Mechanisms of Cu2+ Immobilization Using Carbonyl Iron Powder–Biochar Composites for Remediating Acidic Soils from Copper Sulfide Mining Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Preparation
2.3. Experimental Design
2.3.1. Adsorption Kinetics and Isotherm Studies
2.3.2. Soil Incubation Trial
2.4. Chemical Analysis
2.4.1. Performance Characterization
2.4.2. Data Processing
3. Results and Discussion
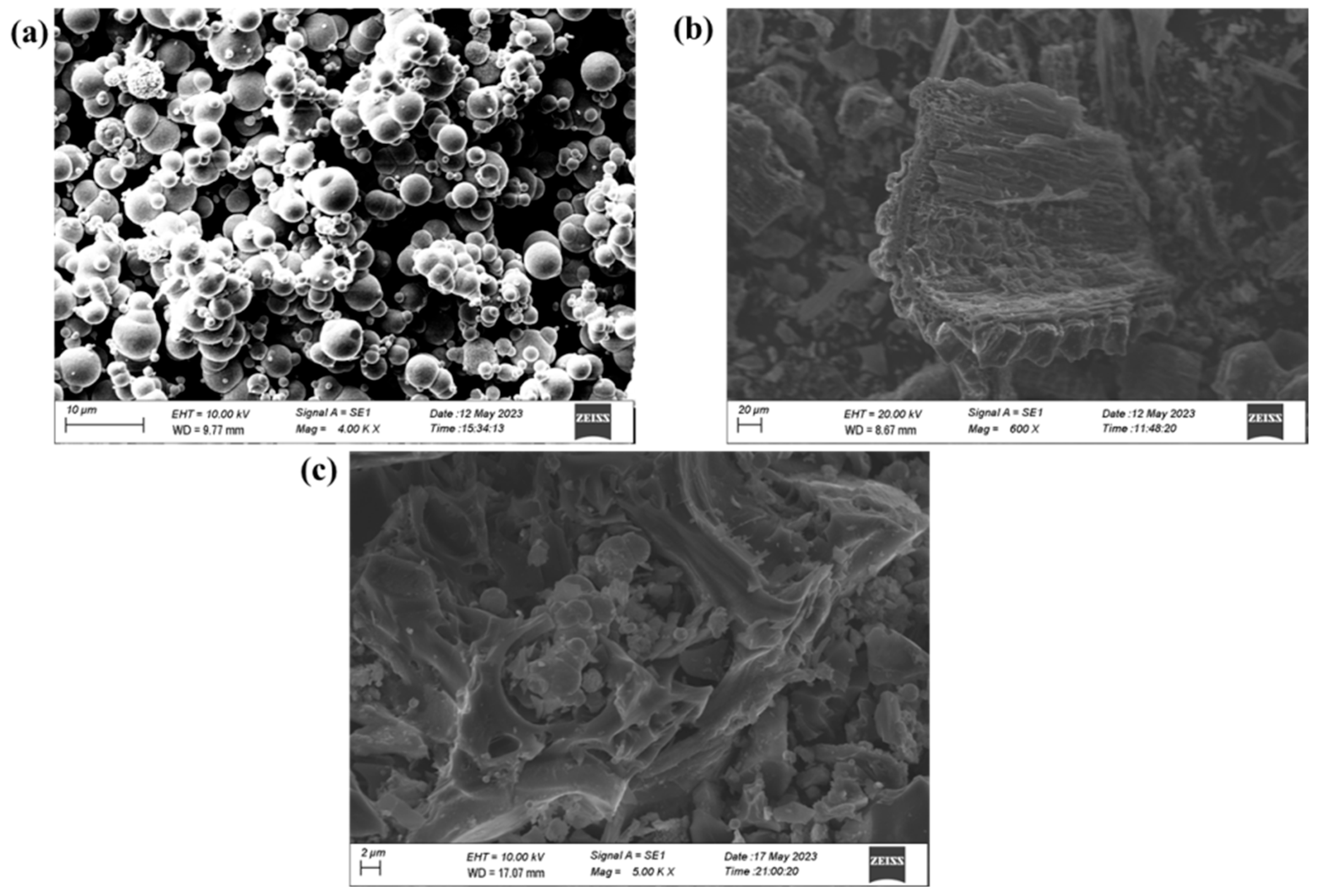
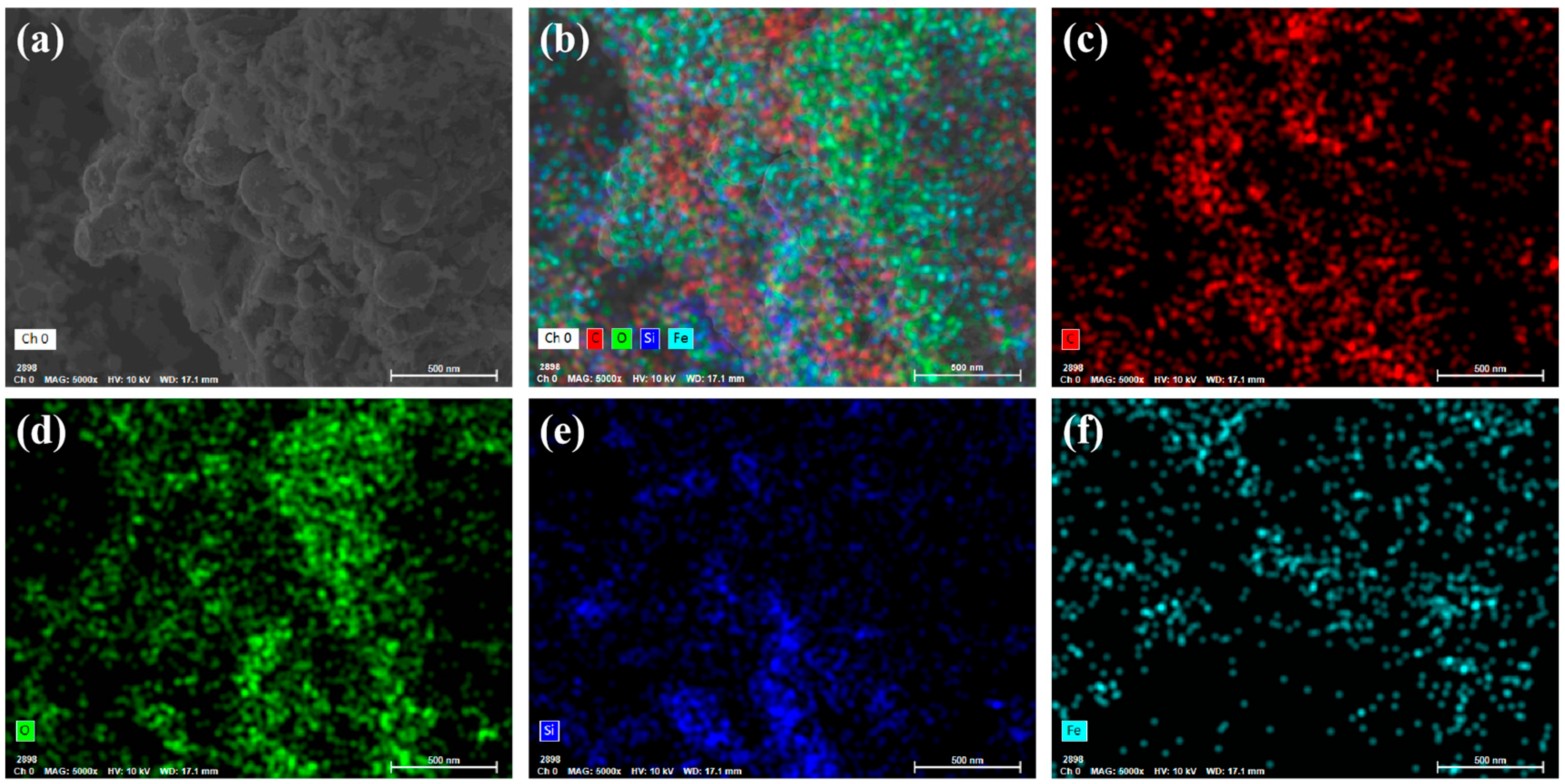
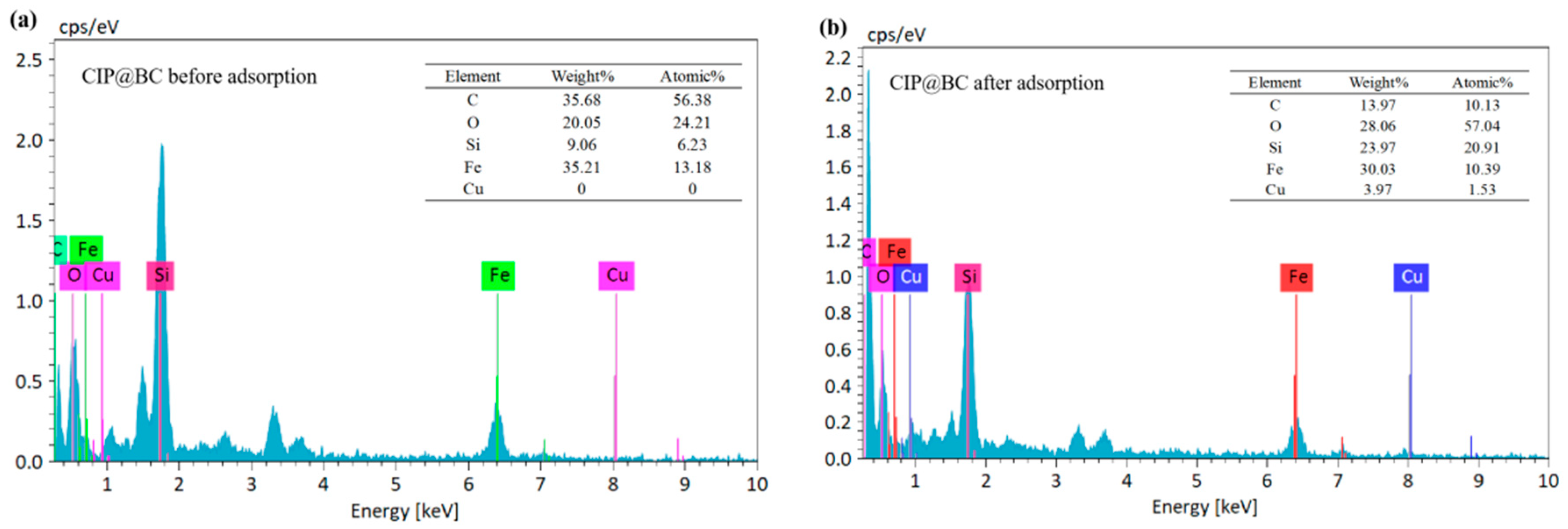
3.1. Characterization Analysis of CIP@BC
3.1.1. Micromorphology
3.1.2. Element Composition
3.1.3. Phase and Crystal Structure
3.1.4. Functional Groups
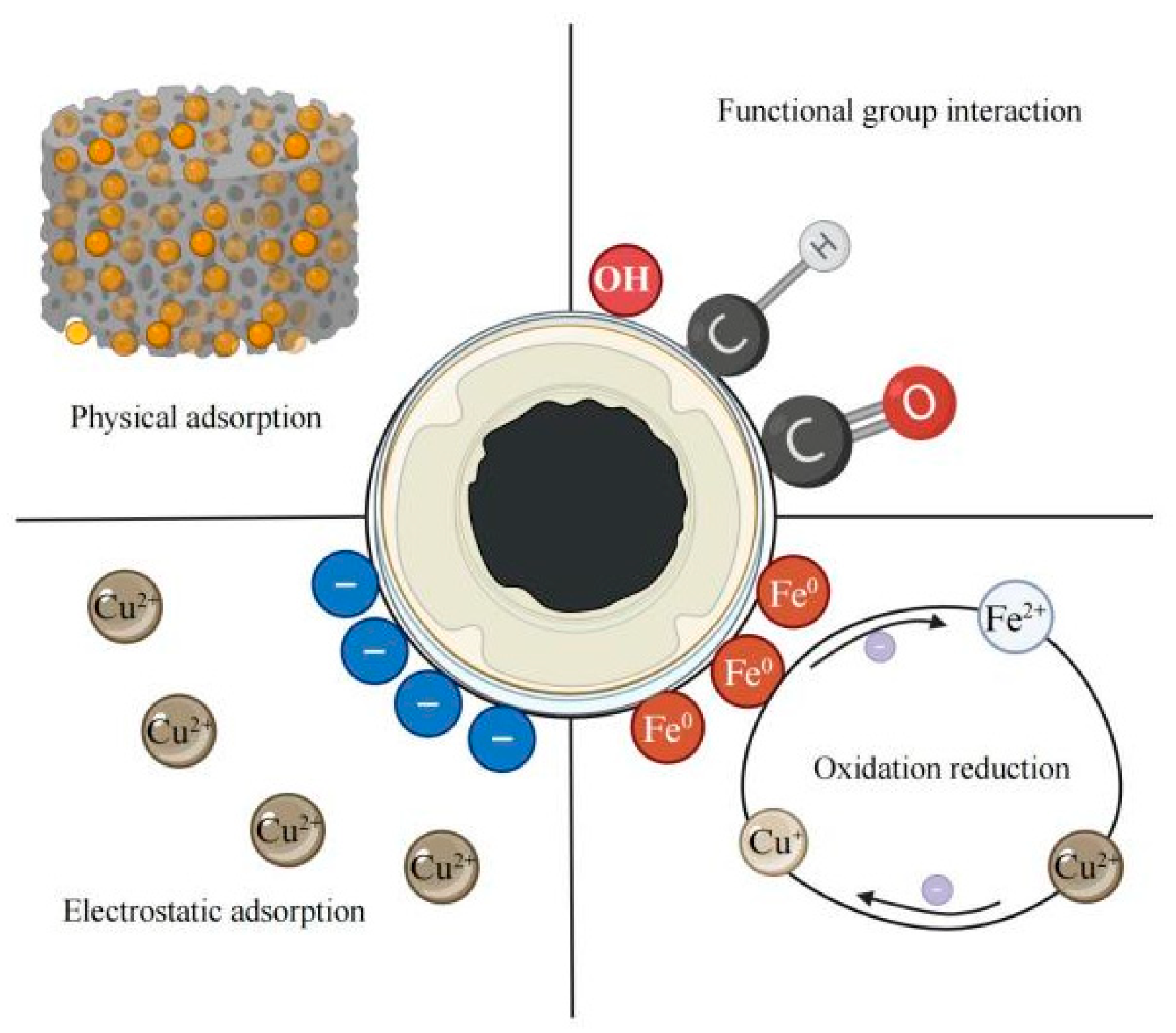
3.2. Analysis of Cu2+ Adsorption Properties of CIP@BC Composite
3.2.1. Cu2+ Adsorption Kinetics Analysis of CIP@BC Composite
3.2.2. Isothermal Adsorption Analysis of Cu2+ for CIP@BC Composite
3.3. CIP@BC Influence on Physical and Chemical Properties of Soil
3.3.1. Analysis of Soil pH and Conductivity Changes
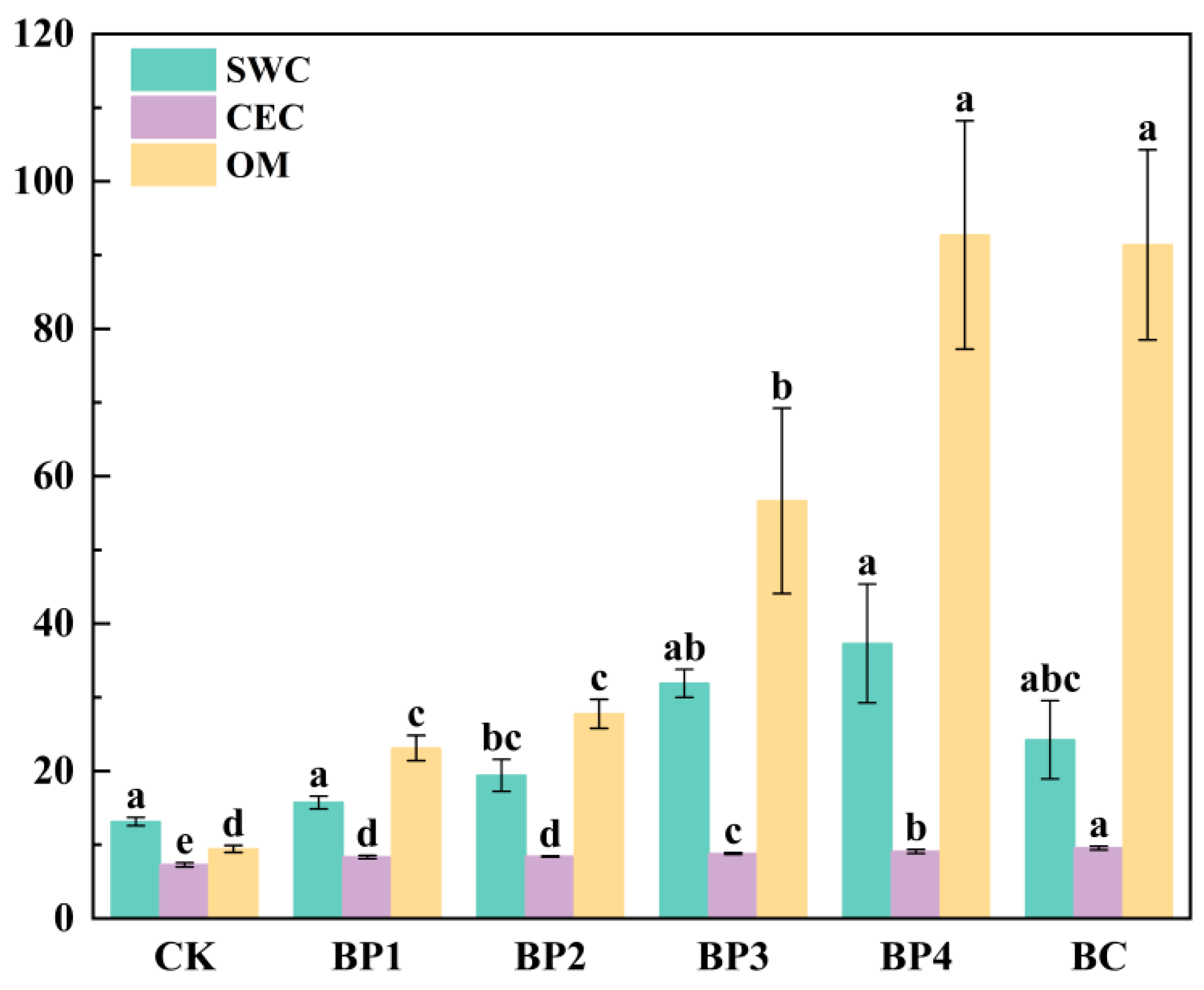
3.3.2. Analysis of Soil Moisture Content, Cation Exchange Capacity, and Organic Matter Change
3.4. Effect of CIP@BC Composite on Soil Cu Content
3.4.1. Analysis of Soil Total Cu Content Change
3.4.2. Analysis of Changes in Soil Cu Content of Different Forms
3.4.3. Determination of Available Copper Speciation in Soil
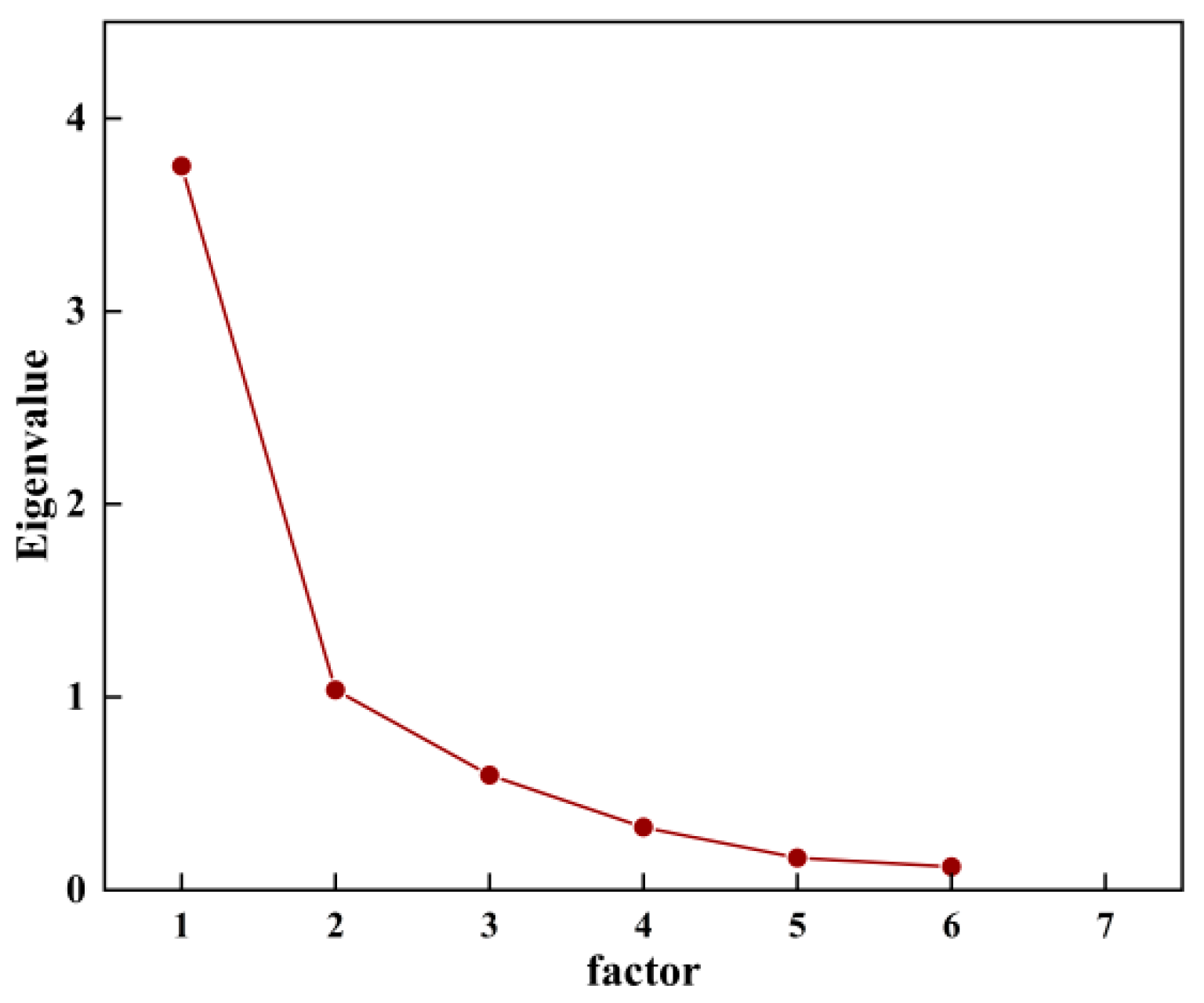
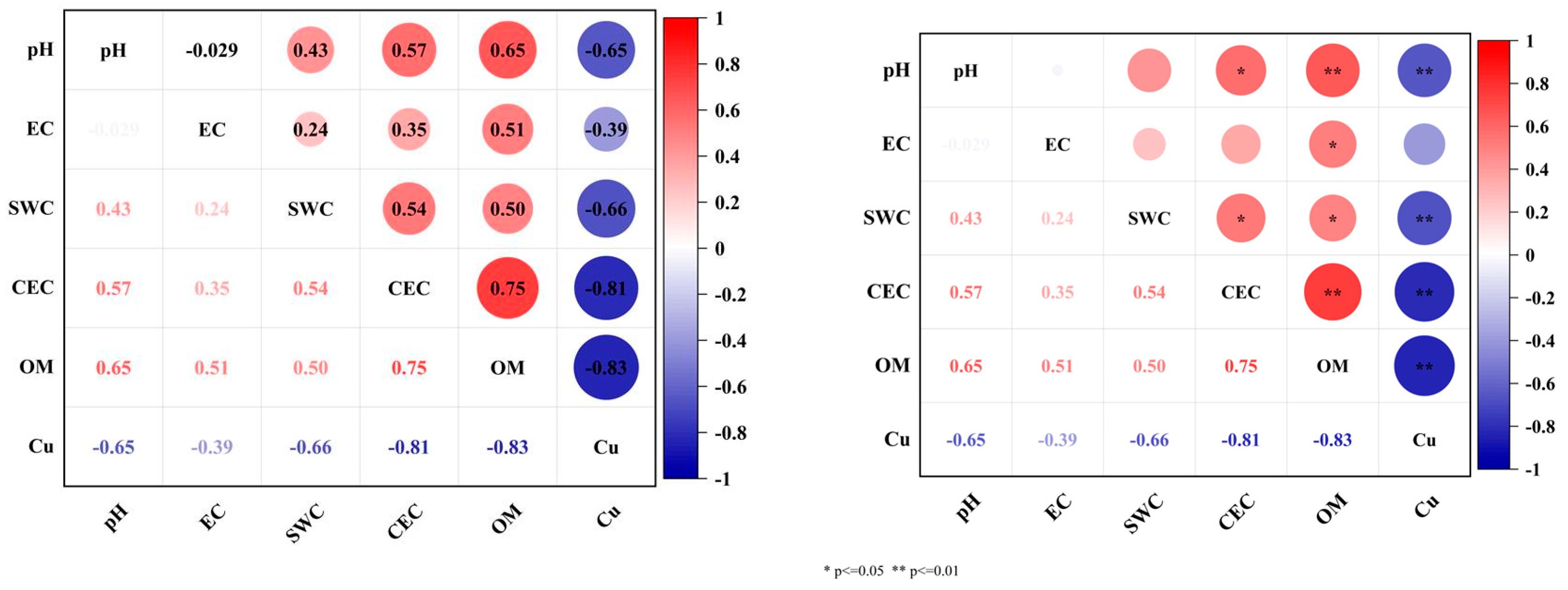
3.4.4. Analysis of the Relationship Between Soil Physical and Chemical Properties and Soil Cu Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | rice husk biochar |
| CIP | carbonyl iron powder |
| CIP@BC | carbonyl iron powder–biochar composite |
| OM | organic matter |
| SWC | soil water content |
| EC | electrical conductivity |
| CEC | cation exchange capacity |
| F1 | acid-soluble Cu |
| F2 | reducible Cu |
| F3 | oxidizable Cu |
| F4 | residual Cu |
References
- Wu, Q.H.; Zhou, H.C.; Tam, N.F.Y.; Tian, Y.; Tan, Y.; Zhou, S.; Li, Q.; Chen, Y.H.; Leung, J.Y.S. Contamination, toxicity and speciation of heavy metals in an industrialized urban river: Implications for the dispersal of heavy metals. Mar. Pollut. Bull. 2016, 104, 153–161. [Google Scholar] [CrossRef]
- Zhao, X.X.; Zhang, J.X.; Ma, R.J.; Luo, H.; Wan, T.; Yu, D.Y.; Hong, Y.Q. Worldwide Examination of Magnetic Responses to Heavy Metal Pollution in Agricultural Soils. Agriculture 2024, 14, 702. [Google Scholar] [CrossRef]
- Modis, K.; Vatalis, K.I. Assessing the Risk of Soil Pollution around an Industrialized Mining Region Using a Geostatistical Approach. Soil Sediment Contam. 2013, 23, 63–75. [Google Scholar] [CrossRef]
- Hou, D.Y.; Jia, X.Y.; Wang, L.; McGrath, S.P.; Zhu, Y.G.; Hu, Q.; Zhao, F.J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Poch, R.M.; Lobb, D.A.; Bhattacharyya, R.; Alloush, G.; Eudoxie, G.D.; Anjos, L.H.C.; Castellano, M.; Ndzana, G.M.; Chenu, C.; et al. Status of the World’s Soils. Annu. Rev. Environ. Resour. 2024, 49, 73–104. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yin, Y.Y.; Zhang, Y.; Shi, S.G. Legal System of Soil Pollution Remediation in China and Its Regulation and Guidance to Soil Pollution Remediation. Sustainability 2023, 15, 11504. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Khan, M.; Nizamani, M.M.; Asif, M.; Kamran, A.; He, G.D.; Li, X.Y.; Yang, S.W.; Xie, X. Comprehensive approaches to heavy metal bioremediation: Integrating microbial insights and genetic innovations. J. Environ. Manag. 2025, 374, 123969. [Google Scholar] [CrossRef]
- Wang, X.J.; Xue, J.C.; He, M.; Qi, H.; Wang, S.T. The Effects of Vermicompost and Steel Slag Amendments on the Physicochemical Properties and Bacterial Community Structure of Acidic Soil Containing Copper Sulfide Mines. Appl. Sci. 2024, 14, 1289. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Fraudentali, I.; Rodrigues-Pousada, R.A.; Angelini, R.; Ghuge, S.A.; Cona, A. Plant Copper Amine Oxidases: Key Players in Hormone Signaling Leading to Stress-Induced Phenotypic Plasticity. Int. J. Mol. Sci. 2021, 22, 5136. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar]
- Karimi, B.; Masson, V.; Guilland, C.; Leroy, E.; Pellegrinelli, S.; Giboulot, E.; Maron, P.A.; Ranjard, L. Ecotoxicity of copper input and accumulation for soil biodiversity in vineyards. Environ. Chem. Lett. 2021, 19, 2013–2030. [Google Scholar] [CrossRef]
- Helling, B.; Reinecke, S.A.; Reinecke, A.J. Effects of the Fungicide Copper Oxychloride on the Growth and Reproduction of Eisenia fetida (Oligochaeta). Ecotoxicol. Environ. Saf. 2000, 46, 108–116. [Google Scholar] [CrossRef]
- Hou, D.Y.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [PubMed]
- Teschke, R. Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury. Int. J. Mol. Sci. 2024, 25, 6662. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yan, Q.S.; Shi, Y.; Long, M. Copper Toxicity in Animals: A Review. Biol. Trace Elem. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Anggayasti, W.L.; Salamah, L.N.; Rizkymaris, A.; Yamamoto, T.; Kurniawan, A. The role of ion charge density and solubility in the biosorption of heavy metals by natural biofilm matrix of polluted freshwater: The cases of Mg(II), Cr(VI), and Cu(II). Environ. Pollut. Bioavailab. 2023, 35, 2220571. [Google Scholar] [CrossRef]
- Cain, D.; Croteau, M.N.; Luoma, S. Bioaccumulation dynamics and exposure routes of Cd and Cu among species of aquatic mayflies. Environ. Toxicol. Chem. 2011, 30, 2532–2541. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Shi, J.H.; Liu, W.Y.; Luo, Y.; Gao, S.; Liu, J.X. Copper overload induces apoptosis and impaired proliferation of T cell in zebrafish. Aquat. Toxicol. 2024, 267, 106808. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.F.; Skjolding, L.M.; Selck, H.; Baun, A. A “point-of-entry” bioaccumulation study of nanoscale pigment copper phthalocyanine in aquatic organisms. Environ. Sci. Nano 2021, 8, 554–564. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chang, L.; Liu, H.J.; Alquiza, M.D.P.; Li, Y.F. Biochar application to soils can regulate soil phosphorus availability: A review. Biochar 2025, 7, 13. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, F.F.; Peng, J.B.; Ji, M.F.; Li, B.L. A Comprehensive Review of the Application and Potential of Straw Biochar in the Remediation of Heavy Metal-Contaminated Soil. Toxics 2025, 13, 69. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, Y.B.; Xia, H.J.; Han, H.; Li, M.S.; Gong, P.; Wang, C.X.; Li, Y.F.; Li, P.F.; Liu, H.G. Mitigation of soil salinity by biochar and halophytes. Geoderma 2025, 454, 117191. [Google Scholar] [CrossRef]
- Huang, T.Z.; Imran. Mitigating cadmium contamination in soil using Biochar, sulfur-modified Biochar, and other organic amendments. Int. J. Phytoremediation 2025, 27, 874–887. [Google Scholar] [CrossRef]
- Su, J.Z.; Guo, Z.L.; Zhang, M.Y.; Xie, Y.M.; Shi, R.; Huang, X.F.; Tuo, Y.; He, X.H.; Xiang, P. Mn-modified bamboo biochar improves soil quality and immobilizes heavy metals in contaminated soils. Environ. Technol. Innov. 2024, 34, 103630. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Kumar, D.; Sinha, G.R.; Moon, A.P. Carbonyl iron powders as absorption material for microwave interference shielding: A review. J. Alloys Compd. 2021, 853, 157251. [Google Scholar] [CrossRef]
- Li, L.Y.; Gao, Z.; Li, A.K.; Yi, J.H.; Ge, Y.C. Fabrication of carbonyl iron powder/SiO2-reduced iron powder/SiO2 soft magnetic composites with a high resistivity and low core loss. J. Magn. Magn. Mater. 2018, 464, 161–167. [Google Scholar] [CrossRef]
- Peng, J.; Wang, D.W.; Cheng, Z.Y.; Yang, M.F.; Liu, J.T.; Wang, M. Improving microwave absorption performance of carbonyl iron powder by regulating geometric dimensions and electromagnetic-dielectric synergism. Compos. Part A Appl. Sci. Manuf. 2025, 190, 108719. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, F.; Zhang, Z.; Liu, Z.M.; Zhang, J.L.; Jiang, K.Y. Effect of Ball-Milling Process on Microwave Absorption Behaviors of Flaky Carbonyl Iron Powders. Materials 2023, 16, 4397. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.N.; Xu, X.N.; Kuniyoshi, R.; Zhou, H.H.; Zhu, Y.F. Electromagnetic and mechanical properties of carbonyl iron powders-PLA composites fabricated by fused deposition modeling. Mater. Res. Express 2018, 5, 115303. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Teng, Y.; Liu, Y. A study on soil-environmental quality criteria and standards of arsenic. Appl. Geochem. 2017, 77, 158–166. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, C.Q.; Yu, L.; Hou, H.Q.; Sun, W.M.; Ke, F. Synthesis of benzimidazole by mortar-pestle grinding method. Green Chem. Lett. Rev. 2021, 14, 610–617. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, J.C.; Han, H.Q.; Wang, Y. Study on improvement of copper sulfide acid soil properties and mechanism of metal ion fixation based on Fe-biochar composite. Sci. Rep. 2024, 14, 247. [Google Scholar] [CrossRef]
- Nicolaou, E.; Philippou, K.; Anastopoulos, I.; Pashalidis, I. Copper Adsorption by Magnetized Pine-Needle Biochar. Processes 2019, 7, 903. [Google Scholar] [CrossRef]
- Gai, C.; Gai, C.; Guo, Y.; Guo, Y.; Peng, N.; Peng, N.; Liu, T.; Liu, T.; Liu, Z.; Liu, Z. N-Doped biochar derived from co-hydrothermal carbonization of rice husk and Chlorella pyrenoidosa for enhancing copper ion adsorption. RSC Adcances 2016, 6, 53713–53722. [Google Scholar] [CrossRef]
- Haladu, S.A.; Al-Hamouz, O.C.S.; Ali, S.A. Adsorption of Cd2+ and Cu2+ ions from aqueous solutions by a cross-linked polysulfonate-carboxylate resin. Arab. J. Chem. 2019, 12, 2597–2607. [Google Scholar] [CrossRef]
- Kumar, D. Analytical Techniques and Instrumental Methods in Soil, Plant and Water Analysis, 1st ed.; NIPA: New Delhi, India, 2022; p. 138. [Google Scholar]
- Li, Y.P.; Zhao, M.Y.; Yan, H.Q.; Chen, Y.N.; Liu, Y.H.; Jiang, H.J.; Chen, L.; Hou, S.Z.; Chi, N.P.; Jia, S.Y. Silica from rice husk for sludge-based biochar modification: As a novel adsorbent for lead. J. Water Process Eng. 2024, 60, 105218. [Google Scholar] [CrossRef]
- Cuong, D.V.; Liu, N.L.; Nguyen, V.A.; Hou, C.H. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal. Sci. Total Environ. 2019, 692, 844–853. [Google Scholar] [CrossRef]
- Carrasco, J.; Klimes, J.; Michaelides, A. The role of van der Waals forces in water adsorption on metals. J. Chem. Phys. 2013, 138, 024708. [Google Scholar] [CrossRef]
- Hou, D.B.; Cui, X.D.; Liu, M.; Qie, H.T.; Tang, Y.M.; Leng, W.P.; Luo, N.; Luo, H.L.; Lin, A.J.; Yang, W.J.; et al. Degradation of trichloroethylene by biochar supported nano zero-valent iron (BC-nZVI): The role of specific surface area and electrochemical properties. Sci. Total Environ. 2024, 908, 168341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.H.; Wang, L.P.; Zhang, Q.Y.; Cao, Y.; Zhang, Y.A.; Kang, X.D. Enhanced Cr(VI) removal by biochar-loaded zero-valent iron coupled with weak magnetic field. J. Water Process Eng. 2022, 47, 102732. [Google Scholar] [CrossRef]
- Wang, M.; Tafti, N.D.; Wang, J.J.; Wang, X.D. Effect of pyrolysis temperature on Si release of alkali-enhanced Si-rich biochar and plant response. Biochar 2021, 3, 469–484. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhai, M.; Guo, H.K.; Panahi, A.; Dong, P.; Levendis, Y.A. High-temperature pyrolysis of biomass pellets: The effect of ash melting on the structure of the char residue. Fuel 2021, 285, 119084. [Google Scholar] [CrossRef]
- Wang, Y.Y.; You, L.C.; Lyu, H.H.; Liu, Y.X.; He, L.L.; Hu, Y.D.; Luo, F.C.; Yang, S.M. Role of biochar-mineral composite amendment on the immobilization of heavy metals for Brassica chinensis from naturally contaminated soil. Environ. Technol. Innov. 2022, 28, 102622. [Google Scholar] [CrossRef]
- Xie, H.; Zhou, Y.Y.; Ren, Z.W.; Wei, X.; Tao, S.P.; Yang, C.Q. Enhancement of electromagnetic interference shielding and heat-resistance properties of silver-coated carbonyl iron powders composite material. J. Magn. Magn. Mater. 2020, 499, 166244. [Google Scholar] [CrossRef]
- Krumpolec, R.; Homola, T.; Cameron, D.C.; Humlícek, J.; Caha, O.; Kuldová, K.; Zazpe, R.; Prikryl, J.; Macak, J.M. Structural and Optical Properties of Luminescent Copper(I) Chloride Thin Films Deposited by Sequentially Pulsed Chemical Vapour Deposition. Coatings 2018, 8, 369. [Google Scholar] [CrossRef]
- Tarekegn, M.M.; Hiruy, A.M.; Dekebo, A.H. Nano zero valent iron (nZVI) particles for the removal of heavy metals (Cd2+, Cu2+ and Pb2+) from aqueous solutions. RSC Adv. 2021, 11, 27084. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kurosu, S.; Yamaguchi, R.; Kawase, Y. A phenomenological reaction kinetic model for Cu removal from aqueous solutions by zero-valent iron (ZVI). Chemosphere 2018, 200, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.J.; Li, J.X.; Bao, Q.Q.; Li, L.N.; Guan, X.H. Role of dissolved oxygen in metal(loid) removal by zerovalent iron at different pH: Its dependence on the removal mechanisms. RSC Adv. 2016, 6, 50144–50152. [Google Scholar] [CrossRef]
- Li, S.L.; Wang, W.; Liang, F.P.; Zhang, W.X. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef]
- Jaiswal, J.; Dhayal, M. Preparation of 2D coatings of functionally graded chitosan-gold nanocomposite through in-situ reduction in cationic and anionic environments: Application for inhibiting hepatocellular carcinoma cells response. Mater. Chem. Phys. 2020, 243, 122663. [Google Scholar] [CrossRef]
- Wu, B.; Peng, D.H.; Hou, S.Y.; Tang, B.C.; Wang, C.; Xu, H. Dynamic study of Cr(VI) removal performance and mechanism from water using multilayer material coated nanoscale zerovalent iron. Environ. Pollut. 2018, 240, 717–724. [Google Scholar] [CrossRef]
- Wang, C.J.; Qiao, J.X.; Yuan, J.D.; Tang, Z.T.; Chu, T.Z.; Lin, R.F.; Wen, H.T.; Zheng, C.; Chen, H.L.; Xie, H.J.; et al. Novel chitosan-modified biochar prepared from a Chinese herb residue for multiple heavy metals removal: Characterization, performance and mechanism. Bioresour. Technol. 2024, 402, 130830. [Google Scholar] [CrossRef]
- Su, H.J.; Fang, Z.Q.; Tsang, P.E.; Fang, J.Z.; Zhao, D.Y. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ. Pollut. 2016, 214, 94–100. [Google Scholar] [CrossRef]
- Wang, H.L.; Tian, L.; Kang, K.; Zhang, B.; Li, G.M.; Zhang, K.Q. Characterization of Ultrasonic-Induced Wettability Alteration under Subsurface Conditions. Langmuir 2022, 38, 514–522. [Google Scholar] [CrossRef]
- Qian, L.B.; Zhang, W.Y.; Yan, J.C.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M.F. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Asadi, H.; Ghorbani, M.; Rezaei-Rashti, M.; Abrishamkesh, S.; Amirahmadi, E.; Chen, C.R.; Gorji, M. Application of Rice Husk Biochar for Achieving Sustainable Agriculture and Environment. Rice Sci. 2021, 28, 325–343. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.Z.; Huang, Z.L.; Wang, H.; Jiang, L.B.; Huang, J.; Tan, M.J.; Li, H. Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 579, 123642. [Google Scholar] [CrossRef]
- Sun, M.; Ma, M.M.; Lv, B.; Yao, Q.X.; Gao, J.W.; Wang, R.C.; Zhang, Y.J.; Ma, X.X. Pyrolysis characteristics of ethanol swelling Shendong coal and the composition distribution of its coal tar. J. Anal. Appl. Pyrolysis 2019, 138, 94–102. [Google Scholar] [CrossRef]
- Dave, D.; Chauhan, K.; Khimani, A.; Soni, K.; Vaidya, Y. Photocatalytic degradation of low-density polythene using protein-coated titania nanoparticles and Lactobacillus plantarum. Environ. Technol. 2023, 44, 619–630. [Google Scholar] [CrossRef]
- Ahmad, S.Z.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Wang, G.F.; Su, X.; Hua, Y.Y.; Ma, S.J.; Wang, J.; Xue, X.Q.; Tao, Q.; Komarneni, S. Kinetics and thermodynamic analysis of the adsorption of hydroxy-Al cations by montmorillonite. Penn. State Univ. Dept Ecosyst. 2016, 129, 79–87. [Google Scholar] [CrossRef]
- Regazzoni, A.E. Adsorption kinetics at solid/aqueous solution interfaces: On the boundaries of the pseudo-second order rate equation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124093. [Google Scholar] [CrossRef]
- Wu, Z.; Ji, Y.; Zhang, K.; Jing, L.; Zhao, T.Y. On the Use of the Multi-Site Langmuir Model for Predicting Methane Adsorption on Shale. Energies 2024, 17, 4990. [Google Scholar] [CrossRef]
- Wei, B.L.; Peng, Y.C.; Jeyakumar, P.; Lin, L.X.; Zhang, D.L.; Yang, M.Y.; Zhu, J.N.; Lin, C.S.K.; Wang, H.L.; Wang, Z.T.; et al. Soil pH restricts the ability of biochar to passivate cadmium: A meta-analysis. Environ. Res. 2023, 219, 115110. [Google Scholar] [CrossRef]
- Arwenyo, B.; Varco, J.J.; Dygert, A.; Brown, S.; Pittman, C.U.; Mlsna, T. Contribution of modified P-enriched biochar on pH buffering capacity of acidic soil. J. Environ. Manag. 2023, 339, 117863. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, K.Y.; Zhao, Y.Z.; Li, S.H.; Wu, Q.F.; Liang, C.F.; Sun, X.; Xu, Q.F.; Chen, J.H.; Qin, H. Change in composition and function of microbial communities in an acid bamboo (Phyllostachys praecox) plantation soil with the addition of three different biochars. For. Ecol. Manag. 2020, 473, 118336. [Google Scholar] [CrossRef]
- Ellery, S.; Ellery, W.; Tsikos, H.; Dunlevey, J. Depression wetland formation by redox-driven iron and silica cycling. Wetl. Ecol. Manag. 2024, 32, 191–206. [Google Scholar] [CrossRef]
- Chathura de Alwis, C.; Perrine, K.A. In Situ PM-IRRAS at the Air/Electrolyte/Solid Interface Reveals Oxidation of Iron to Distinct Minerals. J. Phys. Chem. A 2020, 124, 6735–6744. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ren, M.; Tang, Y.M.; Cui, X.D.; Cui, J.; Xu, C.B.; Qie, H.T.; Tan, X.; Liu, D.P.; Zhao, J.S.; et al. Immobilization on anionic metal(loid)s in soil by biochar: A meta-analysis assisted by machine learning. J. Hazard. Mater. 2022, 438, 129442. [Google Scholar] [CrossRef] [PubMed]
- Manirakiza, E.; Gagnon, B.; Ziadi, N. Soil and plant cations as affected by application of wood ash, biochar, and papermill biosolids. Agron. J. 2025, 117, e21714. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compos. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Wang, C.S.; Wang, H.Q.; Wang, W.; Liang, C.Z.; Liu, H.M.; Wang, L.X. The salt secretion of leaves promotes the competitiveness of Reaumuria soongarica in a desert grassland. BMC Plant Biol. 2022, 22, 85. [Google Scholar] [CrossRef]
- Luo, K.; Li, Y.Q.; Guan, X.Y.; Zhang, X.F. Effects of Biochar with Different Particle Sizes on Soil Physicochemical Properties and Vertical Transport of Selenium and Cadmium. Eurasian Soil Sci. 2023, 56, 1999–2008. [Google Scholar] [CrossRef]
- Bian, X.; Ren, Z.L.; Zeng, L.L.; Zhao, F.Y.; Yao, Y.K.; Li, X.Z. Effects of biochar on the compressibility of soil with high water content. J. Clean. Prod. 2024, 434, 140032. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Konvalina, P. Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas. Agronomy 2024, 14, 533. [Google Scholar] [CrossRef]
- Gao, C.; Fan, J.; Zhang, X.J.; Gong, Z.W.; Tan, Z.Y. Sediment metals adhering to biochar enhanced phosphorus adsorption in sediment capping. Water Sci. Technol. 2021, 84, 2057–2067. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, B.; Shafi, M.; Ma, J.W.; Guo, J.; Wu, J.S.; Ye, Z.Q.; Liu, D.; Jin, H.X. Effects of biochar on growth, and heavy metals accumulation of moso bamboo (Phyllostachy pubescens), soil physical properties, and heavy metals solubility in soil. Chemosphere 2019, 219, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Xue, J.C.; Qi, H.; Wang, S.T.; Wang, Y. Remediation of acidic soils in copper sulfide mines through combined plant amendments and their effects on soil bacterial community structure. Water Air Soil Pollut. 2024, 235, 141. [Google Scholar] [CrossRef]
- Šimanský, V.; Horák, J.; Lukac, M. Addition of Biochar and Fertiliser Drives Changes in Soil Organic Matter and Humic Substance Content in Haplic Luvisol. Land 2024, 13, 481. [Google Scholar] [CrossRef]
- Song, P.P.; Liu, J.; Ma, W.J.; Gao, X.Y. Remediation of cadmium-contaminated soil by biochar-loaded nano-zero-valent iron and its microbial community responses. J. Environ. Chem. Eng. 2024, 12, 112311. [Google Scholar] [CrossRef]
- Zheng, X.M.; Xu, W.H.; Dong, J.; Yang, T.; Shangguan, Z.C.; Qu, J.; Li, X.; Tan, X.F. The effects of biochar and its applications in the microbial remediation of contaminated soil: A review. J. Hazard. Mater. 2022, 438, 129557. [Google Scholar]
- Madibekov, A.; Ismukhanova, L.; Opp, C.; Saidaliyeva, Z.; Zhadi, A.; Sultanbekova, B.; Kurmanova, M. Spatial Distribution of Cu, Zn, Pb, Cd, Co, Ni in the Soils of Ili River Delta and State Natural Reserve “Ili-Balkhash”. Appl. Sci. 2023, 13, 5996. [Google Scholar] [CrossRef]
- Tan, Z.X.; Yuan, S.N.; Hong, M.F.; Zhang, L.M.; Huang, Q.Y. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Wang, X.; Cheng, T.; Dong, J.; Feng, K. The Mechanism of Cu2+ Sorption by Rice Straw Biochar and Its Sorption–Desorption Capacity to Cu2+ in Soil. Bull. Environ. Contam. Toxicol. 2022, 109, 562–570. [Google Scholar] [CrossRef]
- Zheng, R.X.; Zhao, W.L.; Li, H.Q.; Hu, H.B.; Lin, T.; Chen, W.; Song, Y.F. Pyrazole-3,5-dicarboxylic acid intercalated LDH: A highly selective structured adsorbents for Cu2+ ions removal. Sep. Purif. Technol. 2024, 346, 127532. [Google Scholar] [CrossRef]
- Saracoglu, S.; Soylak, M.; Elçi, L. Extractable Trace Metals Content of Dust from Vehicle Air Filters as Determined by Sequential Extraction and Flame Atomic Absorption Spectrometry. J. Aoac Int. 2009, 92, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Dong, Z.J.; Zhu, B.; Yan, X.M.; Huang, J.H.; Xie, X.; Chang, Z.F.; Tian, S.L.; Ning, P. Feasibility and solidification mechanism study of self-sustaining smoldering remediation for copper and lead-contaminated soil. Environ. Res. 2024, 250, 118498. [Google Scholar] [CrossRef]
- Jan, S.; Bhardwaj, S.; Singh, B.; Kapoor, D. Silicon efficacy for the remediation of metal contaminated soil. 3 Biotech 2024, 14, 212. [Google Scholar]
- Yang, W.C.; Li, Q.; He, Y.H.; Xi, D.D.; Arinzechi, C.; Zhang, X.M.; Liao, Q.; Yang, Z.H.; Si, M.Y. Synergistic Cr(VI) reduction and adsorption of Cu(II), Co(II) and Ni(II) by zerovalent iron-loaded hydroxyapatite. Chemosphere 2023, 313, 137428. [Google Scholar] [CrossRef]
- Sun, Q.W.; Yang, H.F.; Feng, X.D.; Liang, Y.H.; Gao, P.; Song, Y.L. A novel iron-based composite modified by refinery sludge for fixing Pb, Zn, Cu, Cd, and As in heavy metal polluted soil: Preparation, remediation process and feasibility analysis. J. Environ. Chem. Eng. 2023, 11, 111233. [Google Scholar] [CrossRef]
- Frank, T.; Zimmermann, I.; Horn, R. Lime application in marshlands of Northern Germany-Influence of liming on the physicochemical and hydraulic properties of clayey soils. Soil Tillage Res. 2020, 204, 104730. [Google Scholar] [CrossRef]
- Keenan, C.R.; Goth-Goldstein, R.; Lucas, D.; Sedlak, D.L. Oxidative Stress Induced by Zero-Valent Iron Nanoparticles and Fe(II) in Human Bronchial Epithelial Cells. Environ. Sci. Technol. 2009, 43, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Yirsaw, B.D.; Mayilswami, S.; Megharaj, M.; Chen, Z.L.; Naidu, R. Effect of zero valent iron nanoparticles to Eisenia fetida in three soil types. Environ. Sci. Pollut. Res. 2016, 23, 9822–9831. [Google Scholar] [CrossRef]
- Yang, Y.F.; Cheng, Y.H.; Liao, C.M. In situ remediation-released zero-valent iron nanoparticles impair soil ecosystems health: A C. elegans biomarker-based risk assessment. J. Hazard. Mater. 2016, 317, 210–220. [Google Scholar] [CrossRef]
- Gong, X.M.; Huang, D.L.; Liu, Y.G.; Zeng, G.M.; Wang, R.Z.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W.J. Stabilized Nanoscale Zerovalent Iron Mediated Cadmium Accumulation and Oxidative Damage of Boehmeria nivea (L.) Gaudich Cultivated in Cadmium Contaminated Sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, Y.F.; Zheng, C.L.; Tang, C.L.; Guan, J.; Guo, Y.G. Sulfidated nanoscale zero valent iron for in situ immobilization of hexavalent chromium in soil and response of indigenous microbes. Chemosphere 2023, 344, 140343. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Cajthaml, T.; Semerád, J.; Filipová, A.; Klementová, M.; Skála, R.; Vítková, M.; Michálková, Z.; Teodoro, M.; Wu, Z.X.; et al. Nano zero-valent iron aging interacts with the soil microbial community: A microcosm study. Environ. Sci. Nano 2019, 6, 1189–1206. [Google Scholar] [CrossRef]
- Singh, R.; Misra, V.; Singh, R.P. Removal of Cr(VI) by Nanoscale Zero-valent Iron (nZVI) From Soil Contaminated with Tannery Wastes. Bull. Environ. Contam. Toxicol. 2012, 88, 210–214. [Google Scholar] [CrossRef]






| Project | Detection Value |
|---|---|
| Soil pH | 4.27 |
| Moisture content (%) | 13.15 |
| Electrical conductivity (μS·cm−1) | 774.60 |
| Organic matter (mg·kg−1) | 8.87 |
| Cation exchange capacity (cmol+·kg−1) | 8.65 |
| Total Cu content (mg·kg−1) | 2398.29 |
| Acid-soluble Cu content (mg·kg−1) | 349 |
| Reducible Cu content (mg·kg−1) | 252 |
| Oxidizable Cu content (mg·kg−1) | 801 |
| Residual Cu content (mg·kg−1) | 582 |
| Adsorbent | Experimental Conditions (pH; Temperature; Dosage; Initial Cu2+ Concentration Range) | Adsorption Capacity (mg·g−1) | References |
|---|---|---|---|
| Fe–biochar composite | 5.5; 25 °C; 2 g·L−1; 10–1000 mg·L−1 | 276.12 | [38] |
| Magnetized pine-needle biochar | 2–10; 23 ± 2 °C; 0.01 g; 10−5–9 × 10−3 mol·L−1 | 63.5 | [39] |
| Rice husk biochar | 5; 30 °C; 1–10 g·L−1; 20 mg·L−1 | 13.12 | [40] |
| N-doped biochar derived from co-hydrothermal carbonization of rice husk and Chlorella pyrenoidosa | 29.11 | ||
| Rice husk biochar | 6; 25 °C; 1 g·L−1; 1 g·L−1 | 633.5 | This research |
| Carbonyl iron powder | 218.25 | ||
| Composite material prepared by wet grinding when the mass ratio of biochar to carbonyl iron powder is 1:1 | 651 | ||
| Composite material prepared by dry grinding when the mass ratio of biochar and carbonyl iron powder is 1:1 | 623.25 | ||
| Composite material prepared by wet grinding when the mass ratio of biochar to carbonyl iron powder is 5:1 | 243 | ||
| Composite material prepared by dry grinding when the mass ratio of biochar and carbonyl iron powder is 5:1 | 718.5 | ||
| Composite material prepared by wet grinding when the mass ratio of biochar to carbonyl iron powder is 10:1 | 910.5 | ||
| Composite material prepared by dry grinding when the mass ratio of biochar and carbonyl iron powder is 10:1 (CIP@BC) | 860.25 |
| Group | BC (w/w) | CIP@BC (w/w) |
|---|---|---|
| CK | 0 | 0 |
| BP1 | 0 | 1 |
| BP2 | 0 | 2 |
| BP3 | 0 | 4 |
| BP4 | 0 | 6 |
| BC | 6 | 0 |
| Matter | Experimental Maximum Adsorption Capacity/(mg·g−1) | Quasi-First-Order Kinetic Model | Quasi-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| Qe | k1 | R2 | Qe | k2 | R2 | ||
| BC | 26.505 | 1.920 | 1.460 × 10−3 | 0.804 | 26.546 | 0.003 | 0.999 |
| CIP@BC | 43.380 | 2.862 | 7.547 × 10−4 | 0.912 | 43.271 | 0.002 | 0.999 |
| Matter | Langmuir Isothermal Adsorption Model | Freundlich Isothermal Adsorption Model | ||||
|---|---|---|---|---|---|---|
| Qm | kL | R2 | kF | 1/n | R2 | |
| BC | 44.583 | 0.483 | 0.980 | 0.241 | 19.450 | 0.489 |
| CIP@BC | 442.478 | 0.036 | 0.988 | 0.623 | 19.407 | 0.957 |
| Groups | CK | BP1 | BP2 | BP3 | BP4 | BC |
|---|---|---|---|---|---|---|
| DTPA-Cu | 147.51 ± 11.62 a | 100.84 ± 9.93 b | 90.40 ± 4.46 b | 67.59 ± 7.21 c | 48.30 ± 2.16 d | 50.33 ± 3.91 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xue, J.; He, M.; Wang, X.; Qi, H. Mechanisms of Cu2+ Immobilization Using Carbonyl Iron Powder–Biochar Composites for Remediating Acidic Soils from Copper Sulfide Mining Areas. Sustainability 2025, 17, 4281. https://doi.org/10.3390/su17104281
Wang S, Xue J, He M, Wang X, Qi H. Mechanisms of Cu2+ Immobilization Using Carbonyl Iron Powder–Biochar Composites for Remediating Acidic Soils from Copper Sulfide Mining Areas. Sustainability. 2025; 17(10):4281. https://doi.org/10.3390/su17104281
Chicago/Turabian StyleWang, Shuting, Jinchun Xue, Min He, Xiaojuan Wang, and Hui Qi. 2025. "Mechanisms of Cu2+ Immobilization Using Carbonyl Iron Powder–Biochar Composites for Remediating Acidic Soils from Copper Sulfide Mining Areas" Sustainability 17, no. 10: 4281. https://doi.org/10.3390/su17104281
APA StyleWang, S., Xue, J., He, M., Wang, X., & Qi, H. (2025). Mechanisms of Cu2+ Immobilization Using Carbonyl Iron Powder–Biochar Composites for Remediating Acidic Soils from Copper Sulfide Mining Areas. Sustainability, 17(10), 4281. https://doi.org/10.3390/su17104281






