Manganese Ferrite Nanoparticle-Assisted Enhancement of Photosynthetic Carbon Sequestration in Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Sources of Algae Species and Culture Media
2.1.2. Experimental Reagents
2.1.3. Main Instrumentation of the Experiment
2.2. Construction of the Experimental Setup
2.3. Experimental Design
2.4. Analysis and Calculations
2.4.1. Methods of Biomass Determination
2.4.2. Measurement of Growth Rate
2.4.3. Determination of Carbon Dioxide Fixation Rate
2.4.4. Determination of pH
2.4.5. Measurement Method of Chlorophyll Content
2.4.6. Determination Method of Chlorophyll Fluorescence Parameters
2.4.7. Fluorescence Quantitative PCR Assays
Selection of Target Genes
Fluorescence Quantitative PCR Procedure
3. Results
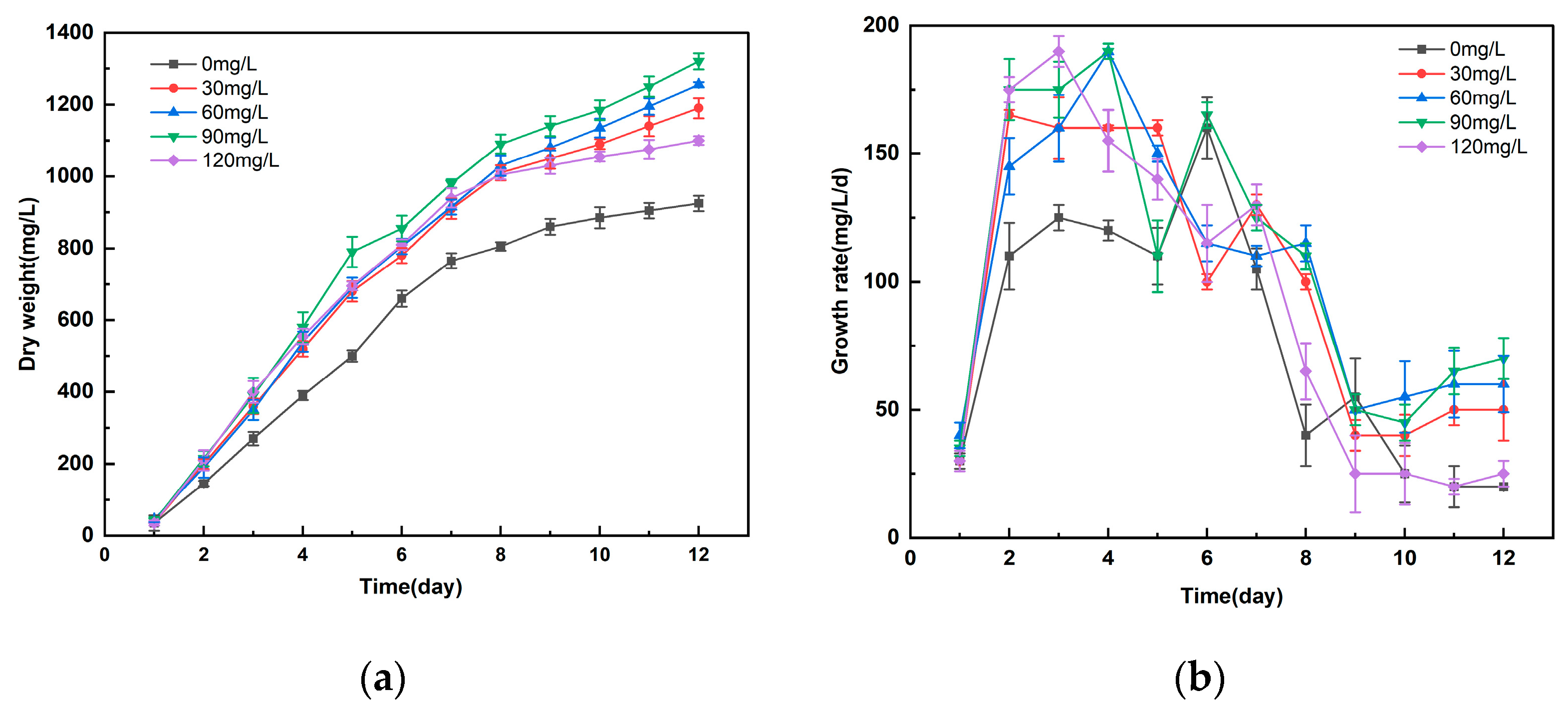
3.1. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on the Growth of Microalgae
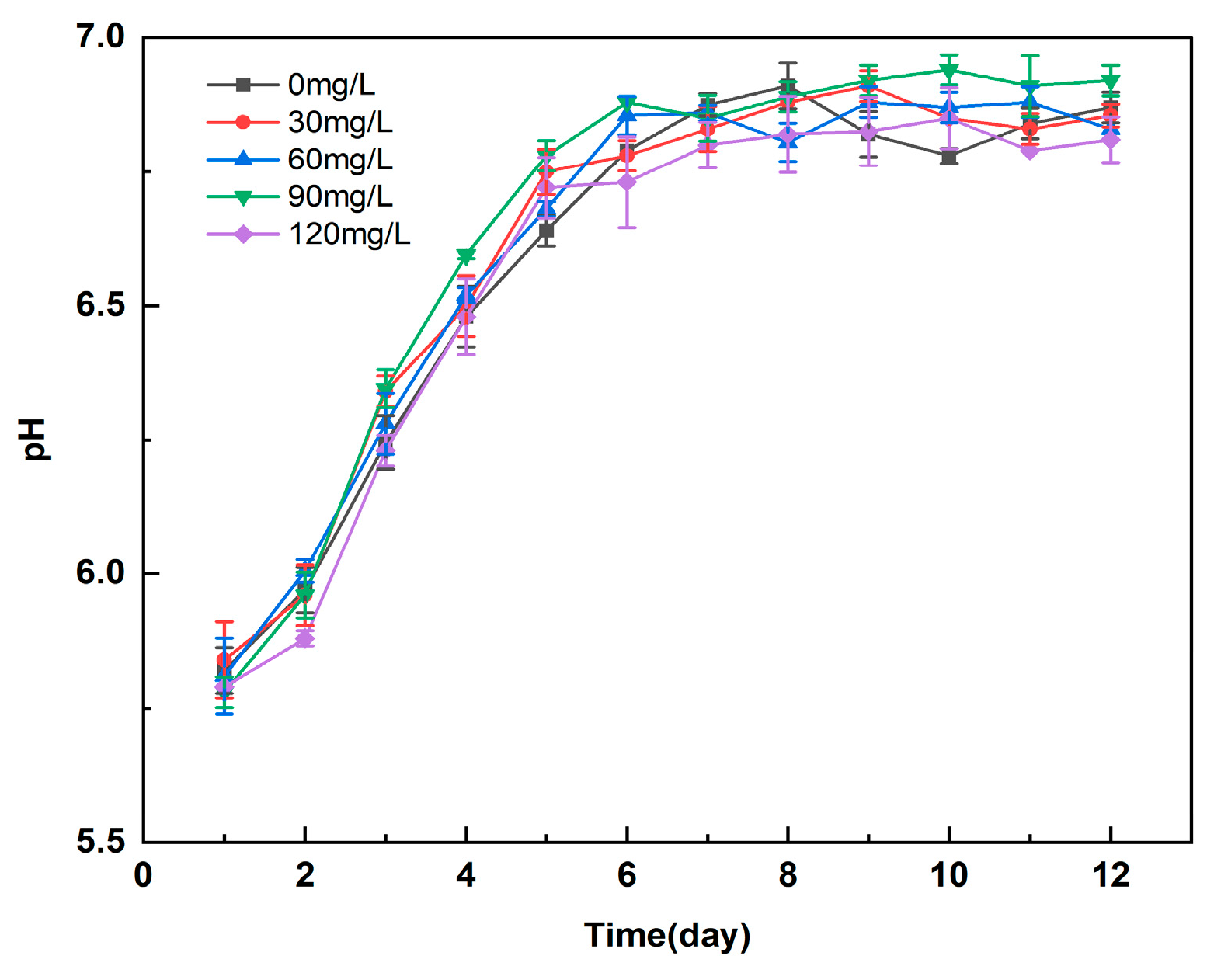
3.2. Effect of Different Concentrations of Manganese Ferrite Nanoparticles on the pH of Microalgal Algal Blooms
3.3. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on the Carbon Sequestration Rate of Microalgae
3.4. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on Chlorophyll Accumulation in Microalgae
3.5. Study on the Effect of Different Nanomaterials on Chlorophyll Fluorescence Parameters of Microalgae
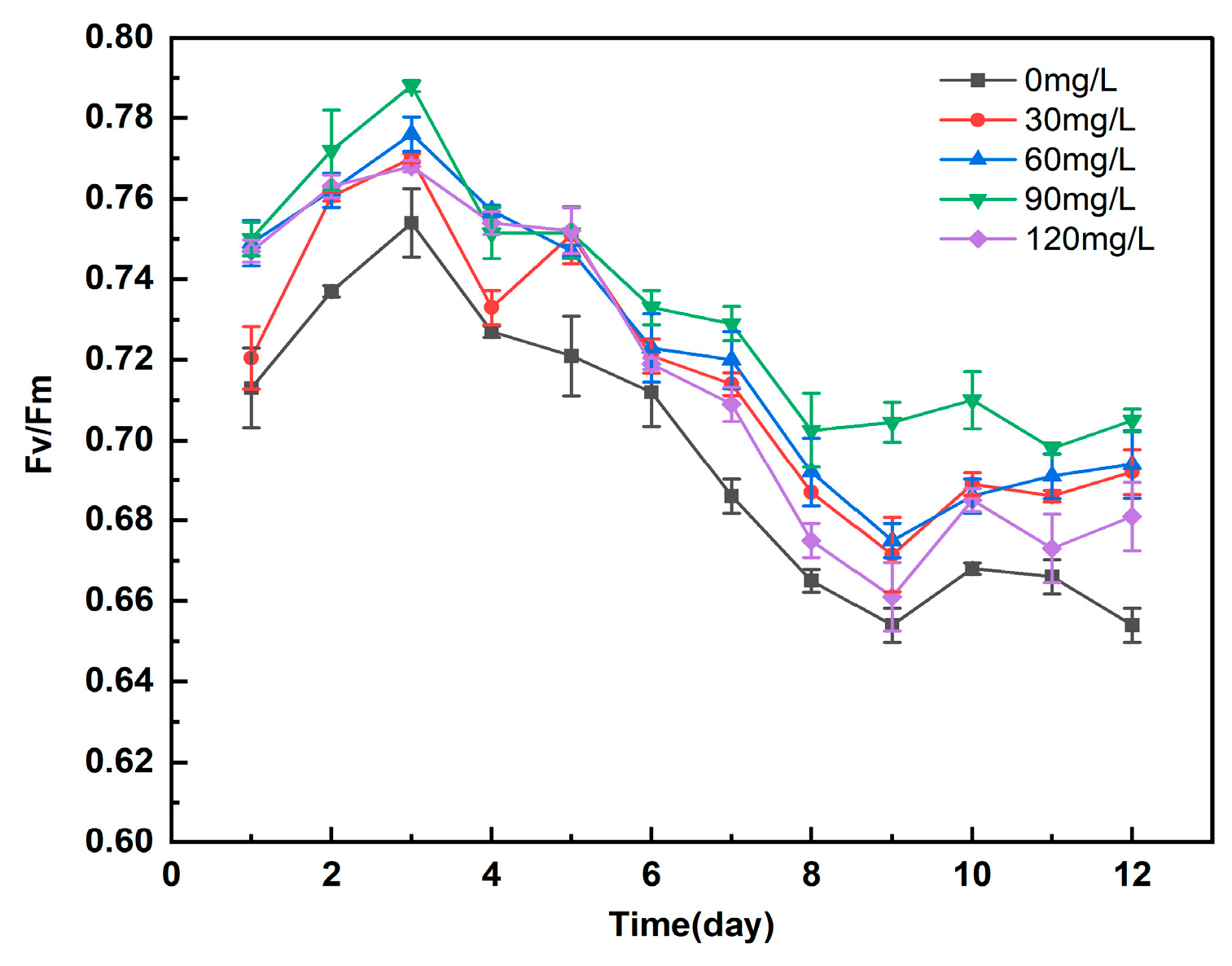
3.5.1. Study on the Effect of Different Concentrations of Manganese Ferrite Nanomaterials on Fv/Fm of Microalgae
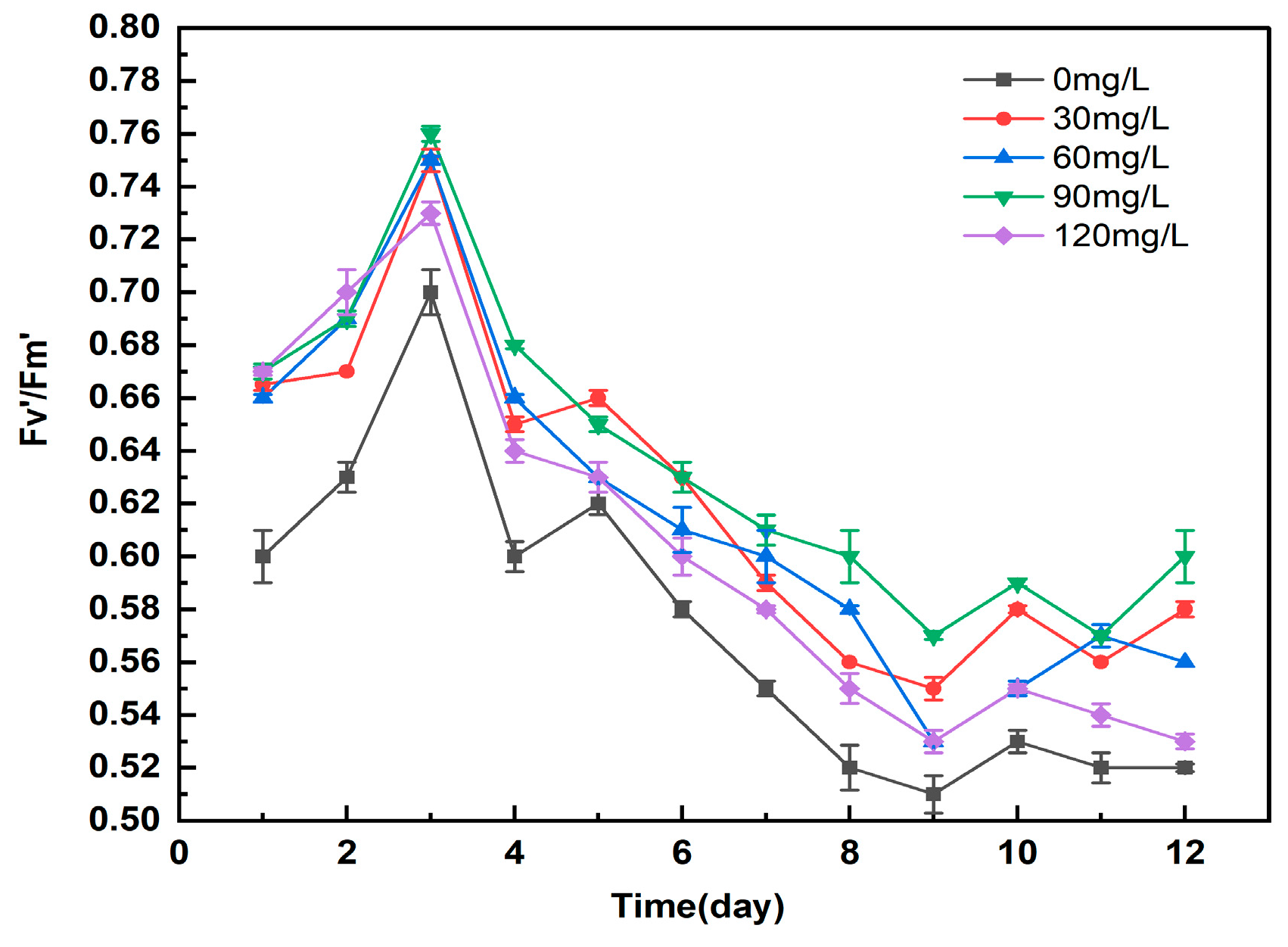
3.5.2. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on Microalgae Fv′/Fm′
3.5.3. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on Microalgae qP
3.5.4. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on NPQ of Microalgae
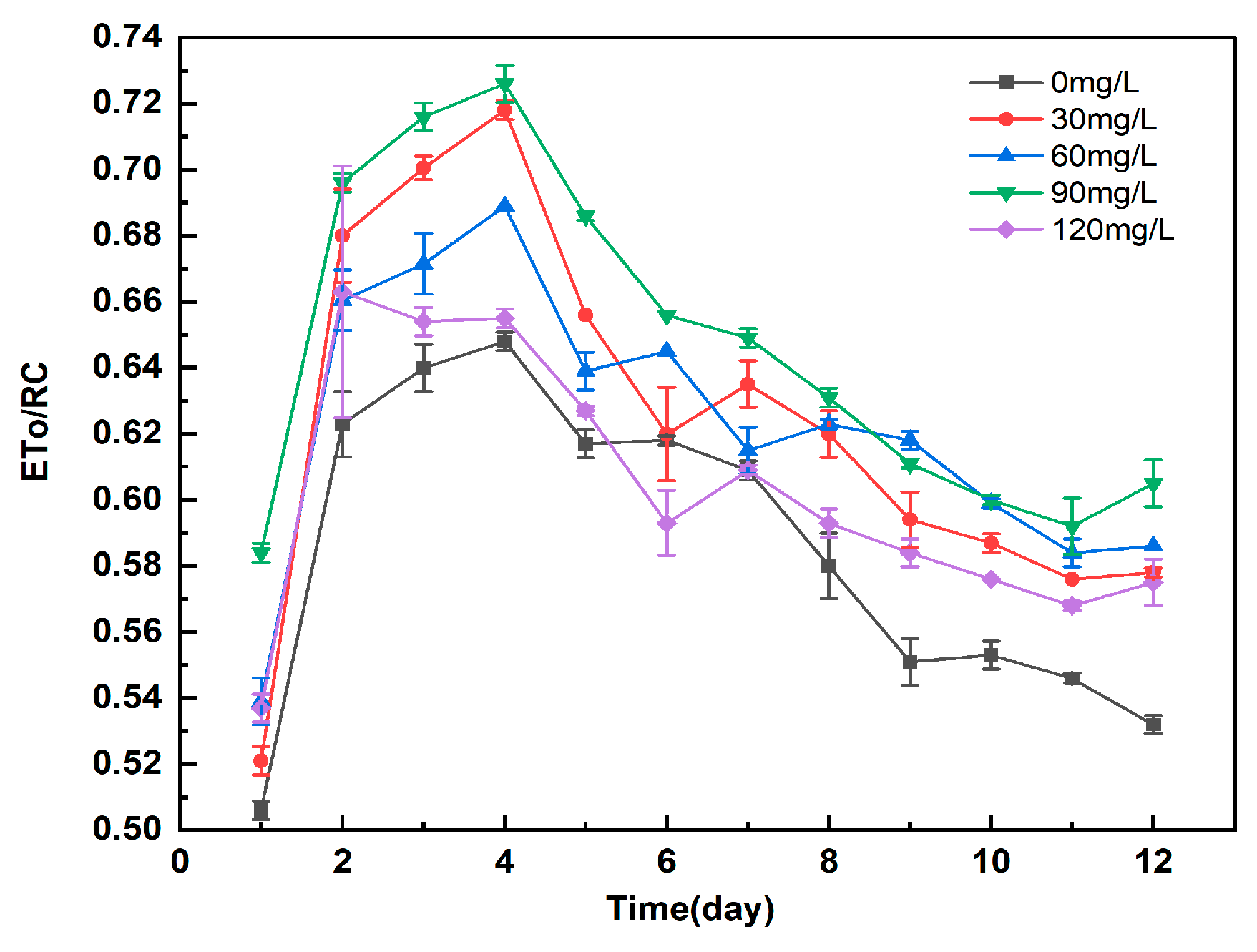
3.5.5. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on ETo/RC of Microalgae
3.6. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on Gene Expression of Microalgae
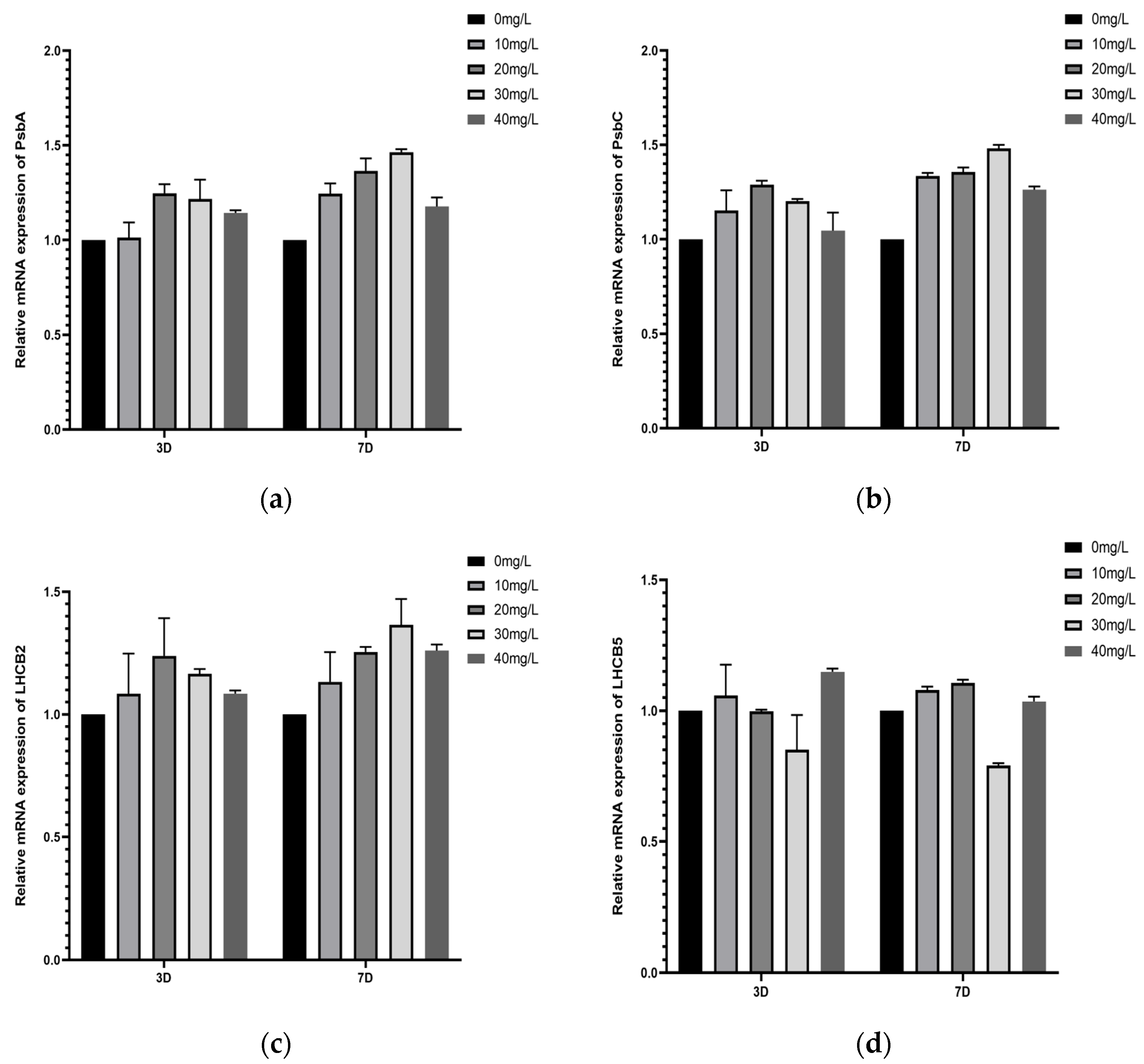
3.6.1. Study on the Effect of Two Nanomaterials on the Expression of Key Genes of Microalgae Photoresponsive PSII
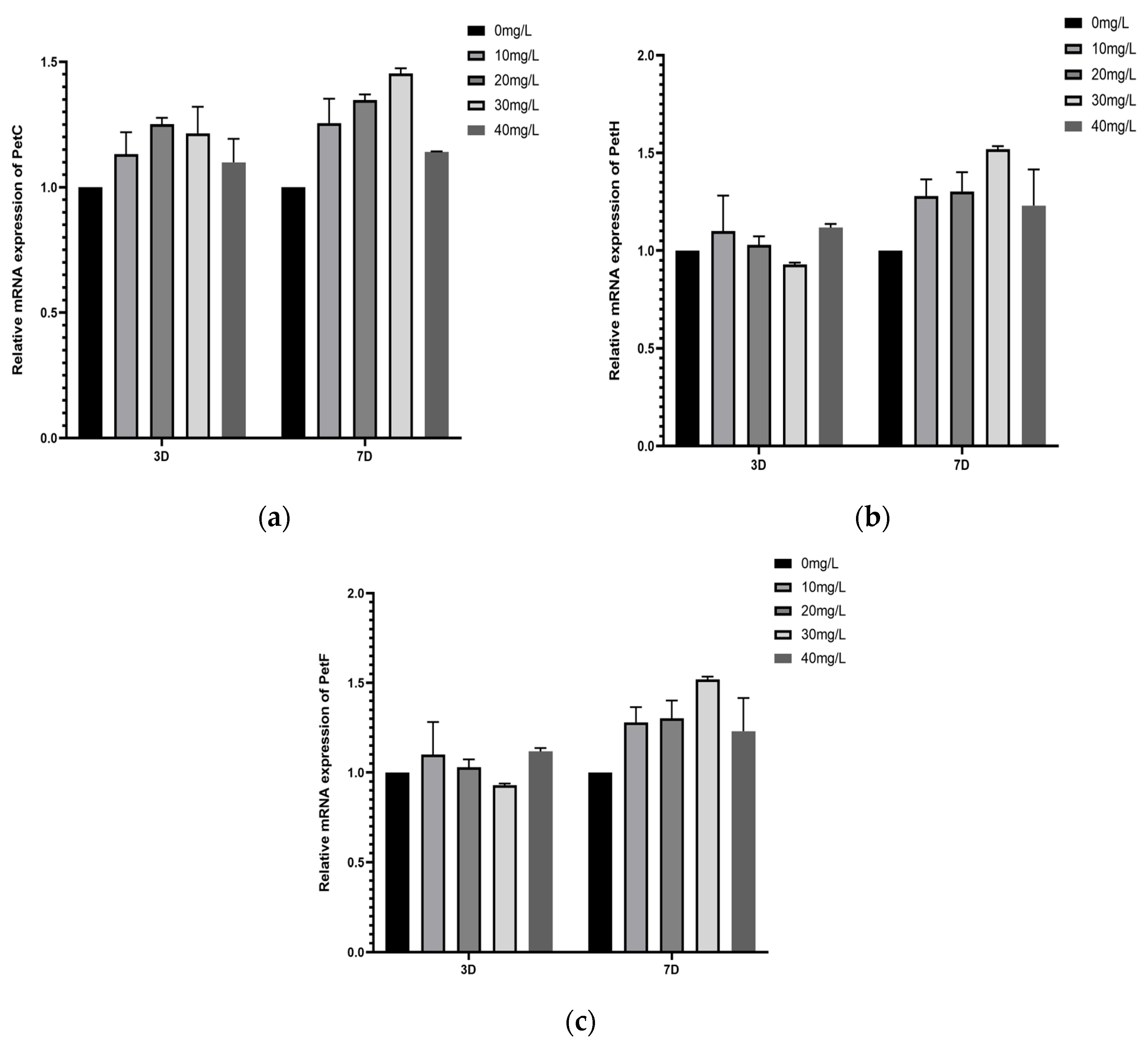
3.6.2. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on the Expression of Key Genes for Light-Responsive Electron Transfer in Microalgae
3.6.3. Effects of Different Concentrations of Manganese Ferrite Nanoparticles on the Expression of Key Genes of Microalgae Photoresponsive PSI
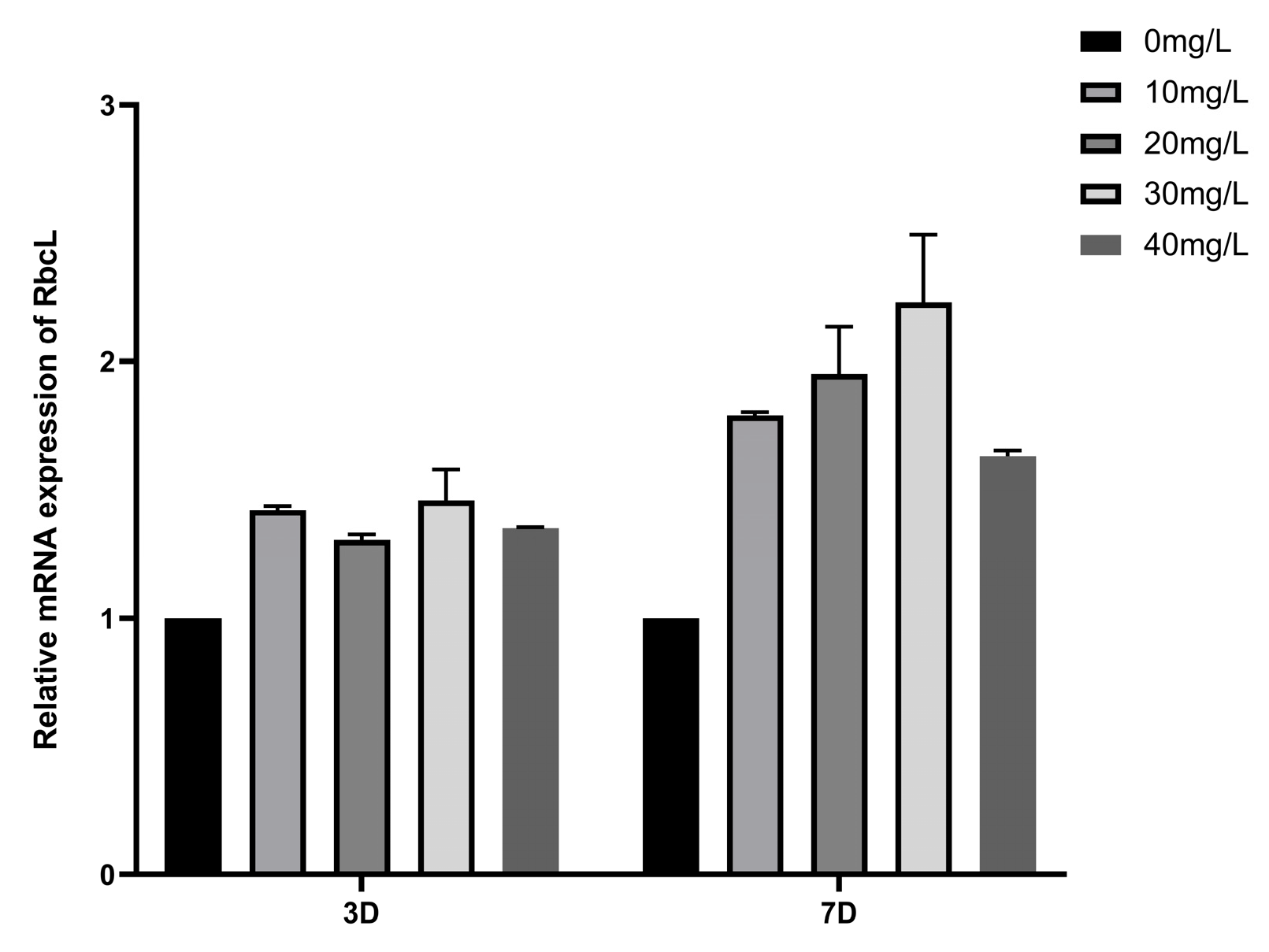
3.6.4. Study on the Effect of Different Concentrations of Manganese Ferrite Nanoparticles on the Expression of Key Rubisco Genes for Carbon Sequestration in Microalgae
4. Conclusions
5. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the Bio-Prospective of Microalgae by Different Light Systems and Photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The Technological and Economic Prospects for CO2 Utilization and Removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Nagappan, S.; Bhosale, R.R.; Tsai, P.-C.; Natarajan, S.; Devendran, S.; Al-Haj, L.; Ponnusamy, V.K.; Kumar, G. Various Potential Techniques to Reduce the Water Footprint of Microalgal Biomass Production for Biofuel—A Review. Sci. Total Environ. 2020, 749, 142218. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Wang, R.L.; Yang, Y.W.; Chen, J.X. Numerical and Experimental Analysis of Optimized Conical Flask Photobioreactor Structures to Improve Liquid–Gas Two-Phase Distribution and Microalgae Carbon Sequestration. Appl. Therm. Eng. 2020, 180, 115855. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A Review of High Value-Added Molecules Production by Microalgae in Light of the Classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating Micro-Algae into Wastewater Treatment: A Review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Zhe, L.; Jie, W.; Yuan, H. The Effect of Place Attachment of Geographical Indication Agricultural Products on Repurchase Intention. J. Retail. Consum. Serv. 2023, 72, 103266. [Google Scholar] [CrossRef]
- He, M.; Yan, Y.; Pei, F.; Wu, M.; Gebreluel, T.; Zou, S.; Wang, C. Improvement on Lipid Production by Scenedesmus Obliquus Triggered by Low Dose Exposure to Nanoparticles. Sci. Rep. 2017, 7, 15526. [Google Scholar] [CrossRef]
- Pádrová, K.; Lukavský, J.; Nedbalová, L.; Čejková, A.; Cajthaml, T.; Sigler, K.; Vítová, M.; Řezanka, T. Trace Concentrations of Iron Nanoparticles Cause Overproduction of Biomass and Lipids during Cultivation of Cyanobacteria and Microalgae. J. Appl. Phycol. 2015, 27, 1443–1451. [Google Scholar] [CrossRef]
- Rudic, V.; Cepoi, L.; Gutsul, T.; Rudi, L.; Chiriac, T.; Miscu, V.; Sadovnic, D.; Nicorici, A. Red Algae Porphyridium cruentum Growth Stimulated by CdSe Quantum Dots Covered with Thioglycerol. J. Nanoelectron. Optoelectron. 2012, 7, 681–687. [Google Scholar] [CrossRef]
- Petrou, K.; Kranz, S.A.; Doblin, M.A.; Ralph, P.J. Photophysiological Responses of Fragilariopsis Cylindrus (Bacillariophyceae) to Nitrogen Depletion at Two Temperatures. J. Phycol. 2012, 48, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-S.; Park, S.E.; Ahn, B.; Kim, Y.-K. Enhancement of Biodiesel Production in Chlorella Vulgaris Cultivation Using Silica Nanoparticles. Biotechnol. Bioprocess Eng. 2017, 22, 136–141. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Mao, X.; Lao, Y.; Chen, F. Enhanced Photosynthesis of Carotenoids in Microalgae Driven by Light-Harvesting Gold Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 7600–7608. [Google Scholar] [CrossRef]
- Yang, L.; Su, Q.; Si, B.; Zhang, Y.; Zhang, Y.; Yang, H.; Zhou, X. Enhancing Bioenergy Production with Carbon Capture of Microalgae by Ultraviolet Spectrum Conversion via Graphene Oxide Quantum Dots. Chem. Eng. J. 2022, 429, 132230. [Google Scholar] [CrossRef]
- Eroglu, E.; Eggers, P.K.; Winslade, M.; Smith, S.M.; Raston, C.L. Enhanced Accumulation of Microalgal Pigments Using Metal Nanoparticle Solutions as Light Filtering Devices. Green Chem. 2013, 15, 3155. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Liao, Q.; Xia, A.; Fu, Q.; Zhu, X.; Fu, J. Boosting Nannochloropsis oculata Growth and Lipid Accumulation in a Lab-Scale Open Raceway Pond Characterized by Improved Light Distributions Employing Built-in Planar Waveguide Modules. Bioresour. Technol. 2018, 249, 880–889. [Google Scholar] [CrossRef]
- Ooms, M.D.; Jeyaram, Y.; Sinton, D. Wavelength-Selective Plasmonics for Enhanced Cultivation of Microalgae. Appl. Phys. Lett. 2015, 106, 063902. [Google Scholar] [CrossRef]
- Estime, B.; Ren, D.; Sureshkumar, R. Effects of Plasmonic Film Filters on Microalgal Growth and Biomass Composition. Algal Res. 2015, 11, 85–89. [Google Scholar] [CrossRef]
- Li, D.; Dong, H.; Cao, X.; Wang, W.; Li, C. Enhancing Photosynthetic CO2 Fixation by Assembling Metal-Organic Frameworks on Chlorella pyrenoidosa. Nat. Commun. 2023, 14, 5337. [Google Scholar] [CrossRef]
- Hajinajaf, N.; Rabbani, Y.; Mehrabadi, A.; Tavakoli, O. Experimental and Modeling Assessment of Large-Scale Cultivation of Microalgae Nannochloropsis Sp. PTCC 6016 to Reach High Efficiency Lipid Extraction. Int. J. Environ. Sci. Technol. 2022, 19, 5511–5528. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Guo, W.; Johnston-Peck, A.C.; Hu, Y.; Song, X.; Wei, W.D. Modulating Multi-Hole Reaction Pathways for Photoelectrochemical Water Oxidation on Gold Nanocatalysts. Energy Environ. Sci. 2020, 13, 1501–1508. [Google Scholar] [CrossRef]
- Da Silva Vaz, B.; Alberto Vieira Costa, J.; Greque De Morais, M. Physical and Biological Fixation of CO2 with Polymeric Nanofibers in Outdoor Cultivations of Chlorella Fusca LEB 111. Int. J. Biol. Macromol. 2020, 151, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Ermis, H.; Guven-Gulhan, U.; Cakir, T.; Altinbas, M. Effect of Iron and Magnesium Addition on Population Dynamics and High Value Product of Microalgae Grown in Anaerobic Liquid Digestate. Sci. Rep. 2020, 10, 3510. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.; Wang, Y.; Yang, W.; Park, J.-Y.; Kim, H.; Xu, L. Strengthening CO2 Dissolution with Zeolitic Imidazolate Framework-8 Nanoparticles to Improve Microalgal Growth in a Horizontal Tubular Photobioreactor. Chem. Eng. J. 2021, 405, 126062. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, Z.; Wang, Q.; Li, J.; Qiu, X. Experimental Research on Chemical Desorption Based on CO2-Rich Absorption Solutions. Int. J. Greenh. Gas Control 2021, 109, 103356. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Pronina, N.A.; Los, D.A. Adapting from Low to High: An Update to CO2-Concentrating Mechanisms of Cyanobacteria and Microalgae. Plants 2023, 12, 1569. [Google Scholar] [CrossRef]
- Gu, W.; Wu, S.; Liu, X.; Wang, L.; Wang, X.; Qiu, Q.; Wang, G. Algal-Bacterial Consortium Promotes Carbon Sink Formation in Saline Environment. J. Adv. Res. 2024, 60, 111–125. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Qian, J.; Wang, B.; Liu, J.; Xu, R.; Chen, P.; Zhou, W. Recent Advances in CO2 Fixation by Microalgae and Its Potential Contribution to Carbon Neutrality. Chemosphere 2023, 319, 137987. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; Lavoie, J.-M. Effect of Photoperiods and CO2 Concentrations on the Cultivation of Carbohydrate-Rich P. Kessleri Microalgae for the Sustainable Production of Bioethanol. J. CO2 Util. 2022, 58, 101934. [Google Scholar] [CrossRef]
- Liu, B.; Kang, C.; Wang, X.; Bao, G. Physiological and Morphological Responses of Leymus Chinensis to Saline-Alkali Stress. Grassl. Sci. 2015, 61, 217–226. [Google Scholar] [CrossRef]
- You, X.; Chen, C.; Yang, L.; Xia, X.; Zhang, Y.; Zhou, X. Physiological and Morphological Responses of Chlorella pyrenoidosa to Different Exposure Methods of Graphene Oxide Quantum Dots. Sci. Total Environ. 2023, 854, 158722. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhou, D.; Xiao, L.; Li, Y. Chlorella Vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing. Gels 2022, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Comitre, A.A.; da Silva Vaz, B.; Costa, J.A.V.; de Morais, M.G. Renewal of Nanofibers in Chlorella Fusca Microalgae Cultivation to Increase CO2 Fixation. Bioresour. Technol. 2021, 321, 124452. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.; Chen, G.-Y.; Zhou, W. Research Progress in Plant RuBisCO. Sci. Sin. Vitae 2023, 53, 1213–1229. [Google Scholar] [CrossRef]
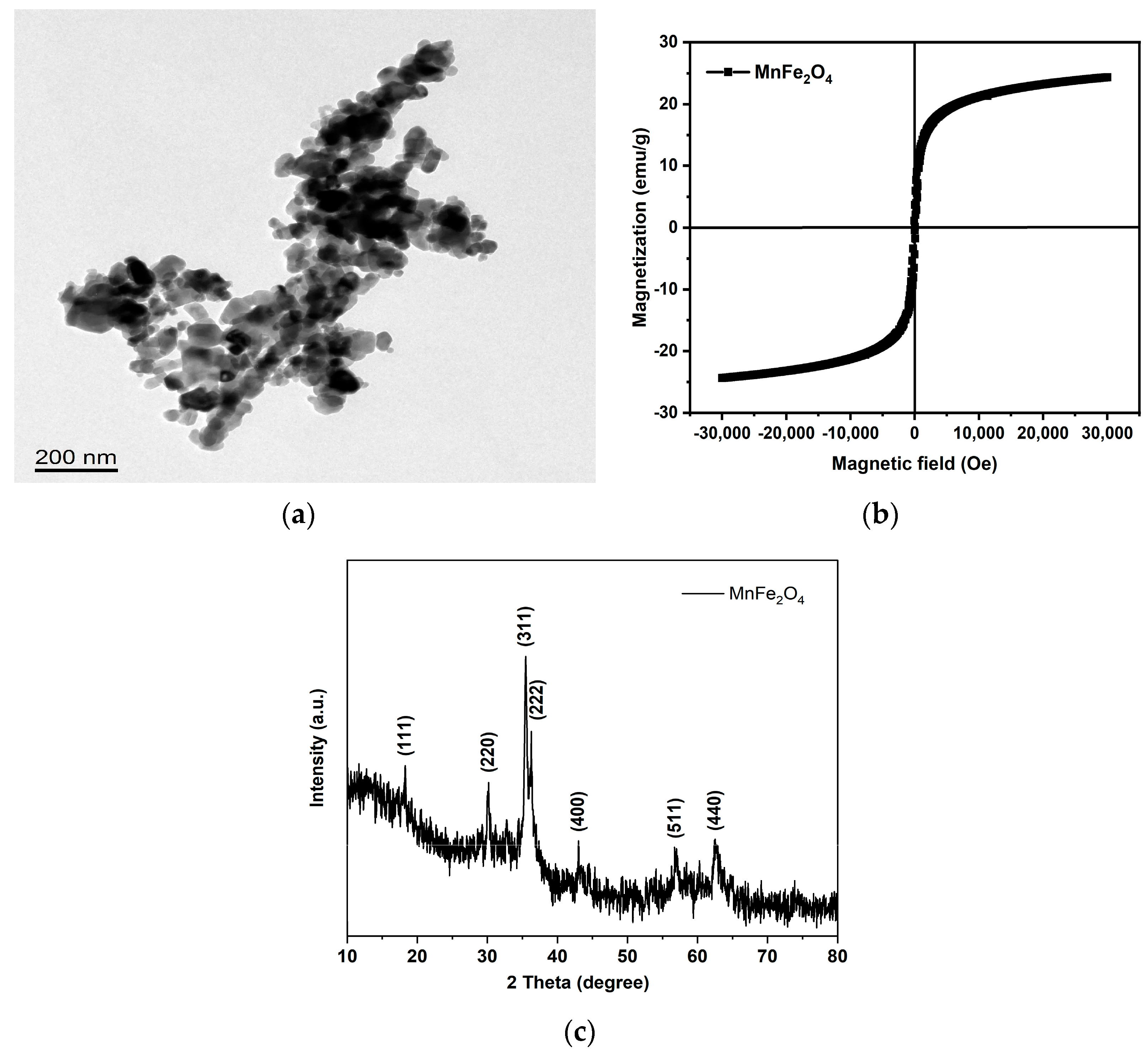






| Microalgae Species | Nanomaterials | Property | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Material | Size (nm) | Concentration (mg/L) | Cost Level | Index | Improve Efficiency (%) | CO2 Tolerance | ||
| Scenedesmus obliquus | MgO | <50.0 | 40.0 | Low | Lipid | 18.50 | 10–15% | [8] |
| Desmodesmus subspicatus | Zero-vzlent iron | 50.0 | 5.1 | Low | Lipid | 58.33 | 0–5% | [9] |
| Porphyridium cruentum | CdSe | 3.5 | 6.0 | High | Biomass | 47.50 | 0–5% | [10] |
| Microcystis aeruginosa | TiO2 | - | 0.8 | Low | Growth rate | 50.00 | 0–5% | [11] |
| Chlorella vulgaris | SiO2 | 200.0 | 0.2 wt% | Low | Growth rate | 177.00 | 10–15% | [12] |
| C.zofingiensis | Au | 5.0 | 25.0 | High | Carotenoid | 42.70 | 0–5% | [13] |
| C. pyrenoidosa | GOQDs | - | 100.0 | High | Electron transport rate | 50.00 | 2–10% | [14] |
| C. reinhardtii | Ag | - | 20.0 | High | Growth rate | 30.00 | 0–5% | [15] |
| Raphidocelis subcapitata | Zero-vzlent iron | 50.0 | 5.1 | Low | Lipid | 45.20 | 0–5% | [8] |
| Nannochloropsis oculata | Organosilicon compound | - | Internal waveguide doping | Medium | Lipid | 11.00 | 5–10% | [16] |
| Synechococcus elongatus | Au | 90.0 | PBR | High | Growth rate | 13.00 | 0–2% | [17] |
| Chlamydomonas reinhardtii | Ag | ±12.0 | PBR | High | Biomass | 10.00 | 1–5% | [18] |
| Serial Number | Restoratives | Concentration (g/L) | Dosage (mL/L) |
|---|---|---|---|
| 1 | NaNO3 | 150.0 | 10.0 |
| 2 | K2HPO4 | 4.0 | 10.0 |
| 3 | MgSO4·7H2O | 7.5 | 10.0 |
| 4 | CaCl2·2H2O | 3.6 | 10.0 |
| 5 | Citric acid | 0.6 | 10.0 |
| 6 | Ferric ammonium citrate | 0.6 | 10.0 |
| 7 | EDTA-Na2 | 0.1 | 10.0 |
| 8 | A5 | Table 3 | 1.0 |
| Serial Number | Restoratives | Concentration (g/L) | Dosage (mL/L) |
|---|---|---|---|
| 1 | H3BO3 | 2.9 | 1.0 |
| 2 | MnCl2·4H2O | 1.9 | 1.0 |
| 3 | ZnSO4·7H2O | 0.2 | 1.0 |
| 4 | Na2MoO4·2H2O | 0.4 | 1.0 |
| 5 | CuSO4·5H2O | 0.1 | 1.0 |
| 6 | Co(NO3)2·6H2O | 0.1 | 1.0 |
| Drug Name | Manufacturer |
|---|---|
| Sodium Nitrate | McLean Biotechnology Co., Shanghai, China |
| Dipotassium Hydrogen Phosphate | McLean Biotechnology Co., Shanghai, China |
| Magnesium Sulfate Heptahydrate | McLean Biotechnology Co., Shanghai, China |
| Calcium Chloride Dihydrate | McLean Biotechnology Co., Shanghai, China |
| Citric Acid | McLean Biotechnology Co., Shanghai, China |
| Ammonium Ferric Citrate | McLean Biotechnology Co., Shanghai, China |
| EDTA Salt | McLean Biotechnology Co., Shanghai, China |
| Sodium Carbonate | McLean Biotechnology Co., Shanghai, China |
| Boric Acid | Chemical Factory, Beijing, China |
| Manganese Chloride Tetrahydrate | McLean Biotechnology Co., Shanghai, China |
| Zinc Sulfate Heptahydrate | McLean Biotechnology Co., Shanghai, China |
| Sodium Molybdate Dihydrate | McLean Biotechnology Co., Shanghai, China |
| Copper Sulfate Pentahydrate | McLean Biotechnology Co., Shanghai, China |
| Manganese ferrite Nanometer | Hesimo New Material Co., Zhejiang, China |
| Cobalt Nitrate Hexahydrate | McLean Biotechnology Co., Shanghai, China |
| Anhydrous ethanol | Zhiyuan Chemical Reagent Co., Tianjin, China |
| RNAprep pure Plant Total RNA Extraction Kit (Gene Column Type) | Tiangen Biochemistry Technology Co., Beijing, China |
| FastKing cDNA First Strand Synthesis Kit (de-genomic) | Tiangen Biochemistry Technology Co., Beijing, China |
| SuperReal Fluorescence Pre-mixing Kit (SYBR Green have) | Tiangen Biochemistry Technology Co., Beijing, China |
| Carbon Dioxide | Nanfei Gas Technology Development Co., Beijing, China |
| Instrument | Model | Manufacturer |
|---|---|---|
| Electronic balance | JB/T5374-1991 | Mettler, Zurich, Switzerland |
| Constant temperature | MS7 | IKAC-MAG, Staufen, Germany |
| Autoclave | GR60DR | Xiamen Zhiwei Instrument Co., Xiamen, China |
| High-speed centrifuge | Sorvall Lynx 60000 | Thermo Fisher Scientific, New York, NY, USA |
| Light oscillation incubator | ZQZY-BGF8 | Zhichu Instrument Co., Shanghai, China |
| UV spectrophotometer | 7600 | Jinghua Science and Technology Instrument Co., Shanghai, China |
| Ultra-clean bench | Heraguard ECO 1.8 | Thermo Fisher Scientific, New York, NY, USA |
| TGrade Lite Metal Bath | OSE-DB-05/06 | Tiangen Biochemical Technology Co., Beijing, China |
| Fluorescence Quantitative PCR Instrument | LightCycler 96 | Goldside Biotechnology Co., Shanghai, China |
| Enzyme labeling instrument | Tecan Spark | Tecan Austria Ltd., Grodig, Austria |
| Gas flow indicator | D08-2F | Qixing Huachuang Flow Meter Co., Beijing, China |
| Gas flow controller | D07 | Qixing Huachuang Flow Meter Co., Beijing, China |
| Centrifuge | 3-18ks | Sigma, Osterode am Harz, Germany |
| Drying oven | DZF-6020 | Yiheng Technology Co., Shanghai, China |
| pH meter | PHS-3C | Yidian Scientific Instrument Co., Shanghai, China |
| Chlorophyll fluorescence meter | AP110 | FluorCam, Brno, Czech Republic |
| Parameter | Meaning |
|---|---|
| Fv/Fm | Maximum photosynthetic efficiency under dark conditions in response to the potential photochemical capacity of PSII in algal cells |
| Fv′/Fm′ | Actual light energy conversion efficiency, reflecting the primary light energy capture efficiency of open PSII reaction center |
| ETo/RC | Energy used for electron transfer in the unit reaction center |
| qP | Photochemical quenching coefficient, reflecting the degree of opening of PSII reaction centers |
| NPQ | Non-photochemical quenching coefficient, how much reaction heat is dissipated |
| Step | Temperature | Time | Number of Cycles |
|---|---|---|---|
| Step 1 | 95 °C | 30 s | 1 |
| Step 2 | 95 °C | 5 s | 45 |
| 60 °C | 20 s | ||
| Step 3 | 95 °C | 1 min | 1 |
| 55 °C | |||
| 95 °C |
| Gene | Primer Sequence 5′-3′ | Gene Annotation |
|---|---|---|
| 18s | F: ACTTCTTAGAGGGACTATTGGCG | Growth reference genes |
| R: CCTTGTTACGACTTCTCCTTCCT | ||
| RbcL | F: CTTGGACGACTGTATGGACTG | Rubisco enzyme large subunit gene |
| R: ATACCGTGAGGAGGACCTTG | ||
| PsbA | F: TGCTTGGCCAGTTGTTGGTA | PSII reaction center D1 protein subunit |
| R: ACGCTCGTGCATTACTTCCA | ||
| PsbC | F: AGGT CCAGAAGCATCACA | CP43 protein subunit of PSII reaction center |
| R: AAT CCCAGAAACGCATAG | ||
| LHCB2 | F: CTACCTGACTGGCGAGTTCC | LHCII light-harvesting complex II gene |
| R: CCTCCTGGAAGATCTGAGCA | ||
| LHCB5 | F: GACCTGGACAAGTGGTACGG | LHCII light-harvesting complex II gene |
| R: CAGAGGGTCGTAGCCGTAGT | ||
| PetC | F: TCGGTCACGATCAGGTAGGT | Cytochrome b6-f complex iron–sulfur protein gene |
| R: CCTACGCCCTGTTCTTCGTG | ||
| PetH | F: CCACGTGGAGGTGTACTGAG | Ferredoxin-NADP+ reductase gene |
| GAGTGCCGAAAATAAGCGCC | ||
| PetF | F: GTGATCACTATCAGCTACGA | Iron Oxygen Reduction Protein Gene |
| R: ACCTCCGACTACGCTGTCGT | ||
| LHCA2 | F: GTTGCGGATCTCGTTGGTC | LHCI phototrapping complexl gene |
| R: CCCCTACACGCTGTTCTGG | ||
| LHCA5 | F: TGGGCGACTACGGCTTT | LHCI photoconjugate complexl gene |
| R: CTCCAGGATGGCGAAGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Wang, X.; Zhu, X.; Shao, H.; Wang, W.; Hua, W.; Zhang, N.; Wu, S.; Ruan, R.; Zhou, C.; et al. Manganese Ferrite Nanoparticle-Assisted Enhancement of Photosynthetic Carbon Sequestration in Microalgae. Sustainability 2025, 17, 4303. https://doi.org/10.3390/su17104303
Chen T, Wang X, Zhu X, Shao H, Wang W, Hua W, Zhang N, Wu S, Ruan R, Zhou C, et al. Manganese Ferrite Nanoparticle-Assisted Enhancement of Photosynthetic Carbon Sequestration in Microalgae. Sustainability. 2025; 17(10):4303. https://doi.org/10.3390/su17104303
Chicago/Turabian StyleChen, Tiantian, Xinyi Wang, Xinyue Zhu, Hengxuan Shao, Wanqing Wang, Wei Hua, Na Zhang, Shuang Wu, Roger Ruan, Cheng Zhou, and et al. 2025. "Manganese Ferrite Nanoparticle-Assisted Enhancement of Photosynthetic Carbon Sequestration in Microalgae" Sustainability 17, no. 10: 4303. https://doi.org/10.3390/su17104303
APA StyleChen, T., Wang, X., Zhu, X., Shao, H., Wang, W., Hua, W., Zhang, N., Wu, S., Ruan, R., Zhou, C., & Cheng, Y. (2025). Manganese Ferrite Nanoparticle-Assisted Enhancement of Photosynthetic Carbon Sequestration in Microalgae. Sustainability, 17(10), 4303. https://doi.org/10.3390/su17104303






