Nature-Based Solutions in Sustainable Cities: Trace Metal Accumulation in Urban Forests of Vienna (Austria) and Krakow (Poland)
Abstract
1. Introduction
2. Experimental Part
2.1. Study Area
2.2. The “Wienerwald” Forest, Vienna
2.3. The “Las Wolski” Forest, Krakow
3. Materials
4. Methods
4.1. Chemical Analysis
4.2. Statistical Analysis
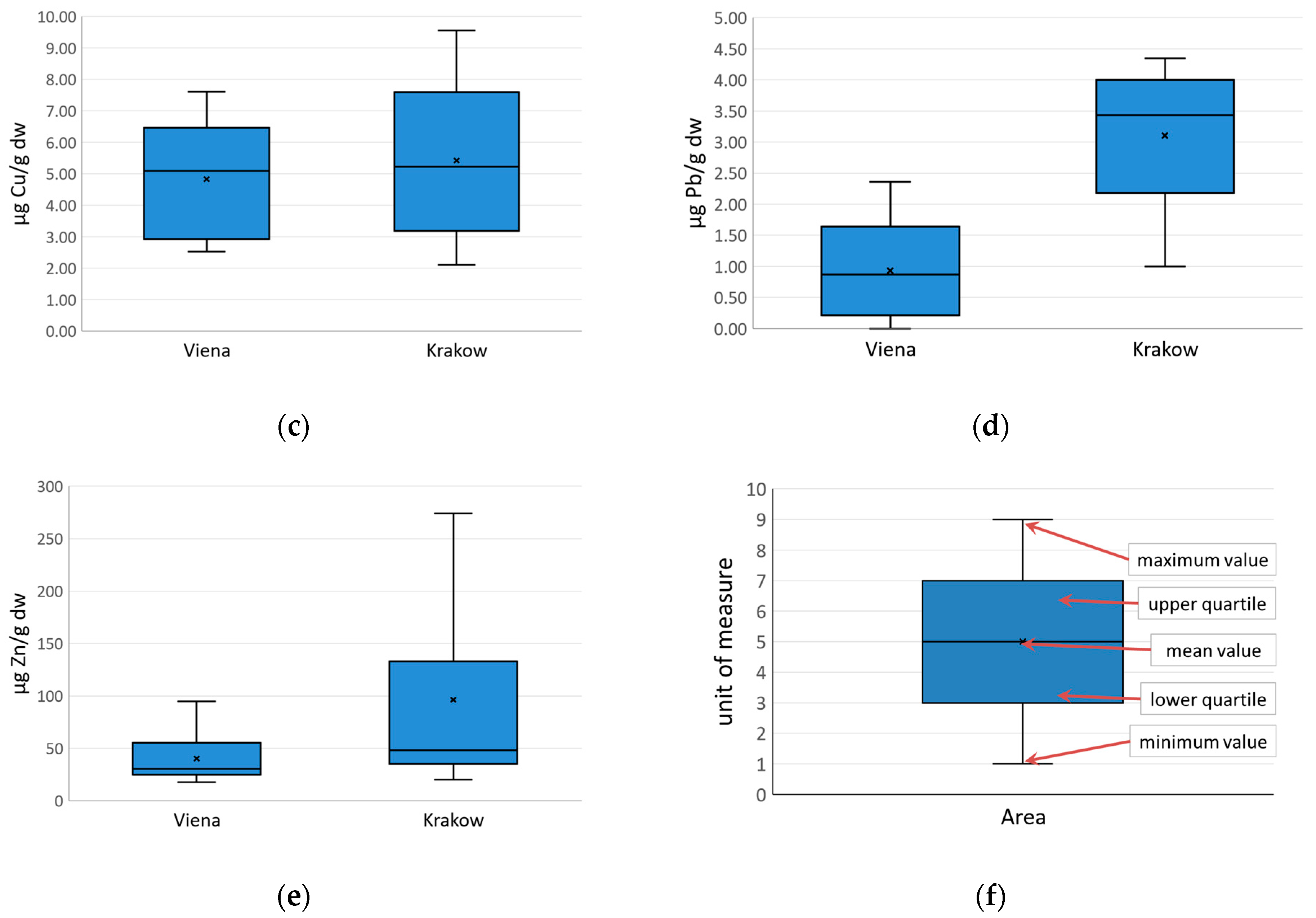
5. Results
5.1. Biomonitor Species Variations
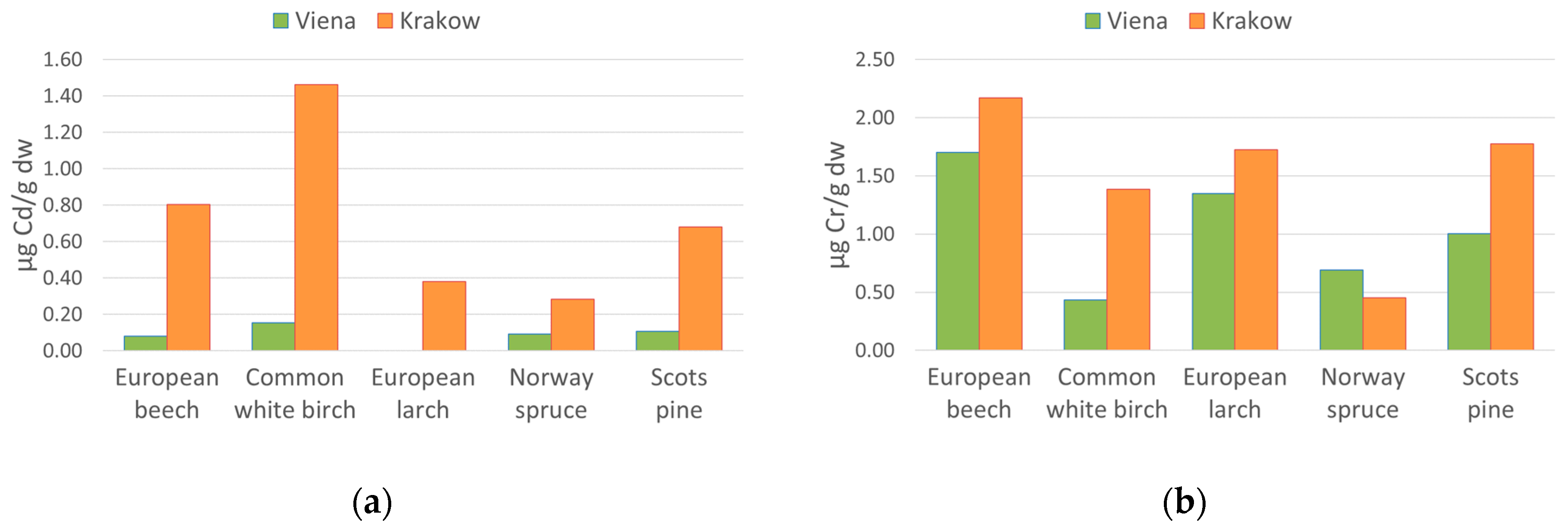
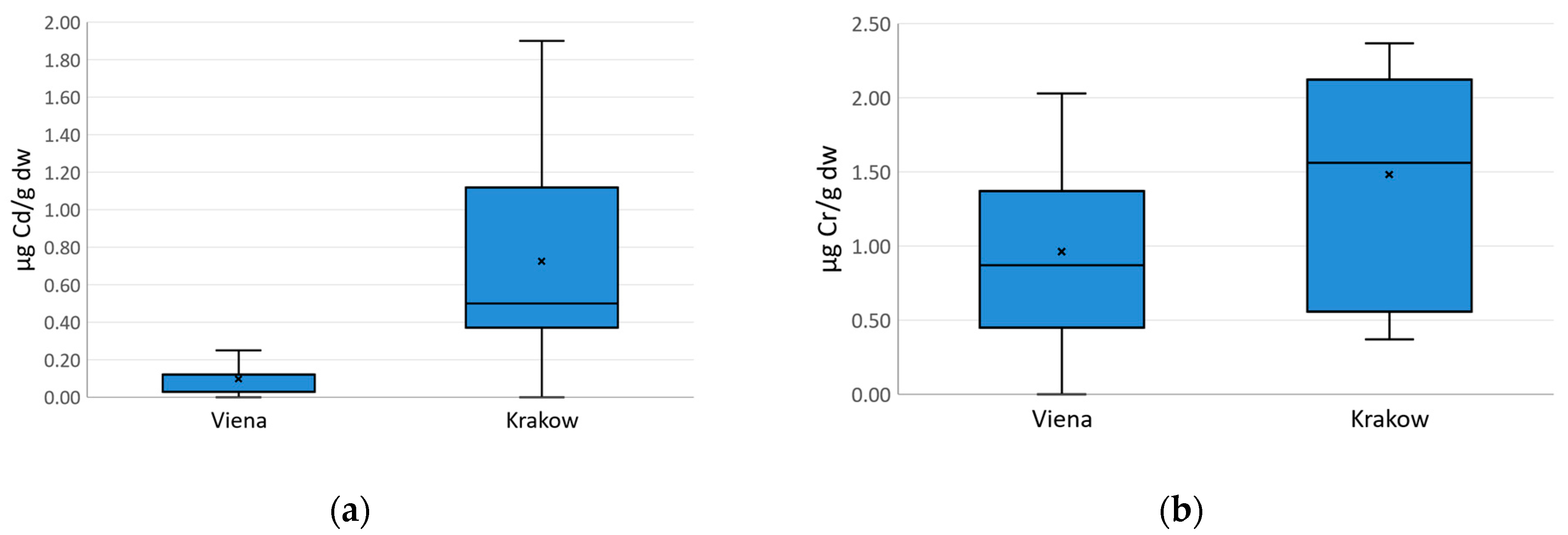
5.2. “Wienerwald” and “Las Wolski” Variations
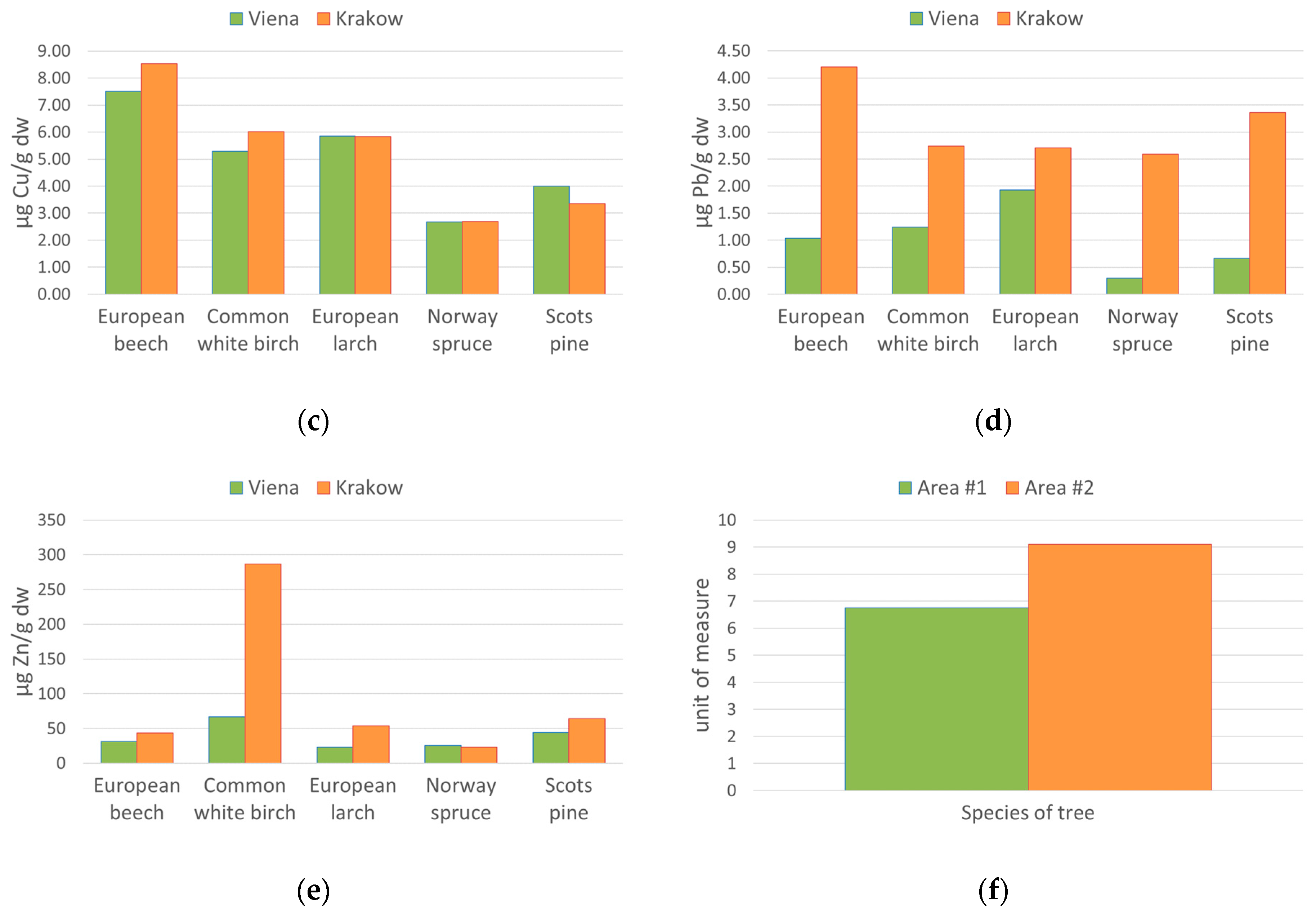
5.3. European Beech (Fagus sylvatica L.)
5.4. Common White Birch (Betula pendula Roth.)
5.5. European Larch (Larix decidua Mill.)
5.6. Scots Pine (Pinus sylvestris L.)
5.7. Norway Spruce (Picea abies (L.) H. Karst)
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakubiak, M.; Urbański, K. Urban planning solutions in the context of dispersion of road pollution. J. Water Land Dev. 2016, 30, 71–78. [Google Scholar] [CrossRef][Green Version]
- Jakubiak, M.; Chmielowski, K. Identification of urban water bodies ecosystem services. Acta Sci. Pol.-Form. Circ. 2020, 19, 73–82. [Google Scholar] [CrossRef]
- United Nations Human Settlements Programme. World Cities Report 2022: Envisaging the Future of Cities; UN-Habitat: Nairobi, Kenya, 2022; p. 422. [Google Scholar]
- Jakubiak, M.; Bojarski, B. Impact of point source pollutants on the distribution of selected water parameters in the Vistula River in Puławy, Poland. J. Water Land Dev. 2021, 51, 50–55. [Google Scholar] [CrossRef]
- Steenberg, J.W.N.; Duinker, P.N.; Nitoslawski, S.A. Ecosystem-based management revisited: Updating the concepts for urban forests. Landsc. Urban Plann. 2019, 186, 24–35. [Google Scholar] [CrossRef]
- Capotorti, G.; Del Vico, E.; Anzellotti, I.; Celesti-Grapow, L. Combining the conservation of biodiversity with the provision of ecosystem services in urban green infrastructure planning: Critical features arising from a case study in the metropolitan area of Rome. Sustainability 2017, 9, 10. [Google Scholar] [CrossRef]
- Fan, C.; Johnston, M.; Darling, L.; Scott, L.; Liao, F.H. Land use and socio-economic determinants of urban forest structure and diversity. Landsc. Urban Plann. 2019, 181, 10–21. [Google Scholar] [CrossRef]
- Gerstenberg, T.; Baumeister, C.F.; Schraml, U.; Plieninger, T. Hot routes in urban forests: The impact of multiple landscape features on recreational use intensity. Landsc. Urban Plann. 2020, 203, 103888. [Google Scholar] [CrossRef]
- Soroka, A.; Wojciechowska-Solis, J. Expectations of the Polish society related to recreational and tourist activities in the forest environment. Sylwan 2020, 164, 513–520. [Google Scholar] [CrossRef]
- da Schio, N.; Phillips, A.; Fransen, K.; Wolff, M.; Haase, D.; Ostoic, S.K.; Zivojinovic, I.; Vuletic, D.; Derks, J.; Davies, C.; et al. The impact of the COVID-19 pandemic on the use of and attitudes towards urban forests and green spaces: Exploring the instigators of change in Belgium. Urban For. Urban Green. 2021, 65, 127305. [Google Scholar] [CrossRef]
- Beckmann-Wübbelt, A.; Fricke, A.; Sebesvari, Z.; Yakouchenkova, I.A.; Fröhlich, K.; Saha, S. High public appreciation for the cultural ecosystem services of urban and peri-urban forests during the COVID-19 pandemic. Sustain. Cities Soc. 2021, 74, 103240. [Google Scholar] [CrossRef]
- Gálicz, I.V.; Magda, R.; Dávid, L.D. Archaeological Parks in the Service of Tourism—A Comparative Analysis of Hungarian and Western-European Archaeological Parks. Sustainability 2024, 16, 3313. [Google Scholar] [CrossRef]
- Branny, A.; Møller, M.; Steen, K.; Korpilo, S.; McPhearson, T.; Gulsrud, N.; Olafsson, A.S.; Raymond, C.M.; Andersson, E. Smarter greener cities through a social-ecological-technological systems approach. Curr. Opin. Environ. Sustain. 2022, 55, 101168. [Google Scholar] [CrossRef]
- Urbański, K.; Jakubiak, M. Impact of land use on soils microbial activity. J. Water Land Dev. 2017, 35, 249–257. [Google Scholar] [CrossRef]
- El-Khatib, A.; Abd El-Rahman, A.E.-R.; Elsheikh, O. Leaf geometric design of urban trees: Potentiality to capture airborne particle pollutants. J. Environ. Stud. 2011, 7, 49–59. [Google Scholar] [CrossRef]
- Smidt, S.; Jandl1, R.; Bauer, H.; Fürst, A.; Mutsch, F.; Zechmeister, H.; Seidel, C. Trace Metals and Radionuclides in Austrian Forest Ecosystems. In The Biosphere; Ishwaran, N., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 93–118. [Google Scholar] [CrossRef][Green Version]
- Diana Grecia, A.-M.; Sergio Arturo, T.-S.; Marlenne, G.-R. Review: Implications of air pollution on trees located in urban areas. Earth 2025, 6, 38. [Google Scholar] [CrossRef]
- Sawidis, T.; Breuste, J.; Mitrovic, M.; Pavlovic, P.; Tsigaridas, K. Trees as bioindicator of heavy metal pollution in three European cities. Environ. Pollut. 2011, 159, 3560–3570. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Panek, E. Testing of selected phytoindicators for the environmental assessment of areas under various levels of pollution. Geomat. Environ. Eng. 2012, 6, 73–81. [Google Scholar] [CrossRef][Green Version]
- Cruz-Huerta, C.; Martínez-Trinidad, T.; Correa-Díaz, A.; Vargas-Hernández, J.J.; Gómez-Guerrero, A.; Villanueva-Díaz, J.; Beramendi-Orosco, L.E. Temporal analysis of pollutant metals in trees of three parks in Mexico City’s Metropolitan Area. iForest 2025, 18, 138–145. [Google Scholar] [CrossRef]
- Śliwka, M.; Jakubiak, M. Application of laser stimulation of some hydrophytes species for more efficient biogenic elements phytoremediation. Proc. ECOpole 2010, 4, 205–211. [Google Scholar]
- Tomašević, M.; Vukmirović, Z.; Rajšić, S.; Tasić, M.; Stevanović, B. Contribution to biomonitoring of some trace metals by deciduous tree leaves in urban areas. Environ. Monit. Assess. 2008, 137, 393–401. [Google Scholar] [CrossRef]
- Maňkovská, B.; Godzik, B.; Badea, O.; Shparyk, Y.; Moravčík, P. Chemical and morphological characteristics of key tree species of the Carpathian Mountains. Environ. Pollut. 2004, 130, 41–54. [Google Scholar] [CrossRef]
- Reimann, C.; Arnoldussen, A.; Boyd, R.; Finne, T.E.; Koller, F.; Nordgulen, Ø.; Englmaier, P. Element contents in leaves of four plant species (birch, mountain ash, fern and spruce) along anthropogenic and geogenic concentration gradients. Sci. Total Environ. 2007, 377, 416–433. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M.F.; Varone, L. Long-term monitoring of metal pollution by urban trees. Atmos. Environ. 2008, 42, 8273–8277. [Google Scholar] [CrossRef]
- Sumalan, R.L.; Nescu, V.; Berbecea, A.; Sumalan, R.M.; Crisan, M.; Negrea, P.; Ciulca, S. The impact of heavy metal accumulation on some physiological parameters in Silphium perfoliatum L. Plants grown in hydroponic systems. Plants 2023, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Tarish, M.; Ali, R.T.; Shan, M.; Amjad, Z.; Rui, Q.; Akher, S.A.; Al Mutery, A. Plant tissues as biomonitoring tools for environmental contaminants. Int. J. Plant Biol. 2024, 15, 375–396. [Google Scholar] [CrossRef]
- Baycu, G.; Tolunay, D.; Özden, H.; Günebakan, S. Ecophysiological and seasonal variations in Cd, Pb, Zn, and Ni concentrations in the leaves of urban deciduous trees in Istanbul. Environ. Pollut. 2006, 143, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, M.; Drozdowski, I. Monitoring of biodiversity in the core zones of Biosphere Reserve Wienerwald. In Proceedings of the 5th Symposium Conference Volume for Research in Protected Areas, Nationalpark Hohe Tauern—Conference Volume, Mittersill, Austria, 10–12 June 2013; pp. 737–738. [Google Scholar]
- Köck, G.; Koch, G.; Diry, C. The UNESCO Biosphere Reserve ‘Biosphärenparka Wienerwald’ (Vienna Woods)—A Long History of Conservation. Eco.mont 2009, 1, 51–56. [Google Scholar] [CrossRef]
- Daryaei, A.; Lechner, M.; Iglseder, A.; Waser, L.T.; Immitzer, M. Sentinel-2 vs. PlanetScope: Comparison and combination for tree species classification in two central European forest ecosystems. Remote Sens. Appl. Soc. Environ. 2025, 38, 101617. [Google Scholar] [CrossRef]
- Haluza, D.; Kersten, P.; Lazic, T.; Steinparzer, M.; Godbold, D. Unlocking the Power of Nature: Insights from a 20-Minute Forest Visit on Well-Being. Forests 2025, 16, 792. [Google Scholar] [CrossRef]
- Wydział Kształtowania Środowiska Urzędu Miasta Krakowa. Kierunki Rozwoju i Zarządzania Terenami Zieleni w Krakowie na Lata 2017–2030 [Directions of Development and Management of Green Areas in Krakow for the Years 2017–2030]; UMK: Krakow, Poland, 2019; p. 126. [Google Scholar]
- Kostrakiewicz-Gierałt, K.; Pliszko, A.; Gmyrek, K. The Effect of Informal Tourist Trails on the Abiotic Conditions and Floristic Composition of Deciduous Forest Undergrowth in an Urban Area. Forests 2021, 12, 423. [Google Scholar] [CrossRef]
- Krommer, V.; Zechmeister, H.G.; Roder, I.; Scharf, S.; Hanus-illnar, A. Monitoring atmospheric pollutants in the biosphere reserve Wienerwald by a combined approach of biomonitoring methods and technical measurements. Sci. Total Environ. 2007, 67, 1956–1966. [Google Scholar] [CrossRef]
- Kiesewetter, G.; Borken-Kleefeld, J.; Schöpp, W.; Heyes, C.; Thunis, P.; Bessagnet, B.; Terrenoire, E.; Fagerli, H.; Nyiri, A.; Amann, M. Modelling street level PM10 concentrations across Europe: Source apportionment and possible futures. Atmos. Chem. Phys. 2015, 15, 1539–1553. [Google Scholar] [CrossRef]
- Hutter, H.P.; Poteser, M.; Moshammer, H.; Lemmerer, K.; Mayer, M.; Weitensfelder, L.; Wallner, P.; Kundi, M. Air Pollution Is Associated with COVID-19 Incidence and Mortality in Vienna, Austria. Int. J. Environ. Res. Public Health 2020, 17, 9275. [Google Scholar] [CrossRef]
- Khomenko, S.; Nieuwenhuijsen, M.; Ambròs, A.; Wegener, S.; Mueller, N. Is a liveable city a healthy city? Health impacts of urban and transport planning in Vienna, Austria. Environ. Res. 2020, 183, 109238. [Google Scholar] [CrossRef]
- Danek, T.; Weglinska, E.; Zareba, M. The influence of meteorological factors and terrain on air pollution concentration and migration: A geostatistical case study from Krakow, Poland. Sci. Rep. 2022, 12, 11050. [Google Scholar] [CrossRef]
- Oleniacz, R.; Gorzelnik, T.; Bogacki, M. Impact of urban, suburban and industrial background on air pollution levels of dust substances in North-Eastern part of Krakow (Poland). In IOP Conference Series: Earth and Environmental Science, Vol. 642, 3rd International Conference on the Sustainable Energy and Environmental Development 16–18 October 2019, Kraków, Poland; IOP Publishing: Bristol, UK, 2021; Volume 642, p. 012013. [Google Scholar] [CrossRef]
- Bokwa, A. Environmental impacts of long-term air pollution changes in Kraków, Poland. Pol. J. Environ. Stud. 2008, 17, 673–686. [Google Scholar]
- Traczyk, P.; Gruszecka-Kosowska, A. The condition of air pollution in Krakow, Poland, in 2005–2020, with health risk assessment. Int. J. Environ. Res. Public Health 2020, 17, 6063. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister, H.G.; Hohenwallner, D.; Riss, A.; Hanus-Illnar, A. Estimation of element deposition derived from road traffic sources by using mosses. Environ. Pollut. 2005, 138, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Piron-Frenet, M.; Bureau, F.; Pineau, A. Lead accumulation in surface roadside soil: Its relationship to traffic density and meteorological parameters. Sci. Total Environ. 1994, 144, 297–304. [Google Scholar] [CrossRef]
- Steinnes, E.; Friedland, A.J. Metal contamination of natural surface soils from long-range atmospheric transport: Existing and missing knowledge. Environ. Rev. 2006, 14, 169–186. [Google Scholar] [CrossRef]
- Lehndorff, E.; Schwark, L. Accumulation histories of major and trace elements on pine needles in the Cologne Conurbation as function of air quality. Atmos. Environ. 2008, 42, 833–845. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 413. [Google Scholar]
- Løbersli, E.M.; Steinnes, E. Metal uptake in plants from a birch forest area near a copper smelter in Norway. Water Air Soil Pollut. 1988, 37, 25–39. [Google Scholar] [CrossRef]
- Čeburnis, D.; Steinnes, E. Conifer needles as biomonitors of atmospheric heavy metal deposition: Comparison with mosses and precipitation, role of the canopy. Atmos. Environ. 2000, 34, 4265–4271. [Google Scholar] [CrossRef]
- Samecka-Cymerman, A.; Kosior, G.; Kempers, A.J. Comparison of the moss Pleurozium schreberi with needles and bark of Pinus sylvestris as biomonitors of pollution by industry in Stalowa Wola (southeast Poland). Ecotoxicol. Environ. Saf. 2006, 65, 108–117. [Google Scholar] [CrossRef] [PubMed]
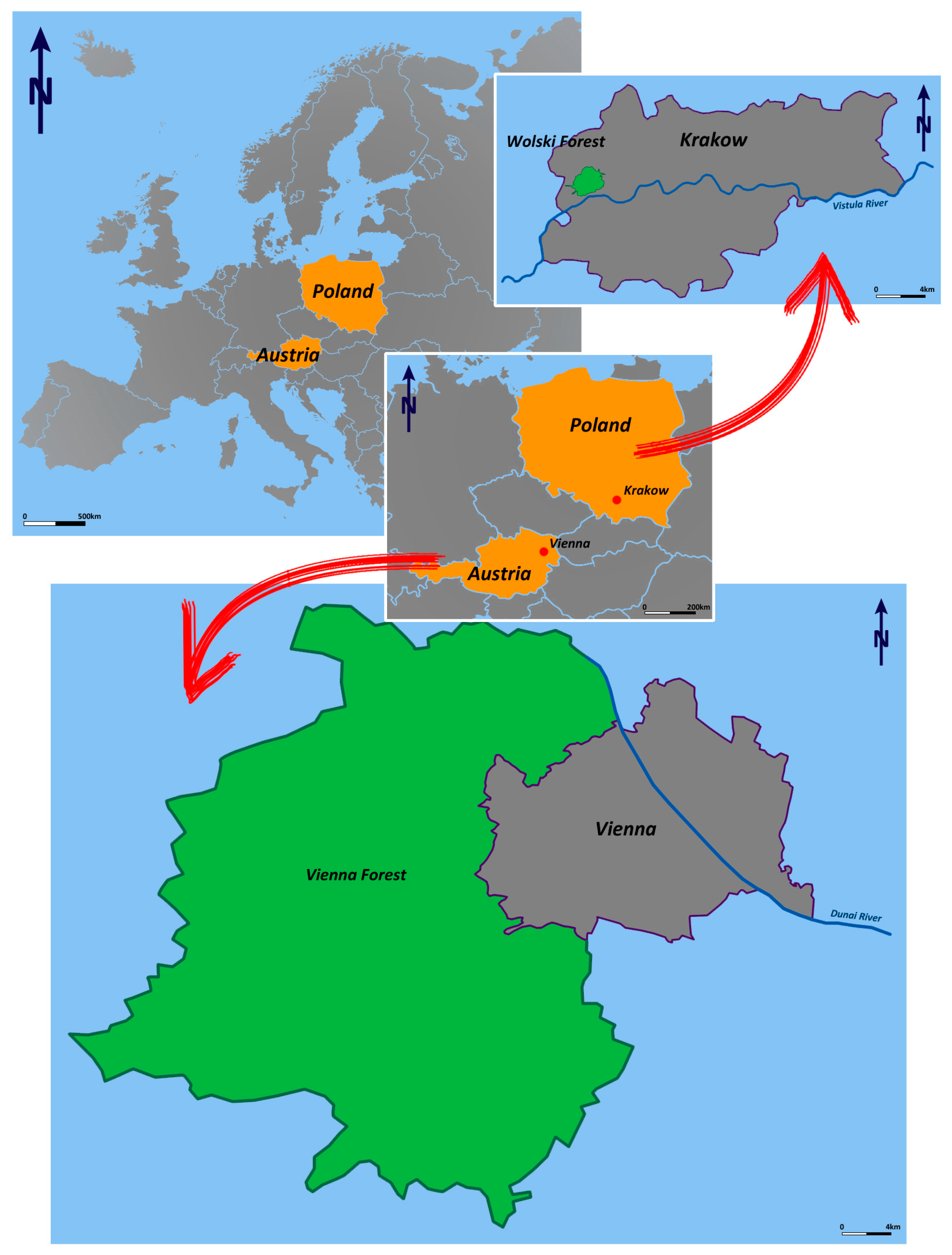
| Sampling Site | Altitude a.s.l. [m] | Geographical Coordinates | |
|---|---|---|---|
| The “Wienerwald” Forest, Vienna: | |||
| 1 | The 19th district Döbling–Latisberg between Cobenzl and Kahlenberg | 320–380 | 48°15′47″ N, 16°18′12″ E |
| 2 | The 18th district Währing, including Pötzleinsdorfer Park | 320–400 | 48°15′47″ N, 16°18′12″ E |
| 3 | The 3rd district Hietzing, a neighborhood of the Lainzer Tiergarten protected area (in the vicinity of Hermesstrasse Street) | 340–400 | 48°10′41″ N, 16°14′08″ E |
| 4 | The 23rd district Liesing, the valley of the Reiche Liesing and Gütenbach streams, north of Breitenfurterstrasse Street | 320–360 | 48°08′57″ N, 16°14′24″ E |
| The “Las Wolski” Forest, Krakow: | |||
| 5 | Pustelnik Hill | 340–380 | 50°03′10″ N, 19°50′52″ E |
| 6 | Sowiniec Hill | 340–370 | 50°03′34″ N, 19°50′54″ E |
| 7 | Sikornik Hill | 280–310 | 50°03′26″ N, 19°52′48″ E |
| Species | Location | Metal Concentration (μg/g d.w. Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Pb | Zn | ||
| European beech (Fagus sylvatica L.) | Vienna | 0.08 ±0.07 | 1.70 ±0.32 | 7.51 ±0.15 | 1.04 ±0.64 | 31.11 ±2.83 |
| Krakow | 0.80 ±0.31 | 2.17 ±0.19 | 8.53 ±0.99 | 4.21 ±0.13 | 43.52 ±8.74 | |
| Median for Vienna and Krakow | 0.31 | 2.00 | 7.60 | 2.92 | 36.11 | |
| Common white birch (Betula pendula Roth.) | Vienna | 0.15 ±0.07 | 0.44 ±0.41 | 5.29 ±1.34 | 1.25 ±0.98 | 66.70 ±20.23 |
| Krakow | 1.46 ±0.40 | 1.39 ±0.91 | 6.01 ±0.72 | 2.74 ±1.45 | 286.76 ±12.26 | |
| Median for Vienna and Krakow | 0.25 | 0.37 | 5.61 | 1.50 | 94.86 | |
| European larch (Larix decidua Mill.) | Vienna | 0.00 ±0.01 | 1.35 ±0.03 | 5.86 ±0.33 | 1.93 ±0.25 | 22.67 ±1.51 |
| Krakow | 0.38 ±0.13 | 1.72 ±0.56 | 5.83 ±1.58 | 2.70 ±1.02 | 53.88 ±11.81 | |
| Median for Vienna and Krakow | 0.25 | 1.37 | 5.62 | 2.11 | 42.30 | |
| Scots pine (Pinus sylvestris L.) | Vienna | 0.11 ±0.11 | 1.01 ±0.88 | 4.01 ±1.14 | 0.67 ±0.69 | 44.49 ±21.76 |
| Krakow | 0.68 ±0.44 | 1.78 ±0.47 | 3.35 ±1.74 | 3.36 ±0.86 | 64.35 ±30.79 | |
| Median for Vienna and Krakow | 0.19 | 1.41 | 3.82 | 1.21 | 46.45 | |
| Norway spruce (Picea abies (L.) H.Karst | Vienna | 0.09 ±0.03 | 0.69 ±0.15 | 2.69 ±0.14 | 0.30 ±0.20 | 25.33 ±2.03 |
| Krakow | 0.28 ±0.25 | 0.45 ±0.14 | 2.70 ±0.64 | 2.59 ±1.40 | 23.04 ±3.00 | |
| Median for Vienna and Krakow | 0.10 | 0.62 | 2.62 | 0.56 | 26.09 | |
| N | Average | Median | Min | Max | Variance | Std. Deviation | |
|---|---|---|---|---|---|---|---|
| Descriptive statistics for all 7 sampling sites (Viena + Krakow) | |||||||
| Cd | 31 | 0.38065 | 0.12000 | 0.00000 | 1.9000 | 0.219 | 0.46827 |
| Cr | 31 | 1.19645 | 1.37000 | 0.00000 | 2.3700 | 0.538 | 0.73357 |
| Cu | 31 | 5.09968 | 5.10000 | 2.10000 | 9.5600 | 4.368 | 2.08998 |
| Pb | 31 | 1.91161 | 1.74000 | 0.00000 | 4.3500 | 2.075 | 1.44059 |
| Zn | 31 | 65.64677 | 39.73000 | 17.84000 | 298.5000 | 5798.283 | 76.14645 |
| Descriptive statistics for all 1–4 sampling sites (Viena) | |||||||
| Cd | 17 | 0.09706 | 0.12000 | 0.00000 | 0.25000 | 0.0057 | 0.07540 |
| Cr | 17 | 0.96118 | 0.87000 | 0.00000 | 2.03000 | 0.4053 | 0.63666 |
| Cu | 17 | 4.83353 | 5.09000 | 2.53000 | 7.61000 | 3.4423 | 1.85535 |
| Pb | 17 | 0.93000 | 0.87000 | 0.00000 | 2.36000 | 0.5992 | 0.77406 |
| Zn | 17 | 40.27882 | 30.43000 | 17.84000 | 94.86000 | 456.8067 | 21.37304 |
| Descriptive statistics for all 5–7 sampling sites (Krakow) | |||||||
| Cd | 14 | 0.72500 | 0.50000 | 0.00000 | 1.9000 | 0.27 | 0.5159 |
| Cr | 14 | 1.48214 | 1.56000 | 0.37000 | 2.3700 | 0.58 | 0.7633 |
| Cu | 14 | 5.42286 | 5.22500 | 2.10000 | 9.5600 | 5.64 | 2.3745 |
| Pb | 14 | 3.10357 | 3.43500 | 1.00000 | 4.3500 | 1.26 | 1.1232 |
| Zn | 14 | 96.45071 | 48.01500 | 20.10000 | 298.5000 | 10,955.01 | 104.6662 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubiak, M.; Panek, E.; Urbański, K.; Victória, S.S.; Lach, S.; Maciuk, K.; Kopacz, M. Nature-Based Solutions in Sustainable Cities: Trace Metal Accumulation in Urban Forests of Vienna (Austria) and Krakow (Poland). Sustainability 2025, 17, 7042. https://doi.org/10.3390/su17157042
Jakubiak M, Panek E, Urbański K, Victória SS, Lach S, Maciuk K, Kopacz M. Nature-Based Solutions in Sustainable Cities: Trace Metal Accumulation in Urban Forests of Vienna (Austria) and Krakow (Poland). Sustainability. 2025; 17(15):7042. https://doi.org/10.3390/su17157042
Chicago/Turabian StyleJakubiak, Mateusz, Ewa Panek, Krzysztof Urbański, Sónia Silva Victória, Stanisław Lach, Kamil Maciuk, and Marek Kopacz. 2025. "Nature-Based Solutions in Sustainable Cities: Trace Metal Accumulation in Urban Forests of Vienna (Austria) and Krakow (Poland)" Sustainability 17, no. 15: 7042. https://doi.org/10.3390/su17157042
APA StyleJakubiak, M., Panek, E., Urbański, K., Victória, S. S., Lach, S., Maciuk, K., & Kopacz, M. (2025). Nature-Based Solutions in Sustainable Cities: Trace Metal Accumulation in Urban Forests of Vienna (Austria) and Krakow (Poland). Sustainability, 17(15), 7042. https://doi.org/10.3390/su17157042







