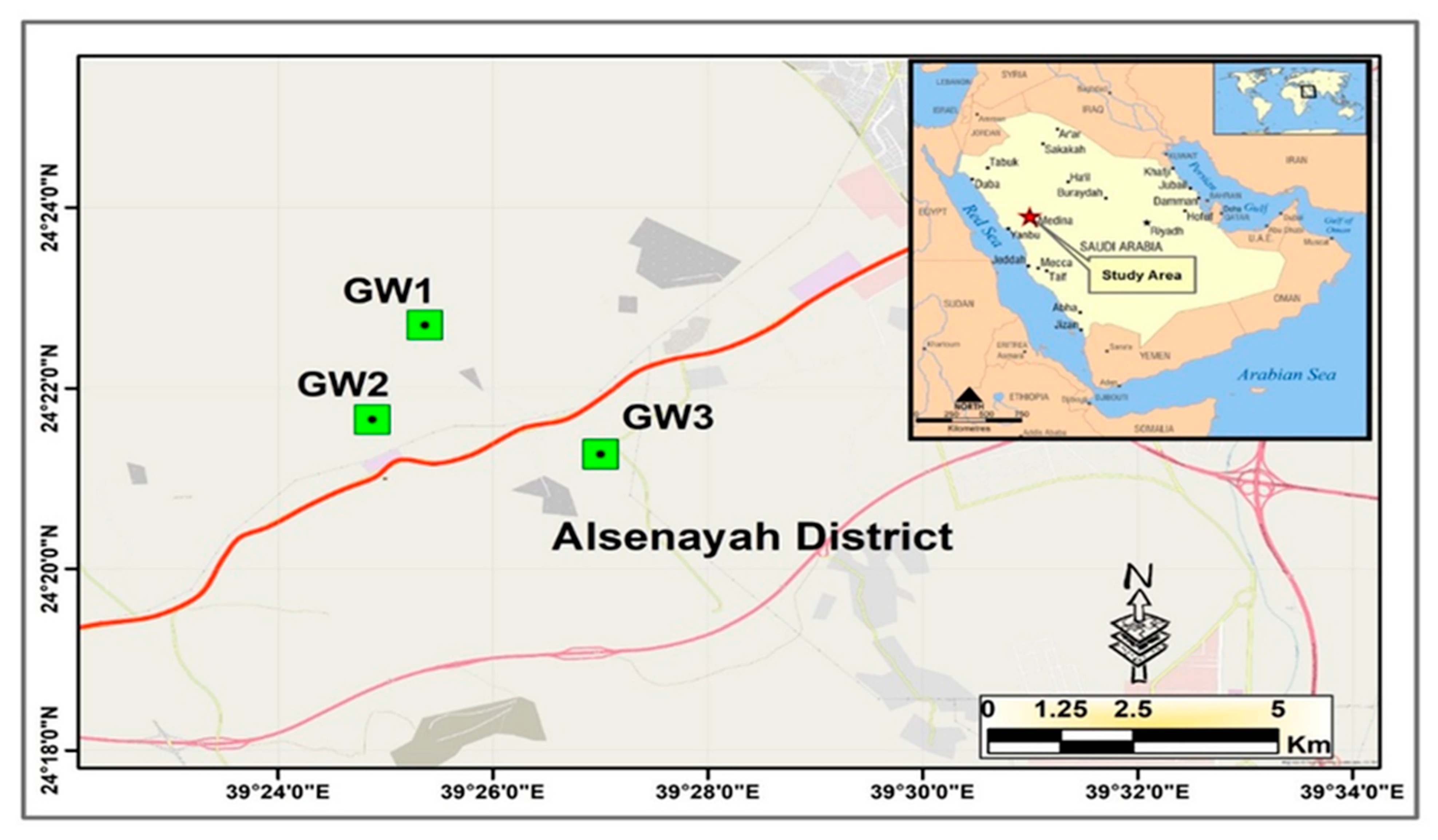
3.9. Method Applications: Analysis of Real Groundwater Samples
3.10. Reusability and Stability of AC-PTA Chelating Resin
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPE | Solid-phase extraction |
| LLE | Liquid–liquid extraction |
| AC | Activated Carbon |
| SCW | Spent coffee waste |
| ICP-MS | Inductively coupled plasma–mass spectra |
| AC-PTA | Activated Carbon functionalized with1,10-Phenanthroline-5-Amine |
References
- Wang, Y.; Sun, L.; An, Z.; Xue, J.; An, Z.; Zhang, N.; Lin, L.; Liu, W. Detection of Ultra-Trace Heavy Metals in Aerosols with Pg^m3 Sensitivity Using Filament-Induced Fluorescence Spectroscopy. arXiv 2025, arXiv:2506.09295. [Google Scholar]
- Williams, M.L.; Olomukoro, A.A.; Emmons, R.V.; Godage, N.H.; Gionfriddo, E. Matrix Effects Demystified: Strategies for Resolving Challenges in Analytical Separations of Complex Samples. J. Sep. Sci. 2023, 46, 2300571. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, R.; Jiang, Z.; Shen, Z.; Yan, M. Determination of Fe, Ni, Cu, Zn, Cd and Pb in Seawater by Isotope Dilution Automatic Solid-Phase Extraction—ICP-MS. Acta Oceanol. Sin. 2022, 41, 129–136. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Luo, D. Separation and Recovery of Vanadium and Aluminum from Oxalic Acid Leachate of Shale by Solvent Extraction with Aliquat 336. Sep. Purif. Technol. 2020, 249, 116867. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Griffin, S.T.; Hartman, D.H.; Rogers, R.D. Liquid/Liquid Extraction of Metal Ions in Room Temperature Ionic Liquids. Sep. Sci. Technol. 2001, 36, 785–804. [Google Scholar] [CrossRef]
- Gebreslassie, G.; Desta, H.G.; Dong, Y.; Zheng, X.; Zhao, M.; Lin, B. Advanced Membrane-Based High-Value Metal Recovery from Wastewater. Water Res. 2024, 265, 122122. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Essilfie-Dughan, J.; Hendry, M.J. Characterization and Environmental Implications of Selenate Co-Precipitation with Barite. Environ. Res. 2020, 186, 109607. [Google Scholar] [CrossRef] [PubMed]
- Camel, V. Solid Phase Extraction of Trace Elements. Spectrochim. Acta Part. B At. Spectrosc. 2003, 58, 1177–1233. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, Y.; Tian, H.; Qu, J.-H.; Wang, Q.; Wang, J.; Jiang, Z. Recent Advances in Accelerating Solid-Phase Extraction. J. Chromatogr. Open 2024, 6, 100156. [Google Scholar] [CrossRef]
- Chu, S.; Feng, X.; Liu, C.; Wu, H.; Liu, X. Advances in Chelating Resins for Adsorption of Heavy Metal Ions. Ind. Eng. Chem. Res. 2022, 61, 11309–11328. [Google Scholar] [CrossRef]
- Oshita, K.; Motomizu, S. Development of Chelating Resins and Their Ability of Collection and Separation for Metal Ions. BUNSEKI KAGAKU 2008, 57, 291–311. [Google Scholar] [CrossRef]
- De La Calle, I.; Lavilla, I.; Bartolomé-Alonso, H.; Bendicho, C. Solid-Phase Extraction of Hg(II) Using Cellulose Filters Modified with Silver Nanoparticles Followed by Pyrolysis and Detection by a Direct Mercury Analyzer. Spectrochim. Acta Part B At. Spectrosc. 2019, 161, 105697. [Google Scholar] [CrossRef]
- Mulugeta, M.; Wibetoe, G.; Engelsen, C.J.; Lund, W. Overcoming Matrix Interferences in Ion-Exchange Solid Phase Extraction of As, Cr, Mo, Sb, Se and V Species from Leachates of Cement-Based Materials Using Multiple Extractions. Talanta 2010, 82, 158–163. [Google Scholar] [CrossRef]
- Tekin, Z.; Özdoğan, N.; Bakırdere, S. Zirconium Nanoparticles Based Solid Phase Extraction-Slotted Quartz Tube-Flame Atomic Absorption Spectrophotometry for the Determination of Cadmium in Wastewater Samples and Evaluation of Green Profile. Int. J. Environ. Anal. Chem. 2022, 102, 935–944. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Yılmaz, V.; Kartal, Ş.; Delibaş, A.; Soykan, C. Synthesis of a Novel Chelating Resin and Its Use for Selective Separation and Preconcentration of Some Trace Metals in Water Samples. J. Hazard. Mater. 2009, 169, 593–598. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Singh, B.; Acharya, B. A Comprehensive Review on Activated Carbon from Pyrolysis of Lignocellulosic Biomass: An Application for Energy and the Environment. Carbon. Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-08-044463-5. [Google Scholar]
- Biegun, M.; Dymerska, A.; Chen, X.; Mijowska, E. Study of the Active Carbon from Used Coffee Grounds as the Active Material for a High-Temperature Stable Supercapacitor with Ionic-Liquid Electrolyte. Materials 2020, 13, 3919. [Google Scholar] [CrossRef]
- Mukherjee, A.; Saha, B.; Niu, C.; Dalai, A.K. Preparation of Activated Carbon from Spent Coffee Grounds and Functionalization by Deep Eutectic Solvent: Effect of Textural Properties and Surface Chemistry on CO2 Capture Performance. J. Environ. Chem. Eng. 2022, 10, 108815. [Google Scholar] [CrossRef]
- Campbell, R.; Xiao, B.; Mangwandi, C. Production of Activated Carbon from Spent Coffee Grounds (SCG) for Removal of Hexavalent Chromium from Synthetic Wastewater Solutions. J. Environ. Manag. 2024, 366, 121682. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of Wood. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Babel, S. Low-Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Pittman, C.U.; He, G.-R.; Wu, B.; Gardner, S.D. Chemical Modification of Carbon Fiber Surfaces by Nitric Acid Oxidation Followed by Reaction with Tetraethylenepentamine. Carbon 1997, 35, 317–331. [Google Scholar] [CrossRef]
- Li, Z.; Chang, X.; Zou, X.; Zhu, X.; Nie, R.; Hu, Z.; Li, R. Chemically-Modified Activated Carbon with Ethylenediamine for Selective Solid-Phase Extraction and Preconcentration of Metal Ions. Anal. Chim. Acta 2009, 632, 272–277. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Deng, B. Ethylenediamine-Modified Activated Carbon for Aqueous Lead Adsorption. Env. Chem. Lett. 2010, 8, 277–282. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, S.; Hu, Z.-J.; Liu, X.-H.; Gao, H.-W. Solid-Phase Extraction of Lead(II) Ions Using Multiwalled Carbon Nanotubes Grafted with Tris(2-Aminoethyl)Amine. Microchim. Acta 2011, 174, 107–113. [Google Scholar] [CrossRef]
- Tehrani, M.S.; Azar, P.A.; Namin, P.E.; Dehaghi, S.M. Removal of Lead Ions from Wastewater Using Functionalized Multiwalled Carbon Nanotubes with Tris(2-Aminoethyl)Amine. J. Environ. Prot. 2013, 04, 529–536. [Google Scholar] [CrossRef]
- Vuković, G.; Marinković, A.; Obradović, M.; Radmilović, V.; Čolić, M.; Aleksić, R.; Uskoković, P.S. Synthesis, Characterization and Cytotoxicity of Surface Amino-Functionalized Water-Dispersible Multi-Walled Carbon Nanotubes. Appl. Surf. Sci. 2009, 255, 8067–8075. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Yilmaz, E.; Habila, M.; Soylak, M. Separation and Preconcentration of Lead(II), Cobalt(II), and Nickel(II) on EDTA Immobilized Activated Carbon Cloth Prior to Flame Atomic Absorption Spectrometric Determination in Environmental Samples. Turk. J. Chem. 2015, 39, 1038–1049. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A Review on Experimental Chemically Modified Activated Carbon to Enhance Dye and Heavy Metals Adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Berka, L.H.; Gagne, R.R.; Philippon, G.E.; Wheeler, C.E. Transition Metal Complexes of 1,10-Phenanthroline and 2,2′-Bipyridine. Inorg. Chem. 1970, 9, 2705–2709. [Google Scholar] [CrossRef]
- Brandt, W.W.; Dwyer, F.P.; Gyarfas, E.D. Chelate Complexes of 1,10-Phenanthroline and Related Compounds. Chem. Rev. 1954, 54, 959–1017. [Google Scholar] [CrossRef]
- Fortune, W.B.; Mellon, M.G. Determination of Iron with O-Phenanthroline: A Spectrophotometric Study. Ind. Eng. Chem. Anal. Ed. 1938, 10, 60–64. [Google Scholar] [CrossRef]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric Determination of Iron(II) with 1,10-Phenanthroline in the Presence of Large Amounts of Iron(III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Yin, X.; Shen, H.; Ye, Y.; Gu, X. 1,10-Phenanthroline as a Complexing Agent for on-Line Sorbent Extraction/Preconcentration for Flow Injection–Flame Atomic Absorption Spectrometry. Anal. Chim. Acta 1999, 392, 283–289. [Google Scholar] [CrossRef]
- Mikuła, B.; Puzio, B.; Feist, B. Application of 1,10-Phenanthroline for Preconcentration of Selected Heavy Metals on Silica Gel. Microchim. Acta 2009, 166, 337–341. [Google Scholar] [CrossRef]
- Feist, B.; Pilch, M.; Nycz, J. Graphene Oxide Chemically Modified with 5-Amino-1,10-Phenanthroline as Sorbent for Separation and Preconcentration of Trace Amount of Lead(II). Microchim. Acta 2019, 186, 91. [Google Scholar] [CrossRef] [PubMed]
- AlSuhaimi, A.O.; AlMohaimadi, K.M.; AlAlawi, B.N.; Ali, I. A Novel Porous Silica Monolith Functionalized with 5-Amino-1,10-Phenanthroline for SPE of Metal Ions in Groundwater Samples Prior to Their Analysis Using ICP-MS. Anal. Methods 2018, 10, 2337–2346. [Google Scholar] [CrossRef]
- AlMohaimadi, K.M.; Albishri, H.M.; Althumayri, K.; AlSuhaimi, A.O.; Hussein, B.H.M. Preparation of Phenanthroline-2-Carbaldehyde Functionalized Mesoporous Silica Nanoparticles as Nanochelator for Solid Phase Extraction of Trace Metals from Wastewater. Arab. J. Chem. 2025, 18, 792024. [Google Scholar] [CrossRef]
- Liu, P.; Sun, S.; Huang, S.; Wu, Y.; Li, X.; Wei, X.; Wu, S. KOH Activation Mechanism in the Preparation of Brewer’s Spent Grain-Based Activated Carbons. Catalysts 2024, 14, 814. [Google Scholar] [CrossRef]
- Rios, R.R.A.; Alves, D.E.; Dalmázio, I.; Bento, S.F.V.; Donnici, C.L.; Lago, R.M. Tailoring Activated Carbon by Surface Chemical Modification with O, S, and N Containing Molecules. Mat. Res. 2003, 6, 129–135. [Google Scholar] [CrossRef]
- Vassileva, E.; Furuta, N. Application of Iminodiacetate Chelating Resin Muromac A-1 in on-Line Preconcentration and Inductively Coupled Plasma Optical Emission Spectroscopy Determination of Trace Elements in Natural Waters. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 1541–1552. [Google Scholar] [CrossRef]
- Salatein, N.M.; Shaaban, M.; Fahim, I.S. Comparing Low-Cost Activated Carbon Made from Coffee Waste and Bagasse to Remove Heavy Metals and Methylene Blue Dye. Results Chem. 2025, 13, 102020. [Google Scholar] [CrossRef]
- Albishri, H.M.; Marwani, H.M. Chemically Modified Activated Carbon with Tris(Hydroxymethyl)Aminomethane for Selective Adsorption and Determination of Gold in Water Samples. Arab. J. Chem. 2016, 9, S252–S258. [Google Scholar] [CrossRef]
- Tu, Z.; He, Q.; Chang, X.; Hu, Z.; Gao, R.; Zhang, L.; Li, Z. 1-(2-Formamidoethyl)-3-Phenylurea Functionalized Activated Carbon for Selective Solid-Phase Extraction and Preconcentration of Metal Ions. Anal. Chim. Acta 2009, 649, 252–257. [Google Scholar] [CrossRef]
- Chowdhry, A.; Kumar, A.; Sharma, A.; Kumar, R.; Kumar, D. Characterization of Functionalized Multi-Walled Carbon Nanotubes and Their Applications in Sensors and Biomedical Systems. Heliyon 2019, 5, e03668. [Google Scholar]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świątkowski, A. The Characterization of Activated Carbons with Oxygen and Nitrogen Surface Groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R.J. Surface Modification and Characterisation of a Coal-Based Activated Carbon. Carbon 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Leventis, H.C.; Davies, I.J.; Crossley, A.; Lawrence, N.S.; Jiang, L.; Jones, T.G.J.; Compton, R.G. Graphite Powder Derivatized with Poly-l-Cysteine Using “Building-Block” Chemistry—A Novel Material for the Extraction of Heavy Metal Ions. J. Mater. Chem. 2005, 15, 2375. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases, HSAB, Part 1: Fundamental Principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Nabid, M.R.; Sedghi, R.; Bagheri, A.; Behbahani, M.; Taghizadeh, M.; Abdi Oskooie, H.; Heravi, M.M. Preparation and Application of Poly(2-Amino Thiophenol)/MWCNTs Nanocomposite for Adsorption and Separation of Cadmium and Lead Ions via Solid Phase Extraction. J. Hazard. Mater. 2012, 203–204, 93–100. [Google Scholar] [CrossRef]
- Gao, R.; Hu, Z.; Chang, X.; He, Q.; Zhang, L.; Tu, Z.; Shi, J. Chemically Modified Activated Carbon with 1-Acylthiosemicarbazide for Selective Solid-Phase Extraction and Preconcentration of Trace Cu(II), Hg(II) and Pb(II) from Water Samples. J. Hazard. Mater. 2009, 172, 324–329. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Szczepańska, N.; De La Guardia, M.; Namieśnik, J. Miniaturized Solid-Phase Extraction Techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Bulman, R.A. Chelating Agents and the Regulation of Metal Ions. Met. Based Drugs 1994, 1, 87–106. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef]
- AlQadhi, N.F.; AlSuhaimi, A.O. Chemically Functionalized Activated Carbon with 8-Hydroxyquinoline Using Aryldiazonium Salts/Diazotization Route: Green Chemistry Synthesis for Oxins-Carbon Chelators. Arab. J. Chem. 2020, 13, 1386–1396. [Google Scholar] [CrossRef]
- Alharbi, H.N.; Alsuhaimi, A.O. Functionalization of Amberlite XAD-4 with 8-Hydroxyquinoline Chelator Using Aryldiazonium Radical Reaction and Its Application for Solid Phase Extraction of Trace Metals from Groundwater Samples. Glob. NEST Int. J. 2020, 22, 306–314. [Google Scholar] [CrossRef]
- Awadh, O.; AlSuhaimi, A.A. Preparation of Silica-4-(2-Pyridylazo) Resorcinol Chelator for Solid Phase Extraction of Transition Metals from Groundwater. JCS Pak. 2019, 41, 151. [Google Scholar] [CrossRef]
- Pietrzak, R.; Nowicki, P.; Wachowska, H. The Influence of Oxidation with Nitric Acid on the Preparation and Properties of Active Carbon Enriched in Nitrogen. Appl. Surf. Sci. 2009, 255, 3586–3593. [Google Scholar] [CrossRef]


| Substrate | Analyte | Ref. | |||
|---|---|---|---|---|---|
| Mn(II) | Cd(II) | Ni(II) | Pb(II) | ||
| Activated Carbon | 13.5 | 8.4 | 13.3 | 8.5 | This work |
| Graphene Oxide | - | - | - | 548 | [37] |
| Silica Monolith | - | - | 8.22 | - | [38] |
| Mesoporous silica NPs | - | 116 | 132 | 121 | [39] |
| Calibration Parameters | Metal Ions (ng mL−1) | |||
|---|---|---|---|---|
| Cd(II) | Pb(II) | Mn(II) | Ni(II) | |
| Conc. range (ng mL−1) (n = 7) | 0–10 | 0–10 | 0–25 | 0–25 |
| RSD at 2 ng mL−1 (n = 7) | 1.14 | 1.22 | 1.18 | 1.92 |
| RSD at 10 ng mL−1 (n = 7) | 1.73 | 1.29 | 1.57 | 2.13 |
| Correlation coefficient, R2 | 0.9990 | 0.9950 | 0.9988 | 0.9980 |
| Sensitivity, CPS ratio/ng mL−1 | 3144.1 | 35,119.0 | 20,038.0 | 283.95 |
| LOD/ng mL−1 | 0.013 | 0.033 | 0.086 | 0.072 |
| Metals | ||||
|---|---|---|---|---|
| Cd(II) | Pb(II) | Mn(II) | Ni(II) | |
| Certified | 0.164 | 1.67 | - | 9.11 |
| Fund | 0.153 ± 0.018 | 1.71 ± 0.05 | 2.31 ± 0.16 | 9.97 ± 1.67 |
| Recovery% | 93.29% | 102.39% | - | 109.44% |
| Sample ID | Metals Concentrations (Recovery %) | |||
|---|---|---|---|---|
| Pb(II) | Ni(II) | Mn(II) | Cd(II) | |
| G1 | 1.420 ± 0.35 | 3.02 ± 0.76 | 8.37 ± 1.67 | 1.36 ± 0.14 |
| G1 Spike | 6.290 ± 0.82 (97.40%) | 8.04 ± 1.82 (99.40%) | 12.74 ± 1.74 (87.40%) | 6.18 ± 0.16 (96.40%) |
| G2 | 1.75 ± 0.62 | 2.66 ± 1.03 | 6.25 ± 1.34 | 1.50 ± 0.28 |
| G2 Spike | 6.64 ± 0.62 (97.80%) | 7.96 ± 1.25 (106.00%) | 11.67 ± 1.13 (108.40%) | 6.35 ± 0.32 (97.00%) |
| G3 | 1.53 ± 0.38 | 1.04 ± 1.21 | 8.17 ± 1.67 | 1.43 ± 0.27 |
| G3 Spike | 6. 87 ± 0.52 (106.80%) | 6.16 ± 1.19 (101.4%) | 13.81 ± 1.42 (112.80%) | 6.50 ± 0.40 (101.34%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSuhaimi, A.O. Sustainable Solid-Phase Extractant Based on Spent Coffee Waste-Derived Activated Carbon Functionalized with 1,10-Phenanthroline-5-Amine for Trace Metals from Groundwater Samples. Sustainability 2025, 17, 8404. https://doi.org/10.3390/su17188404
AlSuhaimi AO. Sustainable Solid-Phase Extractant Based on Spent Coffee Waste-Derived Activated Carbon Functionalized with 1,10-Phenanthroline-5-Amine for Trace Metals from Groundwater Samples. Sustainability. 2025; 17(18):8404. https://doi.org/10.3390/su17188404
Chicago/Turabian StyleAlSuhaimi, Awadh O. 2025. "Sustainable Solid-Phase Extractant Based on Spent Coffee Waste-Derived Activated Carbon Functionalized with 1,10-Phenanthroline-5-Amine for Trace Metals from Groundwater Samples" Sustainability 17, no. 18: 8404. https://doi.org/10.3390/su17188404
APA StyleAlSuhaimi, A. O. (2025). Sustainable Solid-Phase Extractant Based on Spent Coffee Waste-Derived Activated Carbon Functionalized with 1,10-Phenanthroline-5-Amine for Trace Metals from Groundwater Samples. Sustainability, 17(18), 8404. https://doi.org/10.3390/su17188404






