Adsorption Potential and Mechanism of Sludge-Based Activated Carbon Modified with Fly Ash for Removal of Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Sludge and Fly Ash
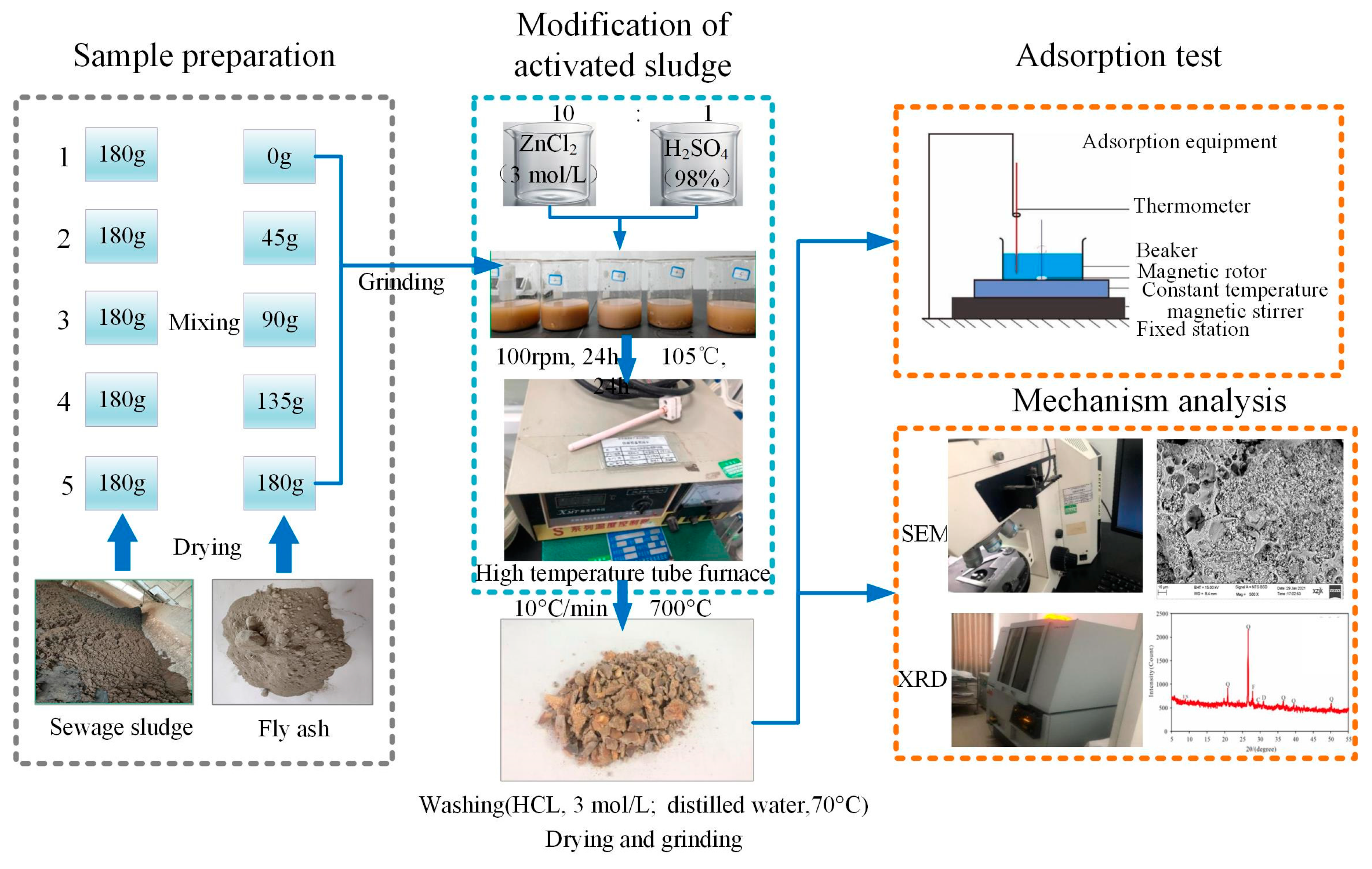
2.2. Preparation of Modified Sludge Adsorbent (MSA)
2.3. Characteristics of Modified Samples
2.3.1. Adsorption of Heavy Metals
2.3.2. Characterization of Physical and Chemical Properties
2.3.3. Data Calculation
3. Results
3.1. Properties of Sludge
3.1.1. Chemical Characteristics
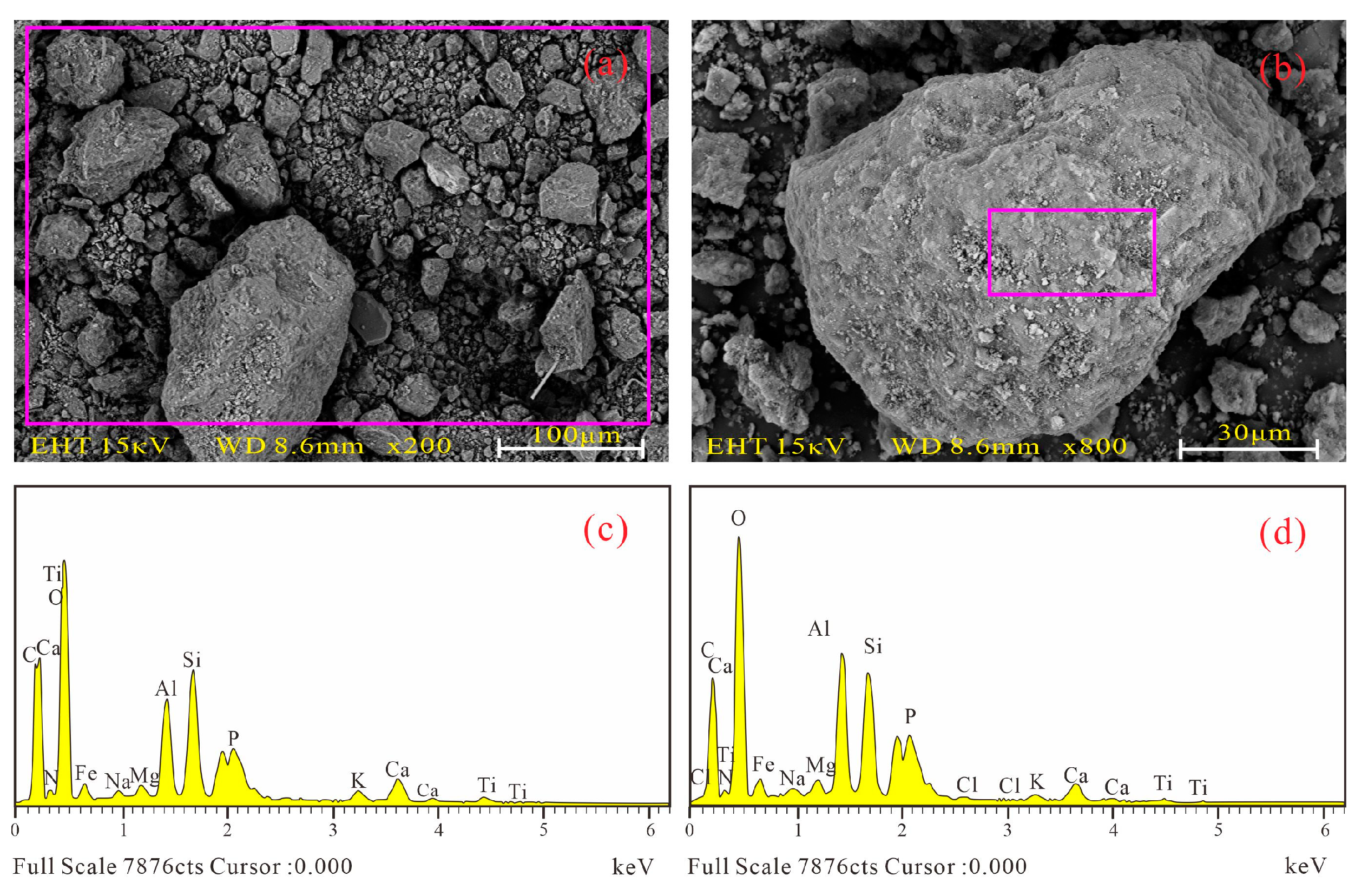
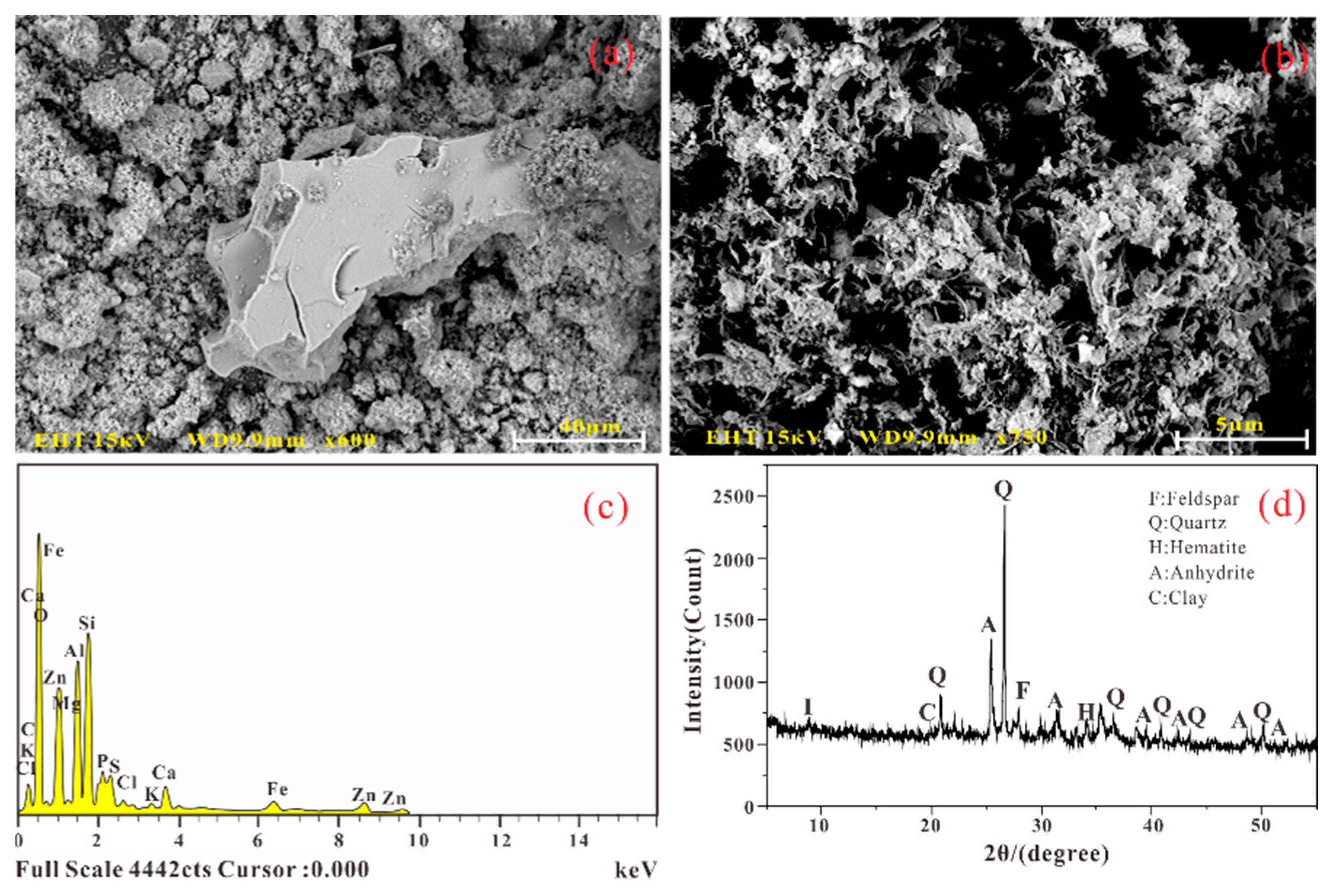
3.1.2. Microstructure and Composition
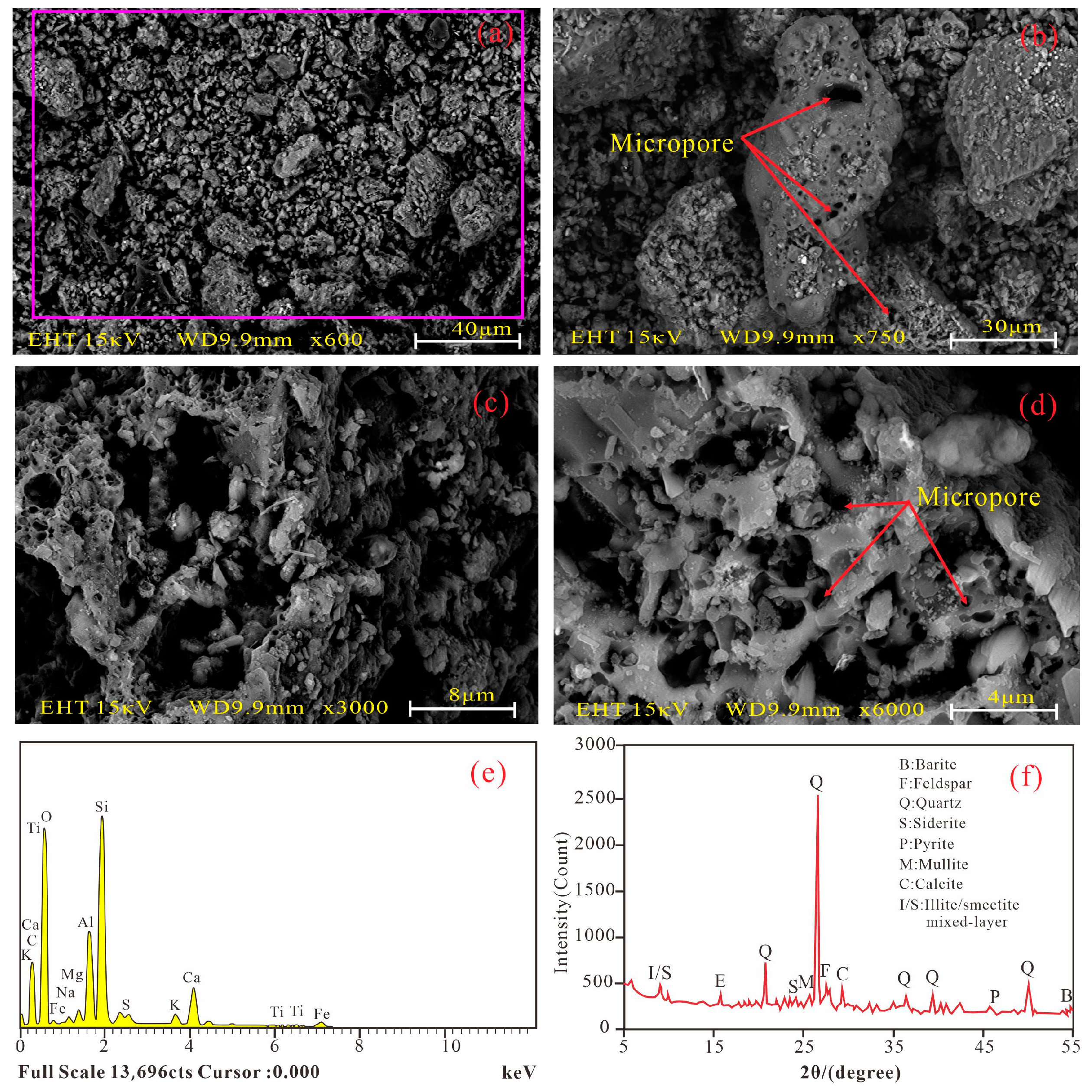
3.2. Properties of Fly Ash
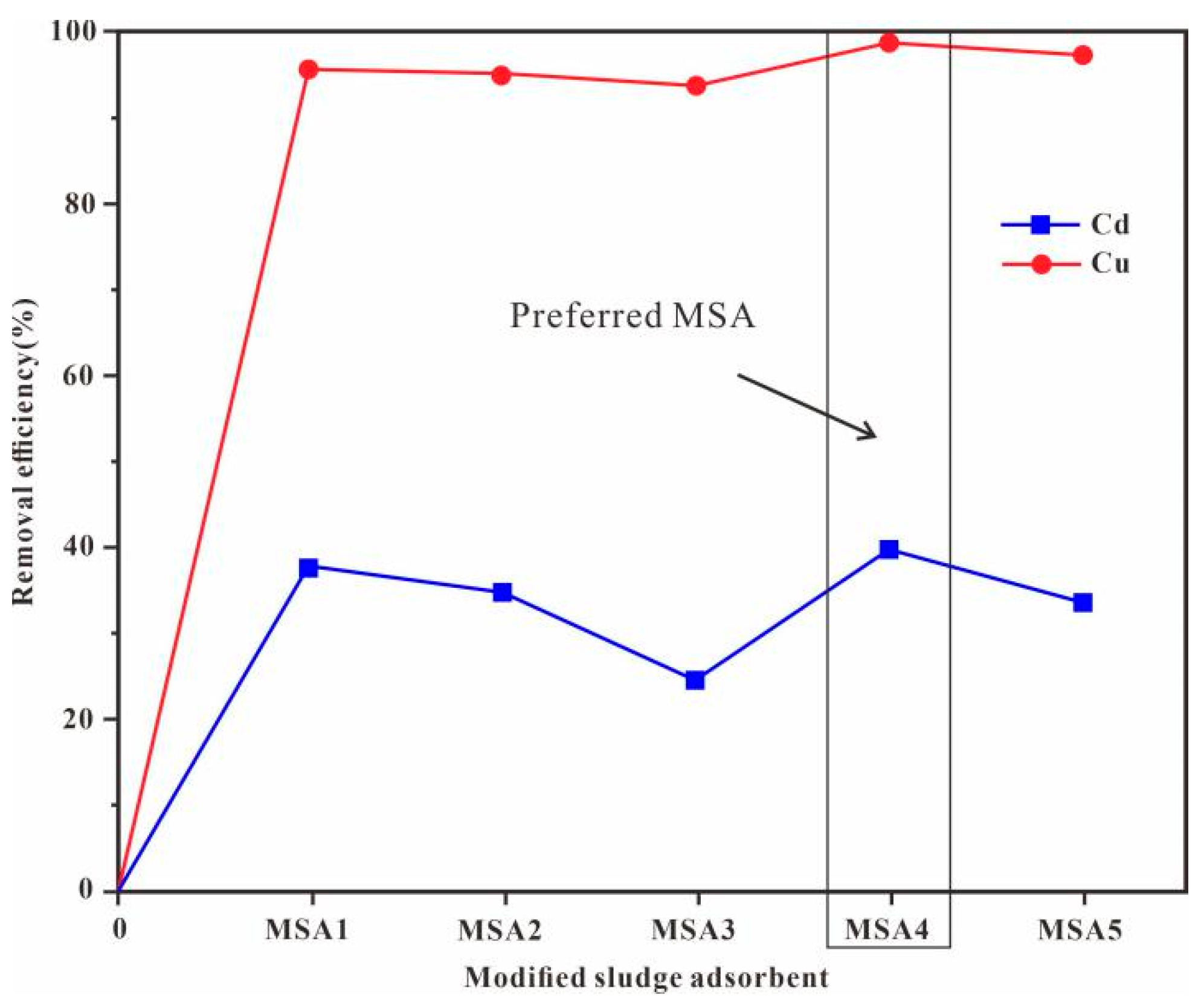
3.3. Adsorption Experiment Results
4. Discussion
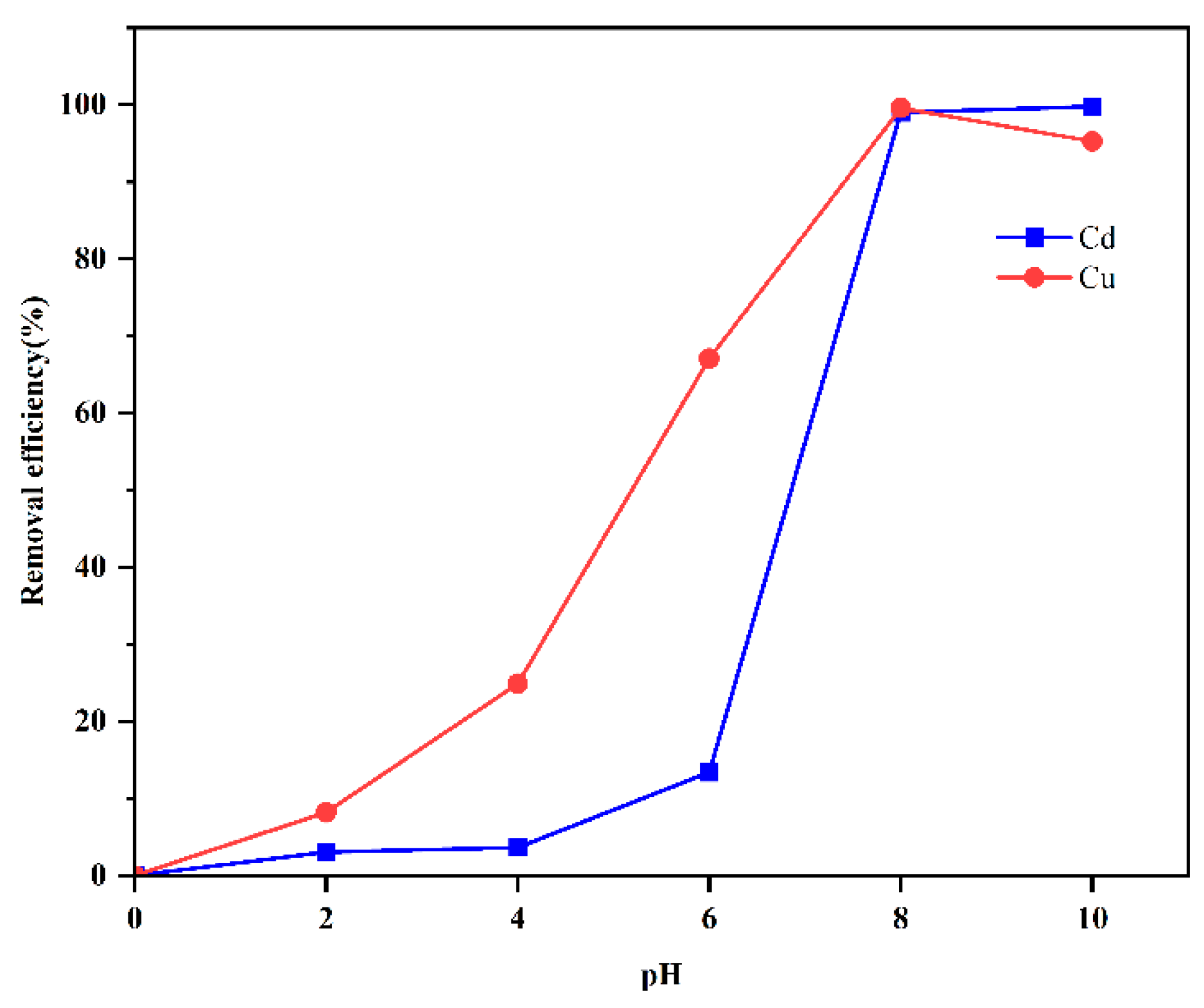
4.1. Influence of Fly Ash Content and pH
4.2. Micro Components and Structure
4.3. Adsorption Mechanism
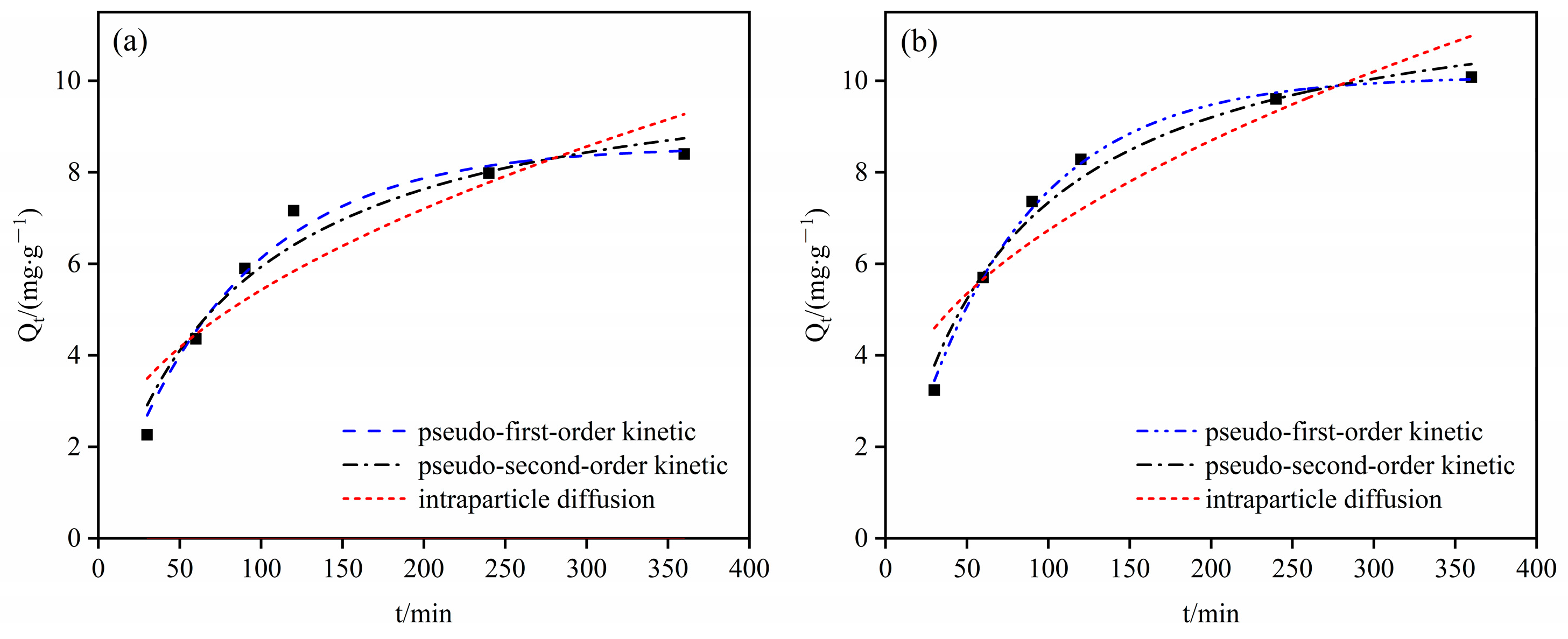
4.3.1. Adsorption Kinetic
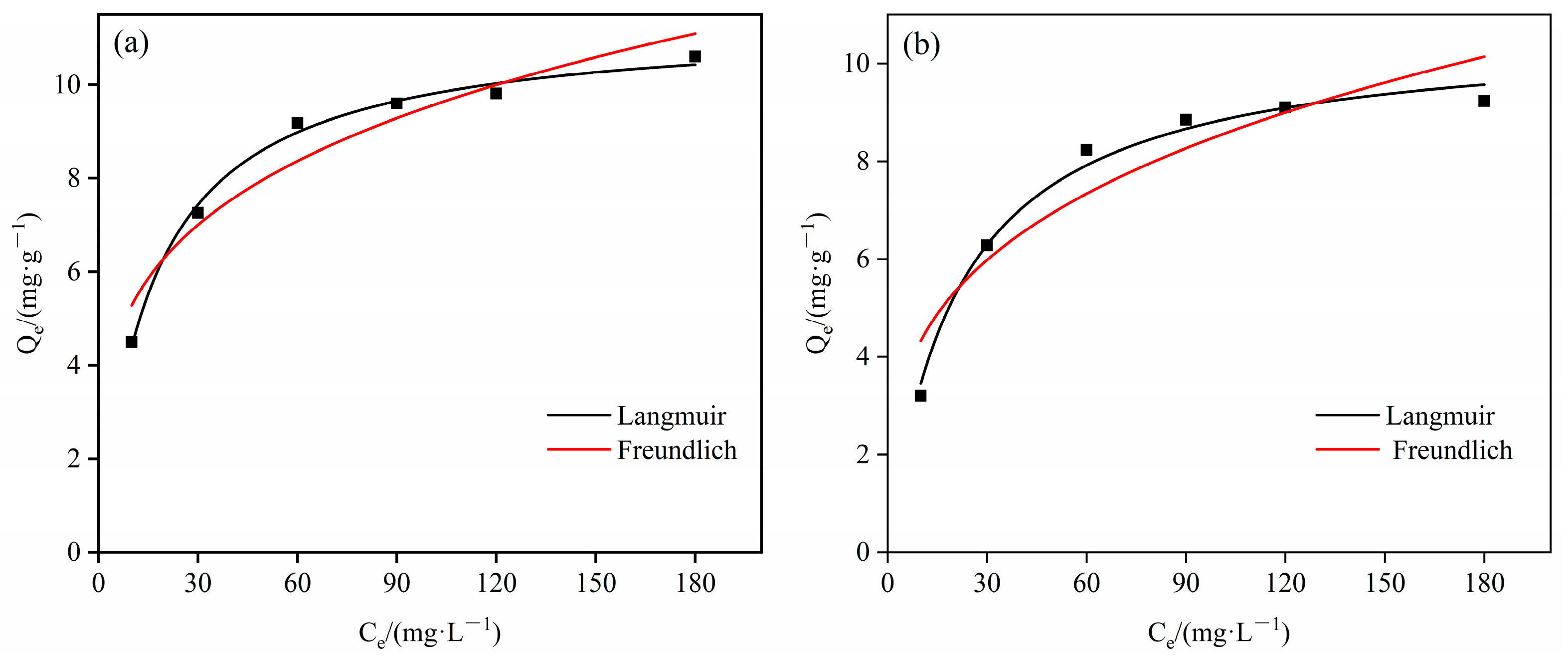
4.3.2. Adsorption Isotherm
4.3.3. Metal Sorption Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poumanyvong, P.; Kaneko, S. Does urbanization lead to less energy use and lower CO2 emissions? A cross-country analysis. Ecol. Econ. 2010, 70, 434–444. [Google Scholar] [CrossRef]
- Cantinho, P.; Matos, M.; Trancoso, M.A.; dos Santos, M.M.C. Behaviour and fate of metals in urban wastewater treatment plants: A review. Int. J. Environ. Sci. Technol. 2015, 13, 359–386. [Google Scholar] [CrossRef]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy Metals in Wastewater and Sewage Sludge from Selected Municipal Treatment Plants in Eastern Cape Province, South Africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Geng, Y.M.; Zhang, C.B.; Zhang, D.D.; Yan, S.X.; Sun, T.F.; Chen, L.; Wang, J.; Mao, Y.X. Speciation and ecological risk assessment of heavy metal (loid)s in the municipal sewage sludge of China. Environ. Sci. 2021, 42, 4834–4843. [Google Scholar]
- Mao, H.; Zhang, Y.; Wang, H.; Cui, K.; Yu, L.; Tan, T. Recycling sewage sludge into ceramic materials: A review. Environ. Chem. Lett. 2023, 21, 1659–1672. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Namieśnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar]
- Tang, J.; He, J.; Liu, T.; Xin, X. Removal of heavy metals with sequential sludge washing techniques using saponin: Optimization conditions, kinetics, removal effectiveness, binding intensity, mobility and mechanism. RSC Adv. 2017, 7, 33385–33401. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A sustainable paradigm of sewage sludge biochar: Valorization, opportunities, challenges and future prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Bolzonella, D.; Fatone, F.; Pavan, P.; Cecchi, F. Poly-chlorinated dibenzo-p-dioxins, dibenzo-furans and dioxin-like poly-chlorinated biphenyls occurrence and removal in conventional and membrane activated sludge processes. Bioresour. Technol. 2010, 101, 9445–9454. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, X.T.; Gao, Y.X.; Ma, Z.W.; Wang, Y.; Ying, C.Y.; Xie, J.H.; Zhou, F.H.; Jiang, H.Y.; Ma, Y.H. Effects of partial replacement of chemical fertilizer with sludge compost on maize growth in chromium-contaminatedfarmland. J. Agro-Environ. Sci. 2023, 42, 2453–2463. [Google Scholar]
- Jafaripour, A.; Rowson, N.A.; Ghataora, G.S. Utilisation of residue gas sludge (BOS sludge) for removal of heavy metals from acid mine drainage (AMD). Int. J. Miner. Process. 2015, 144, 90–96. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Kuisma, M.; Ketoja, E.; Salo, T.; Heikkinen, J. Phosphorus in manure and sewage sludge more recyclable than in soluble inorganic fertilizer. Environ. Sci. Technol. 2015, 49, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Zhang, L.; Seo, S.; Lee, Y.W.; Jahng, D. Protein recovery from excess sludge for its use as animal feed. Bioresour. Technol. 2008, 99, 8949–8954. [Google Scholar] [CrossRef]
- Wu, M.; Wang, X.Q.; Mao, L.X.; Wang, X.F.; Zhu, S.F.; Zhang, G.C.; Xun, N.C.; Qin, Y.H. Experimental study on Cd(II) and Cu(II) of adsorbent prepared by mixing fly ash and domestic sludge. Appl. Chem. Ind. 2023, 52, 689–696. [Google Scholar]
- Du, W.J.; Lu, J.Y.; Hu, Y.R.; Xiao, J.X.; Yang, C.; Wu, J.; Huang, B.C.; Cui, S.; Wang, Y.; Li, W.W. Spatiotemporal pattern of greenhousegas emissions in China’s wastewater sector and pathways towardscarbon neutrality. Nat. Water 2023, 1, 166–175. [Google Scholar] [CrossRef]
- Sun, M.; Yang, J.L.; Xiao, P.Y.; Li, J.S.; Wang, Q.B.; Liu, G.; Huo, M.X. Progress and new trend of low carbon and resource recovery technologies for municipal wastewater treatmentplants. Chin. J. Environ. Eng. 2023, 17, 1748–1760. [Google Scholar]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Velghe, I.; Carleer, R.; Yperman, J.; Schreurs, S.; D’Haen, J. Characterisation of adsorbents prepared by pyrolysis of sludge and sludge/disposal filter cake mix. Water Res. 2012, 46, 2783–2794. [Google Scholar] [CrossRef]
- Kante, K.; Qiu, J.; Zhao, Z.; Cheng, Y.; Bandosz, T.J. Development of surface porosity and catalytic activity in metal sludge/waste oil derived adsorbents: Effect of heat treatment. Chem. Eng. J. 2008, 138, 155–165. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Optimized and functionalized paper sludge activated with potassium fluoride for single and binary adsorption of reactive dyes. J. Ind. Eng. Chem. 2014, 20, 830–840. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Block, K. Effect of pyrolysis temperature and time on catalytic performance of sewage sludge/industrial sludge-based composite adsorbents. Appl. Catal. B 2006, 67, 77–85. [Google Scholar] [CrossRef]
- Chiang, H.L.; Lin, K.H.; Chiu, H.H. Exhaust characteristics during the pyrolysis of ZnCl2 immersed biosludge. J. Hazard. Mater. 2012, 229–230, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Improvement in performance of sludge-based adsorbents by controlling key parameters by activation/modification: A critical review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1704–1743. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Patra, K.C.; Rautray, T.R.; Nayak, P. Analysis of grains grown on fly ash treated soils. Appl. Radiat. Isot. 2012, 70, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; MacKenzie, K.J.D. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Ram, L.C.; Srivastava, N.K.; Jha, S.K.; Sinha, A.K.; Masto, R.E.; Selvi, V.A. Management of lignite fly ash for improving soil fertility and crop productivity. Environ. Manag. 2007, 40, 438–452. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, Y.B.; Lee, C.H.; Hong, C.O.; Kim, P.J.; Yu, C. Characteristics of boron accumulation by fly ash application in paddy soil. Bioresour. Technol. 2008, 99, 5928–5932. [Google Scholar] [CrossRef] [PubMed]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Characterization of sintered coal fly ashes. Fuel 2008, 87, 1334–1340. [Google Scholar] [CrossRef]
- Wang, S. Application of solid ash based catalysts in heterogeneous catalysis. Environ. Sci. Technol. 2008, 42, 7055–7063. [Google Scholar] [CrossRef] [PubMed]
- Khatri, C.; Mishra, M.K.; Rani, A. Synthesis and characterization of fly ash supported sulfated zirconia catalyst for benzylation reactions. Fuel Process. Technol. 2010, 91, 1288–1295. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A study on removal characteristics of heavy metals from aqueous solution by fly ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; He, X.W.; Wang, C.R.; Li, J.W.; Zhang, C.H. Cadmium adsorption characteristic of alkali modified sewage sludge. Bioresour. Technol. 2012, 121, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Matheswaran, M.; Karunanithi, T. Adsorption of Chrysoidine R by using fly ash in batch process. J. Hazard. Mater. 2007, 145, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Singh, N. Adsorption of herbicides on coal fly ash from aqueous solutions. J. Hazard. Mater. 2009, 168, 233–237. [Google Scholar] [CrossRef]
- Mao, L.X.; Zhu, S.F.; Wu, M.; Zhao, Q.; Wang, P.C.; Qin, Y.H. Distribution and environmental risk assessment of heavy metals in coal-fired products of power plants. China Sci. 2023, 18, 1028–1034. [Google Scholar]
- Yu, L.; Zhong, Q. Preparation of adsorbents made from sewage sludges for adsorption of organic materials from wastewater. J. Hazard. Mater. 2006, 137, 359–366. [Google Scholar] [CrossRef]
- Gomez-Pacheco, C.V.; Rivera-Utrilla, J.; Sanchez-Polo, M.; Lopez-Penalver, J.J. Optimization of the preparation process of biological sludge adsorbents for application in water treatment. J. Hazard. Mater. 2012, 217–218, 76–84. [Google Scholar] [CrossRef]
- Ping, F.; Chaoping, C.; Dingsheng, C.; Zhixiong, T. Carbonaceous adsorbents prepared from sewage sludge and its application for Hg0 adsorption in simulated flue gas. Chin. J. Chem. Eng. 2010, 18, 231–238. [Google Scholar]
- Kutchko, B.; Kim, A. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Rio, S.; Faur-Brasquet, C.; Le Coq, L.; Courcoux, P.; Le Cloirec, P. Experimental design methodology for the preparation of carbonaceous sorbents from sewage sludge by chemical activationa––Application to air and water treatments. Chemosphere 2005, 58, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Gascó, G.; Méndez, A.; Gascó, J.M. Preparation of carbon-based adsorbents from sewage sludge pyrolysis to remove metals from water. Desalination 2005, 180, 245–251. [Google Scholar] [CrossRef]
- Hu, Q.; Bin, L.; Li, P.; Fu, F.; Guan, G.; Hao, X.; Tang, B. Highly efficient removal of dyes from wastewater over a wide range of pH value by a self-adaption adsorbent. J. Mol. Liq. 2021, 331, 115719. [Google Scholar] [CrossRef]
- Wei, S.S.; Zhao, Z.Z.; Wu, Y.H.; Liu, H.; Wang, L. Study on adsorption behavior of Cu2+ in water by fibrous sepiolite adsorbent. Ind. Water Treat. 2022, 42, 67–72. [Google Scholar]
- El-Azeem, A.; Samy, A.; Ahmad, M.; Usman, A.R.; Kim, K.-R.; Oh, S.-E.; Lee, S.S.; Ok, Y.S. Changes of biochemical properties and heavy metal bioavailability in soil treated with natural liming materials. Environ. Earth Sci. 2013, 70, 3411–3420. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef]
- Wongrod, S.; Simon, S.; van Hullebusch, E.D.; Lens, P.N.L.; Guibaud, G. Changes of sewage sludge digestate-derived biochar properties after chemical treatments and influence on As(III and V) and Cd(II) sorption. Int. Biodeterior. Biodegrad. 2018, 135, 96–102. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of several metal ions onto a low-cost biosorbent: Kinetic and equilibrium studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.-Q.; Chen, C.; Cui, H.-J.; Fu, M.-L. Enhanced removal of Cd(II) from aqueous solution using CaCO3 nanoparticle modified sewage sludge biochar. RSC Adv. 2017, 7, 16238–16243. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]

| Parameter | Sludge Types | Unit | |
|---|---|---|---|
| DSS | IWS | ||
| pH | 7.15 | 8.50 | \ |
| Tp | 12,620 | 278 | mg/kg |
| Ap | 236 | 194 | mg/kg |
| Om | 25.29 | 2.89 | % |
| Cu | 77.5 | 8.16 | μg/g |
| Cr | 143 | 60.8 | μg/g |
| Zn | 1344 | 141 | μg/g |
| Cd | 3.87 | 0.21 | μg/g |
| Pb | 32.8 | 6.70 | μg/g |
| Mn | 294 | 2648 | μg/g |
| Ni | 43.2 | 42.9 | μg/g |
| Metal Ions | Adsorption Isotherm | Fitting Equations | Parameter 1 | Parameter 2 | R2 |
|---|---|---|---|---|---|
| Cd2+ | Langmuir | Qe = 0.5104Ce/(1 + 0.0478Ce) | Qm = 10.6778 | KL = 0.0478 | 0.9820 |
| Freundlich | Qe = 2.1973 Ce0.2945 | Kf = 2.1973 | 1/n = 0.2945 | 0.9562 | |
| Cu2+ | Langmuir | Qe = 0.7193Ce/(1 + 0.0634Ce) | Qm = 11.3439 | KL = 0.0634 | 0.9972 |
| Freundlich | Qe = 2.9243 Ce0.2568 | Kf = 2.9243 | 1/n = 0.2568 | 0.9323 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Wu, M.; Zhu, S.; Wang, X.; Zhang, J.; Qin, Y. Adsorption Potential and Mechanism of Sludge-Based Activated Carbon Modified with Fly Ash for Removal of Heavy Metals. Sustainability 2024, 16, 2972. https://doi.org/10.3390/su16072972
Mao L, Wu M, Zhu S, Wang X, Zhang J, Qin Y. Adsorption Potential and Mechanism of Sludge-Based Activated Carbon Modified with Fly Ash for Removal of Heavy Metals. Sustainability. 2024; 16(7):2972. https://doi.org/10.3390/su16072972
Chicago/Turabian StyleMao, Lixin, Meng Wu, Shifei Zhu, Xinfu Wang, Jing Zhang, and Yunhu Qin. 2024. "Adsorption Potential and Mechanism of Sludge-Based Activated Carbon Modified with Fly Ash for Removal of Heavy Metals" Sustainability 16, no. 7: 2972. https://doi.org/10.3390/su16072972
APA StyleMao, L., Wu, M., Zhu, S., Wang, X., Zhang, J., & Qin, Y. (2024). Adsorption Potential and Mechanism of Sludge-Based Activated Carbon Modified with Fly Ash for Removal of Heavy Metals. Sustainability, 16(7), 2972. https://doi.org/10.3390/su16072972






