Habitat Selection of Sympatric Lontra felina and L. provocax in Chilean Patagonia: Toward Sustainable Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Period
- -
- Coastal edges protected from wind and tide, dominated by zonal vegetation that sometimes completely covers the shoreline.
- -
- Coastal edges are exposed to wind and tide, with shrubby and herbaceous vegetation characterized by species such as Escallonia rubra, Fuchsia magellanica, Gunnera tinctorea, G. magellanica, Acaena ovalifolia, Blechnum chilense, Asplenium obtusatum, and Fascicularia bicolor.
- -
- Coastal dunes found at estuary mouths of the Palena, Bahía Mala, and Santo Domingo Rivers, with high cover of Fragaria chiloensis, and terraces of variable width dominated by grasses, Cyperaceae, and Juncaceae.
2.2. Wave Exposition
2.3. Oceanography
2.4. Climate and Rainfall
2.5. Otter Species Identification and Records
2.6. Handling of Data from Different Sources
- 2020–2022: Turismo de Mamíferos Marinos, Oportunidad de Conservación y Desarrollo”, Gobierno Regional de Aysén, Fondo de Innovación y Competitividad (FIC).
- 2024–2025. Monitoreo ciudadano de fauna marina en el Área de Conservación de Múltiples Usos Pitipalena Añihue, FAO/PNUD-GEF Gobernanza Marítima.
2.7. Habitat Characterization
2.8. Data Analysis
3. Results
3.1. Species Presence and Distribution Patterns
3.2. Habitat Attributes of Sightings
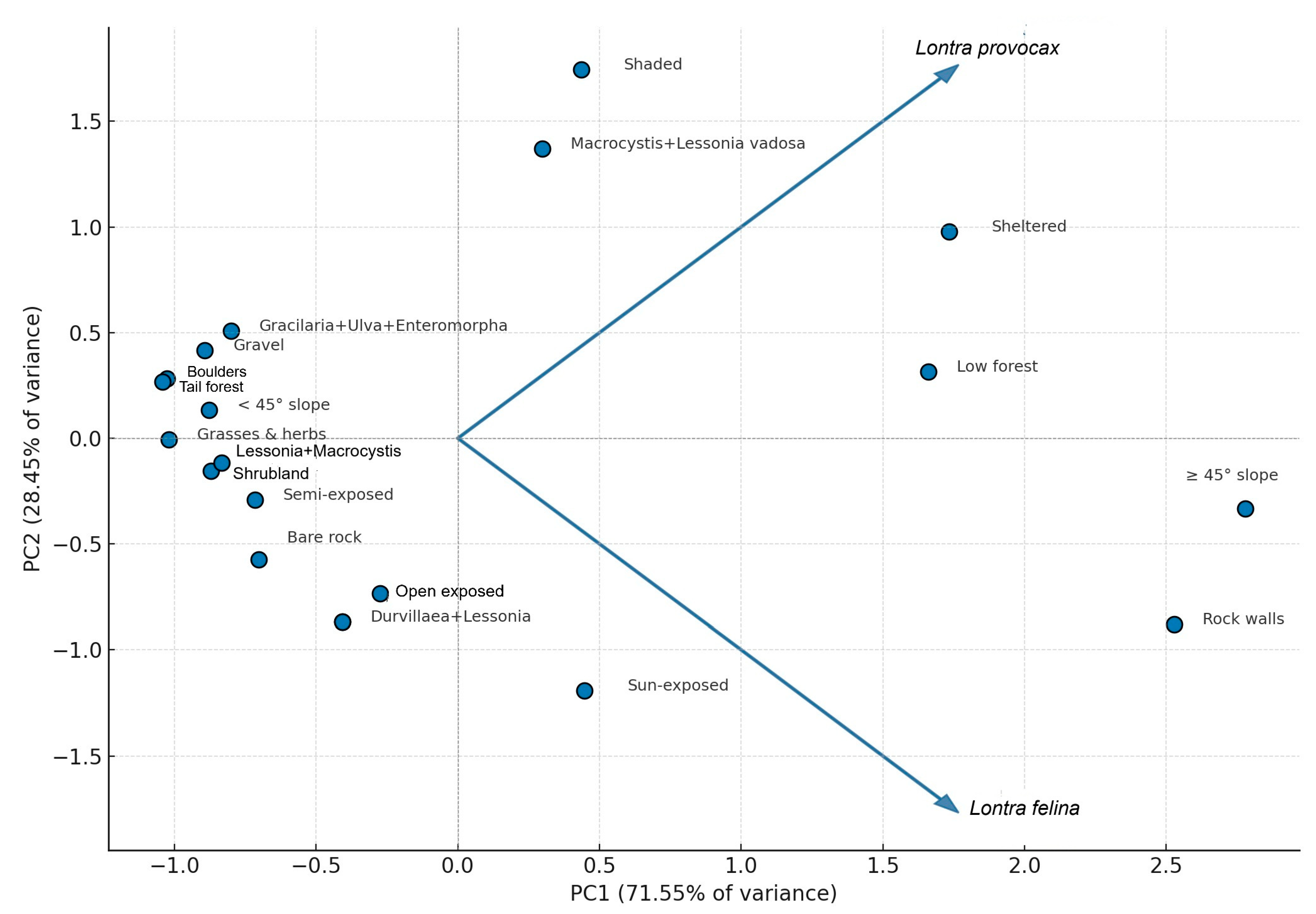
3.3. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wozencraft, W. Mammal Species of the World: A Taxonomic and Geographic Reference; Johns Hopkins University Press: Baltimore, MD, USA, 2005; p. 532. [Google Scholar]
- de Jong, C.v.Z. A Systematic Review of the Nearctic and Neotropical River otters (Genus Lutra, Mustelidae Carnivora); Royal Ontario Museum: Toronto, ON, Canada, 1972. [Google Scholar]
- Kruuk, H. Otters: Ecology, Behaviour and Conservation; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Estes, J.A. Adaptations for aquatic living by carnivores. In Carnivore Behavior, Ecology, and Evolution; Springer: Boston, MA, USA, 1989; pp. 242–282. [Google Scholar]
- Sielfeld, W. Mamíferos marinos de Chile. Ediciones de la Universidad de Chile. Santiago 1983, 1989, 103–108. [Google Scholar]
- Sielfeld, W.; Castilla, J.C. Estado de conservación y conocimiento de las nutrias en Chile scientific. Estud. Oceanol. 1999, 18, 69–79. [Google Scholar]
- Alfaro-Shigueto, J.; Valqui, J.; Mangel, J.C. Nuevo registro de la nutria marina Lontra felina (Molina, 1782) al norte de su distribución actual. Ecol. Apl. 2011, 10, 87–91. [Google Scholar] [CrossRef][Green Version]
- Valle-Rubio, S.; Indacochea, A.G. Environmental Components of the Marine Otter Habitat of Peru. In Marine Otter Conservation; Ayala, L., Sánchez-Scaglioni, R., Medina-Vogel, G., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–16. [Google Scholar]
- Castilla, J. Nuevas observaciones sobre conducta, ecología y densidad de Lutra felina (Molina 1782) (Carnivora: Mustelidae) en Chile. Publicación Ocas. Mus. Nac. Hist. Nat. 1982, 38, 197–206. [Google Scholar][Green Version]
- Castilla, J.C.; Bahamondes, I. Observaciones conductuales y ecológicas sobre Lutra felina (Molina) 1782 (Carnivora: Mustelidae) en las zonas central y centro-norte de Chile. Arch. Biol. Med. Exp. 1979, 12, 119–132. [Google Scholar][Green Version]
- Medina-Vogel, G.; Merino, L.; Monsalve Alarcón, R.; Vianna, J.d.A. Coastal–marine discontinuities, critical patch size and isolation: Implications for marine otter conservation. Anim. Conserv. 2008, 11, 57–64. [Google Scholar] [CrossRef]
- Núñez, J.A.U. La nutria marina (Lontra felina) en los Andes del sur del Peru. Cienc. Desarro. 2021, 20, 71–77. [Google Scholar] [CrossRef]
- Medina, G. Conservation and status of Lutra provocax in Chile. Pac. Conserv. Biol. 1995, 2, 414–419. [Google Scholar] [CrossRef]
- Eisenberg, J.F.; Redford, K.H. Mammals of the Neotropics, Volume 2: The Southern Cone: Chile, Argentina, Uruguay, Paraguay; University of Chicago Press: Chicago, IL, USA, 1989. [Google Scholar]
- Chehébar, C.E.; Gallur, A.; Giannico, G.; Gottelli, M.D.; Yorio, P. A survey of the Southern river otter Lutra provocax in Lanin, Puelo and Los Alerces National Parks, Argentina, and evaluation of its conservation status. Biol. Conserv. 1986, 38, 293–304. [Google Scholar] [CrossRef]
- Ebensperger, L.A.; Botto-Mahan, C. Use of habitat, size of prey, and food-niche relationships of two sympatric otters in southernmost Chile. J. Mammal. 1997, 78, 222–227. [Google Scholar] [CrossRef]
- Cursach, J.A.; Rau, J.R.; Ther, F.; Vilugrón, J.; Tobar, C.N. Sinantropía y conservación marina: El caso del chungungo Lontra felina en el sur de Chile. Rev. Biol. Mar. Oceanogr. 2012, 47, 593–597. [Google Scholar] [CrossRef]
- Muanis, M.C.; Oliveira, L.F.B. Habitat use and food niche overlap by Neotropical otter, Lontra longicaudis, and giant otter, Pteronura brasiliensis, in the Pantanal wetland, Brazil. Otter Spec. Group Bull. 2011, 28, 76–85. [Google Scholar]
- Kenyon, K.W. The Sea Otter in the Eastern Pacific Ocean; US Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1969; No. 68. [Google Scholar]
- Raha, A.; Hussain, S.A. Factors affecting habitat selection by three sympatric otter species in the southern Western Ghats, India. Acta Ecol. Sin. 2016, 36, 45–49. [Google Scholar] [CrossRef]
- Krupa, H.; Borker, A.; Gopal, A. Photographic record of sympatry between asian small-clawed otter and smooth-coated otter in the Northern Western Ghats, India. IUCN Otter Spec. Group Bull. 2017, 34, 51–57. [Google Scholar]
- Virdana, S.; Andeska, F.; Aadrean, A.; Kubontubuh, C.; Wahyudi, G.; Eveisca, N. Records of using the same habitat of three species of otters Lutra lutra, Lutra sumatrana and Aonyx cinereus in the Dharmasraya Sumatran Tiger Rehabilitation Centre Area, West Sumatra, Indonesia. IUCN Otter Spec. Group Bull. 2024, 41, 64–70. [Google Scholar]
- Medina-Vogel, G.; Vivar, D.N.; Calvo-Mac, C. Assessment of the Distribution and Coexistence of Two Sympatric Otter Species in the Chiloé Archipelago, Chile, Using Photo-Identification. Aquat. Mamm. 2024, 50, 423–429. [Google Scholar] [CrossRef]
- Vianna, J.A.; Medina-Vogel, G.; Chehébar, C.; Sielfeld, W.; Olavarría, C.; Faugeron, S. Phylogeography of the Patagonian otter Lontra provocax: Adaptive divergence to marine habitat or signature of southern glacial refugia? BMC Evol. Biol. 2011, 11, 53. [Google Scholar] [CrossRef]
- Sielfeld, W. Características del hábitat de Lutra felina (Molina) y L. provocax Thomas (Carnivora, Mustelidae) en Fuego-Patagonia. Investig. Cienc. Tecnol. Ser. Cienc. Mar 1990, 1, 30–36. [Google Scholar]
- Medina-Vogel, G.; Kaufman, V.S.; Monsalve, R.; Gomez, V. The influence of riparian vegetation, woody debris, stream morphology and human activity on the use of rivers by southern river otters in Lontra provocax in Chile. Oryx 2003, 37, 422–430. [Google Scholar] [CrossRef]
- Medina-Vogel, G.; Bartheld, J.L.; Pacheco, R.A.; Rodríguez, C.D. Population assessment and habitat use by marine otter Lontra felina in southern Chile. Wildl. Biol. 2006, 12, 191–199. [Google Scholar] [CrossRef]
- Villegas, M.J.; Aron, A.; Ebensperger, L.A. The influence of wave exposure on the foraging activity of marine otter, Lontra felina (Molina, 1782) (Carnivora: Mustelidae) in northern Chile. J. Ethol. 2007, 25, 281–286. [Google Scholar] [CrossRef]
- Sielfeld, W.; Capellla, J.; Acevedo, J.; Aguayo, A. The southern river otter, huillín Lontra provocax (Thomas, 1908) and the marine otter, chungungo Lontra felina (Molina, 1782) (Mustelidae: Lutrinae) in the Southern Patagonian fjord and channel system: Distribution and conservation problems. An. Inst. Patagon. 2024, 52. [Google Scholar] [CrossRef]
- Sanino, G.P.; Meza, M.I. Ecología trófica y simpatría de nutrias (Lontra felina y Lontra provocax) en la reserva Añihué, Patagonia chilena. Boletín Mus. Nac. Hist. Nat. 2016, 65, 279–289. [Google Scholar] [CrossRef]
- Valqui, J.; Rheingantz, M.L. Lontra felina. The IUCN Red List of Threatened Species. e.T12303A95970132. Available online: https://www.iucnredlist.org/species/12303/95970132 (accessed on 21 May 2021).
- Sepúlveda, M.; Valenzuela, A.; Pozzi, C.; Medina-Vogel, G.; Chehébar, C. Lontra provocax. The IUCN Red List of Threatened Species 2015, e. T12305A21938042. Available online: https://www.researchgate.net/profile/Alejandro-Valenzuela-3/publication/291161582_Lontra_provocax/links/569e713d08ae4af525446311/Lontra-provocax.pdf (accessed on 12 May 2015).
- Ostfeld, R.; Ebensperger, L.; Klosterman, L.; Castilla, J. Foraging, Activity Budget, and Social-Behavior of the South-American Marine Otter Lutra-Felina (Molina 1782). Natl. Geogr. Res. 1989, 5, 422–438. [Google Scholar]
- Mangel, J.C.; Whitty, T.; Medina-Vogel, G.; Alfaro-Shigueto, J.; Cáceres, C.; Godley, B.J. Latitudinal variation in diet and patterns of human interaction in the marine otter. Mar. Mammal Sci. 2011, 27, E14–E25. [Google Scholar] [CrossRef]
- Choupay, U. Dieta de la Nutria de río Sudamericana (Lontra provocax Thomas 1908) (Mammalia, Carnivora, Mustelidae), en el Parque Nacional Laguna San Rafael [Diet of the South American River Otter (Lontra provocax Thomas 1908) (Mammalia, Carnivora, Mustelidae), in Laguna San Rafael National Park]. Bachelor’s Thesis, Facultad de Ciencias del Mar, Universidad de Valparaíso, Viña del Mar, Chile, 2003. [Google Scholar]
- Biffi, D.; Iannacone, J. Variabilidad trófica de Lontra felina (Molina 1782) (Carnivora: Mustelidae) en dos poblaciones de Tacna (Perú) entre agosto y diciembre de 2006. Mastozoología Neotrop. 2010, 17, 11–17. [Google Scholar]
- Córdova, O.; Rau, J.R.; Suazo, C.G.; Arriagada, A. Estudio comparativo de la ecología alimentaria del depredador de alto nivel trófico Lontra felina (Molina, 1782) (Carnivora: Mustelidae) en Chile. Rev. Biol. Mar. Oceanogr. 2009, 44, 429–438. [Google Scholar] [CrossRef]
- Medina-Vogel, G.; Rodriguez, C.D.; Ricardo, P.; Alvarez, E.; Jose Luis Bartheld, V. Feeding ecology of the marine otter (Lutra felina) in a rocky seashore of the south of Chile. Mar. Mammal Sci. 2004, 20, 134–144. [Google Scholar] [CrossRef]
- Poblete, A.A.; Górski, K.; Moscoso, J. Estimación de dietas del chungungo Lontra felina (Molina, 1782) en dos localidades de la región del Biobío, Chile. Gayana Concepción 2019, 83, 1–9. [Google Scholar] [CrossRef]
- González, C.; Medina-Vogel, G. Dieta del huillín en el humedal de Boroa, IX Región de Chile. In El Huillin Lontra Provocax: Investigaciones Sobre una Nutria Patagonica en Peligro de Extinción; Casini, M.H., Sepúlveda, M.A., Eds.; Serie Fauna Neotropical 1; Publicación de la Organización PROFAUNA: Buenos Aires, Argentina, 2006; Chapter 9; pp. 72–77. [Google Scholar]
- Ministerio del Medio Ambiente. Diagnostico de Información de Línea de Base en Área Propuesta para Conservación en el Fiordo Pitipalena y Áreas Marinas Adyacentes; ONG de Desarrollo Conservación Marina: Santiago, Chile, 2013. [Google Scholar]
- Raimilla, V. Objetivo Específico 3: Evaluación de la composición y estructura de las poblaciones mamíferos marinos presentes en el AMCP-MPU Pitipalena-Añihué. In Informe II, Programa de Monitoreo de la Calidad Ambiental y Objetos de Conservación del AMCP-MU Pitipalena–Añihué; Molinet, C., Ed.; Ministerio del Medio Ambiente, Universidad Austral de Chile, Sede Puerto Montt: Puerto Montt, Chile, 2020. [Google Scholar]
- Molinet, C.; Díaz, M.; González, A.; Henríquez, J.; Matamala, T.; Boldt, J.; Brito, N.; Espinoza, K.; Lafón, A.; Merino, P.; et al. Exploring biodiversity patterns in a marine and coastal protected area: Implications for management within and outside MCPAs. Estuar. Coast. Shelf Sci. 2023, 294, 108540. [Google Scholar] [CrossRef]
- Luebert, F.; Pliscoff, P. Clasificación de pisos de vegetación y análisis de representatividad ecológica de áreas propuestas para la protección en la ecorregión Valdiviana. In Valdivia: Serie de Publicaciones WWF Programa Ecorregión Valdiviana; Valdivia, Chile, 2004; p. 10. Available online: https://wwflac.awsassets.panda.org/downloads/informe_ecorregion_valdiviana_luebert_pliscoff.pdf (accessed on 23 June 2025).
- Dayton, P.K. Ecology of kelp communities. Annu. Rev. Ecol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Fariña, J.M.; Palma, A.T.; Ojeda, F.P. Subtidal kelp associated communities off the temperate Chilean coast. In Food Webs and the Dynamics of Marine Reefs; Oxford Academic: New York, NY, USA, 2008; pp. 79–102. [Google Scholar]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Vásquez, J. Lessonia trabeculata, a subtidal bottom kelp in northern Chile: A case study for a structural and geographical comparison. In Coastal Plant Communities of Latin America; Elsevier: Amsterdam, The Netherlands, 1992; pp. 77–89. [Google Scholar]
- Buschmann, A.H.; Osorio, P.; Reyes, E.; Vega, A.; Vásquez, J.A.; Filún, L.; Hernández-González, M.C. The effect of water movement, temperature and salinity on abundance and reproductive patterns of Macrocystis spp. (Phaeophyta) at different latitudes in Chile. Mar. Biol. 2004, 145, 849–862. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Moreno, C.; Vásquez, J.A.; Hernández-González, M.C. Reproduction strategies of Macrocystis pyrifera (Phaeophyta) in Southern Chile: The importance of population dynamics. J. Appl. Phycol. 2006, 18, 575–582. [Google Scholar] [CrossRef]
- Vásquez, J.; Vega, J. Ecosistemas marinos costeros del Parque Nacional Bosque Fray Jorge. Hist. Nat. Parq. Nac. Bosque Fray Jorge. Ediciones Univ. De La Serena La Serena Chile 2004, 13, 235–252. [Google Scholar]
- Vásquez, J.A.; Vega, J.A. El Niño 1997–1998 en el norte de Chile: Efectos en la estructura y en la organización de comunidades submareales dominadas por algas pardas. El Niño-La Niña 1997, 2000, 119–135. [Google Scholar]
- Asensi, A.; de Reviers, B. Illustrated catalogue of types of species historically assigned to Lessonia (Laminariales, Phaeophyceae) preserved at PC, including a taxonomic study of three South-American species with a description of L. searlesiana sp. nov. and a new lectotypification of L. flavicans. Cryptogam. Algol. 2009, 30, 209. [Google Scholar]
- Rosenfeld, S.; Mendez, F.; Calderon, M.S.; Bahamonde, F.; Rodríguez, J.P.; Ojeda, J.; Marambio, J.; Gorny, M.; Mansilla, A. A new record of kelp Lessonia spicata (Suhr) Santelices in the Sub-Antarctic Channels: Implications for the conservation of the “huiro negro” in the Chilean coast. PeerJ 2019, 7, e7610. [Google Scholar] [CrossRef]
- Martin, P.; Zuccarello, G.C. Molecular phylogeny and timing of radiation in Lessonia (Phaeophyceae, Laminariales). Phycol. Res. 2012, 60, 276–287. [Google Scholar] [CrossRef]
- Macaya, E.C.; Zuccarello, G.C. DNA Barcoding and genetic divergence in the Giant Kelp Macrocystis (Laminariales). J. Phycol. 2010, 46, 736–742. [Google Scholar] [CrossRef]
- Macaya, E.C.; Zuccarello, G.C. Genetic structure of the giant kelp Macrocystis pyrifera along the southeastern Pacific. Mar. Ecol. Prog. Ser. 2010, 420, 103–112. [Google Scholar] [CrossRef]
- Plana, J.; Mansilla, A.; Palacios, M.; Navarro, N.P. Estudio poblacional de Macrocystis pyrifera (L.) C. Agardh (Laminariales: Phaeophyta) en ambientes protegido y expuesto al oleaje en Tierra del Fuego. Gayana Concepción 2007, 71, 66–75. [Google Scholar] [CrossRef]
- Almanza, V.; Buschmann, A.H. The ecological importance of Macrocystis pyrifera (Phaeophyta) forests towards a sustainable management and exploitation of Chilean coastal benthic co-management areas. Int. J. Environ. Sustain. Dev. 2013, 12, 341. [Google Scholar] [CrossRef]
- Vanella, F.A.; Fernández, D.A.; Romero, M.C.; Calvo, J. Changes in the fish fauna associated with a sub-Antarctic Macrocystis pyrifera kelp forest in response to canopy removal. Polar Biol. 2007, 30, 449–457. [Google Scholar] [CrossRef]
- Ríos, C.; Mutschke, E. Contribution to the knowledge of Macrocystis pyrifera: Bibliographic review of the kelp forests distributed in the Magellan region. In Anales del Instituto de la Patagonia; Universidad de Magallanes: Punta Arenas, Chile, 2009; Volume 37, No. 1; pp. 97–102. [Google Scholar]
- Alveal, K. Estudios ficoecológicos en la región costera de Valparaíso. Rev. Biol. Mar 1970, 14, 7–88. [Google Scholar]
- Sielfeld, W. Sobreposición de nicho y patrones de distribución de Lutra felina y L. provocax (Mustelidae: Carnivora) en el medio marino de Sudamérica austral. An. Del Mus. De Hist. Nat. De Valparaíso 1989, 20, 103–108. [Google Scholar]
- Cáceres, M.; Valle-Levinson, A.; Molinet, C.; Castillo, M.; Bello, M.; Moreno, C. Lateral variability of flow over a sill in a channel of southern Chile. Ocean Dyn. 2006, 56, 352–359. [Google Scholar] [CrossRef]
- Molinet, C.; Valle-Levinson, A.; Moreno, C.; Cáceres, M.; Bello, M.; Castillo, M. Effects of sill processes on the distribution of epineustonic competent larvae in a stratified system of Southern Chile. Mar. Ecol. Prog. Ser. 2006, 324, 95–104. [Google Scholar] [CrossRef][Green Version]
- Díaz, P.; Molinet, C.; Cáceres, M.A.; Valle-Levinson, A. Seasonal and intratidal distribution of Dinophysis spp. in a Chilean fjord. Harmful Algae 2011, 10, 155–164. [Google Scholar] [CrossRef]
- Reuther, C.; Dolch, D.; Green, R.; Jahrl, J.; Jefferies, D.; Krekemeyer, A.; Kucerova, M.; Madsen, A.B.; Romanowski, J.; Roche, K. Surveying and monitoring distribution and population trends of the Eurasian otter (Lutra lutra). Habitat 2000, 12, 148. [Google Scholar]
- Osgood, W.H. The Mammals of Chile; Publication (Field Museum of Natural History: 1909), Zoological Series; Smithsonian Institution: Washington, DC, USA, 1943; Volume 30. [Google Scholar]
- Sielfeld, W. Biología y conservación del huillín en los canales magallánicos de Chile. In El huillin Lontra Provocax: Investigaciones Sobre una Nutria Patagonica en Peligro de Extinción; Casini, M.H., Sepúlveda, M.A., Eds.; Serie Fauna Neotropical 1; Publicación de la Organización PROFAUNA: Buenos Aires, Argentina, 2006; Chapter 6; pp. 46–53. [Google Scholar]
- Acevedo, J.F.A.; Sielfeld, W.; Aguayo-Lobo, A.; Esquivel, M.; Quilahuilque, G. Estandarización Metodológica para el Desarrollo de Líneas Base y Seguimientos Ambientales de Mamíferos Marinos en Aguas Jurisdiccionales Chilenas; FIPA 2018–42; FIPA: Valparaíso, Chile, 2019. [Google Scholar]
- Marine Mammal Protection Act. Available online: https://www.fisheries.noaa.gov/action/mmpa-list-fisheries-2021 (accessed on 13 May 2021).
- Delfín-Alonso, C.C.; Gallina-Tessaro, S.A.; López-González, C.A. El hábitat: Definición, dimensiones y escalas de evaluación para la fauna silvestre. In Manual de Técnicas Para El Estudio de la Fauna; Gallina, C., López-González, C., Eds.; INE–Semarnat: Mexico City, Mexico, 2012; ISBN 978-607-7740-98-8. [Google Scholar]
- Greene, H.G.; Yoklavich, M.M.; Starr, R.M.; O’COnnell, V.M.; Wakefield, W.; E Sullivan, D.; E McRea, J.; Cailliet, G.M. A classification scheme for deep seafloor habitats. Oceanol. Acta 1999, 22, 663–678. [Google Scholar] [CrossRef]
- Krausman, P.R. Some basic principles of habitat use. In Grazing Behavior of Livestock and Wildlife; University of Idaho: Moscow, Russia, 1999; Volume 70, pp. 85–90. [Google Scholar]
- Morrison, M.L.; Block, W.M.; Strickland, M.D.; Collier, B.A.; Peterson, M.J. Wildlife Study Design; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Kruuk, H.; Moorhouse, A.; Conroy, J.; Durbin, L.; Frears, S. An estimate of numbers and habitat preferences of otters Lutra lutra in Shetland, UK. Biol. Conserv. 1989, 49, 241–254. [Google Scholar] [CrossRef]
- Prenda, J.; Granado-Lorencio, C. The relative influence of riparian habitat structure and fish availability on otter Lutra lutra L. sprainting activity in a small Mediterranean catchment. Biol. Conserv. 1996, 76, 9–15. [Google Scholar] [CrossRef]
- Anoop, K.; Hussain, S. Factors affecting habitat selection by smooth-coated otters (Lutra perspicillata) in Kerala, India. J. Zool. 2004, 263, 417–423. [Google Scholar] [CrossRef]
- Carrillo-Rubio, E.; Lafón, A. Neotropical river otter micro-habitat preference in West-central Chihuahua, Mexico. IUCN Otter Spec. Group Bull. 2004, 21, 10–15. [Google Scholar]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Crozzoli, L.; Batalla, R.J. Aplicación de la fotografía al análisis granulométrico de ríos con lecho de gravas. Rev. C&G 2003, 17, 29–39. [Google Scholar]
- Kondolf, G.M.; Piégay, H. Tools in Fluvial Geomorphology. Problem Statement and Recent Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Aonken Consultores Ltda. Catastro y Evaluación de Recursos Parques Nacionales Hernando de Magallanes, Alberto de Agostini y Reserva Forestal Holanda; Informe Técnico; Corporación Nacional Forestal (CONAF) XII Región; Aonken Consultores Ltda.: Punta Arenas, Chile, 1982; 204p. [Google Scholar]
- Aonken Consultores Ltda. Catastro y Evaluación de Recursos Parques Nacionales Reserva Forestal Alacalufes y Reserva Forestal Isla Riesco; Informe Técnico; Corporación Nacional Forestal (CONAF) XII Región; Aonken Consultores Ltda.: Punta Arenas, Chile, 1982; 185p. [Google Scholar]
- John, D.M.; Paterson, G.L.J.; Evans, N.J.; Ramírez, M.E.; Spencer Jones, M.; Báez, P.D.; Ferrero, T.J.; Valentine, C.A.; Reid, D.G. Manual de Biotopos Marinos de la Región de Aysén, Sur de Chile; Goverment Printing Office: Washington, DC, USA, 2003. [Google Scholar]
- Santelices, B.; Ojeda, F. Population dynamics of coastal forests of Macrocystis pyrifera in Puerto Toro, Isla Navarino, southern Chile. Mar. Ecol. Prog. Ser. 1983, 14, 175–183. [Google Scholar] [CrossRef]
- Alveal, K.; Romo, H.; Valenzuela, J. Consideraciones ecológicas de las regiones de Valparaíso y de Magallanes. Rev. Biol. Mar. 1973, 15, 1–29. [Google Scholar]
- Alveal, K.; Romo, H. Estudios de distribución vertical de la biota costera en el Seno de Reloncavi-Chile. Gayana Miscelánea 1977, 7, 3–28. [Google Scholar] [CrossRef]
- Romo, H.; Alveal, K. Las comunidades del litoral rocoso de Punta Ventanilla Bahía de Quintero-Chile. Gayana Miscelánea 1977. [Google Scholar] [CrossRef]
- Santelices, B. Phytogeographic characterization of the temperate coast of Pacific South America. Phycologia 1980, 19, 1–12. [Google Scholar] [CrossRef]
- González, A.; Beltrán, J.; Hiriart-Bertrand, L.; Flores, V.; de Reviers, B.; Correa, J.A.; Santelices, B. Identification of cryptic species in the Lessonia nigrescens complex (Phaeophyceae, Laminariales). J. Phycol. 2012, 48, 1153–1165. [Google Scholar] [CrossRef]
- Searles, R. The genus Lessonia Bory (Phaeophyta, Laminariales) in Southern Chile and Argentina. Br. Phycol. J. 1978, 13, 361–381. [Google Scholar] [CrossRef]
- Mansilla, A.; Gérard, K.; Boo, G.H.; Ramirez, M.E.; Ojeda, J.; Rosenfeld, S.; Murcia, S.; Marambio, J.; Gonzalez-Wevar, C.; Calderon, M.S.; et al. Populations of a new morphotype of corrugate Lessonia Bory in the Beagle Channel, sub-Antarctic Magellanic ecoregion: A possible case of on-going speciation. Cryptogam. Algol. 2020, 41, 105–119. [Google Scholar] [CrossRef]
- Scrosati, R.A. Estudios anatómicos en Lessonia vadosa (Phaeophyta, Laminariales) de la Argentina. Bol. Soc. Argent. Bot. Bol. 1991, 27, 165–171. [Google Scholar]
- R Core Team. R: A Language and Enviroment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 5 April 2025).
- Cabello, C. La nutria de mar en la isla de Chiloé; CONAF: Santiago, Chile, 1983. [Google Scholar]
- Ebensperger, L.A.; Castilla, J.C. Selección de hábitat en tierra por la nutria marina, Lutra felina, en Isla Pan de Azúcar, Chile. Rev. Chil. Hist. Nat. 1992, 65, 429–434. [Google Scholar]
- Espinosa, M.I. Dieta y Uso de Hábitat del Huillín (Lontra provocax) en Ambientes de Agua Dulce y su Relación con Comunidades Locales en el Bosque Templado Lluvioso, Isla Grande de Chiloé, Chile. Bachelor’s Thesis, Universidad Mayor, Santiago, Chile, 2012. [Google Scholar]
- Sepúlveda, M.A.; Bartheld, J.L.; Monsalve, R.; Gómez, V.; Medina-Vogel, G. Habitat use and spatial behaviour of the endangered Southern river otter (Lontra provocax) in riparian habitats of Chile: Conservation implications. Biol. Conserv. 2007, 140, 329–338. [Google Scholar] [CrossRef]
- Astorga, C.; Benavides, M.; Sepúlveda, M.; Bartheld, J.L.; Medina-Vogel, G. Variables de paisaje y su relación con la distribución del huillín en las cuencas del río Toltén y Queule. In El huillin Lontra Provocax: Investigaciones Sobre una Nutria Patagonica en Peligro de Extinción; Casini, M.H., Sepúlveda, M.A., Eds.; Serie Fauna Neotropical 1; Publicación de la Organización PROFAUNA: Buenos Aires, Argentina, 2006; Chapter 10; pp. 79–83. [Google Scholar]
- Medina-Vogel, G.; Boher, F.; Flores, G.; Santibañez, A.; Soto-Azat, C. Spacing behavior of marine otters (Lontra felina) in relation to land refuges and fishery waste in central Chile. J. Mammal. 2007, 88, 487–494. [Google Scholar] [CrossRef]
- Medina-Vogel, G.; Barros, M.; Organ, J.; Bonesi, L. Coexistence between the southern river otter and the alien invasive N orth A merican mink in marine habitats of southern C hile. J. Zool. 2013, 290, 27–34. [Google Scholar] [CrossRef]
- Vianna, J.A.; Ayerdi, P.; Medina-Vogel, G.; Mangel, J.C.; Zeballos, H.; Apaza, M.; Faugeron, S. Phylogeography of the marine otter (Lontra felina): Historical and contemporary factors determining its distribution. J. Hered. 2010, 101, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, M.A.; Singer, R.S.; Silva-Rodríguez, E.A.; Eguren, A.; Stowhas, P.; Pelican, K. Invasive American mink: Linking pathogen risk between domestic and endangered carnivores. EcoHealth 2014, 11, 409–419. [Google Scholar] [CrossRef]
- Villegas, M.J.; Laudien, J.; Sielfeld, W.; Arntz, W.E. Fish Abundances in a Lessonia Trabeculata Kelp Bed at Chipana Bay, Chile; Dataset; Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research: Bremerhaven, Germany, 2018. [Google Scholar] [CrossRef]
- Viviana Berrios, C.; Mauricio Vargas, F. Estructura trófica de la asociación de peces intermareales de la costa rocosa del norte de Chile. Rev. Biol. Trop. 2004, 52, 201–212. [Google Scholar] [CrossRef]
- Vargas, M.; Fuentes, P.; Hernáez, P.; Olivares, A.; Rojas, P. Relaciones tróficas de cinco peces costeros comunes en el área submareal del norte de Chile (20°11′–20°20′ S). Rev. De Biol. Trop. 1999, 47, 601–604. [Google Scholar] [CrossRef]
- Velimirov, B.; Field, J.; Griffiths, C.; Zoutendyk, P. The ecology of kelp bed communities in the Benguela upwelling system: Analysis of biomass and spatial distribution. Helgoländer Wiss. Meeresunters. 1977, 30, 495–518. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management: Selected Readings; Springer: Berlin/Heidelberg, Germany, 1994; pp. 130–147. [Google Scholar]
- Levins, R.; Lewontin, R. The Dialectical Biologist; Harvard University Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Tegner, M.J.; Dayton, P.K. Ecosystem effects of fishing in kelp forest communities. ICES J. Mar. Sci. 2000, 57, 579–589. [Google Scholar] [CrossRef]





| Credits | Site | Record Type | Date | Latitude | Longitude | Species |
|---|---|---|---|---|---|---|
| Raimilla (2020) [42] | Punta Huala | Sighting | 9 November 2019 | 43°43′45.2″ S | 73°02′50.9″ W | L. felina |
| New data | Punta Piti: Los Patos | Sighting | 7 November 2019 | 43°44′34.0″ S | 73°00′09.0″ W | L. felina |
| Raimilla (2020) [42] | Frente Raúl Marín | Sighting | 7 November 2019 | 43°46′11.7″ S | 72°56′37.8″ W | L. felina |
| Parra (2022 Com. Pers) [43] | Piti Palena: Ensenada de las Islas | Video | 26 March 2022 | 43°45′53.7″ S | 72°54′32.9″ W | L. provocax |
| New data | Raúl Marín, Canal Garrao | Sighting | 9 February 2022 | 43°49′22.6″ S | 72°57′03.9″ W | L. provocax |
| New data | Rio Rodriguez, sector Barra | Sighting | 1 November 2021 | 43°46′00.9″ S | 72°49′42.8″ W | L. provocax |
| P. Merino (2022) [43] | Las Hermanas islets; north side | Photo/video | 4 February 2022 | 43°46′08.2″ S | 73°01′48.7″ W | L. felina/L. provocax |
| New data | Las Hermanas islets; south side | Photo | 4 February 2022 | 43°46′27.6″ S | 73°01′47″ W | L. provocax |
| New data | Las Hermanas, islote Oeste | Photo | 4 February 2022 | 43°46′22.1″ S | 73°02′53.1″ W | L. felina |
| Raimilla (2020) [42] | Islote Alleupa | Sighting | 9 November 2019 | 43°49′25.9″ S | 73°01′38.0″ W | L. felina |
| New data | Rio Añihue sector bajo | Sighting | 5 February 2022 | 43°50′10.5″ S | 72°59′57.5″ W | L. provocax |
| New data | Estero sur, Bahía Mala | Sighting | 6 February 2022 | 43°55′40.0″ S | 73°03′31.3″ W | L. provocax |
| Sanino and Meza (2016) [30] | Añihue, Islas Velasco 5 | Photo | January 2015 to April 2016 | 43°52′26.2″ S | 73°03′05.7″ W | L. felina/L. provocax |
| Sanino and Meza (2016) [30] | Añihue, Islas Velasco 6 | Photo | January 2015 to April 2016 | 43°51′28.7″ S | 73°03′02.1″ W | L. felina |
| Sanino and Meza (2016) [30] | Añihue, Islas Velasco 7 | Photo | January 2015 to April 2016 | 43°51′55.7″ S | 73°03′13.0″ W | L. felina |
| Sanino and Meza (2016) [30] | Añihue, Islas Velasco 8 | Photo | January 2015 to April 2016 | 43°52′13.2″ S | 73°03′17.1″ W | L. felina/L. provocax |
| Sanino and Meza (2016) [30] | Bahía Añihue interior 01 | Photo | January 2015 to April 2016 | 43°52′21.1″ S | 73°00′56.2″ W | L. felina/L. provocax |
| Sanino and Meza (2016) [30] | Bahía Añihue interior 03 | Photo | January 2015 to April 2016 | 43°52′26.3″ S | 73°00′59.8″ W | L. felina |
| Sanino and Meza (2016) [30] | Bahía Añihue interior 10 | Photo | January 2015 to April 2016 | 43°52′28.3″ S | 73°01′43.5″ W | L. felina/L. provocax |
| New data | Islotes Los Payos | Sighting | 5 February 2022 | 43°50′48.9″ S | 73°04′00.3″ W | L. felina |
| New data | Isla Refugio, islotes Crujul | Sighting | 29 March 2022 | 43°52′48.7″ S | 73°08′13.6″ W | L. felina |
| New data | Islas Agnus, Puerto Bonito | Sighting | 5 February 2022 | 43°53′09.1″ S | 73°03′39.3″ W | L. provocax |
| New data | Isla Refugio, Punta Melipichún | Sighting | 28 March 2022 | 43°57′31.5″ S | 73°07′25.1″ W | L. provocax |
| New data | Isla Larga, Santo Domingo | Sighting | 6 May 2022 | 43°58′03.1″ S | 73°06′51.2″ W | L. provocax |
| New data | Rio Refugio | Sighting | 6 February 2022 | 43°56′35.1″ S | 73°04′54.4″ W | L. provocax |
| New data | Estero Santo Domingo | Sighting | 6 February 2022 | 43°58′42.5″ S | 73°05′53.6″ W | L. provocax |
| New data | Islas Guaquel 1 | Photo | 5 February 2022 | 44°01′34.1″ S | 73°07′36.1″ W | L. provocax |
| New data | Islas Guaquel 2 | Photo | 6 May 2022 | 44°01′39.2″ S | 73°07′20.5″ W | L. provocax |
| Lontra provocax | Lontra felina | ||||
|---|---|---|---|---|---|
| Attributes | Characters | n | % | n | % |
| Exposition | Exposed | 6 | 37.50 | ||
| Semiexposed | 1 | 6.25 | 3 | 18.75 | |
| Protected | 15 | 93.75 | 7 | 43.75 | |
| Grain type | Cobles | 3 | 18.75 | ||
| Boulders | 2 | 12.5 | |||
| Cliffs | 11 | 68.75 | 16 | 100.00 | |
| Slope | ≥45° | 14 | 87.50 | 15 | 93.75 |
| <45° | 2 | 12.5 | 1 | 6.25 | |
| Vegetation | Evergreen forest of Puyuhuapi | 16 | 100.00 | 10 | 62.50 |
| Oceanic evergreen shrub | 2 | 12.50 | |||
| Shrub strip of the oceanic coastline | 4 | 25.00 | |||
| Physiognomy | Normal high forest | 2 | 11.76 | ||
| Normal low forest | 13 | 76.47 | 9 | 56.25 | |
| Low stunted tres | 1 | 5.88 | 2 | 12.50 | |
| Grases and herb | 1 | 5.88 | 1 | 6.25 | |
| Bare devegetated rock | 4 | 25.00 | |||
| Shore vegetation | Vegetated but shady | 13 | 81.25 | ||
| Vegetated but sunny | 2 | 12.50 | 10 | 62.50 | |
| Open | 1 | 6.25 | 6 | 37.50 | |
| Macroalgae | Durvillaea antartica+Lessonia berteroana/spicata | 6 | 37.50 | ||
| Lessonia berteroana/spicata+Macrocystis integrifolia | 1 | 7.69 | 2 | 12.50 | |
| Macrocystis integtifolia+Lessonia vadosa | 9 | 69.23 | 8 | 5.00 | |
| Gracilaria+Enteromorpha+Ulva | 3 | 23.08 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sielfeld, W.; Bunster, C.; Guzmán, J.A.; Buscaglia, M.; Sánchez Jardón, L.; Clark, A.; Briones, R. Habitat Selection of Sympatric Lontra felina and L. provocax in Chilean Patagonia: Toward Sustainable Management. Sustainability 2025, 17, 8290. https://doi.org/10.3390/su17188290
Sielfeld W, Bunster C, Guzmán JA, Buscaglia M, Sánchez Jardón L, Clark A, Briones R. Habitat Selection of Sympatric Lontra felina and L. provocax in Chilean Patagonia: Toward Sustainable Management. Sustainability. 2025; 17(18):8290. https://doi.org/10.3390/su17188290
Chicago/Turabian StyleSielfeld, Walter, Claudia Bunster, Jonathan A. Guzmán, Marx Buscaglia, Laura Sánchez Jardón, Arturo Clark, and Raúl Briones. 2025. "Habitat Selection of Sympatric Lontra felina and L. provocax in Chilean Patagonia: Toward Sustainable Management" Sustainability 17, no. 18: 8290. https://doi.org/10.3390/su17188290
APA StyleSielfeld, W., Bunster, C., Guzmán, J. A., Buscaglia, M., Sánchez Jardón, L., Clark, A., & Briones, R. (2025). Habitat Selection of Sympatric Lontra felina and L. provocax in Chilean Patagonia: Toward Sustainable Management. Sustainability, 17(18), 8290. https://doi.org/10.3390/su17188290






