Optically Active, Chlorophyll-Based Fluorescent Dye from Calabrian Opuntia ficus-indica Cladodes for Sustainable Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. OFI Samples and Reagents
2.2. Extraction and Preparation of OFI Extract from Cladodes
2.3. Thin Layer Chromatography (TLC) Separation
2.4. Chlorophyll Profiling Through UV–Vis Measurements
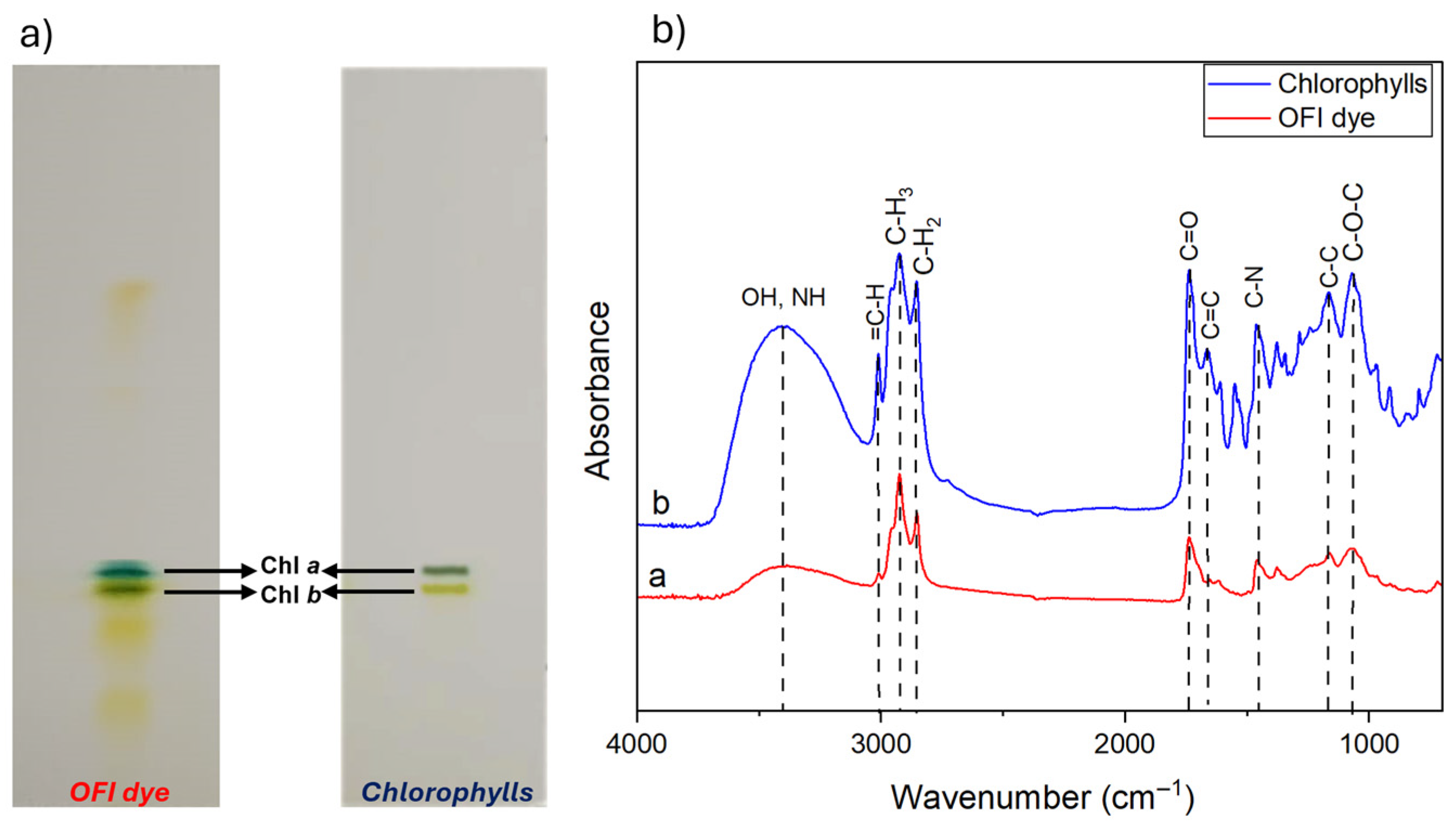
2.5. FTIR Analysis
2.6. Assay of DPPH Radical Scavenging Activity
2.7. Optical Characterization of OFI Dye
2.8. Characterization by Scanning Electron Microscopy
3. Results and Discussion
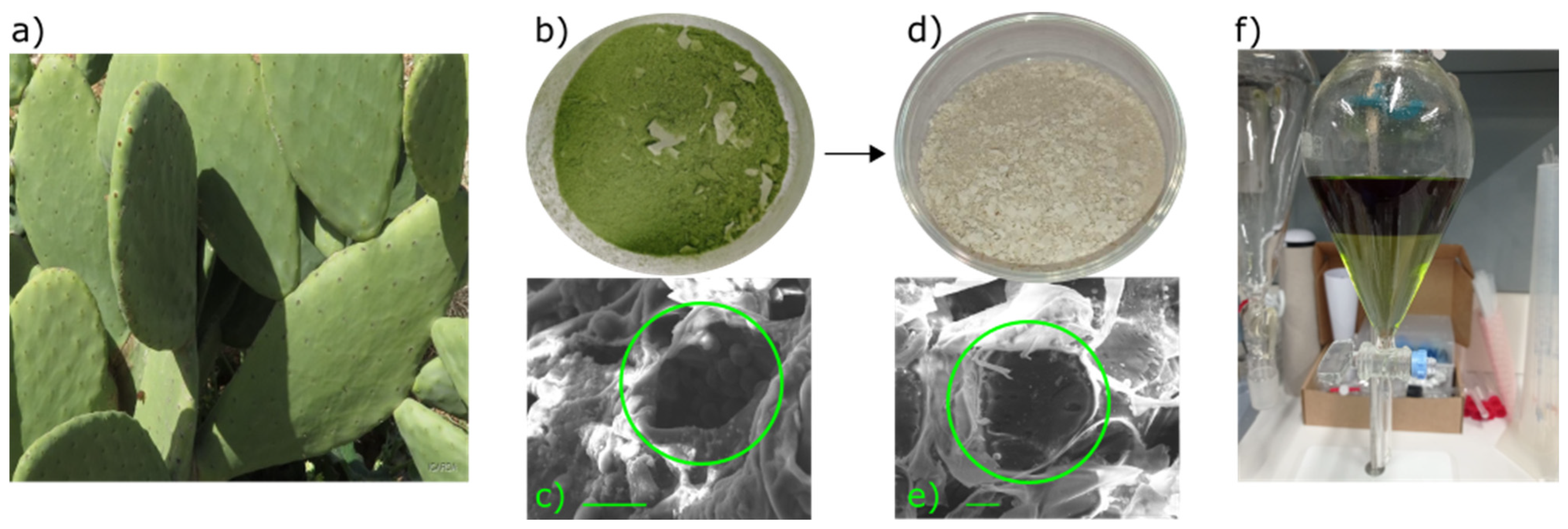
3.1. Extraction and Preparation of OFI Dye from Cladodes
3.2. Qualitative Characterization of OFI Dye
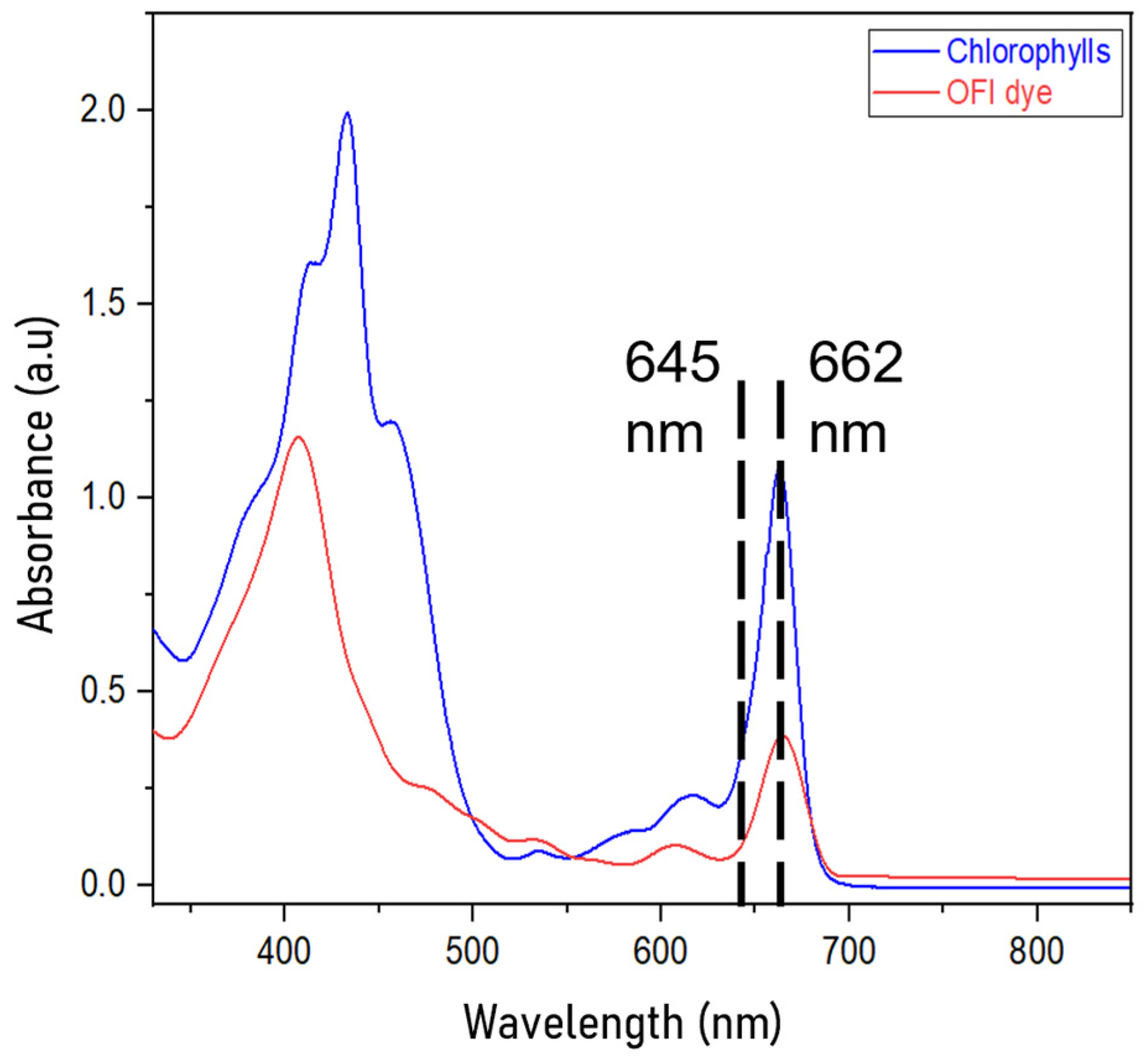
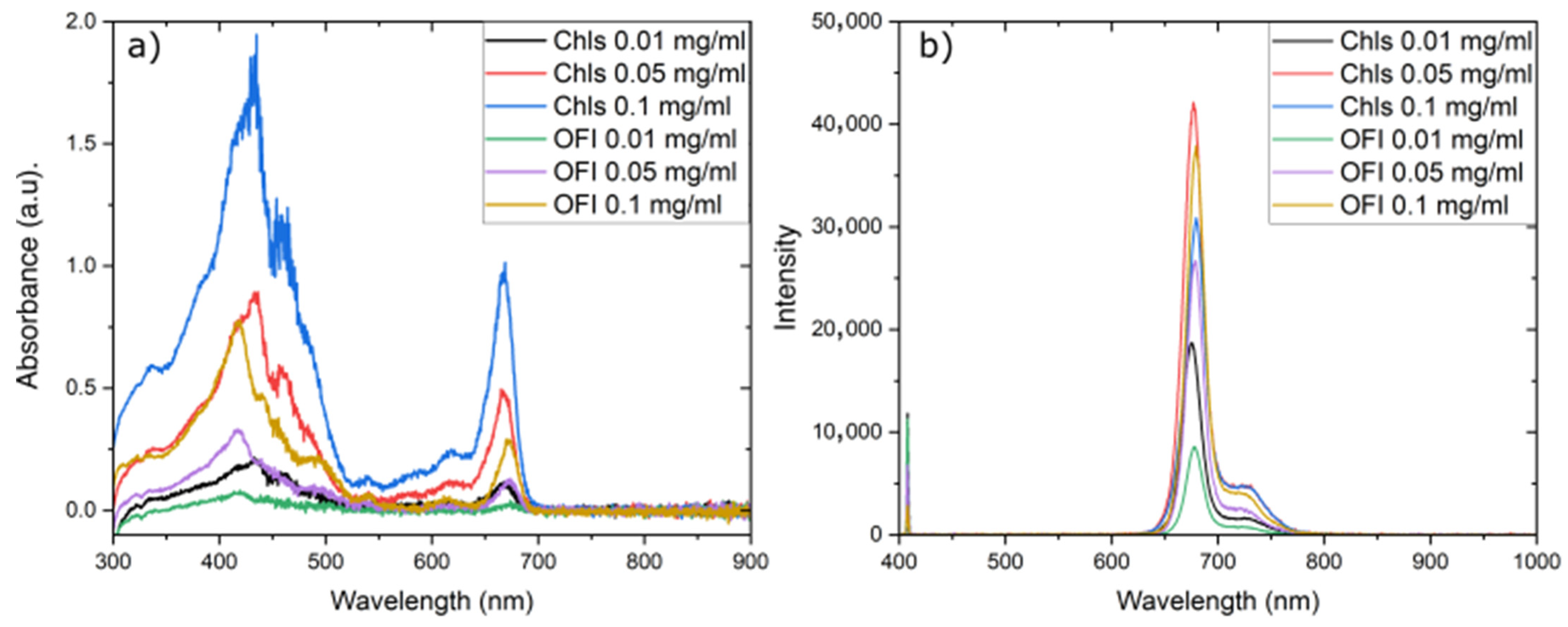
3.3. Chlorophyll Profiling Through UV–Vis Measurements
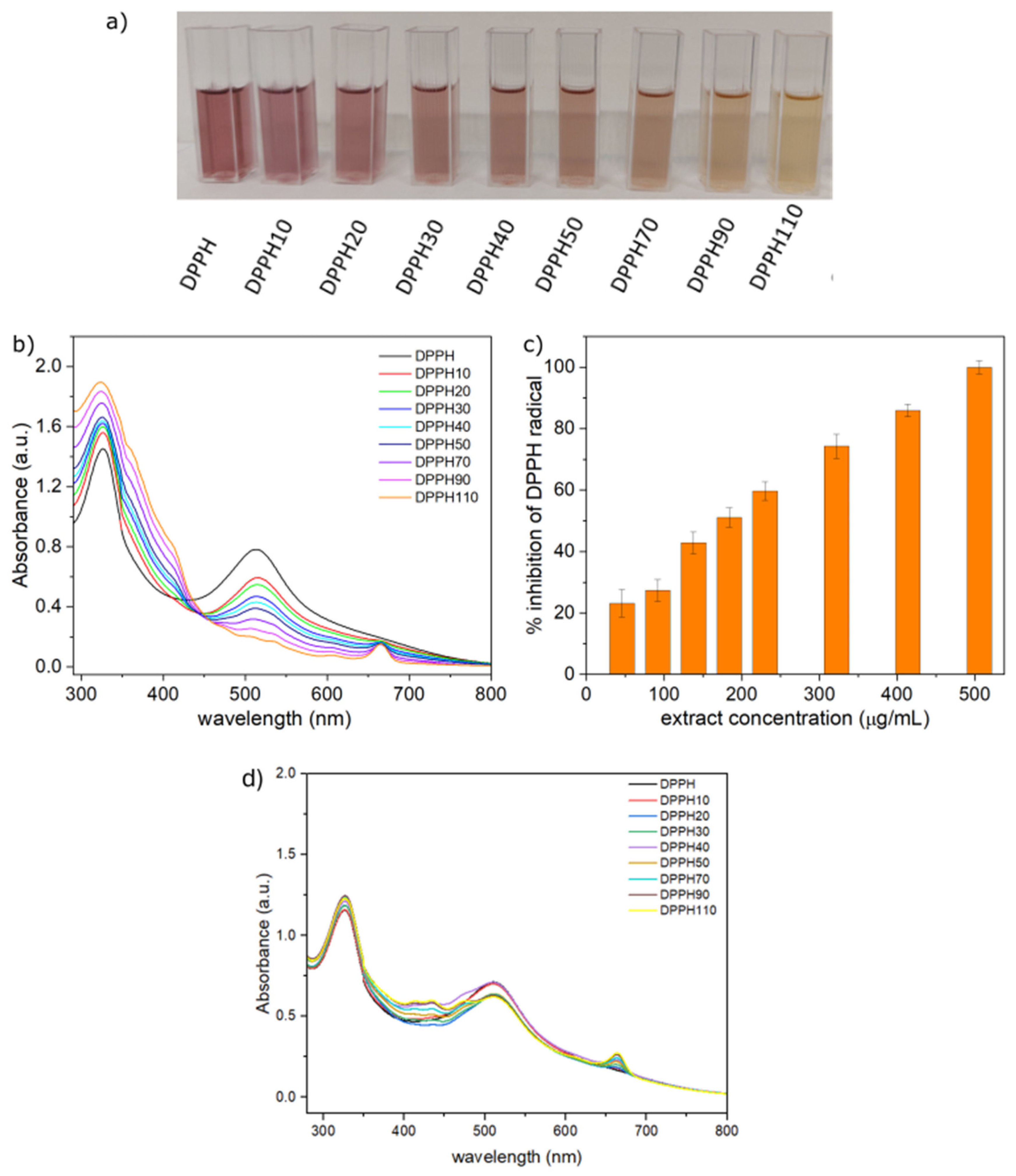
3.4. In Vitro Antioxidant Activity of OFI Extract
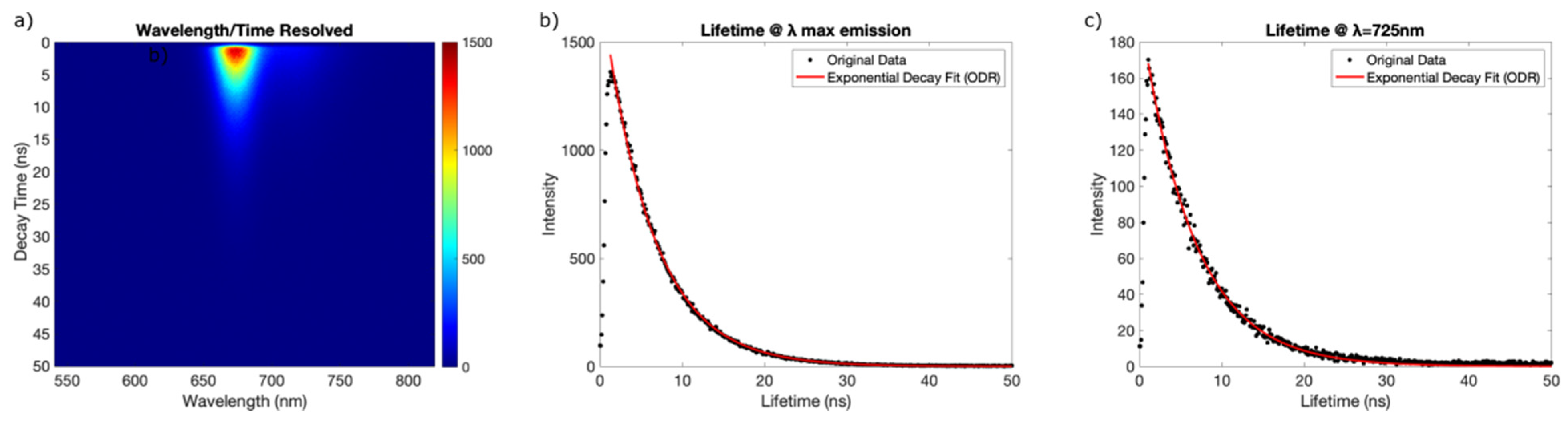
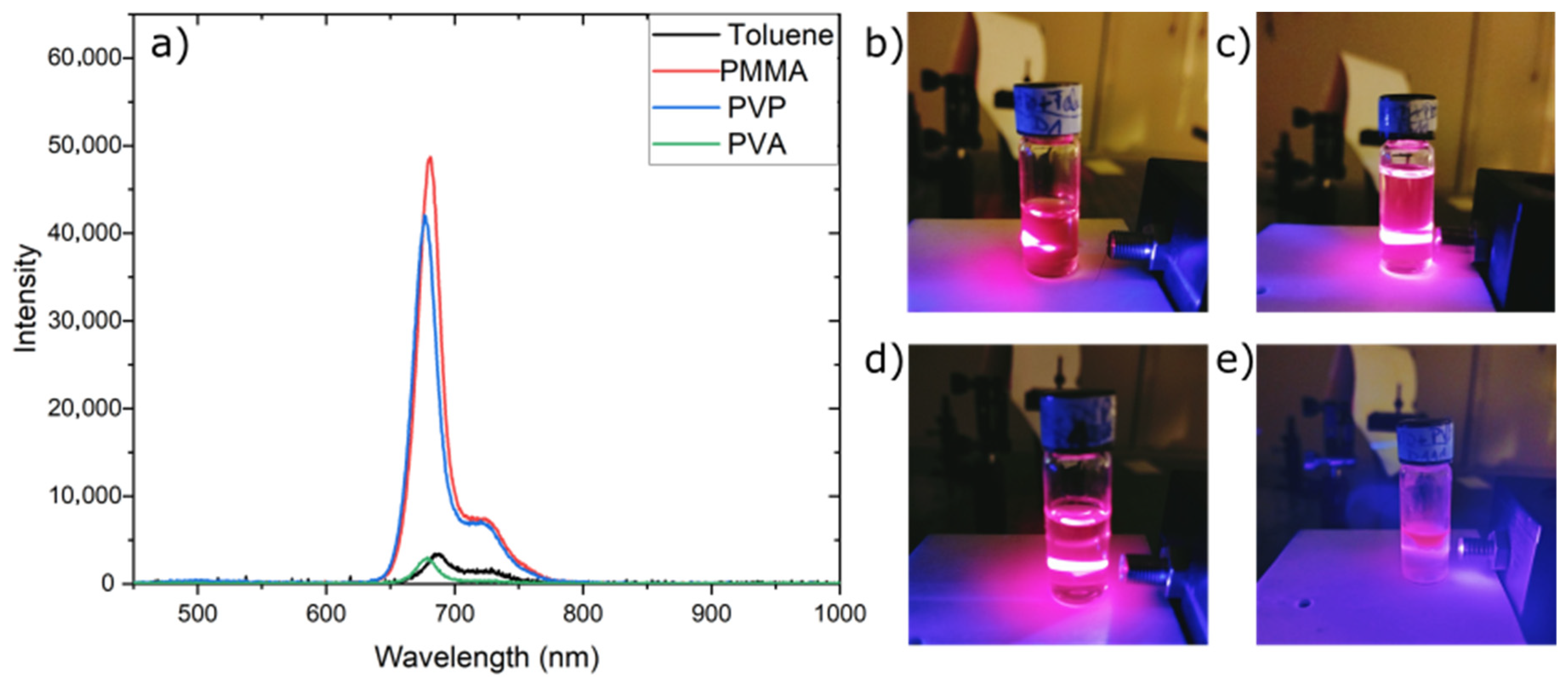
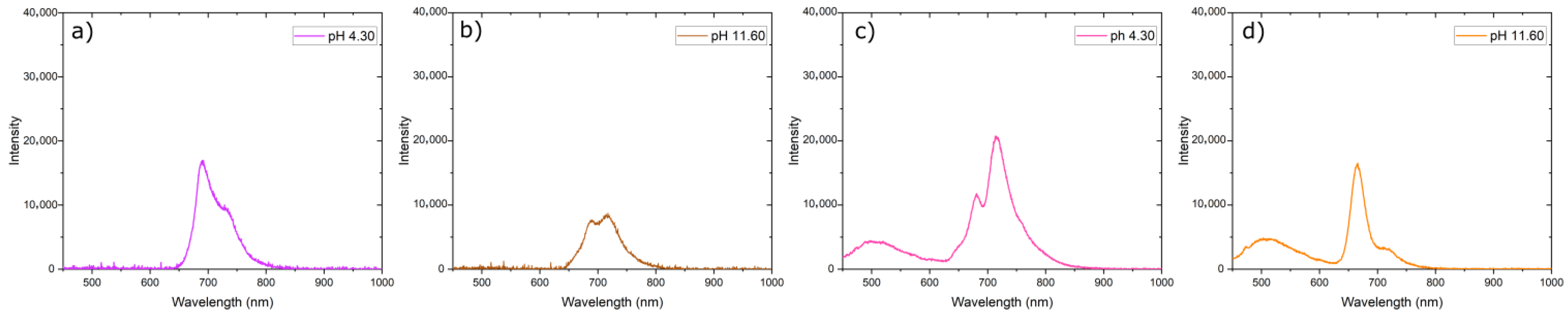
3.5. Optical Properties of OFI Dye
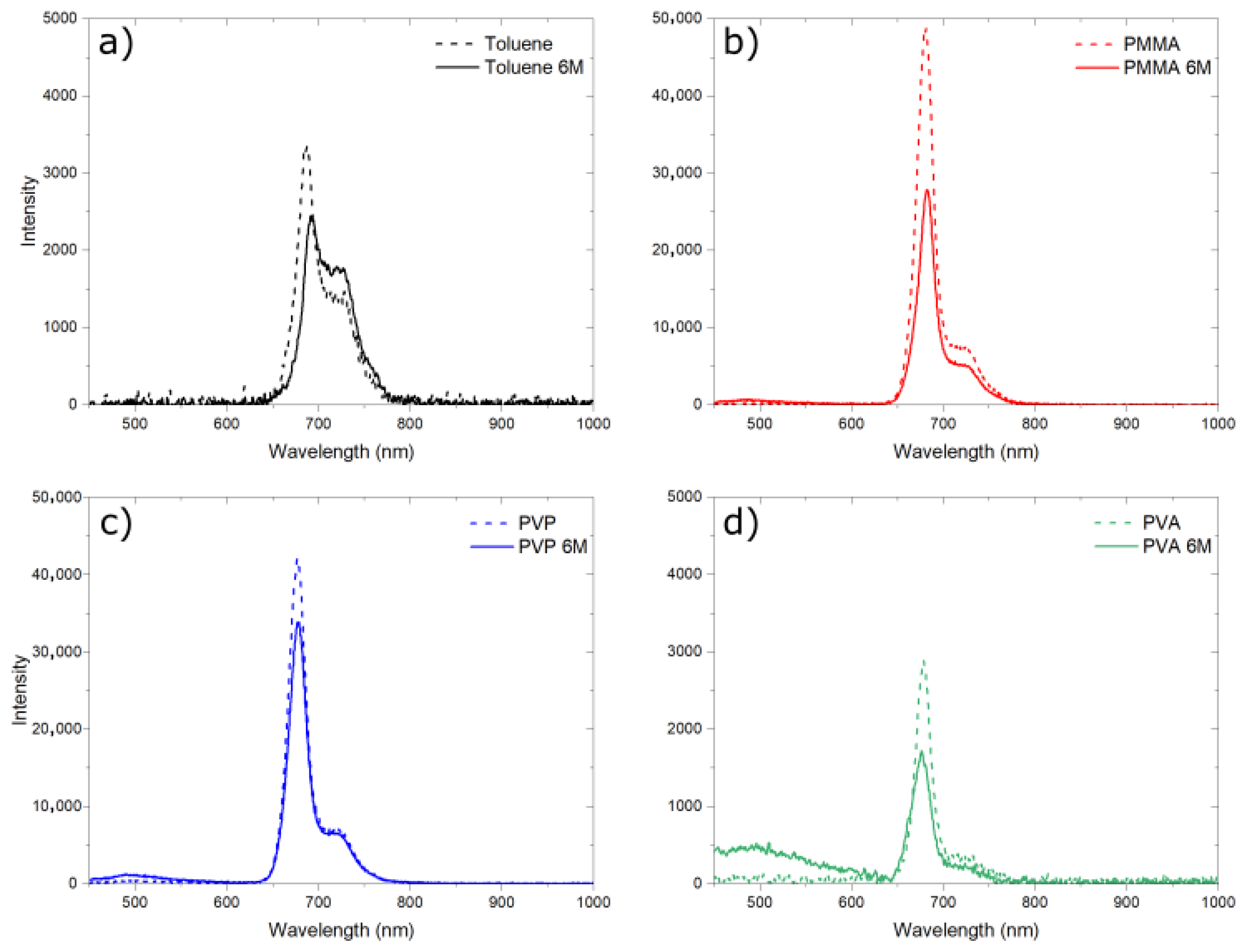
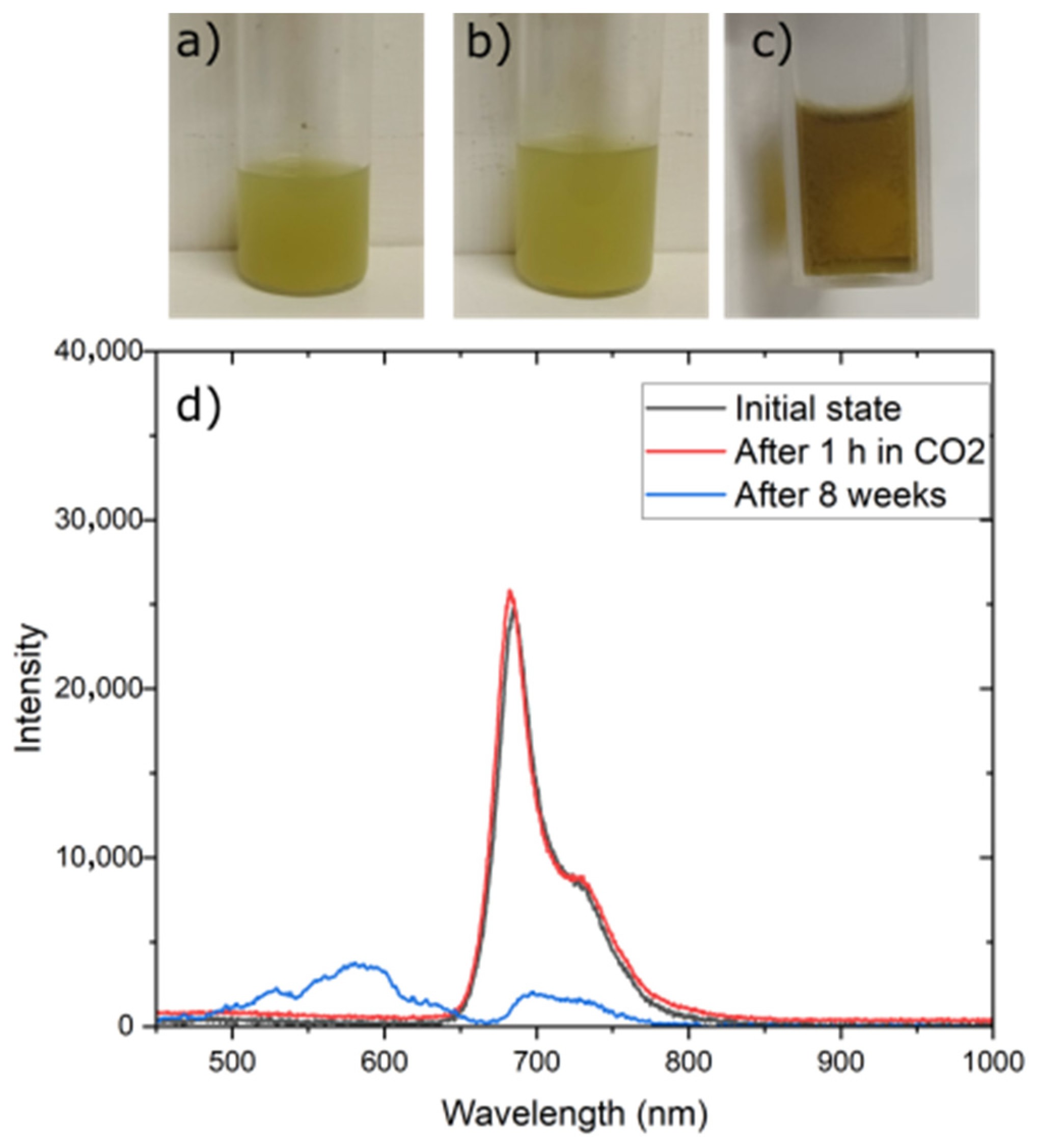
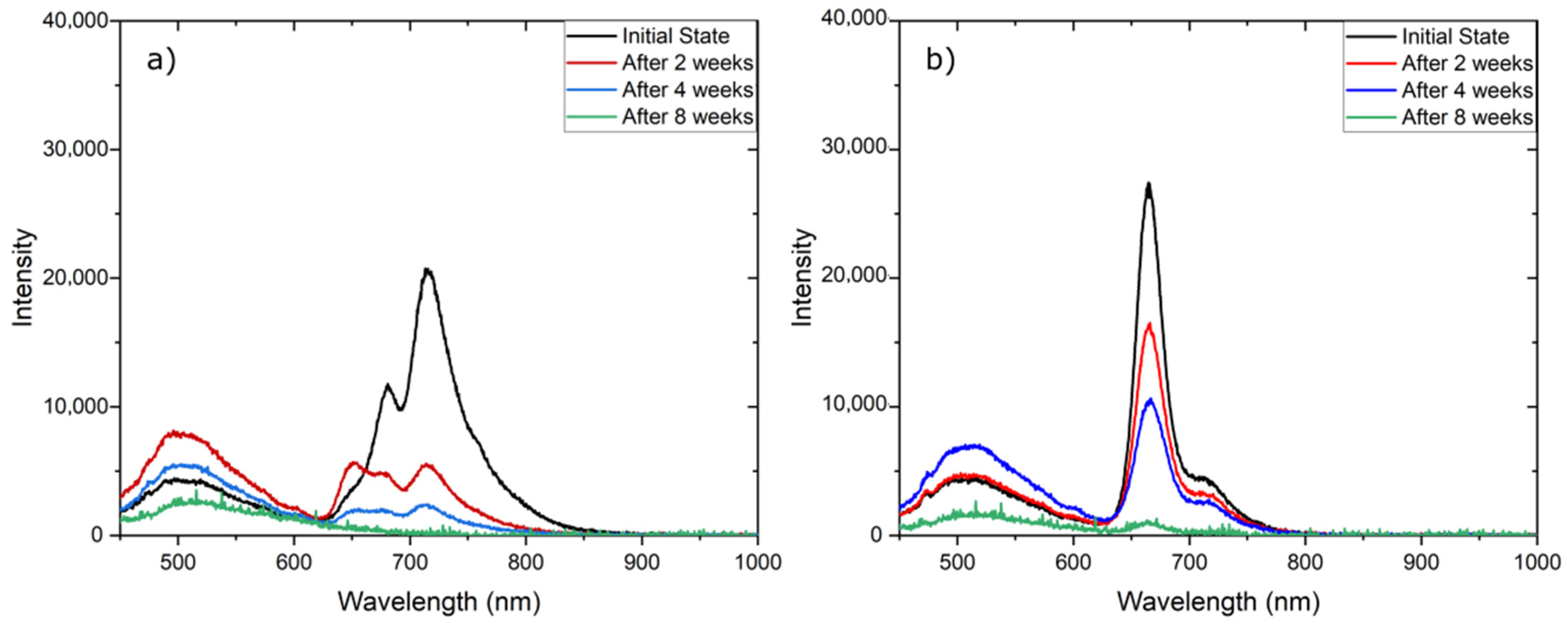
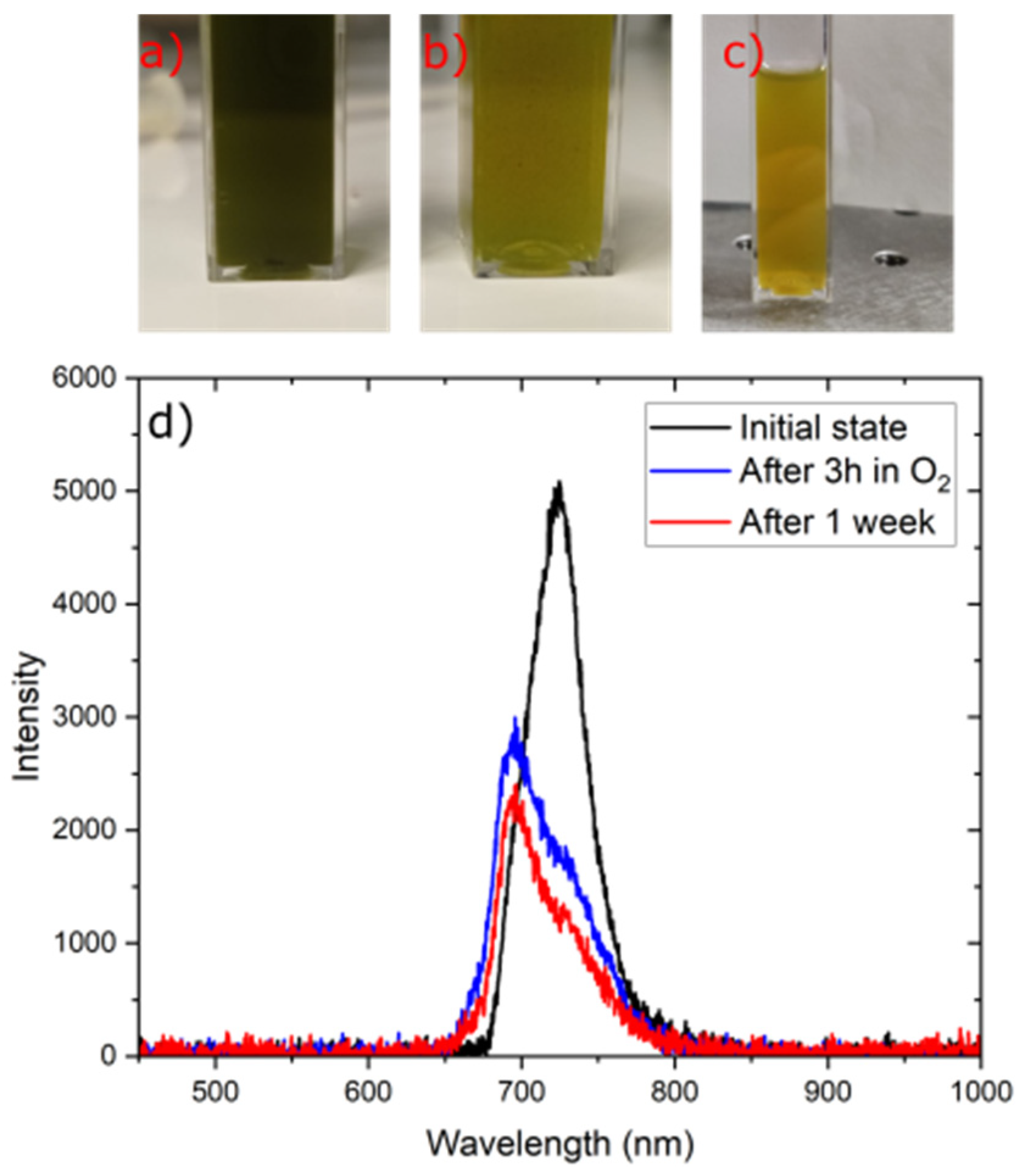
3.6. Stability of Fluorescent Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calogero, G.; Di Marco, G.; Cazzanti, S.; Caramori, S.; Argazzi, R.; Di Carlo, A.; Bignozzi, C.A. Efficient Dye-Sensitized Solar Cells Using Red Turnip and Purple Wild Sicilian Prickly Pear Fruits. Int. J. Mol. Sci. 2010, 11, 254–267. [Google Scholar] [CrossRef]
- Hao, S.; Wu, J.; Huang, Y.; Lin, J. Natural Dyes as Photosensitizers for Dye-Sensitized Solar Cell. Sol. Energy 2006, 80, 209–214. [Google Scholar] [CrossRef]
- Gómez-Ortíz, N.M.; Vázquez-Maldonado, I.A.; Pérez-Espadas, A.R.; Mena-Rejón, G.J.; Azamar-Barrios, J.A.; Oskam, G. Dye-Sensitized Solar Cells with Natural Dyes Extracted from Achiote Seeds. Sol. Energy Mater. Sol. Cells 2010, 94, 40–44. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Nita, R.; Murariu, A.; Miculescu, F.; Iosub, I.; Meghea, A. Encapsulation of Fluorescence Vegetable Extracts within a Templated Sol–Gel Matrix. Opt. Mater. 2010, 32, 711–718. [Google Scholar] [CrossRef]
- Giampaoli, P.; Fernandes, F.F.; Tavares, A.R.; Domingos, M.; Cardoso-Gustavson, P. Fluorescence Emission Spectra of Target Chloroplast Metabolites (Flavonoids, Carotenoids, Lipofuscins, Pheophytins) as Biomarkers of Air Pollutants and Seasonal Tropical Climate. Environ. Sci. Pollut. Res. 2020, 27, 25363–25373. [Google Scholar] [CrossRef]
- Chang, H.; Kao, M.-J.; Chen, T.-L.; Chen, C.-H.; Cho, K.-C.; Lai, X.-R. Characterization of Natural Dye Extracted from Wormwood and Purple Cabbage for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2013, 2013, 159502. [Google Scholar] [CrossRef]
- Azman, N.H.; Khairul, W.M.; Sarbon, N.M. A Comprehensive Review on Biocompatible Film Sensor Containing Natural Extract: Active/Intelligent Food Packaging. Food Control 2022, 141, 109189. [Google Scholar] [CrossRef]
- Das, S.; Roy Maulik, S. Recent Approaches and Advancements in Natural Dyes. In Natural Dyes and Sustainability; Muthu, S.S., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 63–78. ISBN 978-3-031-47471-2. [Google Scholar]
- Bin-Jumah, M.; Alwakeel, S.S.; Moga, M.; Buvnariu, L.; Bigiu, N.; Zia-Ul-Haq, M. Application of Carotenoids in Cosmetics. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 747–756. ISBN 978-3-030-46459-2. [Google Scholar]
- García-Plazaola, J.I.; Fernández-Marín, B.; Duke, S.O.; Hernández, A.; López-Arbeloa, F.; Becerril, J.M. Autofluorescence: Biological Functions and Technical Applications. Plant Sci. 2015, 236, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Reisch, A.; Trofymchuk, K.; Runser, A.; Fleith, G.; Rawiso, M.; Klymchenko, A.S. Tailoring Fluorescence Brightness and Switching of Nanoparticles through Dye Organization in the Polymer Matrix. ACS Appl. Mater. Interfaces 2017, 9, 43030–43042. [Google Scholar] [CrossRef] [PubMed]
- Petrović, S.; Zvezdanović, J.; Marković, D. Chlorophyll Degradation in Aqueous Mediums Induced by Light and UV-B Irradiation: An UHPLC-ESI-MS Study. Radiat. Phys. Chem. 2017, 141, 8–16. [Google Scholar] [CrossRef]
- Galati, E.M.; Tripodo, M.M.; Trovato, A.; d’Aquino, A.; Monforte, M.T. Biological Activity of Opuntia Ficus Indica Cladodes II: Effect on Experimental Hypercholesterolemia in Rats. Pharm. Biol. 2003, 41, 175–179. [Google Scholar] [CrossRef]
- Iacopetta, D.; Baldino, N.; Caruso, A.; Perri, V.; Lupi, F.R.; de Cindio, B.; Gabriele, D.; Sinicropi, M.S. Nutraceuticals Obtained by SFE-CO2 from Cladodes of Two Opuntia ficus-indica (L.) Mill Wild in Calabria. Appl. Sci. 2021, 11, 477. [Google Scholar] [CrossRef]
- Kamwe Sighano, S.; Ritacco, T.; Bruno, M.D.L.; Gennari, O.; Fuscaldo, W.; Zografopoulos, D.C.; Marae-Djouda, J.; Maurer, T.; Beccherelli, R.; Caputo, R.; et al. Optical and Terahertz Anticounterfeiting Tags Via Non-Deterministic Deposition of Fluorescent Opuntia ficus-indica Extract. Adv. Funct. Mater. 2024, 34, 2406632. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Megna, B. Structure-Property Relationship of PLA-Opuntia Ficus Indica Biocomposites. Compos. Part B Eng. 2019, 167, 199–206. [Google Scholar] [CrossRef]
- Abbas, E.Y.; Ezzat, M.I.; El Hefnawy, H.M.; Abdel-Sattar, E. An Overview and Update on the Chemical Composition and Potential Health Benefits of Opuntia ficus-indica (L.) Miller. J. Food Biochem. 2022, 46, e14310. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef]
- Zeb, A. A Simple, Sensitive HPLC-DAD Method for Simultaneous Determination of Carotenoids, Chlorophylls and α-Tocopherol in Leafy Vegetables. Food Meas. 2017, 11, 979–986. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant Activity of Chlorophylls and Their Derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Yadav, S.; Tiwari, K.S.; Gupta, C.; Tiwari, M.K.; Khan, A.; Sonkar, S.P. A Brief Review on Natural Dyes, Pigments: Recent Advances and Future Perspectives. Results Chem. 2023, 5, 100733. [Google Scholar] [CrossRef]
- Pizzicato, B.; Pacifico, S.; Cayuela, D.; Mijas, G.; Riba-Moliner, M. Advancements in Sustainable Natural Dyes for Textile Applications: A Review. Molecules 2023, 28, 5954. [Google Scholar] [CrossRef]
- Quach, H.T.; Steeper, R.L.; Griffin, G.W. An Improved Method for the Extraction and Thin-Layer Chromatography of Chlorophyll a and b from Spinach. J. Chem. Educ. 2004, 81, 385. [Google Scholar] [CrossRef]
- Cazón, P.; Sanches Silva, A. Natural Pigments from Food Wastes: New Approaches for the Extraction and Encapsulation. Curr. Opin. Green Sustain. Chem. 2024, 47, 100929. [Google Scholar] [CrossRef]
- Pooja, B.K.; Sethi, S.; Joshi, A.; Varghese, E.; Kaur, C.; Kumar, R. Shridhar Ultrasound-Assisted Extraction of Chlorophyll from Pea Pod Waste: Optimization, Kinetics, and Stability Study. Food Anal. Methods 2023, 16, 1358–1369. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Wang, H.; Ji, X.; Page, Z.A.; Sessler, J.L. Fluorescent Materials-Based Information Storage. Mater. Chem. Front. 2020, 4, 1024–1039. [Google Scholar] [CrossRef]
- Mandal, B.K.; Ling, Y.-C. Analysis of Chlorophylls/Chlorophyllins in Food Products Using HPLC and HPLC-MS Methods. Molecules 2023, 28, 4012. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, H.; Mu, T. Effects of Different pH, Temperature, and Pressurisation Time on the Stability of Chlorophylls Treated with Zn2+ and High Hydrostatic Pressure. Int. J. Food Sci. Technol. 2023, 58, 4715–4725. [Google Scholar] [CrossRef]
- Vladkova, R. Chlorophyll a Self-Assembly in Polar Solvent–Water Mixtures †. Photochem. Photobiol. 2000, 71, 71–83. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Fluorophores. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 63–95. ISBN 978-0-387-46312-4. [Google Scholar]
- Munawaroh, H.S.H.; Fathur, R.M.; Gumilar, G.; Aisyah, S.; Yuliani, G.; Mudzakir, A.; Wulandari, A.P. Characterization and Physicochemical Properties of Chlorophyll Extract from Spirulina sp. J. Phys. Conf. Ser. 2019, 1280, 022013. [Google Scholar] [CrossRef]
- Barani, H.; Rahmani, F. Dyeing of Wool Yarn with Aqueous Extract of Opuntia ficus-indica Fruit Containing Betalain Colorant Employing Ascorbic Acid as a Bio-Mordant Under Varied Circumstances of Dyeing. Fibers Polym. 2023, 24, 2785–2797. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Reimers, J.R.; Cai, Z.-L.; Kobayashi, R.; Rätsep, M.; Freiberg, A.; Krausz, E. Assignment of the Q-Bands of the Chlorophylls: Coherence Loss via Qx − Qy Mixing. Sci. Rep. 2013, 3, 2761. [Google Scholar] [CrossRef]
- Forster, L.S.; Livingston, R. The Absolute Quantum Yields of the Fluorescence of Chlorophyll Solutions. J. Chem. Phys. 1952, 20, 1315–1320. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Effects of Solvents on Fluorescence Emission Spectra. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1983; pp. 187–215. ISBN 978-1-4615-7658-7. [Google Scholar]
- Rodríguez, A.; Graciani, M.d.M.; Moyá, M.L. Effects of Addition of Polar Organic Solvents on Micellization. Langmuir 2008, 24, 12785–12792. [Google Scholar] [CrossRef]
- Rega, R.; Gennari, O.; Mecozzi, L.; Grilli, S.; Pagliarulo, V.; Ferraro, P. Bipolar Patterning of Polymer Membranes by Pyroelectrification. Adv. Mater. 2016, 28, 454–459. [Google Scholar] [CrossRef]
- Ghoshal, S.K.; Sarkar, S.K.; Kastha, G.S. Effects of Intermolecular Hydrogen-Bonding on the Luminescence Properties of Acetophenone, Characterization of Emission States. Bull. Chem. Soc. Jpn. 1981, 54, 3556–3561. [Google Scholar] [CrossRef]
- Miranda-Quintana, R.A.; Chen, L.; Smiatek, J. Insights into Hildebrand Solubility Parameters – Contributions from Cohesive Energies or Electrophilicity Densities? ChemPhysChem 2024, 25, e202300566. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Pham Van, B.; Dang Van, T.; Nguyen Thi, H. The Optical Properties and Energy Transition Process in Nanocomposite of Polyvinyl-Pyrrolidone Polymer and Mn-Doped ZnS. Opt. Quant. Electron. 2013, 45, 147–159. [Google Scholar] [CrossRef]
- Agostiano, A.; Catucci, L.; Colafemmina, G.; Scheer, H. Role of Functional Groups and Surfactant Charge in Regulating Chlorophyll Aggregation in Micellar Solutions. J. Phys. Chem. B 2002, 106, 1446–1454. [Google Scholar] [CrossRef]
- Plouzeau, M.; Piogé, S.; Peilleron, F.; Fontaine, L.; Pascual, S. Polymer/Dye Blends: Preparation and Optical Performance: A Short Review. J. Appl. Polym. Sci. 2022, 139, e52861. [Google Scholar] [CrossRef]
- Cichelli, A.; Pertesana, G.P. High-Performance Liquid Chromatographic Analysis of Chlorophylls, Pheophytins and Carotenoids in Virgin Olive Oils: Chemometric Approach to Variety Classification. J. Chromatogr. A 2004, 1046, 141–146. [Google Scholar] [CrossRef]
- Pedrós, R.; Moya, I.; Goulas, Y.; Jacquemoud, S. Chlorophyll Fluorescence Emission Spectrum inside a Leaf. Photochem. Photobiol. Sci. 2008, 7, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Gong, N.; Wang, S.; Gao, Y.; Sun, C.; Fang, W.; Men, Z. Effect of pH on Fluorescence and Absorption of Aggregates of Chlorophyll a and Carotenoids. Dyes Pigments 2020, 173, 107975. [Google Scholar] [CrossRef]
- Nishimura, N.; Nakayama, S.; Horiuchi, A.; Kumoda, M.; Miyatake, T. Reversible Aggregation of Chlorophyll Derivative Induced by Phase Transition of Lipid. Langmuir 2019, 35, 7242–7248. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical Constituents and in Vitro Radical Scavenging Activity of Different Aloe Species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, A.; Guzzi, R.; Kamwe Sighano, S.; Nicoletta, G.; Caputo, R.; Cofone, F.; Desiderio, G.; Gennari, O. Optically Active, Chlorophyll-Based Fluorescent Dye from Calabrian Opuntia ficus-indica Cladodes for Sustainable Applications. Sustainability 2025, 17, 7504. https://doi.org/10.3390/su17167504
Ferraro A, Guzzi R, Kamwe Sighano S, Nicoletta G, Caputo R, Cofone F, Desiderio G, Gennari O. Optically Active, Chlorophyll-Based Fluorescent Dye from Calabrian Opuntia ficus-indica Cladodes for Sustainable Applications. Sustainability. 2025; 17(16):7504. https://doi.org/10.3390/su17167504
Chicago/Turabian StyleFerraro, Antonio, Rita Guzzi, Sephora Kamwe Sighano, Giuseppe Nicoletta, Roberto Caputo, Franco Cofone, Giovanni Desiderio, and Oriella Gennari. 2025. "Optically Active, Chlorophyll-Based Fluorescent Dye from Calabrian Opuntia ficus-indica Cladodes for Sustainable Applications" Sustainability 17, no. 16: 7504. https://doi.org/10.3390/su17167504
APA StyleFerraro, A., Guzzi, R., Kamwe Sighano, S., Nicoletta, G., Caputo, R., Cofone, F., Desiderio, G., & Gennari, O. (2025). Optically Active, Chlorophyll-Based Fluorescent Dye from Calabrian Opuntia ficus-indica Cladodes for Sustainable Applications. Sustainability, 17(16), 7504. https://doi.org/10.3390/su17167504








