1. Introduction
Over the decades, the worldwide demand for plastics has experienced exponential growth due to the wide range of properties offered by this class of materials. Their durability, versatility, low cost, and the possibility of adapting their properties to specific needs have made polymers a cornerstone of technological development. Reflecting their importance, a report by PlasticsEurope states that approximately 400 million tons of plastics were produced in 2022, with forecasts to reach 600 million tons in the 2050s [
1].
Among the most widely used thermoplastic polymers is polyvinyl chloride (PVC), valued for its high corrosion resistance, chemical stability, electrical insulation and excellent resistance to water and environmental conditions [
2]. In addition, the performance of PVC can be modified by the addition of additives, making it suitable for a wide range of applications [
3]. In recent years, PVC has become one of the most widely produced polymers worldwide, accounting for 10% of total plastics production in 2021, with primary uses in the healthcare, electrical, and structural sectors [
4].
PVC is synthesized from vinyl chloride monomer (VCM), a compound classified as a carcinogen by the U.S. Environmental Protection Agency and the International Agency for Research on Cancer. Exposure to VCM has been associated with liver damage, functional impairment, and, in some cases, cirrhosis [
5]. In addition to the health hazards associated with VCM, another critical challenge in PVC production is the high water consumption required for the polymerization and post-treatment stages, which affects both environmental sustainability and process profitability.
To solve this problem, water recycling has been implemented in modern PVC production processes in order to significantly reduce the use of freshwater. However, the recirculation of both VCM and water introduces additional safety considerations due to the hazardous nature of the materials and the complexity of the integrated system. This underscores the need for a thorough safety assessment.
The Inherent Safety Index (ISI) analysis, developed by Heikkilä [
6], provides a systematic methodology for assessing chemical and process hazards by evaluating key parameters such as the nature of the substances involved, operating conditions, and type of equipment. This approach allows for the quantitative assessment of whether a process meets the criteria for being intrinsically safe [
7].
Previous studies, such as that of Aguilar-Vazquez [
7], have worked on the safety assessment of the PVC production process based on the considerations and risks it represents. Guardo-Ruiz et al. [
8] evaluate the implementation of direct water recycling, based on parameters such as the fractional water consumption and production yield, showing a reduction in the water consumption index from 2.8 to 2.2, with a 99.8% production yield of PVC, showing substantial improvement. However, the implementation of direct water recycling as a fundamental part of the process has not been evaluated by means of the inherent safety method. The objective of this study is to perform an intrinsic safety analysis of an industrial-scale PVC production process incorporating direct water recycling. The methodology includes hazard identification, parameter evaluation, and assessment of safety control measures, thus contributing to safer and more sustainable polymer manufacturing.
3. Results
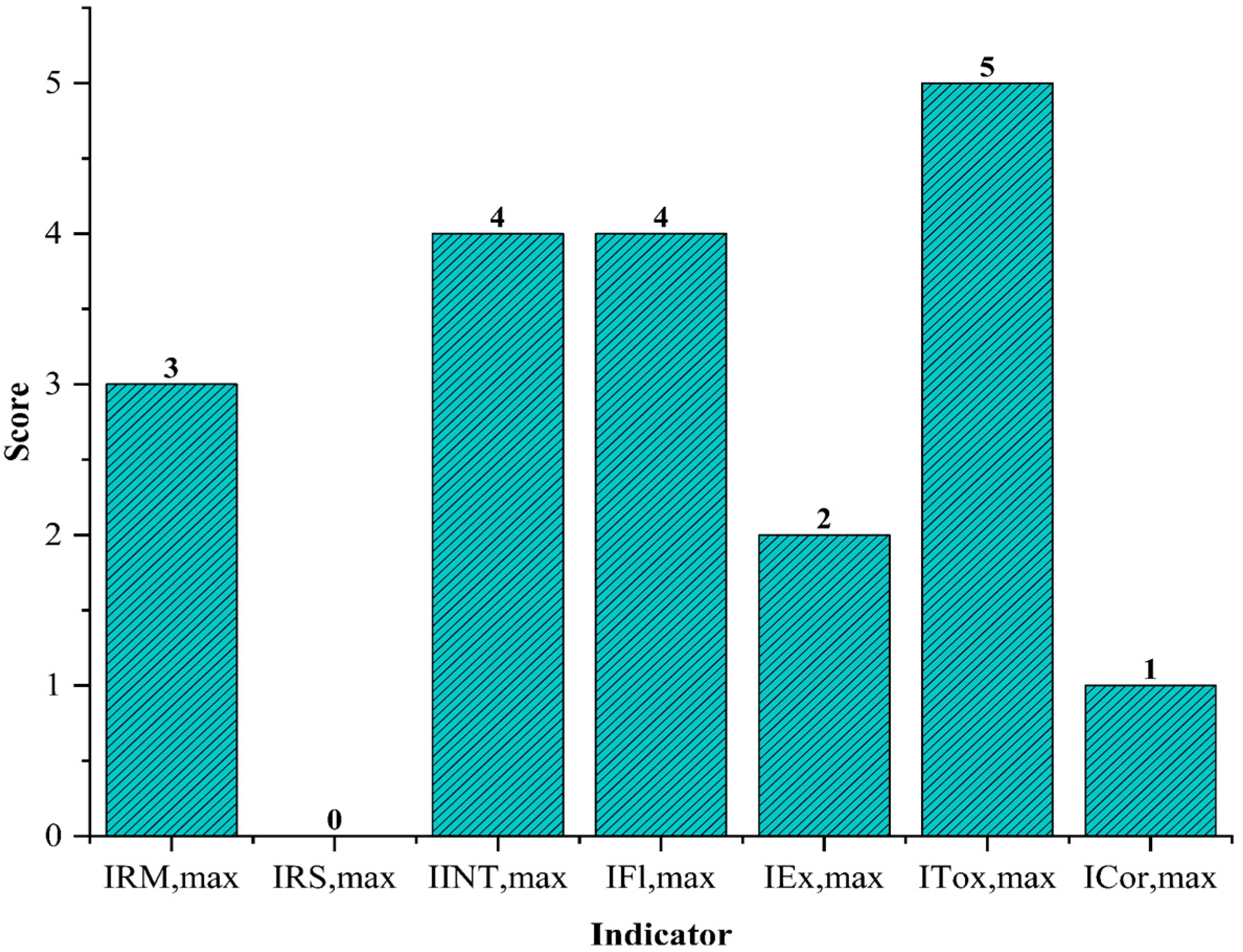
The results of the safety sub-indexes inherent to the chemical substances involved in the production process of PVC in suspension integrated with a direct water recycling system are presented in
Figure 2. PVC production is a representative process in the polymer industry, whose thermal and chemical control is crucial due to the exothermic and complex nature of VCM polymerization. This polymerization reaction, with an enthalpy of approximately −1600 kJ/kg, is a reaction that demands careful management of the heat generated to prevent safety risks and ensure polymer quality. Therefore, the
sub-index was assigned a score of 3, reflecting the need for a continuous and effective cooling system to control the exothermic reaction and avoid episodes of thermal runaway that could trigger serious accidents.
The process is characterized by the absence of significant secondary reactions, which is the reason why the sub-index , corresponding to the heat generated by additional reactions, was qualified with a value of 0. This particularity facilitates the control of the polymeric process, focusing efforts on the stability and safety of the main polymerization reaction.
In the context of polymer research and development, one of the most relevant aspects is the study of adverse chemical interactions that may affect both the process and the final product. In the production system analyzed, important risks associated with the reaction of VCM with atmospheric oxygen and the chemical and thermal degradation of both the monomer and the PVC polymer under the action of free oxygen were identified [
15]. These phenomena, which can compromise the integrity of the polymer chain and reduce the final properties of PVC, are the subject of advanced research in polymer science, where strategies are being sought to improve the thermal and chemical stability of the material. Despite the control mechanisms in place, the
sub-index received a high score of 4, indicating that these risks must be rigorously monitored to avoid incidents such as fires or explosions.
Properties such as toxicity, flammability, and explosiveness were considered to determine whether the substances present in the production environment could be hazardous. These properties were obtained from documents using databases [
16] and safety data sheets. It was determined that vinyl chloride is the most dangerous substance in use, with a score of 11, as shown in
Table 12, encompassing the sub-indexes
,
, and
with scores of 4, 2, and 5, respectively. Unlike other substances in the process, such as the polymer, PVA, or the initiator, which are also toxic and flammable, VCM exists in a solid or liquid state with high flash points.
Table 13 represents the chemical interactions between the compounds involved in the polymerization system, which was designed using CAMEO Chemicals version 3.1.0. The table shows that the compound that presents the highest risk is VCM, and the reason is that VCM does not present any compatible interaction with other chemical compounds involved in the process. The reason for the incompatibility of the VCM with the chemical compounds is the instability in the structure of the monomer; for example, the compatibility between vinyl chloride and oxygen produces explosion for the interaction with the carbon double bond inside of the VCM structure and the oxygen molecule. Additionally, the rest of the compounds can produce toxic gases and liberate heat when reacting with VCM. The generation of heat is influenced by the VCM flashpoint (−78 °C), which is lower than the ambient temperature.
A literature review revealed that the recommended material for use in the suspension PVC production line is stainless steel, as it has proven to be safe and of excellent quality in various similar applications. In contrast, materials such as carbon steel or aluminum alloys are susceptible to degradation in the presence of substances such as water, which can cause corrosion, and vinyl chloride monomer (VCM). When combined with high temperatures and pressures, these conditions may subject the material to significant stress [
17]. In addition, maintaining the purity of the final product, the PVC resin, is crucial due to quality requirements. Therefore, it is essential to use materials that do not alter product characteristics, such as the color. The selected material must be non-reactive and exhibit high sanitary compatibility, ensuring inert and contaminant-free conditions throughout the process. Based on these considerations, the score assigned to the
sub-index is 1.
Figure 3 presents the safety sub-indexes associated with the suspension PVC production process integrated with direct water recycling. For the inventory sub-index, both the ISBL and OSBL were quantified based on the equipment’s capacity during one hour of operation. The OSBL inventory was calculated from the storage capacity of the process’s raw materials, resulting in 37.5 t/h, which corresponds to a score of 1. On the other hand, the ISBL inventory, considering all equipment, amounted to 2891.5 t/h, leading to a score of 5. Consequently, the overall inventory sub-index was assigned a score of 5, as it reflects the highest level of risk.
Regarding the pressure safety sub-index (), the maximum pressure found within the process was 13.7 bar, which corresponds to a score of 1. For the temperature safety sub-index (), the highest recorded temperature was 250 °C at the burner in the drying section; thus, a score of 2 was assigned. Concerning the equipment safety sub-index (), high-risk equipment such as boilers, heaters, and compressors were considered, leading to a score of 4.
A critical aspect in the sustainable production of polymers, particularly in the manufacture of PVC in suspension, is the integration of direct water recycling systems within the process. This practice not only improves operational efficiency and reduces the consumption of water resources but also poses relevant challenges in terms of process safety and final product quality. The presence of contaminants in recycled water can compromise the purity of the PVC produced, generating negative effects on its physicochemical and mechanical properties, which is particularly relevant in applications where high quality standards are required. In addition, such contamination can increase the risks to occupational health, the environment, and consumers of the final product, if adequate treatment and monitoring measures are not implemented.
In the analysis of the structural safety sub-index (), not only should specific accidents occurring in PVC suspension production plants with direct recycling integration be considered but also how such integration, within the framework of a process intensification and sustainability strategy, can influence overall operational safety.
Although no documented accidents have been reported specifically in plants with direct water recycling, the industry’s historical experience has shown that human error and deficiencies in control systems can trigger large-scale incidents. For example, significant accidents have been reported in PVC suspension production plants, such as the explosion and fire in a Formosa Plastics plant, caused by the incorrect opening of a valve that released a reactive mixture with VCM, a highly flammable compound [
17]. Similarly, at the Mexican petrochemical plant, an accident involving VCM caused the death of 32 people after an explosion occurred associated with the handling of the monomer [
18].
Although these events occurred more than a decade ago and it is presumed that stricter safety mechanisms have been implemented since then, their existence underscores the need to maintain a robust safety culture and process engineering focused on prevention. Consequently, and considering both the potential risks of direct recycling integration and experience with this type of facility, the sub-index was assessed with a score of 2.
4. Discussion
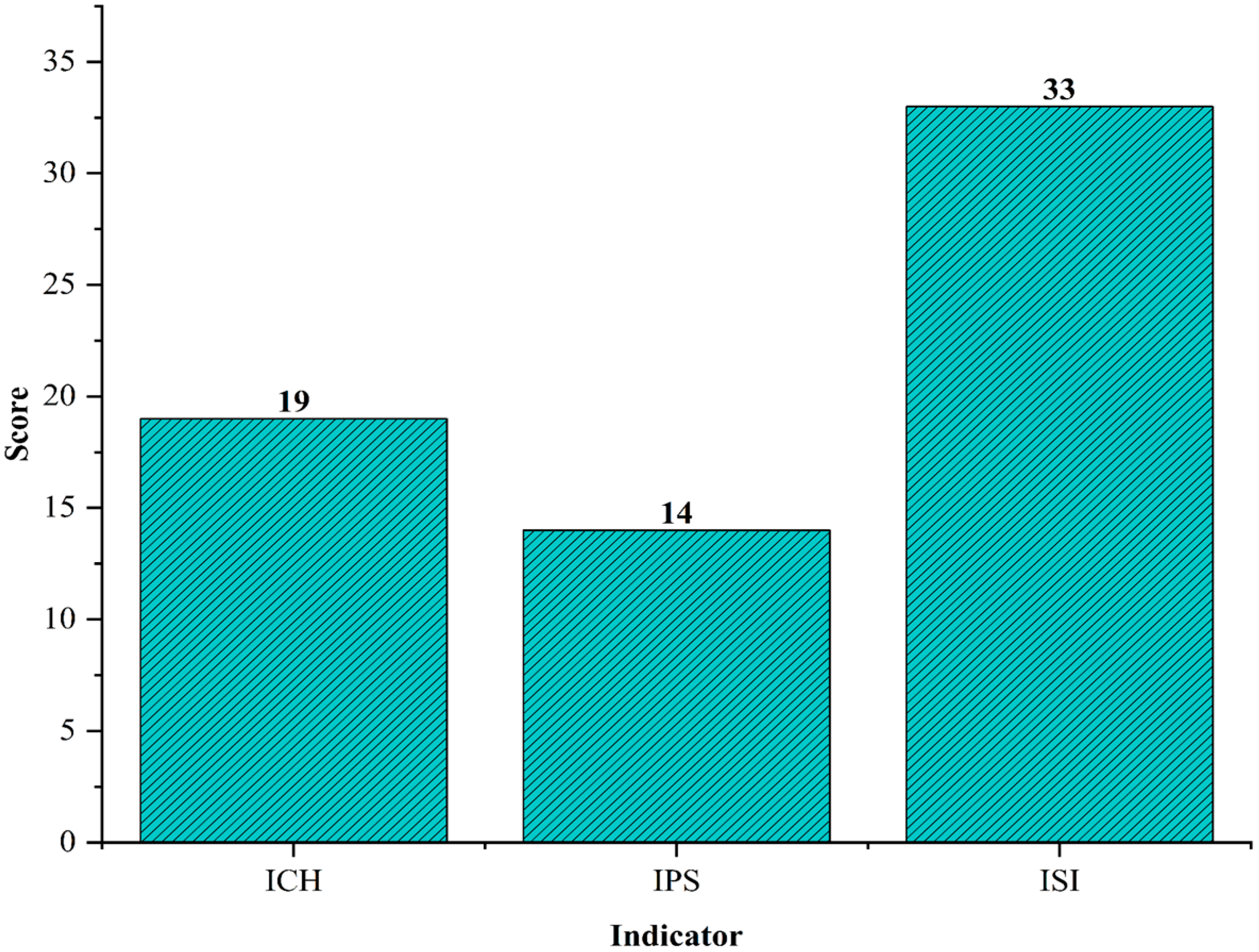
Figure 4 presents the Inherent Safety Index (ISI) for the process, with a process-based score of 14 and a substance-based score of 19, totaling an overall ISI of 33. This value, being significantly above the threshold of 24, confirms that the suspension PVC production process integrated with direct water recycling presents high inherent risks. While the data clearly identify the main contributors to the index, a deeper understanding of their implications is essential.
A critical factor is the contribution of vinyl chloride monomer (VCM), which accounts for over half of the substance-based score. Its classification as highly flammable and toxic positions it as the principal hazard in the system. In contrast to other chemical routes such as the production of acetic acid, where feedstocks can be substituted depending on the chosen pathway, PVC production is rigid due to its exclusive dependence on VCM. The inability to replace VCM with a less hazardous substance reflects a structural limitation of the process, which reinforces the need for robust containment and control strategies rather than substitution-based risk reduction. This aligns with previous discussions in the literature about the trade-offs between material functionality and process safety [
19].
In terms of inventory, the process scored the maximum value due to the large material flows managed, which are nearly triple the threshold of the last scoring range. While this may appear excessive, similar scoring has been observed in standard VCM production settings [
20], suggesting that high-volume handling is a systemic feature of the PVC value chain rather than a specific design flaw. However, recognizing this normativity should not diminish the associated risks. Instead, it points to a need for system-wide innovations in process intensification or real-time inventory control to mitigate the accumulation of hazardous materials. Equipment-related hazards also play a significant role, particularly due to the use of heaters and furnaces, which increase the likelihood of fire or explosion when interacting with flammable materials. Although these units are common in thermal processes, their presence in a context where the raw material is inherently unstable amplifies their criticality. In line with this, measures such as relocating these units outside the battery limits (OSBL) should not be seen merely as optional design improvements but as essential adaptations in inherently high-risk environments.
Interestingly, the operating conditions in this process—250 °C and 13.7 bar—do not significantly elevate the ISI. These parameters are moderate compared to other processes such as benzene and acetone production, which operate under more extreme conditions [
18]. This suggests that thermal and pressure hazards are not the main drivers of risk in this case. However, it is worth noting that the ISI does not capture the full complexity of dynamic risks under real operating conditions. For instance, rapid fluctuations in temperature or pressure in heat exchange units may still trigger dangerous events if not managed with adequate real-time monitoring systems.
The Safe Process Structure sub-index was found to be neutral, consistent with previous studies such as that by Carvalho et al. [
21]. While this may appear reassuring, the neutrality suggests that the current process design does not actively contribute to risk reduction. Given the high hazard level identified in other sub-indexes, this neutrality may represent a missed opportunity for design-based mitigation.
Finally, when interpreting the broader implications, the analysis supports the view that traditional PVC production is constrained not only by material choice but also by systemic features such as large inventories and reliance on thermal equipment. These characteristics pose challenges for the implementation of inherently safer design principles. Therefore, future research should focus on reconfiguring production schemes possibly through modularization, process intensification, or advanced control strategies to achieve meaningful safety improvements without compromising efficiency.
5. Conclusions
We evaluated the inherent safety of the suspension PVC production process with direct water recycling to analyze how mass integration influences the system’s inherent risks. After assigning the corresponding scores to both the process index and the substances involved in production, we found that the overall index reached a score of 33, which indicates a significant level of inherent unsafety. When comparing this system with one that does not include mass integration, we observed only slight variations in the Safe Process Structure index. Based on the above, we consider the process to be relatively neutral in this regard. One of the main risks identified in the process is the handling of VCM, a highly flammable and toxic compound whose presence significantly increases the system’s hazards. In addition, the polymerization reaction involved is highly exothermic, and the process relies on equipment such as furnaces, heaters, and dryers, all of which operate at elevated temperatures and constitute critical risk points. To reduce these hazards, it is essential to lower the inventory of dangerous substances, particularly in units that contain large volumes of VCM. Relocating this equipment outside the battery limits (OSBL) should also be considered when viable. An economic assessment would help determine whether such actions can be implemented without compromising the process’s overall efficiency. Additionally, implementing advanced containment strategies such as double sealed systems, real-time leak detection, and automated emergency shutdowns can further reduce the risks associated with VCM handling and enhance the overall safety profile of the process.
As future research directions, we identified specific methodological gaps in integrated risk assessments that simultaneously combine thermal, dynamic, and inventory analyses, particularly under transient operating conditions. It is also necessary to deepen the study of operational safety during the stages of direct recycling, as their impact on the overall risk profile is still not clearly understood. In addition, there is limited research regarding the behavior of alternative initiators to diacetyl peroxide, whose risk profiles could vary significantly depending on their decomposition mechanisms and interaction with VCM. Furthermore, we recommend advancing the development of real-time monitoring technologies specifically aimed at detecting VCM leaks and controlling its concentrations in enclosed units, along with sensors capable of identifying thermal and pressure deviations at critical points in the system. This would significantly contribute to incident prevention and improve overall process control.