Substrate Properties, Vegetative Growth, Chlorophyll Content Index and Leaf Mineral Content of Sweet Cherry Maiden Trees as Affected by Rootstock and Plant Growth-Promoting Rhizobacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Location
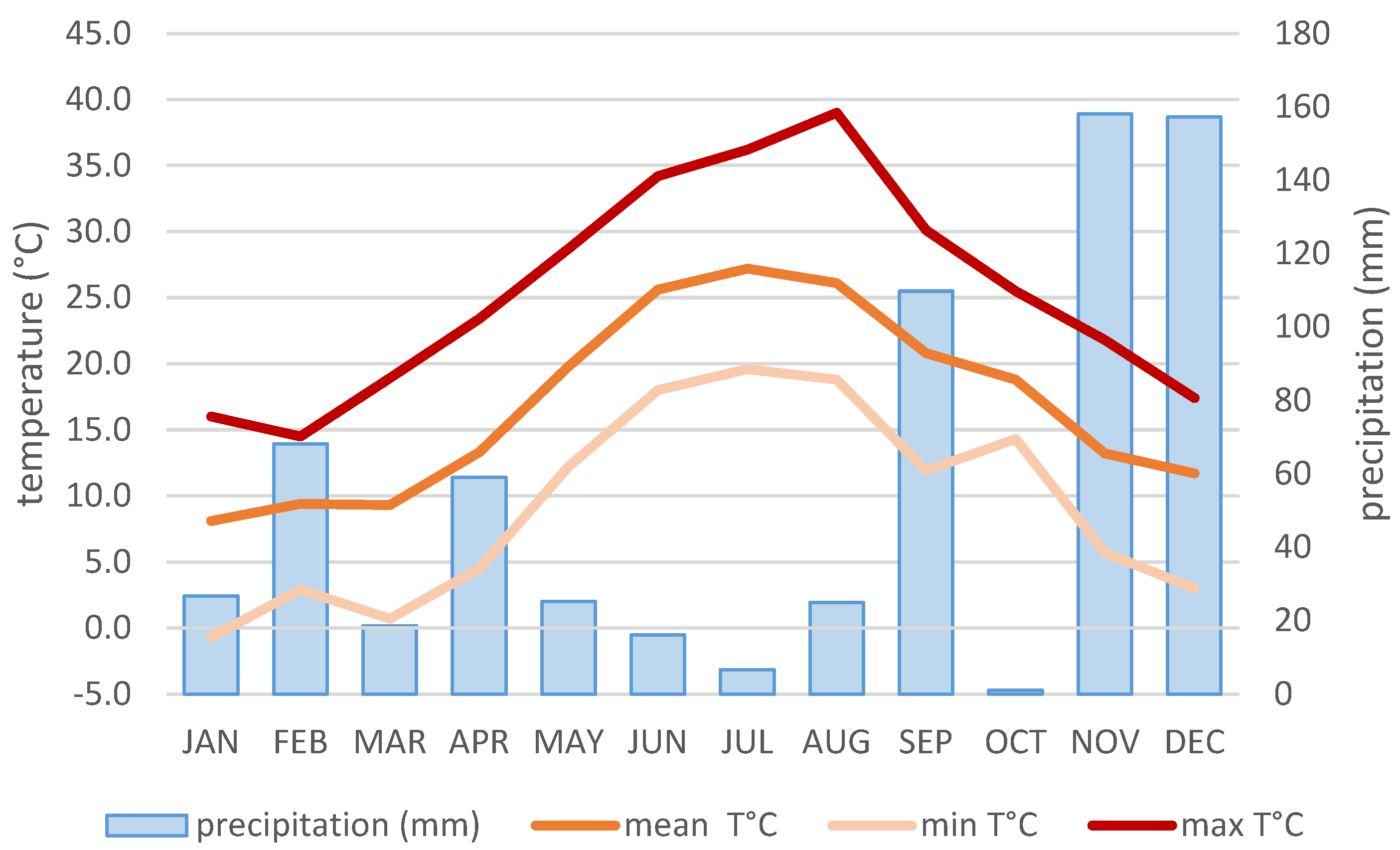
2.2. Climatic Conditions
2.3. Planting Material
2.4. Characteristics of the Substrate
2.5. Experimental Design
2.6. Substrate Analysis
2.7. Vegetative Growth Characteristics
2.8. Chlorophyll Content Index (CCI)
2.9. Analysis of Macronutrients in Leaves
2.10. Statistical Data Analysis
3. Results
3.1. Physicochemical Properties of the Substrate
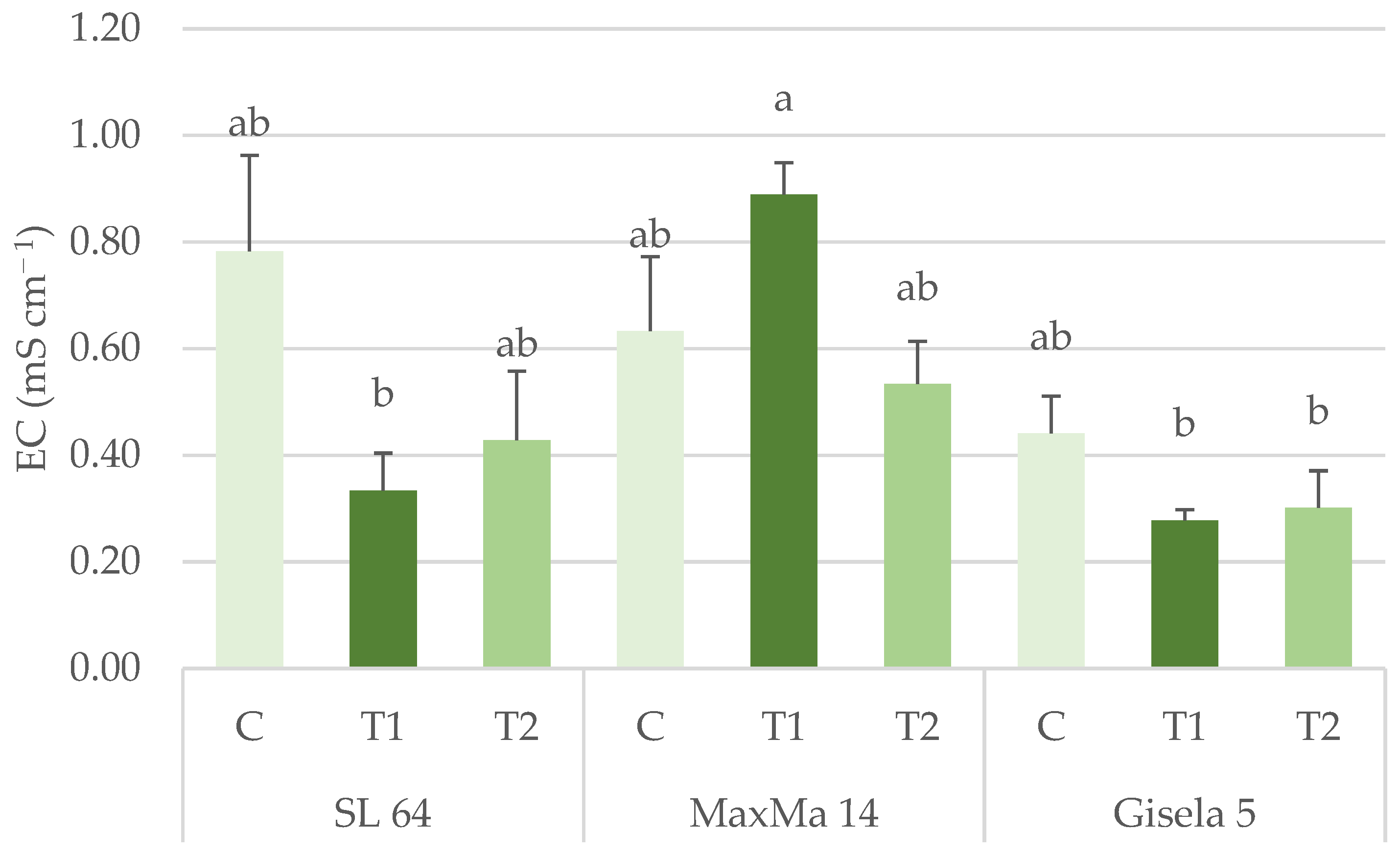
3.1.1. Ammonia, Nitrate and Total Mineral Nitrogen Content
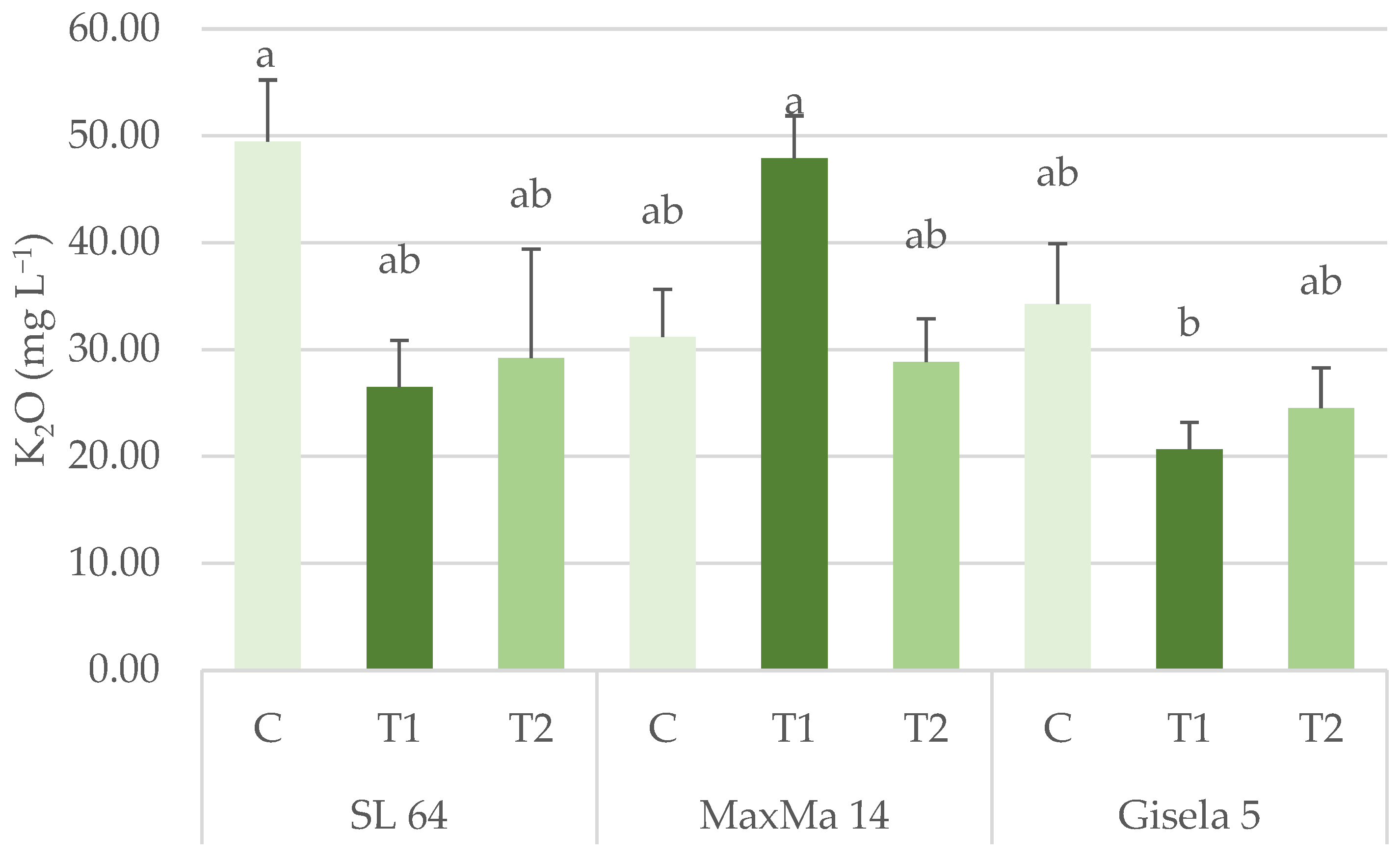
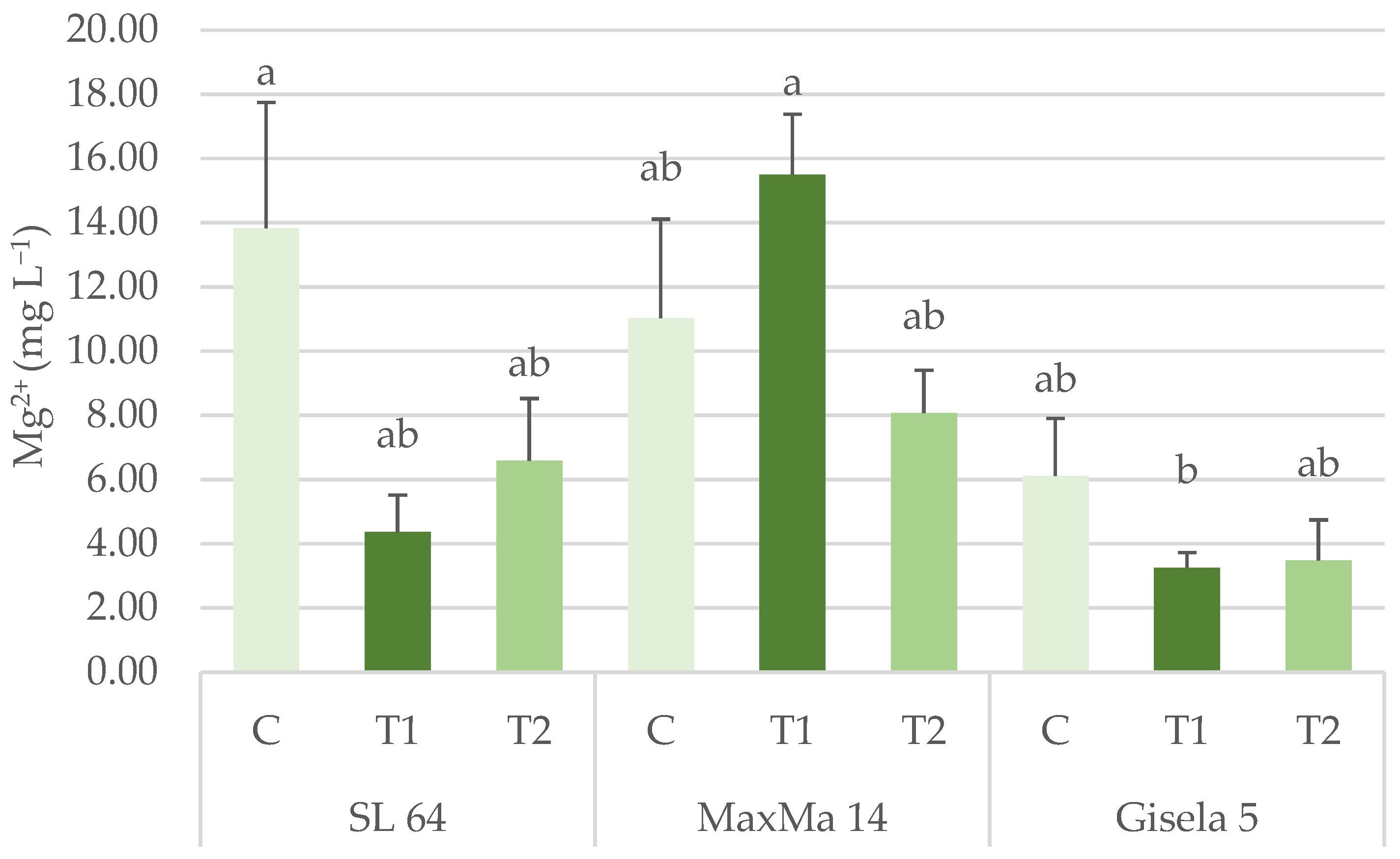
3.1.2. Phosphorus, Potassium, Calcium and Magnesium Content
3.2. Vegetative Characteristics
Growth Dynamics
3.3. Leaf Mineral Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agricultural Organization Statistical Database. Available online: https://www.fao.org/faostat/en/#data (accessed on 23 April 2024).
- Očić, V. Koliko Je Uzgoj Trešnje Isplativ? Gospod. List 2023, 12. Available online: https://gospodarski.hr/rubrike/vocarstvo-rubrike/koliko-je-uzgoj-tresnje-isplativ/ (accessed on 7 December 2024). (In Croatian).
- Čmelik, Z.; Družić, J.; Duralija, B.; Benčić, D. Influence of Clonal Rootstocks on Growth and Cropping of “Lapins” Sweet Cherry. Acta Hortic. 2004, 658, 125–128. [Google Scholar] [CrossRef]
- Gainza, F.; Opazo, I.; Guajardo, V.; Meza, P.; Ortiz, M.; Pinochet, J.; Muñoz, C. Rootstock Breeding in Prunus Species: Ongoing Efforts and New Challenges. Chil. J. Agric. Res. 2015, 75, 6–16. [Google Scholar] [CrossRef]
- Szot, I.; Meland, M. Influence of Rootstocks on Size Distribution and Fruit Quality of Sweet Cherry Cultivars. Int. Agrophys 2001, 15, 207–214. [Google Scholar]
- Duralija, B.; Arko, B.; Zlatko, Č.; Jemri, T.; Šindrak, Z. Influence of cultivar and rootstock on sweet cherry fruit cracking. Pomol. Croat. 2007, 13, 97–106. (In Croatian) [Google Scholar]
- Miljković, I.; Čmelik, Z.; Vrsaljko, A. Podloge Za Trešnju. Pomol. Croat. 2002, 8, 115–134. (In Croatian) [Google Scholar]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernández-Fernández, F.; Harrison, R.J.; Schmidt, S. Contributions of Roots and Rootstocks to Sustainable, Intensified Crop Production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Granatstein, D.; Kupferman, E. Sustainable Horticulture in Fruit Production. In XXVII International Horticultural Congress—IHC2006: International Symposium on Sustainability through Integrated and Organic Horticulture; ISHS Acta Horticulturae: Korbeek, Belgium, 2008; pp. 295–308. [Google Scholar] [CrossRef]
- CTIFL. Porte-Greffe—Porte-Greffe et Variétés du Cerisier. Available online: https://varietes_cerise.ctifl.fr/fiche/porte-greffe/SAINTE_LUCIE_64 (accessed on 5 June 2024).
- Perry, R.L. Progress with Cherry Rootstocks. Compact Fruit Tree 1985, 8, 107–108. [Google Scholar]
- Vujović, T.; Cerović, R.; Ružić, D. Ploidy Level Stability of Adventitious Shoots of Sour Cherry ‘Čačanski Rubin’ and Gisela 5 Cherry Rootstock. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 111, 323–333. [Google Scholar] [CrossRef]
- Moreno, M.A.; Montañés, L.; Tabuenca, M.C.; Cambra, R. The Performance of Adara as a Cherry Rootstock. Sci. Hortic. 1996, 65, 85–91. [Google Scholar] [CrossRef]
- Papachatzis, A. Influence of Rootstock on Growth and Reproductive Characteristics of Cherry Cultivar ‘Stella’during the Period of Complete Fruiting. Sodinink. Darzinink. 2006, 25, 212–217. [Google Scholar]
- Baryła, P.; Kapłan, M.; Krawiec, M. The Effect of Different Types of Rootstock on the Quality of Maiden Trees of Sweet Cherry (Prunus avium L.) Cv. ‘Regina’. Acta Agrobot. 2014, 67, 43–50. [Google Scholar] [CrossRef]
- Gjamovski, V.; Kiprijanovski, M.; Arsov, T. Evaluation of Some Cherry Varieties Grafted on Gisela 5 Rootstock. Turk. J. Agric. For. 2016, 40, 737–745. [Google Scholar] [CrossRef]
- Milinović, B.; Dragović-Uzelac, V.; Kazija, D.H.; Jelačić, T.; Vujević, P.; Čiček, D.; Biško, A.; Čmelik, Z. Influence of Four Different Dwarfing Rootstocks on Phenolic Acids and Anthocyanin Composition of Sweet Cherry (Prunus avium L.) Cvs “Kordia” and “Regina”. J. Appl. Bot. Food Qual. 2016, 89, 29–37. [Google Scholar] [CrossRef]
- Csihon, Á.; Bicskei, K.; Dremák, P.; Gonda, I. Evaluation of the Growing and Fruit Bearing Characteristics of the ‘Lapins’ Sweet Cherry Cultivar Grafted on Rootstocks with Different Vigor. Int. J. Hortic. Sci. 2017, 23, 15–18. [Google Scholar] [CrossRef]
- Zimmermann, A. Gisela 5, a Dwarfing Rootstock for Sweet Cherries from Giessen in a Trial. Obstbau 1994, 19, 62–63. [Google Scholar]
- Blažková, J.; Drahošová, H.; Hlušičková, I. Tree Vigour, Cropping, and Phenology of Sweet Cherries in Two Systems of Tree Training on Dwarf Rootstocks. Hortic. Sci. 2010, 37, 127–138. [Google Scholar] [CrossRef]
- Lane, W.D.; Schmid, H. Lapins and Sunburst Sweet Cherry. Can. J. Plant Sci. 1984, 64, 211–214. [Google Scholar] [CrossRef]
- Alique, R.; Martínez, M.A.; Alonso, J. Metabolic Response to Two Hydrocooling Temperatures in Sweet Cherries Cv Lapins and Cv Sunburst. J. Sci. Food Agric. 2006, 86, 1847–1854. [Google Scholar] [CrossRef]
- De Salvador, F.R.; Di Tommaso, G.; Piccioni, C.; Bonofiglio, P. Performance of New and Standard Cherry Rootstocks in Different Soils and Climatic Conditions. Acta Hortic. 2005, 667, 191–200. [Google Scholar] [CrossRef]
- Godini, A.; Palasciano, M.; Camposeo, S.; Pacifico, A. A Nine-Year Study on the Performance of Twelve Cherry Rootstocks under Non-Irrigated Conditions in Apulia (Southern Italy). Acta Hortic. 2008, 795, 191–198. [Google Scholar] [CrossRef]
- Lanauskas, J.; Uselis, N.; Kviklys, D.; Kviklienė, N.; Buskienė, L. Rootstock Effect on the Performance of Sweet Cherry Cv. Lapins. Hort. Sci. 2012, 2, 55–60. [Google Scholar] [CrossRef]
- Eşitken, A.; Karlidag, H.; Sahin, F. Potential Use of Plant Growth Promoting Rhizobacteria (PGPR) in Organic Apricot Production. In Proceedings of the International Conference on Environmentally Friendly Fruit Growing, Tartu, Estonia, 7–9 January 2005; Volume 2005, pp. 90–97. [Google Scholar]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- O’Connell, P.F. Sustainable Agriculture-a Valid Alternative. Outlook Agric. 1992, 21, 5–12. [Google Scholar] [CrossRef]
- Malusa, E.; Sas-Paszt, L.; Popinska, W.; Zurawicz, E. The Effect of a Substrate Containing Arbuscular Mycorrhizal Fungi and Rhizosphere Microorganisms (Trichoderma, Bacillus, Pseudomonas and Streptomyces) and Foliar Fertilization on Growth Response and Rhizosphere PH of Three Strawberry Cultivars. Int. J. Fruit Sci. 2007, 6, 25–41. [Google Scholar] [CrossRef]
- Głuszek, S.; Sas-Paszt, L.; Derkowska, E.; Sumorok, B.; Sitarek, M. Influence of Various Biofertilizers on Root Growth Dynamics in Sweet Cherry (Prunus avium L.) Cv. “Vanda”. Hortic. Sci. 2021, 48, 105–116. [Google Scholar] [CrossRef]
- Cassán, F.; Vanderleyden, J.; Spaepen, S. Physiological and Agronomical Aspects of Phytohormone Production by Model Plant-Growth-Promoting Rhizobacteria (PGPR) Belonging to the Genus Azospirillum. J. Plant Growth Regul. 2014, 33, 440–459. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture. In PGPR Amelioration in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–157. [Google Scholar]
- Shirkot, C.K.; Sharma, N. Growth Promotion of Apple Seedlings by Plant Growth Promoting Rhizobacterium (Bacillus megaterium). In VII International Symposium on Temperate Zone Fruits in the Tropics and Subtropics-Part Two 696; ISHS Acta Horticulturae: Korbeek, Belgium, 2005; pp. 157–162. [Google Scholar] [CrossRef]
- Yildiz, E.; Yaman, M.; Ercisli, S.; Sumbul, A.; Sonmez, O.; Gunes, A.; Bozhuyuk, M.R.; Kviklys, D. Effects of Rhizobacteria Application on Leaf and Fruit Nutrient Content of Different Apple Scion–Rootstock Combinations. Horticulturae 2022, 8, 550. [Google Scholar] [CrossRef]
- Karakurt, H.; Aslantaş, R. Effects of Some Plant Growth Promoting Rhizobacteria (PGPR) Strains on Plant Growth and Leaf Nutrient Content of Apple. J. Fruit Ornam. Plant Res. 2010, 18, 101–110. [Google Scholar]
- Karakurt, H.; Aslantas, R. Effects of Some Plant Growth Promoting Rhizobacteria Treated Twice on Flower Thining, Fruit Set and Fruit Properties on Apple. Afr. J. Agric. Res. 2010, 5, 384–388. [Google Scholar]
- İpek, M.; Arıkan, Ş.; Eşitken, A.; Pırlak, L.; Turan, M.; Dönmez, M.F. Effects of Some Plant Growth-Promoting Rhizobacteria (PGPR) on Growth and Nutrition of Apple Cv. “Braeburn” under High Lime Soil Condition. Commun. Soil Sci. Plant Anal. 2021, 52, 432–442. [Google Scholar] [CrossRef]
- Pedraza, R.O.; Motok, J.; Salazar, S.M.; Ragout, A.L.; Mentel, M.I.; Tortora, M.L.; Guerrero-Molina, M.F.; Winik, B.C.; Díaz-Ricci, J.C. Growth-Promotion of Strawberry Plants Inoculated with Azospirillum brasilense. World J. Microbiol. Biotechnol. 2010, 26, 265–272. [Google Scholar] [CrossRef]
- Pirlak, L.; Köse, M. Effects of Plant Growth Promoting Rhizobacteria on Yield and Some Fruit Properties of Strawberry. J. Plant Nutr. 2009, 32, 1173–1184. [Google Scholar] [CrossRef]
- Salazar, S.M.; Lovaisa, N.C.; Guerrero-Molina, M.F.; Ragout, A.L.; Kirschbaum, D.S.; Díaz-Ricci, J.C.; Pedraza, R.O. Fruit Yield of Strawberry Plants Inoculated with Azospirillum brasilense RLC1 and REC3 under Field Conditions. Revta. Agron. N. O. Argent. 2012, 32, 63–66. [Google Scholar]
- Ipek, M.; Pirlak, L.; Eşitken, A.; Figen Dönmez, M.; Turan, M.; Sahin, F. Plant Growth-Promoting Rhizobacteria (PGPR) Increase Yield, Growth And Nutrition Of Strawberry Under High-Calcareous Soil Conditions. J. Plant Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Guerrero-Molina, M.F.; Lovaisa, N.C.; Salazar, S.M.; Martínez-Zamora, M.G.; Díaz-Ricci, J.C.; Pedraza, R.O. Physiological, Structural and Molecular Traits Activated in Strawberry Plants after Inoculation with the Plant Growth-Promoting Bacterium Azospirillum brasilense REC3. Plant Biol. 2015, 17, 766–773. [Google Scholar] [CrossRef]
- Rueda, D.; Valencia, G.; Soria, N.; Rueda, B.; Manjunatha, B.; Kundapur, R.; Selvanayagam, M. Effect of Azospirillum Spp. and Azotobacter Spp. on the Growth and Yield of Strawberry (Fragaria vesca) in Hydroponic System under Different Nitrogen Levels. J. Appl. Pharm. Sci. 2016, 6, 48–54. [Google Scholar] [CrossRef]
- Pii, Y.; Graf, H.; Valentinuzzi, F.; Cesco, S.; Mimmo, T. The Effects of Plant Growth-Promoting Rhizobacteria (PGPR) on the Growth and Quality of Strawberries. Acta Hortic. 2018, 1217, 231–238. [Google Scholar] [CrossRef]
- Thakur, D.; Kaushal, R.; Shyam, V. Characterization of Rhizospheric and Endophytic Plant Growth Promoting Rhizobacteria Isolated from Cherry (Prunus avium L.) and Their Effect on the Growth of Cherry Seedlings. Int. J. Farm. Sci. 2017, 7, 73–80. [Google Scholar]
- Eşitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of Floral and Foliar Application of Plant Growth Promoting Rhizobacteria (PGPR) on Yield, Growth and Nutrition of Sweet Cherry. Sci. Hortic. 2006, 110, 324–327. [Google Scholar] [CrossRef]
- Arikan, Ş.; Pirlak, L. Einfluss von Wachstumsfördernden Rhizobacteria (PGPR) Auf Wachstum, Ertrag Und Fruchtqualität Bei Sauerkirschen (Prunus cerasus L.). Erwerbs-Obstbau 2016, 58, 221–226. [Google Scholar] [CrossRef]
- Zhou, W.; Qin, S.; Lyu, D.; Zhang, P. Soil Sterilisation and Plant Growth-Promoting Rhizobacteria Promote Root Respiration and Growth of Sweet Cherry Rootstocks. Arch. Agron. Soil Sci. 2015, 61, 361–370. [Google Scholar] [CrossRef]
- Eşitken, A.; Karlidag, H.; Ercisli, S.; Turan, M.; Sahin, F. The Effect of Spraying a Growth Promoting Bacterium on the Yield, Growth and Nutrient Element Composition of Leaves of Apricot (Prunus armeniaca L. Cv. Hacihaliloglu). Aust. J. Agric. Res. 2003, 54, 377–380. [Google Scholar] [CrossRef]
- Zhou, M.; Li, P.; Wu, S.; Zhao, P.; Gao, H. Bacillus Subtilis CF-3 Volatile Organic Compounds Inhibit Monilinia Fructicola Growth in Peach Fruit. Front. Microbiol. 2019, 10, 470637. [Google Scholar] [CrossRef]
- Salman, M.A.M. Biological Control of Rhizopus Soft Rot on Apple, Pear and Peach by Trichoderma Harzianum. Master’s Thesis, An-Najah National University, Nablus, Palestine, 2005. [Google Scholar]
- Arıkan, Ş.; Eşitken, A.; İpek, M.; Aras, S.; Şahin, M.; Pırlak, L.; Dönmez, M.F.; Turan, M. Effect of Plant Growth Promoting Rhizobacteria on Fe Acquisition in Peach (Prunus persica L.) Under Calcareous Soil Conditions. J. Plant Nutr. 2018, 41, 2141–2150. [Google Scholar] [CrossRef]
- Ipek, M.; Arikan, Ş.; Eşitken, A.; Pirlak, L.; Dönmez, M.F.; Turan, M. Influence of Bacterial Inoculation on Growth and Plant Nutrition of Peach Grafted in Different Rootstocks in Calcareous Soil. Sains Malays. 2021, 50, 2615–2624. [Google Scholar] [CrossRef]
- Al-Hadethi, M.E.A.; Al-Dulaimi, A.S.T.; Almashhadani, B.M.K. Influence of Biofertilizers on Growth and Leaf Mineral Content in Peach Transplants. IOSR J. Agric. Vet. Sci. 2017, 10, 90–93. [Google Scholar] [CrossRef]
- Pedraza, R.O.; Filippone, M.P.; Fontana, C.; Salazar, S.M.; Ramírez-Mata, A.; Sierra-Cacho, D.; Baca, B.E. Azospirillum. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–105. ISBN 9780128234143. [Google Scholar]
- Kumar Sahu, P.; Gupta, A.; Sharma, L.; Bakade, R. Mechanisms of Azospirillum in Plant Growth Promotion. Sch. J. Agric. Vet. Sci. 2017, 4, 338–343. [Google Scholar] [CrossRef]
- Vettori, L.; Russo, A.; Felici, C.; Fiaschi, G.; Morini, S.; Toffanin, A. Improving Micropropagation: Effect of Azospirillum brasilense Sp245 on Acclimatization of Rootstocks of Fruit Tree. J. Plant Interact. 2010, 5, 249–259. [Google Scholar] [CrossRef]
- Singh, Y.; Bhatnagar, P.; Singh, J.; Kumar Sharma, Y.; Singh Bisht, Y.; Arya, C.K.; Singh Rathore, B. The Contribution of Azospirillum Brasilense and Vermicompost to Improving Plant Growth Characteristics, Yield Variables, Physical Characteristics and Maintaining Sustainable Agriculture in Custard Apple Cv. “Balanagar”. Appl. Fruit Sci. 2024, 66, 813–821. [Google Scholar] [CrossRef]
- Coniglio, A.; Mora, V.; Puente, M.; Cassán, F. Azospirillum as Biofertilizer for Sustainable Agriculture: Azospirillum Brasilense AZ39 as a Model of PGPR and Field Traceability. In Microbial Probiotics for Agricultural Systems: Advances in Agronomic Use; Zúñiga-Dávila, D., González-Andrés, F., Ormeño-Orrillo, E., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 45–70. ISBN 978-3-030-17597-9. [Google Scholar]
- Bashan, Y.; Holguin, G. Azospirillum-Plant Relationships: Environmental and Physiological Advances (1990–1996). Can. J. Microbiol. 1997, 43, 103–121. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Ptacek, D.; Vanderleyden, J.; Dutto, P.; Labandera-Gonzalez, C.; Caballero-Mellado, J.; Aguirre, J.F.; Kapulnik, Y.; et al. Responses of Agronomically Important Crops to Inoculation with Azospirillum. Funct. Plant Biol. 2001, 28, 871. [Google Scholar] [CrossRef]
- Omar, M.N.A.; Osman, M.E.H.; Kasim, W.A.; Abd El-Daim, I.A. Improvement of Salt Tolerance Mechanisms of Barley Cultivated Under Salt Stress Using Azospirillum brasilense. In Salinity and Water Stress: Improving Crop Efficiency; Ashraf, M., Ozturk, M., Athar, H., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 133–147. ISBN 978-1-4020-9065-3. [Google Scholar]
- Russo, A.; Vettori, L.; Felici, C.; Fiaschi, G.; Morini, S.; Toffanin, A. Enhanced Micropropagation Response and Biocontrol Effect of Azospirillum brasilense Sp245 on Prunus cerasifera L. Clone Mr.S 2/5 Plants. J. Biotechnol. 2008, 134, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Chattopadhyay, G.N.; Gangwar, S.K.; Ghosh, J.K. Effect of Foliar Application of Azotobacter, Azospirillum and Beijerinckia on Leaf Yield and Quality of Mulberry (Morus alba). J. Agric. Sci. 2000, 134, 227–234. [Google Scholar] [CrossRef]
- Abd-Ella, E.E.-S.K. Effect of Biofertilization on Reducing Chemical Fertilizers, Vegetative Growth, Nutritional Status, Yield and Fruit Quality of Arabi Pomegranate Trees. J. Agric. Environ. Sci. Alex. Univ. Egypt. 2006, 5, 1–23. [Google Scholar]
- Croatian Meteorological and Hydrological Service (CMHS). Available online: https://meteo.hr (accessed on 29 July 2024). (In Croatian).
- Šegota, T.; Filipčić, A. Köppenova Podjela Klima i Hrvatsko Nazivlje. Geoadria 2003, 8, 17–37. (In Croatian) [Google Scholar] [CrossRef]
- Miljković, I. Trešnja; Hrvatsko Agronomsko Društvo: Zagreb, Croatia, 2011. [Google Scholar]
- Gonçalves, B.; Aires, A.; Oliveira, I.; Afonso, S.; Morais, M.C.; Correia, S.; Martins, S.; Silva, A.P. Sweet Cherry. In Temperate Fruits; Apple Academic Press: Williston, VT, USA, 2021; pp. 333–415. [Google Scholar]
- HRN EN 13037:2012; Soil Improvers and Growing Media—Determination of PH. Association Francaise de Normalisation: Saint-Denis, France, 2012.
- HRN EN 13038:2012; Soil Improvers and Growing Media—Determination of Electrical Conductivity. Association Francaise de Normalisation: Saint-Denis, France, 2012.
- BS EN 13654-1:2001; Soil Improvers and Growing Media—Determination of Nitrogen—Part 1: Modified Kjeldahl Method. Association Francaise de Normalisation: Saint-Denis, France, 2001.
- HRN EN 13652:2008; Soil Improvers and Growing Media—Extraction of Water Soluble Nutrients and Elements. Association Francaise de Normalisation: Saint-Denis, France, 2008.
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2015; pp. 806–814. [Google Scholar]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; BBCH Monograph, Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- Ranjbar, A.; Imani, A.; Piraivtlou, S.P.; Abdoosi, V. Effects of Drought Stress on Almond Cultivar’s Responses Grafted on Different Rootstocks. J. Nuts 2019, 10, 9–24. [Google Scholar] [CrossRef]
- Ryan, P.R.; Dessaux, Y.; Thomashow, L.S.; Weller, D.M. Rhizosphere Engineering and Management for Sustainable Agriculture. Plant Soil 2009, 321, 363–383. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, Biogeochemistry and Ecological Relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Aini, H.; Suryatmana, P.; Hindersah, R. Application of Inorganic Fertilizer and Bio-Fertilizer on Chlorophyll Content, PH, and Leaves Number of Pak Choi (Brassica rapa L.). Int. J. Agric. Environ. Bioresearch 2019, 4, 269–278. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Jones, A.L.; Sobiczewski, P.P.; Bird, G.W. Factors Associated with the Decline of Sweet Cherry Trees in Michigan: Nematodes, Bacterial Canker, Nutrition, Soil PH, and Winter Injury. Plant Dis. 1993, 77, 266–271. [Google Scholar] [CrossRef]
- Fulton, A.; Advisor, F.; Counties, S. Primary Plant Nutrients: Nitrogen, Phosphorus, and Potassium. Available online: https://cetehama.ucanr.edu/newsletters/Soil_Testing_Articles-by_Allan_Fulton39345.pdf (accessed on 9 June 2024).
- Qursyna, N.; Yaacob, A. Effects of Liming, Urea and NPK Fertilizers on Availability and Movement of Mineral Nitrogen in Peat Soils under Aerobic and Anaerobic Condition. J. Appl. Sci. Agric. 2014, 9, 292–299. [Google Scholar]
- Neilsen, G.; Kappel, F. “Bing” Sweet Cherry Leaf Nutrition Is Affected by Rootstock. HortScience 1996, 31, 1169–1172. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Phyto-Microbiome in Stress Regulation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 147–203. [Google Scholar]
- Vodovod, D.O.O. Zadar Water Supply System of Zadar County. Available online: https://www.vodovod-zadar.hr/voda/kvaliteta-vode/vodoopskrbni-sustav-zadarskog-vodovoda (accessed on 29 May 2024). (In Croatian).
- Gallart, M.; Paungfoo-Lonhienne, C.; Gonzalez, A.; Trueman, S.J. Nitrogen Source Influences the Effect of Plant Growth-Promoting Rhizobacteria (PGPR) on Macadamia integrifolia. Agronomy 2021, 11, 1064. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root Exudates as Mediators of Mineral Acquisition in Low-Nutrient Environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Pii, Y.; Marastoni, L.; Springeth, C.; Fontanella, M.C.; Beone, G.M.; Cesco, S.; Mimmo, T. Modulation of Fe Acquisition Process by Azospirillum brasilense in Cucumber Plants. Environ. Exp. Bot. 2016, 130, 216–225. [Google Scholar] [CrossRef]
- Jiménez, S.; Garín, A.; Albás, E.S.; Betrán, J.A.; Gogorcena, Y.; Moreno, M.A. Effect of Several Rootstocks on Fruit Quality of ‘Sunburst’ Sweet Cherry. Acta Hortic. 2004, 658, 353–358. [Google Scholar] [CrossRef]
- Aglar, E.; Yıldız, K. Influence of Rootstocks (Gisela 5, Gisela 6, MaxMa, SL 64) on Performance of ‘0900 Ziraat’ Sweet Cherry. J. Basic. Appl. Sci. 2021, 10, 60–66. [Google Scholar] [CrossRef]
- Vizzotto, G.; Lain, O.; Costa, G. Root Restriction and Photosynthetic Response in a Peach Rootstock. HortScience 1993, 28. Available online: https://ricerca.unityfvg.it/entities/publication/a2135677-0501-4cca-8135-30dfbfd3c7ec/details (accessed on 26 November 2024). [CrossRef]
- Girardi, E.A.; de Francisco, A.A.M.F.; Christiano César, D.G.; Olic Fernando, B. Vegetative Growth of Citrus Nursery Trees Related to the Container Volume. Fruits 2005, 60, 101–105. [Google Scholar] [CrossRef][Green Version]
- Akova, V.; Staneva, I.; Dimitrov, A. Effect of Container Volume on the Growth Habits and Nutritional Status of Cherry Plants of Summit Cultivar Produced in Containers. J. Mt. Agric. Balk. 2021, 24, 289–298. [Google Scholar]
- Świerczyński, S. Influence of the Propagation Method of Three Semidwarf Rootstocks on the Growth and Activity of the Physiological Processes of Maiden Sweet Cherry Trees in a Nursery. Acta Sci. Pol-Hortoru 2023, 22, 47–66. [Google Scholar] [CrossRef]
- Domozetova, D.D.; Radomirska, I.S. Growth and Reproductive Behavior of Nine Sweet Cherry Cultivars Grafted on Two Vegetative Rootstocks. Acta Hortic. 2017, 1161, 293–298. [Google Scholar] [CrossRef]
- Lang, G.A. Underlying Principles of High Density Sweet Cherry Production. In IV International Cherry Symposium 667; ISHS Acta Horticulturae: Korbeek, Belgium, 2005; pp. 325–336. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Zhu, T.H. Walnut Growth and Soil Quality after Inoculating Soil Containing Rock Phosphate with Phosphate-Solubilizing Bacteria. Sci. Asia 2014, 40, 21–27. [Google Scholar] [CrossRef]
- De Silva, A.; Patterson, K.; Rothrock, C.; Moore, J. Growth Promotion of Highbush Blueberry by Fungal and Bacterial Inoculants. HortScience 2000, 35, 1228–1230. [Google Scholar] [CrossRef]
- Orhan, E.; Eşitken, A.; Ercisli, S.; Turan, M.; Sahin, F. Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Yield, Growth and Nutrient Contents in Organically Growing Raspberry. Sci. Hortic. 2006, 111, 38–43. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Soleymani, A. The Roles of Plant-Growth-Promoting Rhizobacteria (PGPR)-Based Biostimulants for Agricultural Production Systems. Plants 2024, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf Nitrogen Determination Using Non-Destructive Techniques–A Review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-Based Leaf Nitrogen Estimation Is Impacted by Environmental Factors and Crop Leaf Characteristics OPEN. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, S.K.M.A.; Ahmed Mohamed, A.A.; Mohamed El-Naggar, Y.I. Effect of Melatonin, GA3 and NAA on Vegetative Growth, Yield and Quality of ‘Canino’ Apricot Fruits. Acta Sci. Pol-Hortoru 2019, 18, 167–174. [Google Scholar] [CrossRef]
- Moreno, M.A.; Adrada, R.; Aparicio, J.; BetráN, S. Performance of ‘Sunburst’ Sweet Cherry Grafted on Different Rootstocks. J. Hortic. Sci. Biotechnol. 2001, 76, 167–173. [Google Scholar] [CrossRef]
- Jiménez, S.; Pinochet, J.; Gogorcena, Y.; Betrán, J.A.; Moreno, M.A. Influence of Different Vigour Cherry Rootstocks on Leaves and Shoots Mineral Composition. Sci. Hortic. 2007, 112, 73–79. [Google Scholar] [CrossRef]
- Pérez, C.; Val, J.; Monge, E. Photosynthetic Changes of “Prunus avium L.” Grafted on Different Rootstocks in Relation to Mineral Deficiencies. Acta Hortic. 1997, 448, 81–85. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Kappel, F.; Forge, T. Interaction of Irrigation and Soil Management on Sweet Cherry Productivity and Fruit Quality at Different Crop Loads That Simulate Those Occurring by Environmental Extremes. HortScience 2014, 49, 215–220. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Glišić, I.; Nikolić, R.; Milivojević, J. Early Tree Growth, Productivity, Fruit Quality and Leaf Nutrients Content of Sweet Cherry Grown in a High Density Planting System. Hortic. Sci. 2015, 42, 1–12. [Google Scholar] [CrossRef]
- Gannouni Thouraya, A.; Ali, A.; Campoy, J.A.; Mezni, M.; Ahmed Hela, B.; Youssef, A.; Agr, I.J.; Agri, R. Effect of Soil Mineralogical Composition on Fruit Quality of Sweet Cherry Cultivars. Int. J. Agron. Agric. Res. 2016, 9, 45–56. [Google Scholar]
- Leece, D. Diagnostic Leaf Analysis for Stone Fruit. 5. Sweet Cherry. Aust. J. Exp. Agric. 1975, 15, 118. [Google Scholar] [CrossRef]
- Pilbeam, D.J.; Morley, P.S. Calcium. In Handbook of Plant Nutrition; Barker, V.A., Pilbeam, J.D., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2007; pp. 121–144. [Google Scholar]
- Rutkowski, K.; Łysiak, G.P. Effect of Nitrogen Fertilization on Tree Growth and Nutrient Content in Soil and Cherry Leaves (Prunus cerasus L.). Agriculture 2023, 13, 578. [Google Scholar] [CrossRef]
- Belkhodja, R.; Sanz, M.; Abadía, A.; Abadía, J. Effect of Chlorosis on the Nutrient Concentration in Flowers and Leaves of peach along the Season. Acta Hortic. 1997, 448, 363. [Google Scholar] [CrossRef]
- Fallahi, E.; Mohan, S.K. Influence of Nitrogen and Rootstock on Tree Growth, Precocity, Fruit Quality, Leaf Mineral Nutrients, and Fire Blight in `Scarlet Gala’ Apple. Horttechnology 2000, 10, 589–592. [Google Scholar] [CrossRef]
- Sahin, F.; Çakmakçi, R.; Kantar, F. Sugar Beet and Barley Yields in Relation to Inoculation with N 2-Fixing and Phosphate Solubilizing Bacteria; Spinger: Berlin/Heidelberg, Germany, 2004; Volume 265. [Google Scholar]
- Çakmakçi, R.; Dönmez, F.; Aydin, A.; Şahin, F. Growth Promotion of Plants by Plant Growth-Promoting Rhizobacteria under Greenhouse and Two Different Field Soil Conditions. Soil Biol. Biochem. 2006, 38, 1482–1487. [Google Scholar] [CrossRef]
- Hrotkó, K. Potentials in Prunus Mahaleb L. for Cherry Rootstock Breeding. Sci. Hortic. 2016, 205, 70–78. [Google Scholar] [CrossRef]
- Pirlak, L.; Turan, M.; Sahin, F.; Eşitken, A. Floral and Foliar Application of Plant Growth Promoting Rhizobacteria (PGPR) to Apples Increases Yield, Growth, and Nutrient Element Contents of Leaves. J. Sustain. Agric. 2007, 30, 145–155. [Google Scholar] [CrossRef]
- Hernandez, J.P.; De-Bashan, L.E.; Bashan, Y. Starvation Enhances Phosphorus Removal from Wastewater by the Microalga Chlorella Spp. Co-Immobilized with Azospirillum Brasilense. Enzym. Microb. Technol. 2006, 38, 190–198. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Oberson, A.; Liebisch, F.; Keller, F.; Annaheim, K.E.; Huguenin-Elie, O.; Frossard, E. Rapid Microbial Phosphorus Immobilization Dominates Gross Phosphorus Fluxes in a Grassland Soil with Low Inorganic Phosphorus Availability. Soil Biol. Biochem. 2012, 51, 84–95. [Google Scholar] [CrossRef]
- Güler, S.; Macit, I.; Koc, A.; Ibrikci, H. Estimating Leaf Nitrogen Status of Strawberry by Using Chlorophyll Meter Reading. J. Biol. Sci. 2006, 6, 1011–1016. [Google Scholar] [CrossRef][Green Version]
- Ahemad, M.; Kibret, M. Mechanisms and Applications of Plant Growth Promoting Rhizobacteria: Current Perspective. J. King Saud. Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Johnson, R.S.; Uriu, K. Mineral Nutrition. In Peach, Plum and Nectarine: Growing and Handling for Fresh Market; Larue, J., Johnson, R.S., Eds.; University of California, Division of Agriculture Resource: Oakland, CA, USA, 1989; pp. 68–81. [Google Scholar]
- Hong-Bo, S.; Li-Ye, C.; Ming-An, S. Calcium as a Versatile Plant Signal Transducer under Soil Water Stress. BioEssays 2008, 30, 634–641. [Google Scholar] [CrossRef]
- Reddy, A.S.N.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with Stresses: Roles of Calcium- and Calcium/Calmodulin-Regulated Gene Expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Measuring Unit | Value |
|---|---|---|
| pH | H2O | 7.29 |
| EC | mS cm−1 | 0.19 |
| NH4+ | mg L−1 | 23.85 |
| NO3− | mg L−1 | 15.10 |
| N-min. | mg L−1 | 21.93 |
| P2O5 | mg L−1 | 84.75 |
| K2O | mg L−1 | 36.23 |
| Ca | mg L−1 | 9.09 |
| Mg | mg L−1 | 1.28 |
| Parameter | Rootstock | Treatment | R | T | R × T | ||||
|---|---|---|---|---|---|---|---|---|---|
| SL 64 | MaxMa 14 | Gisela 5 | C | T1 | T2 | p | p | p | |
| pH (H2O) | 6.46 ± 0.15 | 6.22 ± 0.09 | 6.40 ± 0.09 | 6.23 ± 0.09 | 6.40 ± 0.14 | 6.45 ± 0.10 | n.s. | n.s. | n.s. |
| EC (mS cm−1) | 0.52 ± 0.10 ab | 0.69 ± 0.07 a | 0.34 ± 0.09 b | 0.62 ± 0.09 | 0.50 ± 0.10 | 0.42 ± 0.06 | ** | n.s. | * |
| NH4+ (mg L−1) | 4.68 ± 0.67 | 4.48 ± 0.94 | 3.29 ± 0.41 | 5.32 ± 0.66 a | 4.43 ± 0.72 ab | 2.69 ± 0.41 b | n.s. | * | n.s. |
| NO3− (mg L−1) | 18.56 ± 2.31 a | 19.15 ± 1.85 a | 10.99 ± 1.43 b | 18.70 ± 3.18 | 15.57 ± 1.35 | 14.43 ± 1.68 | ** | n.s. | n.s. |
| Mineral N (mg L−1) | 7.82 ± 0.95 a | 7.81 ± 0.86 a | 5.04 ± 0.34 b | 8.36 ± 1.12 a | 6.96 ± 0.65 ab | 5.35 ± 0.40 b | ** | ** | n.s. |
| P2O5 (mg L−1) | 29.19 ± 2.86 a | 22.18 ± 1.91 ab | 19.19 ± 3.03 b | 28.74 ± 3.36 | 22.02 ± 2.27 | 19.80 ± 2.41 | * | n.s. | n.s. |
| K2O (mg L−1) | 35.06 ± 5.11 | 35.97 ± 3.66 | 26.49 ± 2.91 | 38.31 ± 3.88 | 31.68 ± 4.55 | 27.53 ± 3.41 | n.s. | n.s. | * |
| Ca2+ (mg L−1) | 48.98 ± 12.29 ab | 64.50 ± 8.37 a | 25.55 ± 5.59 b | 62.28 ± 11.40 a | 42.33 ± 11.26 ab | 34.42 ± 6.34 b | ** | * | n.s. |
| Mg2+ (mg L−1) | 8.27 ± 1.93 ab | 11.54 ± 1.55 a | 4.29 ± 2.38 b | 13.84 ± 3.91 | 4.38 ± 1.14 | 6.60 ± 1.93 | ** | n.s. | * |
| Parameter | Rootstock | Treatment | R | T | R × T | ||||
|---|---|---|---|---|---|---|---|---|---|
| SL 64 | MaxMa 14 | Gisela 5 | C | T1 | T2 | p | p | p | |
| Maiden height (cm) | 75.42 ± 0.76 b | 80.48 ± 0.50 a | 72.64 ± 0.20 c | 74.58 ± 0.32 | 79.90 ± 0.55 | 74.47 ± 0.21 | *** | n.s. | n.s. |
| Rootstock circumference (mm) | 37.59 ± 0.78 a | 34.56 ± 0.39 b | 31.42 ± 0.32 c | 34.15 ± 0.61 | 33.12 ± 0.52 | 34.62 ± 0.66 | *** | n.s. | n.s. |
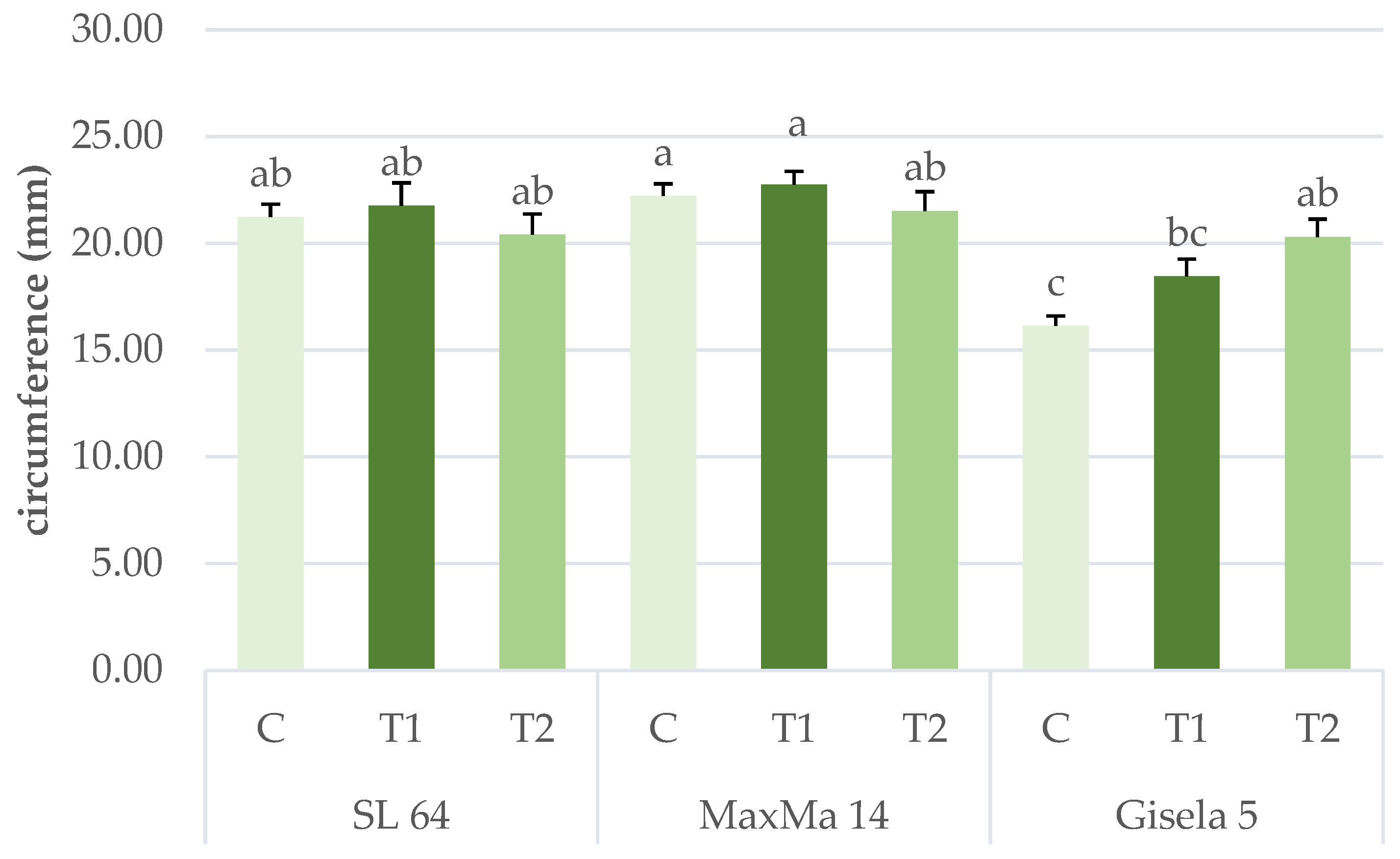
| Shoot circumference (mm) | 21.21 ± 0.49 a | 22.22 ± 0.39 a | 18.36 ± 0.49 b | 19.78 ± 0.56 | 20.74 ± 0.52 | 20.89 ± 0.56 | *** | n.s. | ** |
| CCI | 23.60 ± 1.21 a | 23.61 ± 1.34 a | 19.69 ± 1.01 b | 17.51 ± 0.87 b | 26.98 ± 1.40 a | 22.01 ± 0.68 b | ** | *** | n.s. |
| Stage | Parameter | Rootstock | Treatment | R | T | R × T | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SL 64 | MaxMa 14 | Gisela 5 | C | T1 | T2 | p | p | p | ||
| BBCH 34 | Shoot length (cm) | 7.01 ± 1.28 b | 13.00 ± 1.65 a | 14.81 ± 1.69 a | 9.21 ± 1.34 b | 15.01 ± 1.93 a | 13.24 ± 1.82 ab | ** | * | n.s. |
| Internode n° | 2.13 ± 0.31 b | 3.78 ± 0.41 a | 4.05 ± 0.40 a | 2.97 ± 0.34 | 3.97 ± 0.46 | 3.65 ± 0.45 | ** | n.s. | n.s. | |
| Internode length (cm) | 2.91 ± 0.26 | 3.02 ± 0.19 | 3.22 ± 0.19 | 2.60 ± 0.20 b | 3.22 ± 0.19 ab | 3.49 ± 0.20 a | n.s. | * | n.s. | |
| BBCH 39 | Shoot length (cm) | 60.50 ± 3.09 | 62.18 ± 2.90 | 59.22 ± 3.22 | 57.12 ± 2.85 | 66.02 ± 3.43 | 59.42 ± 3.05 | n.s. | n.s. | n.s. |
| Internode n° | 17.54 ± 0.57 ab | 18.59 ± 0.60 a | 16.07 ± 0.66 b | 17.27 ± 0.58 | 17.87 ± 0.83 | 16.91 ± 0.60 | * | n.s. | n.s. | |
| Internode length (cm) | 3.41 ± 0.10 | 3.29 ± 0.10 | 3.62 ± 0.09 | 3.25 ± 0.11 b | 3.46 ± 0.09 ab | 3.68 ± 0.06 a | n.s. | * | n.s. | |
| BBCH 91 | Shoot length (cm) | 66.42 ± 2.65 | 68.91 ± 1.96 | 61.23 ± 2.81 | 63.26 ± 2.11 | 69.16 ± 2.92 | 63.82 ± 2.76 | n.s. | n.s. | n.s. |
| Internode n° | 18.79 ± 0.60 a | 20.32 ± 0.46 a | 16.59 ± 0.54 b | 18.73 ± 0.56 | 18.55 ± 0.68 | 18.09 ± 0.57 | *** | n.s. | n.s. | |
| Internode length (cm) | 3.53 ± 0.08 | 3.40 ± 0.07 | 3.64 ± 0.09 | 3.39 ± 0.08 b | 3.50 ± 0.08 ab | 3.71 ± 0.08 a | n.s. | * | n.s. | |
| Parameter | Rootstock | Treatment | R | T | R × T | ||||
|---|---|---|---|---|---|---|---|---|---|
| SL 64 | MaxMa 14 | Gisela 5 | C | T1 | T2 | p | p | p | |
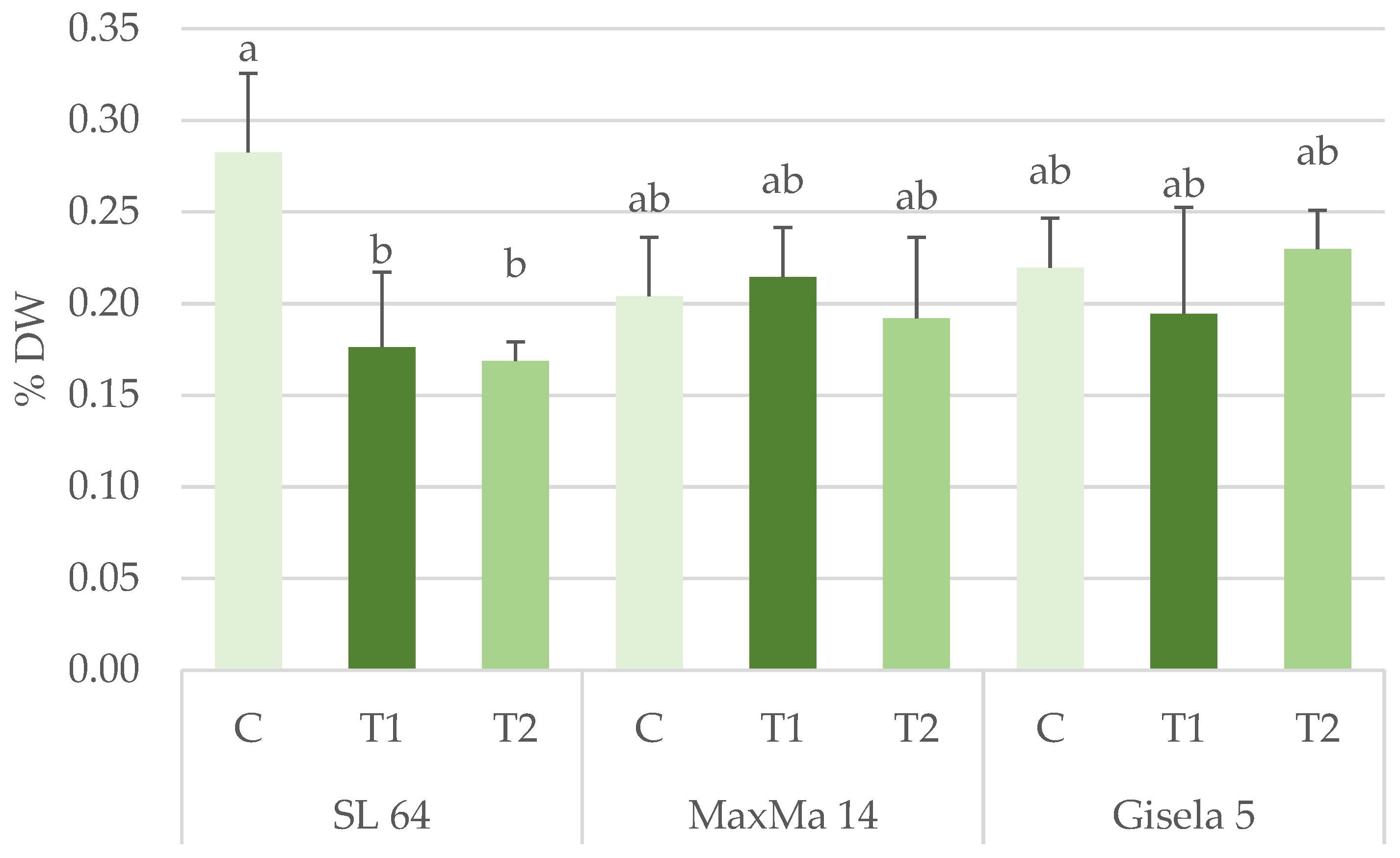
| LDMC (%) | 40.57 ± 0.44 | 38.81 ± 1.58 | 40.32 ± 0.84 | 38.88 ± 1.46 | 40.50 ± 1.85 | 40.31 ± 1.90 | n.s. | n.s. | n.s. |
| N | 1.66 ± 0.07 | 1.77 ± 0.11 | 1.87 ± 0.05 | 1.57 ± 0.05 b | 1.96 ± 0.07 a | 1.77 ± 0.07 ab | n.s. | ** | n.s. |
| P | 0.21 ± 0.02 | 0.20 ± 0.01 | 0.21 ± 0.02 | 0.24 ± 0.05 | 0.20 ± 0.04 | 0.20 ± 0.04 | n.s. | n.s. | * |
| K | 1.66 ± 0.07 b | 1.42 ± 0.06 b | 2.01 ± 0.07 a | 1.81 ± 0.09 | 1.65 ± 0.09 | 1.64 ± 0.13 | *** | n.s. | n.s. |
| Ca | 0.87 ± 0.04 b | 1.15 ± 0.05 a | 0.93 ± 0.05 b | 0.89 ± 0.05 b | 1.01 ± 0.06 ab | 1.05 ± 0.07 a | *** | * | n.s. |
| Mg | 0.47 ± 0.03 b | 0.60 ± 0.04 a | 0.32 ± 0.02 c | 0.40 ± 0.05 b | 0.50 ± 0.05 a | 0.49 ± 0.05 ab | *** | * | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolega, Š.; Kos, T.; Zorica, M.; Marcelić, Š.; Fruk, G. Substrate Properties, Vegetative Growth, Chlorophyll Content Index and Leaf Mineral Content of Sweet Cherry Maiden Trees as Affected by Rootstock and Plant Growth-Promoting Rhizobacteria. Sustainability 2025, 17, 158. https://doi.org/10.3390/su17010158
Kolega Š, Kos T, Zorica M, Marcelić Š, Fruk G. Substrate Properties, Vegetative Growth, Chlorophyll Content Index and Leaf Mineral Content of Sweet Cherry Maiden Trees as Affected by Rootstock and Plant Growth-Promoting Rhizobacteria. Sustainability. 2025; 17(1):158. https://doi.org/10.3390/su17010158
Chicago/Turabian StyleKolega, Šimun, Tomislav Kos, Marko Zorica, Šime Marcelić, and Goran Fruk. 2025. "Substrate Properties, Vegetative Growth, Chlorophyll Content Index and Leaf Mineral Content of Sweet Cherry Maiden Trees as Affected by Rootstock and Plant Growth-Promoting Rhizobacteria" Sustainability 17, no. 1: 158. https://doi.org/10.3390/su17010158
APA StyleKolega, Š., Kos, T., Zorica, M., Marcelić, Š., & Fruk, G. (2025). Substrate Properties, Vegetative Growth, Chlorophyll Content Index and Leaf Mineral Content of Sweet Cherry Maiden Trees as Affected by Rootstock and Plant Growth-Promoting Rhizobacteria. Sustainability, 17(1), 158. https://doi.org/10.3390/su17010158






