Abstract
In this review, an overview was given of the mutual interactions between nematodes and fungi of the genus Trichoderma sp. due to the potential of these fungi to protect plant roots from plant-parasitic nematodes on the one hand and the influence of nematodes (fungivores) on the efficacy of the fungus on the other. In addition, an overview of the advantages of Trichoderma sp. for agricultural production was given. The basis of sustainable agricultural production is the healthy functioning of the soil ecosystem. The diversity of organisms—bacteria, protozoa, algae, metazoans (nematodes) and fungi—improves the quality and performance of the soil by maintaining biological productivity. Root exudates in the rhizosphere support microbial communities that play a key role in regulating the dynamics of organic matter decomposition and the availability of plant nutrients. The microbial activity of organisms in the soil is interconnected and interacts to form a soil food web that reflects the condition, function and health of the soil. The energy in food webs flows through trophic chains of consumers, which are divided into energy channels. Root, bacterial and fungal channels increase soil biomass, carbon (C) and energy flow through the soil food web. The structure of the nematode community is an effective tool for the biological assessment of soil quality. This is due to a number of characteristics that nematodes have, including the following: a great diversity of species, the possibility of subdivision according to different criteria such as trophic groups and c-p groups, the duration of reproduction, the ease of sampling, the identification of genera and preservation, etc. Nematodes are involved in various ecological functions in the soil, of which the interaction between them and fungi is based on antagonism or mutualism, which is the basis for a better understanding of their impact on the ecosystem. Fungi of the genus Trichoderma sp. are successful colonizers of all habitats, secondary opportunists and fast growing.
1. Introduction
In agriculture, the control of plant diseases and pests is a key challenge because achieving high yields and sustainable cultivation requires the rational use and management of the ecosystem. Striving for a healthy soil that maintains biodiversity, productivity, regenerative capacity and the ability to function can overcome the challenges of increasing food demands [1]. Physical, chemical, biological and enzymatic indicators are used to assess the health status of soil [2]. The rhizosphere zone (area around the roots of a plant), which represents a vital habitat for microbial communities, plays a key role in regulating the dynamics of decomposition and the availability of nutrients for the plant [3]. The amount and type of organisms in soil and their interactions maintain biological productivity in order to improve the health of plants and animals.
Organisms in the vicinity of the roots of the plant in connected interactions form the soil food web. The representation of the individual organisms in the soil food web reflects the state and function of the soil, soil health, the ability to supply the plant with necessary nutrients, and the regulation of the number of pests and other opportunistic species [4]. Analysis of the soil food web allows us to better understand the flow of energy through different trophic groups that are formed according to the way of nutrition. According to research, three main energy channels have been established: root, fungal and bacterial. Each of these channels contributes to soil biomass and the flow of carbon and energy through the soil food web. Intensive agricultural production significantly affects the channel structure, reducing the biomass of root and fungal channels and increasing the dominance of the bacterial energy channel [5].
Nematodes are the most diverse and numerous multicellular groups of invertebrates on Earth and vary in their sensitivity to pollutants and environmental disturbances [6]. Soil nematodes are trophically diverse (bacterivores, fungivores, predators and omnivores), and include economically important plant parasites. Due to their trophic diversity, they reflect not only their own condition, but also the condition of bacterial and fungal communities [7].
The interactions of nematodes with fungi (antagonistic or mutualistic) in the soil represent an intriguing area of research with diverse biological implications. Initial research focus on these interactions was focused on their potential in controlling plant-parasitic nematodes and on understanding their role in soil ecosystems [8]. Nematophagous fungi, which act antagonistically towards nematodes, play a key role in maintaining the balance of nematode populations in the soil [9]. In addition, there is also a positive side of the interaction between nematodes and fungi, a mutualistic interaction, for example, between the genus Aphelenchiods sp. and fungi from the genera Glomus and Gigaspora [10], and between entomopathogenic nematodes and entomopathogenic fungi [11].
In the context of sustainable agricultural production, fungi of the genus Trichoderma sp. occupy an important place in biological control against plant parasitic nematodes, providing numerous advantages in the protection of agricultural crops [12]. However, the presence of mycelium-feeding nematodes can reduce the effectiveness of fungus Trichoderma sp. and inhibit its growth and spread [13]. The interaction between Trichoderma sp. and plants results in improved root growth and a higher yield and crop quality. Considering the complexity of the interactions between fungi of genus Trichoderma sp. and nematodes, it is important to study these processes in order to maximize the effectiveness for the biological control of plant parasitic nematodes. The purpose is to improve alternative practices in sustainable agricultural production. Also, it is important to determine whether the interactions between fungi of genus Trichoderma sp. are related only for plant-parasitic nematodes or include other trophic groups.
2. Soil
Soil is the most diverse ecosystem with a great variety of organisms in a very small space [14]. On average, one gram of soil contains 50 to 100 species of protozoa, the same number of species of fungi and 30 to 80 species of invertebrates [15], and it is estimated that bacteria live in it in a density of 20,000 to 40,000 species [16]. The composition and ratio of organisms greatly influence the physical properties, including texture, structure and porosity, and the proportion of pores in the soil. These properties in turn influence the movement of air and water and thus the ability of the soil to maintain its productivity. Agricultural soils usually have 1–6% organic matter, and consist of three different parts: living organisms, fresh residues and decomposed residues. [17]. Organic matter also serves as an important indicator to determine soil fertility and health [18,19]. A more accurate and better soil assessment can be achieved by integrating various factors such as physical, chemical, biological and enzymatic activities. These factors should be used in combination as indicators for soil quality assessment [2]. Soil quality is defined as the ability of soil to fulfil ecological functions, maintain biological productivity and environmental quality, and improve plant and animal health [20]. Natural disturbances and agricultural practices, such as tillage, irrigation, the burning of organic matter and the application of pesticides and fertilizers, lead to an imbalance of physical and chemical parameters such as texture, moisture, soil pH and organic matter, conductivity, etc. [21]. On the other hand, the microbial communities in the soil have a decisive influence on the dynamics of organic matter decomposition and the availability of plant nutrients such as nitrogen and phosphorus [3].
The rhizosphere is a place where numerous biophysical and biochemical processes take place in parallel, maintaining all the other trophic levels of the biosphere. The functioning of the rhizosphere has major implications for food production and climate change in terms of carbon sequestration. In the rhizosphere, the roots and microorganisms shape and organize the physical (pore volume, cross-linking and aggregation) and biogenic structure (surface coatings, carbon associated with minerals) that control infiltration, water storage and aeration at the soil profile level. The release of organic compounds promotes the growth of microorganisms and creates microbial habitats that, depending on the availability of certain substrates and carbon, form a specific microbiome in the rhizosphere. The rhizosphere microbiome, which is influenced by the plant species and soil type, is of fundamental importance for plant health and nutrient cycling in the soil [22,23].
The microbiome of the rhizosphere consists of bacteria, protozoa, algae, metazoans (nematodes) and fungi, and they represent the largest and most diverse biotic group in the soil. Their role in structuring the organic soil layer (humus) is to regulate the dynamics of the decomposition of organic matter and the availability of plant nutrients in the root zone of plants (carbon, nitrogen, phosphorus, etc.) and to improve the physical components of the soil as they loosen and mix the soil through their movement [24].
The substances deposited by the roots in the rhizosphere can be broadly categorized into two main groups: firstly, water-soluble exudates such as sugars, amino acids, organic acids, hormones and vitamins and secondly, water-insoluble substances such as cell walls, exfoliated material and other root debris as well as mucilage, such as lysates, which are released when the cells initiate autolysis. In addition, the carbon dioxide produced during root respiration often accounts for a large proportion of the carbon released from the roots. Exudates such as polymeric carbohydrates and enzymes can also be considered root exudates, depending on the metabolic processes that lead to their release [25]. Soil, root substances and organisms in the root zone are a prerequisite for the existence of a soil food web in ecosystems.
3. Soil Food Web
A soil food web consists of numerous organisms that interact with each other. The main driving force of the interactions between soil organisms starts with the primary producers (plants) and ends with the consumers (predators). The interactions vary depending on the amount of energy channeled through the trophic level and the number of populations of the organisms in the soil. According to Ferris (2001) [4], the soil food web is a community of organisms that exploit each other as a source of carbon and energy, either through predation or by consuming decomposed remains.
Numerous activities of the organisms in the soil lead to various ecological functions or services such as the decomposition of organic matter, cycling, storage, the distribution of minerals and nutrients, the sequestration of carbon and the degradation of pollutants (pesticides). In addition, the activity of the organisms contributes to the modification of soil structure and the biological regulation of various types of pests, which are crucial for maintaining plant production and soil fertility [26].
The energy in food webs flows through trophic groups, which are grouped based on the way they feed on basic resources (e.g., organic material). This is probably the most common way to understand the structure and functioning of belowground food webs [27]. De Vries and Wallenstein (2017) [5] divide the soil food web into a root energy channel, which is supplied by living roots through the activities of the mycorrhizal fungi and nematodes that feed on them, and two energy channels that originate from detritus, namely the fungal energy channel and the bacterial energy channel. All three energy channels increase the soil biomass and thus the flow of carbon and energy through the food web. Their presence in the soil is closely related to the condition above the soil, which influences their stability and function. Intensive agricultural production reduces the biomass of the root and fungal energy channels and increases the energy flow though the bacterial energy channel [5]. It is assumed that the fungal energy channel, which consists of slow growing organisms with weak interactions, is more stable and functional than the bacterial energy channel under various disturbances.
4. Nematodes
Nematodes, roundworms or eelworms belong to the phylum Nematoda, superphylum Ecdysozoa and kingdom Animalia. Numerous recent papers based on molecular data have focused on specific taxonomic groups that may contribute to the further understanding of nematodes and their classification [28]. Nematodes are the most important and most abundant soil invertebrates, occupying a key position at all trophic levels of the soil food web [29]. Probably four out of five organisms on earth are nematodes, and due to their ubiquity in the soil, they have become a hot topic in applied ecology. It has been discovered that some species are ideal for selection experiments in the laboratory such as Caenorhabditis elegans Maupas, 1900 [30] for genetic research, so that the number of described species has risen from 15,000 [31] to over 25,000 [32] within twenty years. This makes nematodes one of the best-known taxonomic groups in soil. The life cycle of nematodes comprises six stages: (a) the egg, (b) the first juvenile stage (J1), (c) the second juvenile stage (J2), (d) the third juvenile stage (J3), (e) the fourth juvenile stage (J4) and (f) the adult. The hatched juvenile looks like an adult, but it is smaller in body size and lacks the gonads that develop during the last molt [33]. The structure of the nematode community provides an effective tool for the biological assessment of soil quality and function because of the following: (a) nematodes are found wherever organic matter is decomposed, (b) their morphology is closely related to their diet, (c) their interactions with other organisms in the soil are numerous, (d) they respond rapidly to changes in the environment, (e) they can be easily isolated from the substrate and (f) genus identification is relatively simple [34]. Each soil sample contains an abundance and diversity of nematodes and therefore has a high information value [7,34,35,36]. Furthermore, as the functional groups of soil nematodes and their feeding habits are clearly related to the oral structure, their trophic affiliation can be easily determined. The authors Yeates et al. (1993) [36] classify soil nematodes according to their feeding habits into (1) plant-parasitic nematodes, (2) fungivores, (3) bacterivores, (4) omnivores, (5) predators, (6) nematodes that feed on unicellular eukaryotes, (7) nematodes that feed on various infective stages of parasites and (8) nematodes that feed on the substrate. In addition to feeding on the roots of higher plants, nematodes also feed on bacteria and fungi or are predators. Changes in the abundance of bacterial or fungal feeders reflect a change in the decomposition process and the breadth of energy channels.
Several life strategies have been developed in nematodes [7]. Colonizers (r-strategists) produce many small eggs and quickly exploit the nutrient-rich habitat. In contrast, persisters (K-strategists) hardly react to temporary conditions with high food availability. Nematodes are classified in groups from 1 to 5 on the scale colonizer (c)-persister (p), and the characteristics of each group are explained by the type of diet, longer or shorter life cycle, size of gonads, number and size of eggs, mobility, sensitivity to various disturbances, sensitivity to various contaminants, etc.
Classification by feeding type (trophic group) allows ecologists to better understand the role of nematodes in the soil food web [37]. Nematodes are a potential tool for assessing soil quality and developing biomonitoring systems. Developed nematological indices make it possible to compare the structure of the nematode community as a bioindicator with the conditions under which nematodes live in the soil ecosystem. The relationships within the nematode community provide valuable information on the ecological status and health of the soil based on soil responses and conditions.
The Development of the Maturity Index (MI) [7], its modification [38] and the further development of nematode-based indices [4,39] represent an important contribution to the study of soil ecosystems.
The Maturity Index (MI) was developed as a measure of environmental disturbance and represents the state of the ecosystem based on the composition of the nematode community. It is calculated as the weighted average value of the c-p scale of the individual nematodes in the community. Another important index, The Plant Parasite Index (PPI), considers only plant-parasitic nematodes, as these nematodes react differently to disturbances [7,31]. The first modification of The Maturity Index (MI) proposed by Yeates (1994) [38] was based on the combination of free-living and plant-parasitic nematodes in order to obtain comprehensive information on environmental conditions and disturbances. By combining The Maturity Index (MI) and The Plant Parasite Index (PPI), a Summarized Maturity Index (Σ MI) was obtained. A further modification to The Maturity Index (MI) proposed by Korthals et al. (1995) [40] and Bongers et al. (1997) [31] is that free-living c-p 1 nematodes (e.g., Rhabditidae) were omitted due to their lack of tolerance to stress from pollution such as heavy metals and other chemicals. With this change, another useful tool is developed, The Maturity Index 2–5 (MI 2–5).
The function of the soil food web in terms of the presence and number of organisms influences the way the soil is managed. The structure of the food web based on analyses of the nematode fauna allows a diagnostic interpretation of the state of the food web [4]. Diagnostic indices of soil food webs, the Basal Index (BI), the Enrichment Index (EI), the Structure Index (SI) and The Channel Index (CI) provide information on the structure of the nematode community under stressed, enriched, stably structured and decomposed conditions and thus provide important information on the dynamics of soil food webs. The Basal Index (BI) in an indicator of disturbance, The Enrichment Index (EI) is an indicator of the conditions created by fast-growing bacterivores, The Channel Index (CI) is an indicator of the dominance of organic matter decomposition by fungi or bacteria and The Structure Index (SI) is an indicator of more structured soil food webs and improved ecological functions due to higher trophic linkages [39].
Ecological Functions of Nematodes
Nematodes have various ecological functions that play an important role in ecosystems. Some examples of the ecological functions of nematodes are the following:
- The decomposition of organic matter; nematodes are important in the decomposition of organic matter in the soil as they feed on dead plant material and animal remains and help to break down these materials into smaller particles, facilitating further decomposition and the recycling of nutrients [4].
- Predation and pest control; nematodes are predators of other organisms, including bacteria, fungi, insects and plant-parasitic nematodes. The best-known predatory nematodes belong to the orders Mononchida, Dorylaimida, Diplogasterida and Aphelenchida, which play an important role in regulating populations of soil pests with their different mouth structure, feeding mechanism and food preferences [41].
- The bioturbation of the soil; by moving through the soil, nematodes contribute to loosening and mixing the soil. This activity helps to improve soil structure, gas exchange and water circulation, facilitates plant root access to nutrients and physically alters the soil through bioturbation [42].
- Biogeochemical cycles (e.g., nitrogen cycle, carbon cycle); nematodes can influence the biogeochemical cycles of nutrients, such as the nitrogen, phosphorus and carbon cycles, through their interactions with microorganisms and plants [43]. Their food, secretions and activities can influence the availability and circulation of nutrients in ecosystems [4].
- Interactions with plants and other organisms (bacteria, fungi, earthworms, etc.); nematodes can form mutualistic [44] or parasitic interactions with plants [45]. Some species of nematodes participate in nitrogen fixation processes in the soil in co-operation with certain plants, while other species can cause disease or damage to plant roots [44,45].
5. Interaction of Nematodes and Fungi
The interaction of nematodes with fungi is of particular interest. Initial interest in the study of interactions between fungi and their nematode hosts centered on their potential for the biological control of plant-parasitic nematodes [8]. Nematophagous fungi have an antagonistic effect on nematodes, as shown in Figure 1, while entomopathogenic fungi together with nematodes form a mutualistic interaction in soil (Figure 1).
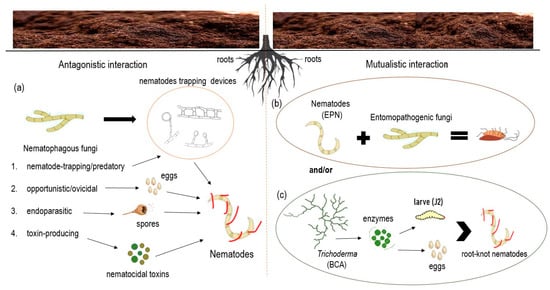
Figure 1.
Interactions between fungi and nematodes. Antagonistic interaction between fungi and nematodes; (a) in antagonistic interaction, nematophagous fungi can be categorized into four main classes according to their infection strategies. Nematophagous fungi use different devices to trap nematodes, e.g., non-constricting rings, adhesive knobs, two-dimensional adhesive net, etc., use spores to colonize (eggs and cysts) and produce nematocidal toxins. Mutualistic interaction between; (b) entomopathogenic fungi and entomophatogenic nematodes (EPN) where nematodes serve as natural parasites of insects, and entomopathogenic fungi fulfil the function of killing and decomposing insects, and/or (c) mutual interaction between the fungus Trichoderma sp. which has potential as a biological control agent (BCA) against plant feeders such as root-knot nematodes because they produce extracellular enzymes such as hitinase and protease that allow direct parasitization of eggs and larvae.
Nematophagous fungi are cosmopolitan microorganisms that can transform their saprophytic behavior into carnivorous behavior, allowing them to feed on nematodes under unfavorable nutritional conditions [9]. There are more than 700 species of nematophagous fungi species from various subdivisions such as Ascomycota, Basidiomycota, Chytridiomycota and Zygomycota. In addition, there are several species from the subdivision Oomycota for which nematophagous activity has been described [46].
Most of the known nematophagous fungi can be categorized into four main classes according to their infection strategies: nematode-trapping/predatory, opportunistic or ovicidal, endoparasitic and toxin-producing fungi.
Predatory nematode-trapping fungi suppress different types of nematodes (free-living, predatory, omnivorous and plant-feeders) and often have an increased predatory ability in nutrient-poor environments [47]. The predatory behavior is due to the need for nitrogen, which is necessary for fungal growth and is not freely available in soils where carbon is abundant [48]. Nematophagous fungi use hyphae to form different devices to trap the nematodes: three-dimensional adhesive networks, two-dimensional adhesive networks, adhesive hyphae, adhesive knobs, adhesive branches, non-differentiated adhesive hyphae, and constricting and non-constricting rings [8,9]. During the infection process, the fungus penetrates the cuticle of the prey, the nematodes are immobilized and the prey is finally attacked and digested by the fungus [49]. Luo et al. (2006) [50] found in their studies that two species belonging to the basidiospores, Stropharia rugosoannulata and Coprinus comatus, produce a special device to attack the nematodes: Acanthocytes, which resemble a sharp sword that can damage the cuticle of the nematode, can cause the leakage of the nematode’s internal material. This result suggests that a mechanical force could be a very important factor in the virulence of these fungi.
Endoparasitic fungi do not use hyphae for predation. These fungi are obligate parasites of nematodes and use spores (conidia, zoospores) for infection, which can adhere to the cuticle of nematodes or be ingested [51].
A group of fungi that normally live as saprophytes but can utilize nematodes as one of their food sources are often referred to as opportunistic nematophagous fungi [52]. These fungi can colonize the reproductive structures of nematodes (eggs, cysts) and female nematodes and penetrate the cuticular barrier to infect and kill the host [8]. There is also a group of fungi that can produce nematocidal toxins to attack nematodes.
Species of Aphelenchoids sp. form a mutualistic relationship with fungi of the genera Glomus and Gigaspora, which form an arbuscular mycorrhiza with the roots of plants. In this interaction, the fungi help to absorb water and nutrients from the soil, while the nematodes receive nutrients from the fungal mycelium. This mutualistic connection increases the plants’ ability to absorb water and nutrients and improves plant growth and stress resistance [10].
The nematodes Steinernema sp. and Heterorhabditis sp. entomopathogenic nematodes (EPN) enter into a mutualistic relationship with entomopathogenic fungi such as Metarhizium sp. These nematodes serve as natural parasites of insects, while the entomopathogenic fungi fulfil the function of killing and decomposing insects (Figure 1). The nematodes introduce the fungi into the body of the insects, where the fungi multiply and kill the host, which in turn provides resources for the growth and reproduction of the nematodes. Ansari et al. (2008) [11] reported the use of the enthomopathogenic nematodes Steinernema feltiae (Filipjev, 1934), Steinernema kraussei (Steiner, 1923) and Heterorhabditis bacteriophora Poinar, 1976 (EPN) with the entomopathogenic fungus Metarhizium anisopliae under laboratory and greenhouse conditions and proved that the combination of the first by the addition of entomopathogenic fungi and after one or two weeks by the addition of entomopathogenic nematodes caused the 100% mortality of the black vine weevil (Figure 1), Otiorhynchus sulcatus (Fabricius, 1775) (Coleoptera: Curculionidae). The interactions that take place in the soil between the nematodes and the fungi, whether negative or positive, offer the potential to find positive solutions for agricultural production which would ultimately reduce the negative impact of agricultural production on ecosystems. One of the positive impacts on agricultural production has an interaction between the genus Trichoderma sp. and nematodes.
Trichoderma sp. and Nematodes
The genus Trichoderma is a genus of fungi that reproduce asexually by spores and can be found in soils in all climatic zones. The genus Trichoderma is a secondary opportunist, a fast-growing fungus, a major producer of spores, a source of enzymes that degrade cell walls and an important producer of antibiotics [53]. According to Herman et al. (2004) [54] the use of Trichoderma sp. in agriculture has numerous advantages: the rapid colonization of the rhizosphere (rhizosphere competence) through the establishment of stable microbial communities in the rhizosphere, biocontrol of pathogens and competent/harmful microflora through various mechanisms, improvement of plant health and stimulation of root growth. Trichoderma strains can be recognized by common morphological characteristics, form a light green conidial pigment on artificial substrates and grow rapidly, and the conidiophores branch multiple times [55]. Molecular methods are now being used to identify these species.
The genus Trichoderma has considerable potential as a biological control agent (BCA) against plant-feeders such as root-knot nematodes (Meloidogyne javanica Treub, 1885 and Meloidogyne incognita Kofoid and White, 1919) and against the cyst nematodes by the direct parasitisation of the eggs and larvae (Heterodera avenae Wollenweber, 1924 and Globodera pallida Stone, 1973). Trichoderma sp. increases the content of extracellular enzymes (Figure 1) such as hitinase and protease, which allow the fungus to penetrate the eggs by acting directly on the eggshell (softening the eggshell), thus reducing the number of eggs that can be hatched and thus the number of infective larvae in stage 2 (J2) [12]. Sahebani and Hadavi (2008) [56] reported in their work that the inoculation of tomato seeds with Trichoderma harzianum under greenhouse conditions significantly reduced the extent of disease caused by M. javanica (Treub, 1885) nematodes by reducing development and multiplication (reduced number of galls per plant, reduced number of eggs per plant) and the number of hatching eggs. Contina et al. (2017) [57] used T. harzianum to study the interaction between the fungus and nematodes of the species G. pallida (Stone, 1973) and confirmed the reduced infection and reproduction of cyst nematodes, with the fungus being able to affect both the cysts and the infective second stage of juveniles (J2). However, no effect of Trichoderma harzianum on G. pallida in the egg stage was observed. The spores of the fungus T. longibrachiatum caused the physiological changes in the cysts of H. avenae Wollenweber, 1924, by producing enzymes, most likely chitinase, and had a strong inhibitory effect on the production of cysts by completely covering their surface and causing their decomposition [58]. The fungus T. longibrachiatum also showed an effect on the females and on the development of eggs and second stage larvae (J2) of H. avenae on wheat [59]. According to the same authors, T. longibrachiatum can be used as a biological control agent for H. avenae in selected crops.
A large number of fungivorous nematode species have been found in various soils. In agricultural soils, the most common fungivorous genera are Aphelenchoides, Aphelenchus, Tylenchus and Ditylenchus, but some species within these genera are plant-parasitic nematodes [60]. Frugivorous have a stylet and feed on the micelles of many different types of fungi in the soil, including both beneficial fungi and plant pathogens. They are normally present in the soil in lower numbers than bacterivorous nematodes, but if there are enough fungi in the soil to feed the fungivore population, their numbers can increase greatly [60]. For example, the potato rot nematode, Ditylenchus destructor Thorne, 1945, fed and reproduced on 64 of the 115 soil-dwelling fungal species represented in 40 genera and 8 orders [61]. Frugivorous nematodes can reduce the growth and efficiency of T. harzianum in the soil. Bae and Knudsen (2000) [13] found in a laboratory study that the presence of Aphelenchoides sp. significantly reduced the radial growth of the hyphae of T. harzianum, and hypothesized that fungivore populations may increase rapidly following the addition of biocontrol fungi in the field, which consequently reduces the growth and reproduction of the added fungi.
6. The Influence of the Genus Trichoderma on Sustainable Agricultural Production
In addition to the biological control of plant-feeding nematodes, the use of Trichoderma sp. in agriculture offers numerous advantages due to its defense mechanisms, beneficial and harmful interactions with hosts, production and excretion of enzymes, good response to environmental conditions and reduction in the biotic and abiotic stress on the plant, which has a positive impact on the quality and yield of agricultural crops.
One way to improve plant production and increase plant resistance to biotic and abiotic stress is to use a beneficial soil-borne microbe as a soil inoculant. Species of the genus Trichoderma play an important role as soil inoculants due to their distribution in all soils and their positive effects on agricultural crops. The effects of the inoculation of plant roots with Trichoderma sp. are not limited to the site of colonization, but affect the entire plant system [62]. The interaction between the plant and Trichoderma sp. leads to improved root growth and higher crop yields due to the cumulative effects that the fungus causes by improving the uptake and transport of nutrients in the plant [63]. These effects are reflected in the ability of the fungus to rapidly colonize and modify the root system of plants by exhibiting high resistance to the phenolic compounds produced by the plants at the time of infection with microorganisms [55,64]. On the other hand, according to Lόpez-Bucio et al. (2015) [65], in the interaction between Trichoderma sp. and the plant root, a phytohormone auxin is responsible for the alteration of the root system, which at low concentrations stimulates the primary growth of the root, while at high concentrations promotes the expansion of the main root and the formation of lateral roots. When investigating the influence of Trichoderma sp. on the yield and quality of tomatoes, Molla et al. (2012) [66] used enriched kitchen compost with T. harzianum (biofertilizer) and demonstrated the increased vegetative capacity of tomatoes, which was reflected in the height of the plant, the number of leaves and branches per plant. Also, when combining enriched kitchen compost with T. harzianum and mineral fertilizer (N: P: K), they demonstrated a better reproductive growth, higher yield and better nutritional quality of tomatoes due to a slower and more uniform release of the nutrients in the plants, ultimately leading to less use of the mineral fertilizer. In addition, the influence of T. harzianum on the germination of the seeds and shoots of tomato seedlings was investigated under laboratory and greenhouse conditions using two inoculation methods: (a) by coating tomato seeds with a suspension of T. harzianum spores and (b) by inoculating T. harzianum on sterilized wheat, which was added to the soil for the seedlings at a ratio of 1:5 after incubation. Significant differences (p < 0.05) were found in the germination of the tomato seeds and the emergence of the seedlings compared to the control. The shoot height, shoot diameter, fresh and dry weight of the shoots, fresh and dry weight of the roots, chlorophyll content and mineral content in the roots also increased compared to the control [67].
The important role of Trichoderma sp. in increasing the yield and resistance of the plant to pathogens (induction) is the production of secondary metabolites. Pascale et al. (2017) [68] observed in the field and greenhouse experiments on grapevines that T. harzianum and T. atroviride with their main secondary metabolites, harzianic acid (HA) and 6-pentyl-α-pyrone (6PP) increased the yield and quality of grapefruit compared to the control and also reduced the pressure of powdery mildew (Uncinula necator) on the leaves of the vine.
In addition to the ability of Trichoderma sp. to colonize and modify the root system, which promotes plant growth, it also plays an important role in inducing the resistance (induced resistance) of the plant to pathogens. This role is considered one of the most important mechanisms by which the fungus protects the plant from biotic stress [68]. The induced resistance of a plant to biotic stress is a complex process based on the interconnected chemical reactions between the fungus and the plant which is characterized by an increase in the number of active enzymes (e.g., xylanase, chitinase, peroxidase, etc.), defensive metabolites [69,70], proteins, phytoalexins and antioxidants [71] produced by fungi of the genus Trichoderma.
Plant resistance to abiotic stress is defined as the ability of the plant to maintain crop yields at an adequate level despite extreme environmental conditions [72]. According to Lόpez-Bucio et al. (2015) [65], the quantity and quality of crop yields are most strongly influenced by temperature, salinity, drought and a lack of precipitation, as well as the presence of heavy metals. These environmental conditions reduce the efficiency of photosynthesis, hinder the transport of nutrients in the plant and inhibit the synthesis of chlorophyll [73]. These changes lead to damage to some cells and tissues, the inhibition of root and shoot growth, chlorosis and the necrosis of plant organs [74]. In addition, abiotic stress impairs the uptake and accumulation of carbon, which is necessary for the synthesis of the cell structures of plant organs [74]. The positive effect of Trichoderma sp. has been shown to reduce abiotic stress for the plant. It regulates the activity of enzymatic and non-enzymatic antioxidants in the plant as well as the production and regulation of phytohormones and osmolytes [75]. The above-aforementioned influences show the enormous importance of Trichoderma sp. for the health status of the plant and thus the improvement of the sustainability of agricultural production and the preservation of the ecosystem.
7. Conclusions
In the investigated interactions between nematodes and fungi of the genus Trichoderma sp., we can conclude that these interactions are extremely complex. Also, we can conclude that they can have a significant impact on the maintenance of soil health, plant health and a generally positive impact on ecosystems.
Positive, mutualistic interactions were observed between fungi of the genus Trichoderma sp., a fungus providing biological control, and nematodes in protecting plants from plant-parasitic nematodes. Also, the interaction between entomopathogenic fungi and entomopathogenic nematodes has been proven in the control and protection from harmful insects, especially their overwintering stages in the soil. This interaction may have a significant potential in sustainable pest management.
On the other hand, the negative, antagonistic interaction between nematophagous fungi and nematodes is reflected in the fungi’s ability to reduce the number of nematodes in soil, especially under unfavorable grazing conditions. Does the interaction between nematodes and fungi Trichoderma sp., which manifests itself in the reduction in the radial growth of hyphae due to grazing by fungivores, belong to a negative or positive interaction?
The interaction between fungi of the genus Trichoderma sp. and plants results in improved root growth and a higher yield and crop quality. This interaction is based on the ability of Trichoderma sp. to colonize plant roots and improve the intake and transport of nutrients in plants. In addition, its role is visible in the reduction in biotic and abiotic stress on plants.
The integration of fungi of the genus Trichoderma sp. into agricultural systems not only enables the effective protection of the plant from pests, but also the improvement of the overall productivity and sustainability of agricultural production. The further research and application of this beneficial fungus can improve agricultural practices and contribute to global efforts to preserve soil health and the environment and ensure a safe and sustainable food supply.
Author Contributions
Conceptualization, A.G.P., T.K. and M.B.; methodology, A.G.P. and M.B.; validation, A.G.P., T.K., J.P., K.V., T.B.-L. and M.B.; resources, T.K. and M.B.; writing—original draft preparation, A.G.P. and J.P.; writing—review and editing A.G.P., T.K., J.P., K.V., T.B.-L. and M.B.; visualization, A.G.P., T.K., J.P. and M.B.; supervision, T.K. and M.B.; project administration, M.B.; funding acquisition, T.K. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by funds of the European Union 90% and Croatia 10%, Submeasure 16.1 “Support for the establishment and work of the operational group of the European Innovation Partnership (EPI) for agricultural productivity and sustainability”—implementation of operation type 16.1.2 “Operational groups” from the Program of Rural development of the Republic of Croatia for the period 2014–2020. The name of the project is “Development of innovative methods for increasing olive yields and olive oil classification”. Funding number: 2014HR06RDNP001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors wish to express gratitude to Tomislav Filipović, owner of the company NIR analysis d.o.o. He is the originator of the research that will provide a PhD thesis. For English proofreading, Stjepan Dokuzović, a native English Canadian speaker read this review. Thanks to them for supporting this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lewandowski, I.; Hardtlein, M.; Kaltschmitt, M. Sustainable Crop Production: Definition and Methodological Approach for Assessing and Implementing Sustainability. Crop Sci. 1999, 39, 184–193. [Google Scholar] [CrossRef]
- Liao, Y.; Min, X.; Yang, Z.; Chai, L.; Zhang, S.; Wang, Y. Physicochemical and biological quality of soil in hexavalent chromium-contaminated soils as affected by chemical and microbial remediation. Environ. Sci. Pollut. Res. 2014, 21, 379–388. [Google Scholar] [CrossRef]
- Gregory, P.J. Roots, rhizosphere and soil: The route to a better understanding of soil science? Eur. J. Soil Sci. 2006, 57, 2–12. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- de Vries, F.T.; Wallenstein, M.D. Below-ground connections underlying above-ground food production: A framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Römbke, J.; Schmelz, R.M.; Scheffczyk, A.; Faber, J.H.; Bloem, J.; Pérès, G.; Cluzeau, D.; Chabbi, A.; Suhadolc, M.; et al. Selecting cost effective and policy-relevant biological indicators for European monitoring of soil biodiversity and ecosystem function. Ecol. Indic. 2016, 69, 213–223. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Niu, X.M.; Zhang, K.Q. Arthrobotrys oligospora: A model organism for understanding the interaction between fungi and nematodes. Mycology 2011, 2, 59–78. [Google Scholar] [CrossRef]
- de Freitas Soares, F.E.; Sufiate, B.L.; de Queiroz, J.H. Nematophagous fungi: Far beyond the endoparasite, predator and ovicidal groups. Agric. Nat. Resour. 2018, 52, 1–8. [Google Scholar] [CrossRef]
- Hua, J.; Jiang, Q.; Bai, J.; Ding, F.; Lin, X.; Yin, Y. Interactions between arbuscular mycorrhizal fungi and fungivorous nematodes on the growth and arsenic uptake of tobacco in arsenic-contaminated soils. Appl. Soil Ecol. 2014, 84, 176–184. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shah, F.A.; Butt, T.M. Combined use of entomopathogenic nematodes and Metarhizium anisopliae as a new approach for black vine weevil, Otiorhynchus sulcatus, control. Entomol. Exp. Appl. 2008, 129, 340–347. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Knudsen, G.R. Influence of a fungus-feeding nematode on growth and biocontrol efficacy of Trichoderma harzianum. Phytopathology 2001, 91, 301–306. [Google Scholar] [CrossRef]
- Scheu, S.; Falca, M. The soil food web of two beech forests (Fagus sylvatica) of contrasting humus type: Stable isotope analysis of a macro-and a mesofauna-dominated community. Oecologia 2000, 123, 285–296. [Google Scholar] [CrossRef]
- Adl, M.S.; Gupta, V.S. Protists in soil ecology and forest nutrient cycling. Can. J. For. Res. 2006, 36, 1805–1817. [Google Scholar] [CrossRef]
- Tiedje, J.M. Approaches to the Comprehensive Evaluation of Prokaryote Diversity of a Habitat. Microbial Diversity and Ecosystem Function; CAB International: Oxon, UK, 1995; pp. 73–87. [Google Scholar]
- Magdoff, F.; van Es, H. Chapter 2.—Organic matter: What it is and why it’s so important. In Building Soils for Better Crops Ecological Management for Healthy Soils; Barba, L., Ed.; National Institute of Food and Agriculture (NIFA): Washington, DC, USA; University of Maryland: College Park, MD, USA, 2021; pp. 13–31. ISBN 9781888626193. [Google Scholar]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef]
- Joimel, S.; Schwartz, C.; Hedde, M.; Kiyota, S.; Krogh, P.H.; Nahmani, J.; Peres, G.; Vergnes, A.; Cortet, J. Urban and industrial land uses have a higher soil biological quality than expected from physicochemical quality. Sci. Total Environ. 2017, 584–585, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, V.E.; Arbeli, Z.; Terán, W.; Lorenz, N.; Dick, R.P.; Roldan, F. Effect of land management and Prosopis juliflora (Sw.) DC trees on soil microbial community and enzymatic activities in intensive silvopastoral systems of Colombia. Agric. Ecosyst. Environ. 2012, 150, 139–148. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Görres, J.H.; Amador, J.A. Book chapter 8—The soil fauna. In Principles and Applications of Soil Microbiology, 3rd ed.; Gentry, T.J., Fuhrmann, J.J., Zuberer, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–212. [Google Scholar] [CrossRef]
- Lynch, J.M.; Brimecombe, M.J.; De Leij, F.A. Rhizosphere. eLS 2001. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M.M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Gongalsky, K.B.; Klarner, B.; Korobushkin, D.I.; et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef]
- van Megen, H.; van den Elsen, S.; Holterman, M.; Karssen, G.; Mooyman, P.; Bongers, T.; Bakker, J.; Holovachov, O.; Helder, J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 2009, 11, 927–950. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.; Schouten, A.J.; Hund-Rinke, K.; Breure, A.M. The use of nematodes in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 278–289. [Google Scholar] [CrossRef]
- Bongers, T.; van der Meulen, H.; Korthals, G. Inverse relationship between the nematode maturity index and plant parasite index under enriched nutrient conditions. Appl. Soil Ecol. 1997, 6, 195–199. [Google Scholar] [CrossRef]
- Geisen, S.; Briones, M.J.I.; Gan, H.; Behan-Pelletier, V.M.; Friman, V.-P.; Groot, G.A.; Hannula, S.E.; Lindo, Z.; Philippot, L.; Tiunov, A.V.; et al. A methodological framework to embrace soil biodiversity. Soil Biol. Biochem. 2019, 136, 107536. [Google Scholar] [CrossRef]
- Jonathan, E.I. Nematology Fundamentals & Applications (2nd Revised & Enlarged Edition); New India Publishing Agency: New Delhi, India, 2022; pp. 41–53. ISBN 9391383378. [Google Scholar]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar] [PubMed]
- Brmež, M. Nematode Communities as Bioindicators of Changes in the Agroecosystem. Ph.D. Dissertation, Faculty of Agriculture in Osijek, Osijek, Croatia, 2004; p. 100. (In Croatia). [Google Scholar]
- Yeates, G.W. Modification and qualification of the nematode maturity index. Pedobiologia (Jena) 1994, 38, 97–101. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Korthals, G.W.; De Goede, R.G.M.; Kammenga, J.E.; Bongers, T. The maturity index as an instrument for risk assessment of soil pollution. In Proceedings of the NATO Advanced Research Workshop on New Approaches to the Development of Bioindicator Systems for Soil Pollution, Moscow, Russia, 24–28 April 1995; pp. 85–93. [Google Scholar]
- Khan, Z.; Kim, Y.H. A review on the role of predatory soil nematodes in the biological control of plant parasitic nematodes. Appl. Soil Ecol. 2007, 35, 370–379. [Google Scholar] [CrossRef]
- Gabet, E.J.; Reichman, O.J.; Seabloom, E.W. The effects of bioturbation on soil processes and sediment transport. Annu. Rev. Earth Planet Sci. 2003, 31, 249–273. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Costello, E.K.; Nemergut, D.R.; Cleveland, C.C.; Reed, S.C.; Weintraub, M.N.; Meyer, A.F.; Martin, A.M. Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 2007, 88, 1379–1385. [Google Scholar] [CrossRef]
- Wood, C.W.; Pilkington, B.L.; Vaidya, P.; Biel, C.; Stinchcombe, J.R. Genetic conflict with a parasitic nematode disrupts the legume–rhizobia mutualism. Evol. Lett. 2018, 2, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Pulavarty, A.; Egan, A.; Karpinska, A.; Horgan, K.; Kakouli-Duarte, T. Plant parasitic nematodes: A review on their behaviour, host interaction, management approaches and their occurrence in two sites in the Republic of Ireland. Plants 2021, 10, 2352. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.-Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Borges-Walmsley, M.I.; Walmsley, A.R. cAMP signalling in pathogenic fungi: Control of dimorphic switching and pathogenicity. Trends Microbiol. 2000, 8, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Barron, G.L. Predatory fungi, wood decay, and the carbon cycle. Biodiversity 2003, 4, 3–9. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, M.; Che, Y. The living strategy of nematophagous fungi. Mycoscience 2009, 50, 20–25. [Google Scholar] [CrossRef]
- Luo, H.; Li, X.; Li, G.; Pan, Y.; Zhang, K. Acanthocytes of Stropharia rugosoannulata function as a nematode-attacking device. AEM 2006, 72, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; de Araújo, J.V. Nematophagous fungi for biological control of gastrointestinal nematodes in domestic animals. Appl. Microbiol. Biotechnol. 2014, 98, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Jansson, H.B.; Lopez-Uorca, L.V. Biology of nematophagous fungi. In Trichomycetes and Other Fungal Groups; Misra, J.K., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 145–174. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Sahebani, N.; Hadavi, N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 2008, 40, 2016–2020. [Google Scholar] [CrossRef]
- Contina, J.B.; Dandurand, L.M.; Knudsen, G.R. Use of GFP-tagged Trichoderma harzianum as a tool to study the biological control of the potato cyst nematode Globodera pallida. Appl. Soil Ecol. 2017, 115, 31–37. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B.; Xue, Y. The parasitic and lethal effects of Trichoderma longibrachiatum against Heterodera avenae. Biol. Control 2014, 72, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Ji, W.; Xu, B.; Hou, B.; Liu, J. Mechanisms and characterization of Trichoderma longibrachiatum T6 in suppressing nematodes (Heterodera avenae) in Wheat. Front. Plant Sci. 2017, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Qi, Y.C. Abundance and diversity of soil nematodes as influenced by different types of organic manure. Helminthologia 2010, 47, 58–66. [Google Scholar] [CrossRef]
- Faulkner, L.R.; Darling, H.M. Pathological histology, hosts, and culture of the potato rot nematode. Phytopathology 1961, 51, 778–786. [Google Scholar]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Al-Ani, L.K.T. Plant microbiome: Stress response. In Trichoderma: Beneficial Role in Sustainable Agriculture by Plant Disease Management; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; pp. 105–126. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Molla, A.H.; Manjurul Haque, M.; Amdadul Haque, M.; Ilias, G.N.M. Trichoderma-enriched biofertilizer enhances production and nutritional quality of tomato (Lycopersicon esculentum Mill.) and minimizes NPK fertilizer use. Agric. Res. 2012, 1, 265–272. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Wo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.C.; Adusumilli, N. Chapter 7—Trichoderma—Its paramount role in agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–83. ISBN 978-0-12-821007-9. [Google Scholar]
- Singh, R.; Anbazhagan, P.; Viswanath, H.S.; Tomer, A. Soil Biology. In Trichoderma: Agricultural Applications and Beyond; Manoharachary, C., Singh, H.B., Varma, A., Eds.; Springer Nature, AG: Cham, Switzerland, 2020; pp. 127–158. ISBN 978-3-030-54757-8. [Google Scholar]
- Urban, L.; Lauri, F.; Ben Hdech, D.; Aarrouf, J. Prospects for increasing the efficacy of plant resistance inducers stimulating salicylic acid. Agronomy 2022, 12, 3151. [Google Scholar] [CrossRef]
- Japanis, F.G.; Vetaryan, S.; Raja, N.K.K.; Mokhtar, M.A.A.; Fishal, E.M.M. The Impact of Trichoderma spp. on Agriculture and Their Identification. Malays. Appl. Biol. 2022, 51, 1–15. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wolna-Maruwka, A.; Pilarska, A.A.; Niewiadomska, A.; Piotrowska-Cyplik, A. Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Appl. Sci. 2023, 13, 6434. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).