Synthesis of Fe(III)-g-C3N4 and Applications of Synergistic Catalyzed PMS with Mn(VII) for Methylene Blue Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials of Test
2.1.1. Reagents
2.1.2. Instruments and Equipment
2.2. Synthesis of Fe(III)-g-C3N4
2.3. Characterization Methods
2.4. Redox Catalytic Activity and Degradation Experiment of Methylene Blue
2.5. Effect of Different Factors on Catalysis
2.6. Degradation Effection for Other Pollutants
2.6.1. The Degradation of Methyl Orange
2.6.2. The Degradation of Rhodamine B
3. Results
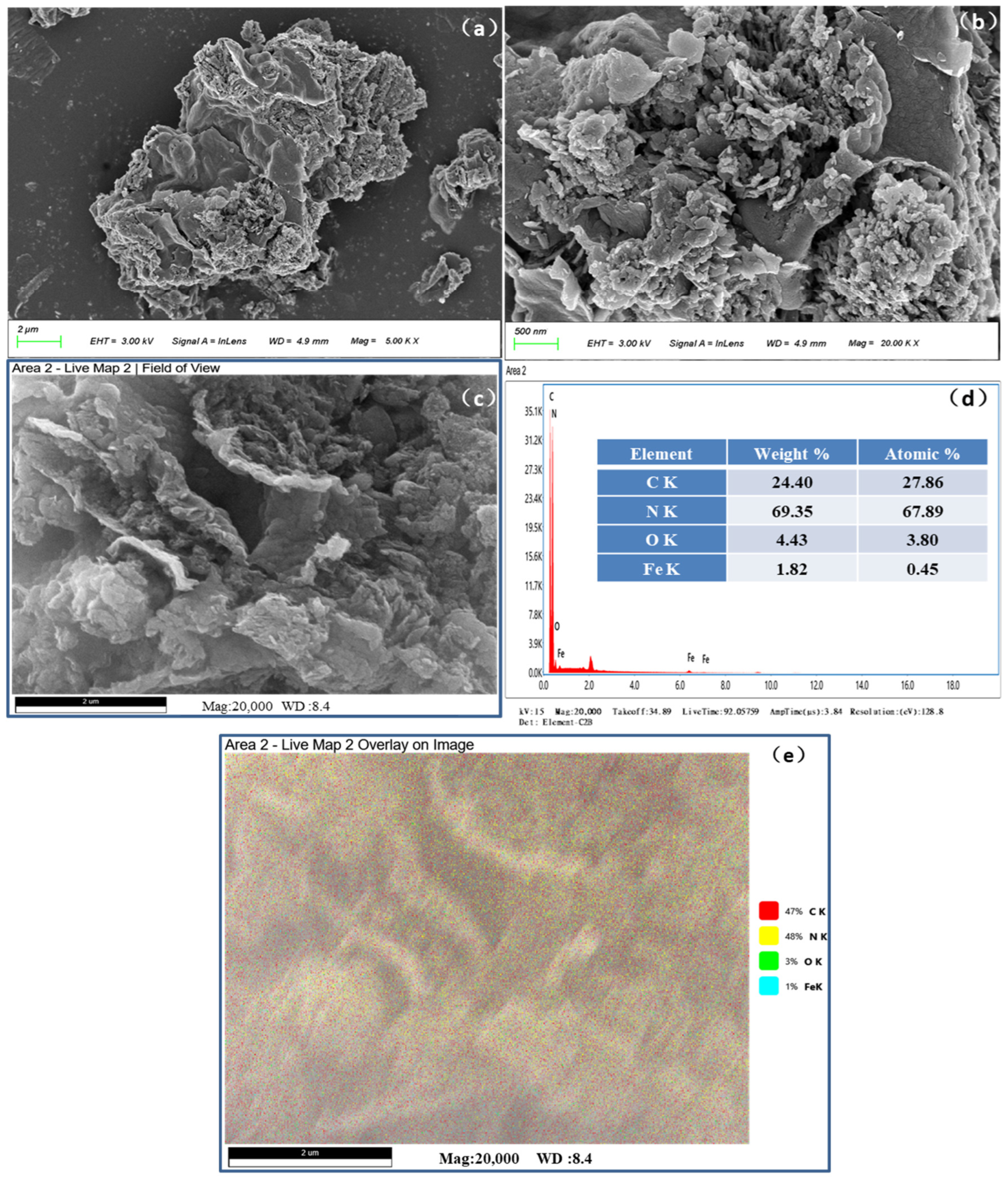
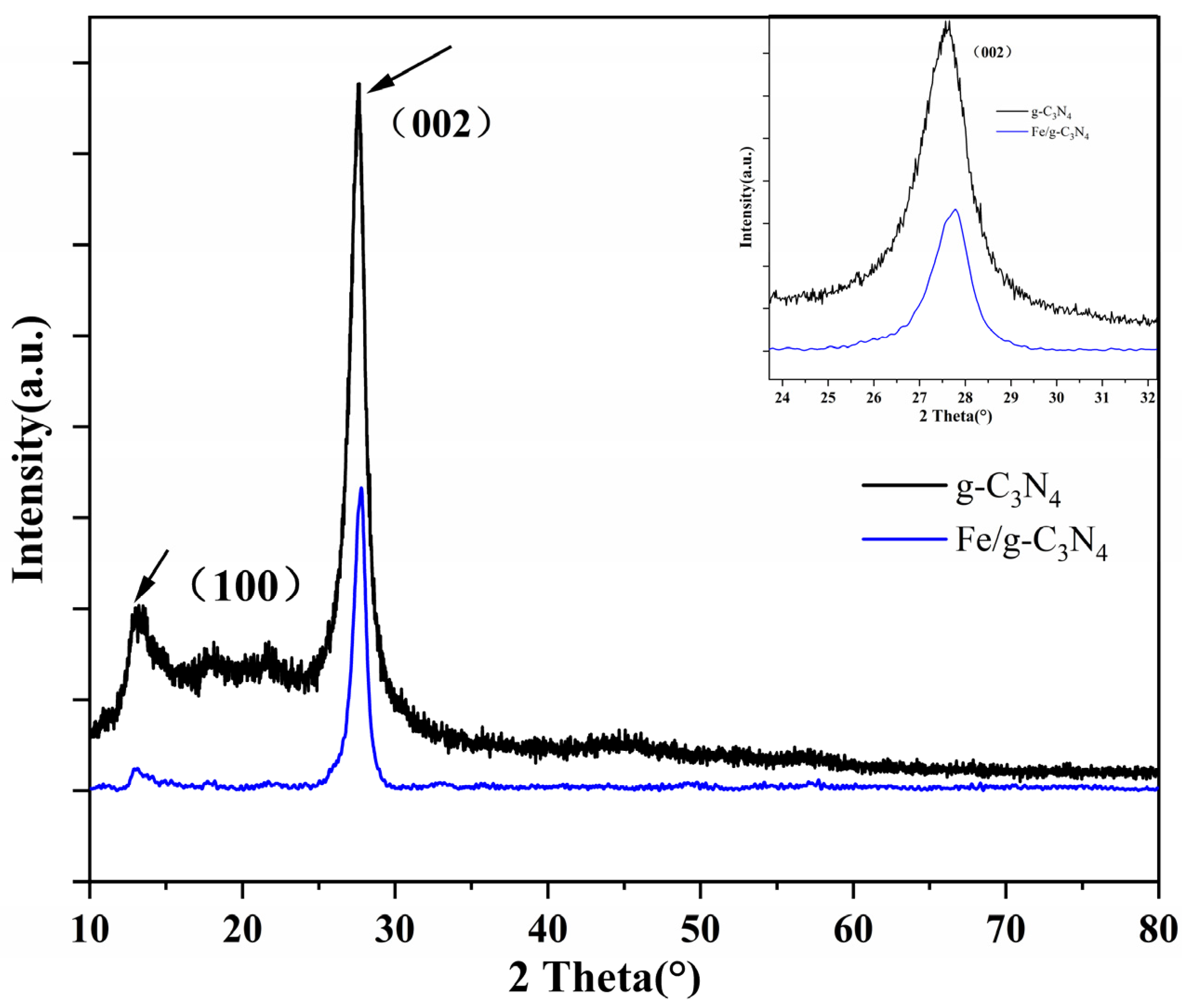
3.1. Characteristics of Structure and Morphology
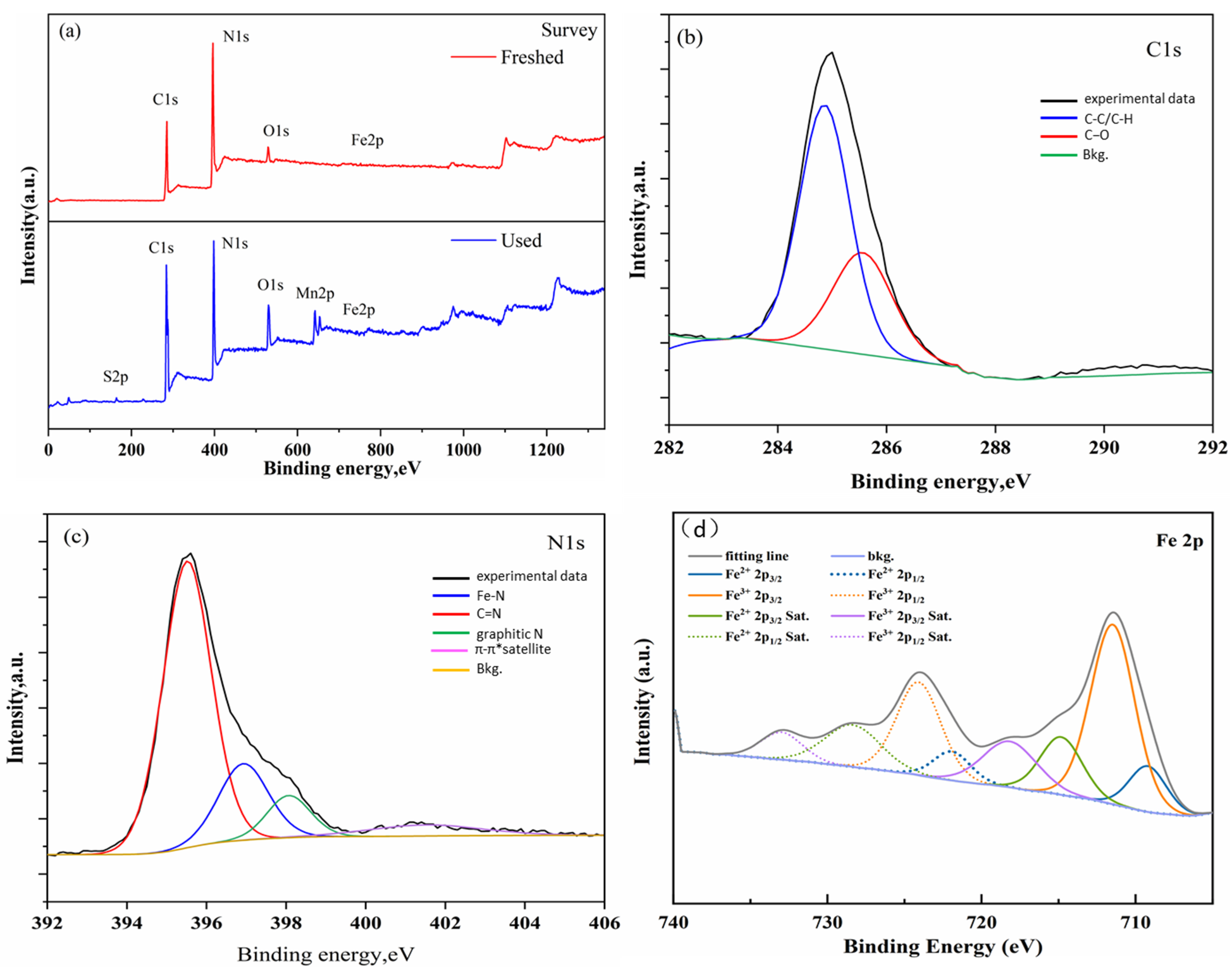
3.2. X-ray Photoelectron Spectroscopy Analysis
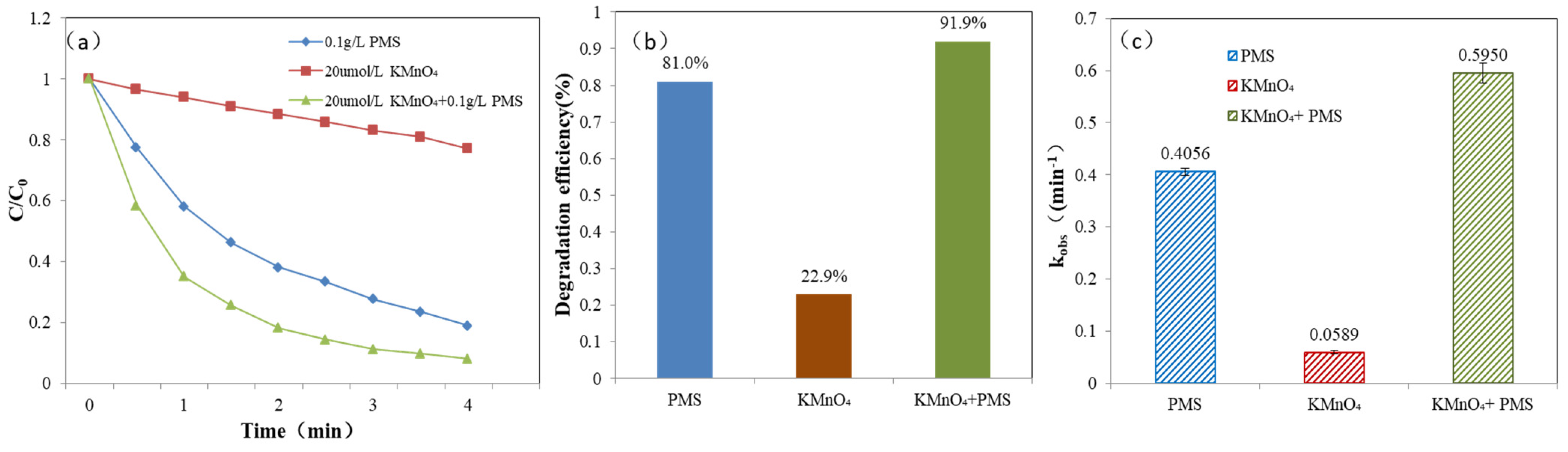
3.3. Redox Catalytic Activity and Degradation Experiment of MB
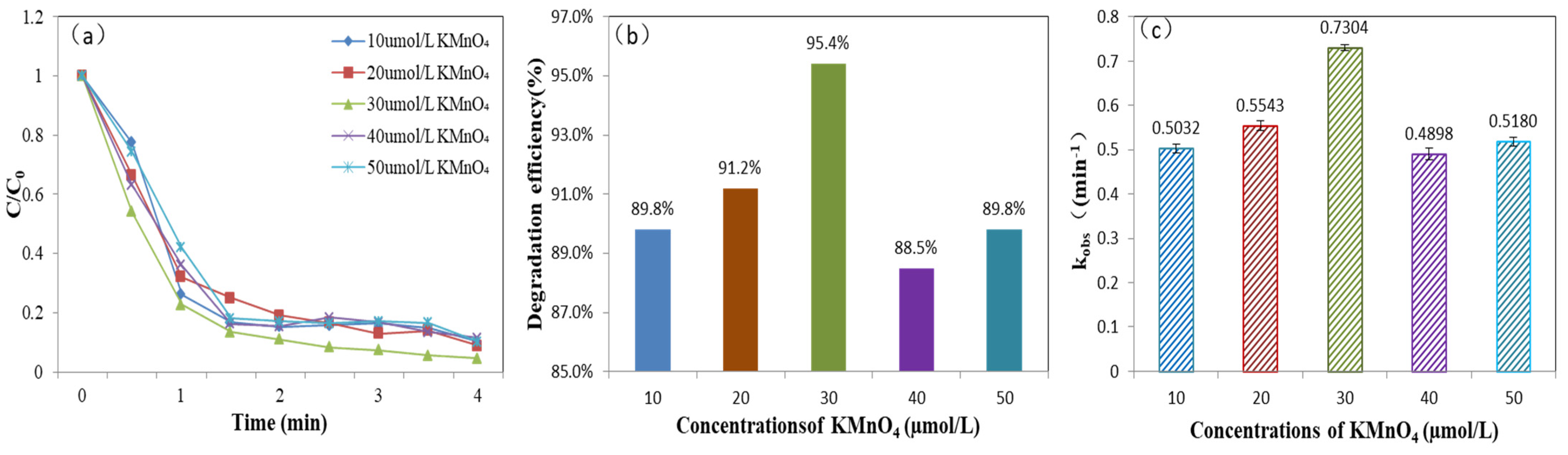
3.4. Degradation of MB by Different Fe(III)/Mn(VII) Ratios
3.5. Effect of Different Factors on Catalysis
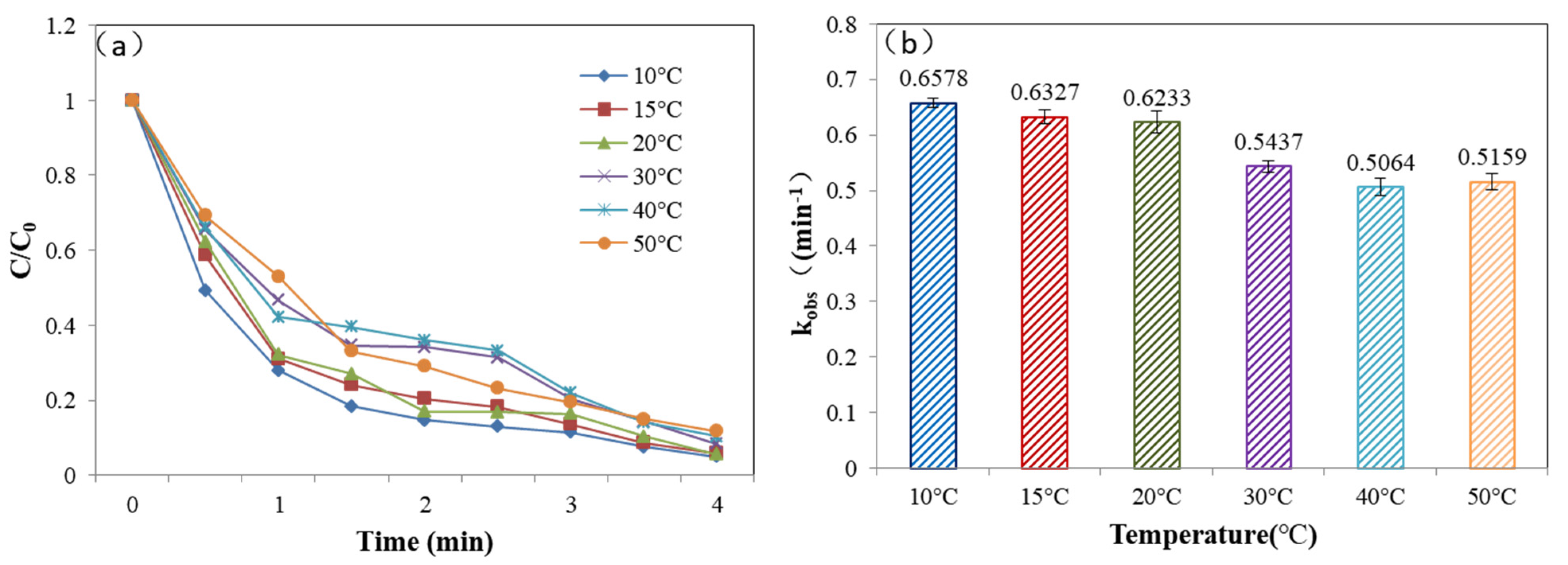
3.5.1. Different Reaction Temperature
3.5.2. pH
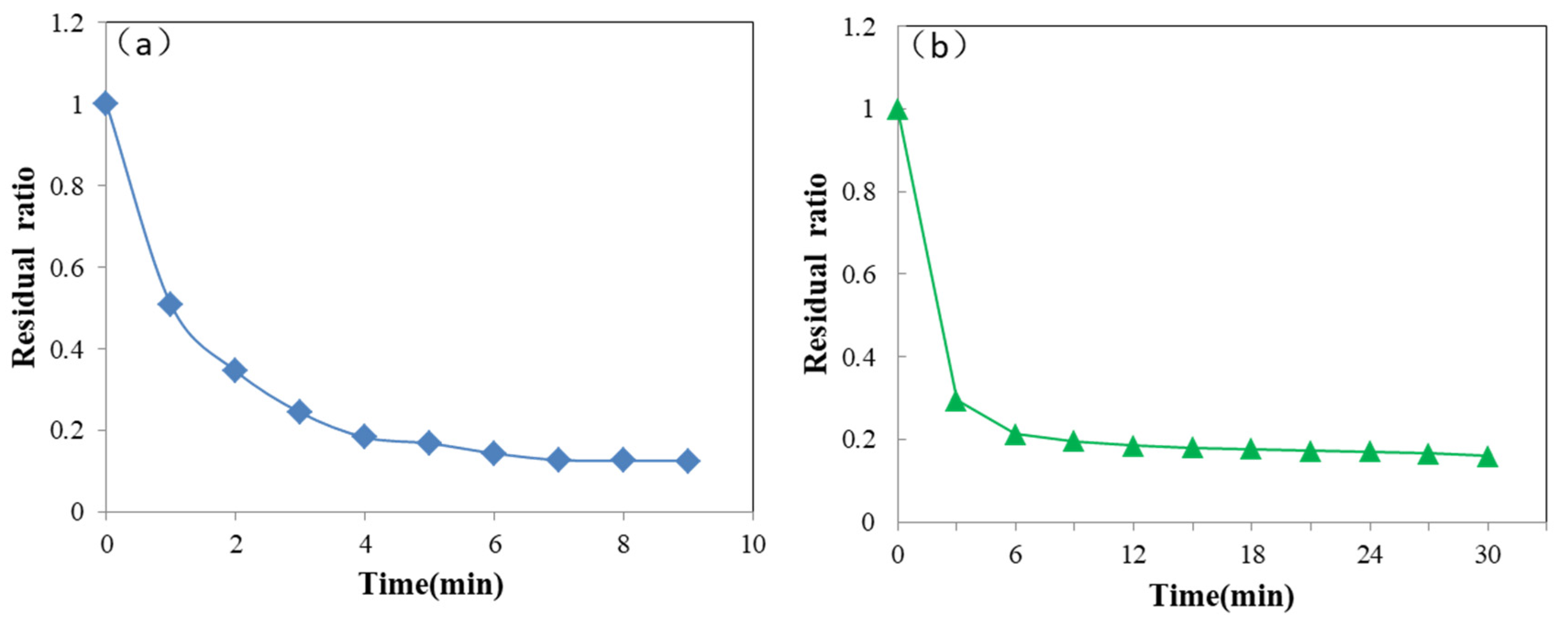
3.6. Degradation Effection for Other Pollutants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Q. Implications of the Thought of Community of Life between Man and Nature for the Innovation of China’s Ecological Governance System. Environ. Eng. 2023, 41, 296. [Google Scholar]
- Wei, Y.; Ye, Z. Theoretical evolution and experience of the Communist Party of China in modernising ecological governance. Seek. Knowl. 2023, 12, 21–24. [Google Scholar]
- Feng, Y.; Liao, C.; Kong, L.; Wu, D.; Liu, Y.; Lee, P.H.; Shih, K. Facile synthesis of highly reactive and stable Fe-doped g-C3N4 composites for peroxymonosulfate activation: A novel nonradical oxidation process. J. Hazard. Mater. 2018, 354, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lv, H.; Ma, L.; Tan, X.; Jin, C.; Wu, H.; Chen, L.; Liu, M.; Wei, H.; Sun, C. Use of catalytic wet air oxidation (CWAO) for pretreatment of high-salinity high-organic wastewater. J. Environ. Sci. 2022, 120, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.M.; Luo, L.T.; Zhang, H.T.; Huang, S.B.; Li, J.; Xiang, Y. Current situation and development trend of harmless treatment technology for dye wastewater infine chemical industry. Sci. Technol. Rev. 2021, 39, 45–54. [Google Scholar]
- Li, Z.; Sun, Y.; Huang, W.; Xue, C.; Zhu, Y.; Wang, Q.; Liu, D. Innovatively employing magnetic CuO nanosheet to activate peroxymonosulfate for the treatment of high-salinity organic wastewater. J. Environ. Sci. 2020, 88, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zeng, H.; Liang, Y.; Cao, Y.; Xiao, Y.; Ma, J. Degradation of bisphenol AF in water by periodate activation with FeS (mackinawite) and the role of sulfur species in the generation of sulfate radicals. Chem. Eng. J. 2021, 407, 126738. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Wu, D.; Zhou, Z.; Deng, Y.; Zhang, T.; Shih, K. Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem. Eng. J. 2015, 280, 514–524. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Pan, B. Fe(III)-Doped g-C3N4 Mediated Activation of Peroxymonosulfate for Selective Degradation of Phenolic Compounds via High valent Iron-oxo Species. Environ. Sci. Technol. 2018, 52, 2197–2205. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, X.; Nie, Y.; Yang, C.; Wang, Y. Enhanced peroxymonosulfate activation for phenol degradation over MnO2 at pH 3.5–9.0 via Cu(II) substitution. J. Hazard. Mater. 2018, 360, 303–310. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, X.; Yang, L.; Chen, Z. Facile synthesis of Fe(III)-doped g-C3N4 and its application in peroxymonosulfate activation for degrading refractory contaminants via nonradical oxidation. J. Mater. Sci. 2021, 56, 17556–17567. [Google Scholar] [CrossRef]
- Zuo, S.; Jin, X.; Wang, X.; Lu, Y.; Zhu, Q.; Wang, J.; Liu, W.; Du, Y.; Wang, J. Sandwich structure stabilized atomic Fe catalyst for highly efficient Fenton-like reaction at all pH values. Appl. Catal. B Environ. 2021, 282, 119551. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 78–188. [Google Scholar] [CrossRef]
- Yuan, G.; Pi, R.; Wu, Z.; Sun, X. Oxidative degradation of atrazine in water by ferrate-sulfite system. Chem. Ind. Eng. Prog. 2020, 39, 3794–3800. [Google Scholar]
- Jia, Y.; Xue, D.; Liu, Q.; Zhang, H.; Li, Z.; Zhang, L. Sulfite activation technology and its application in wastewater treatment. Chem. Ind. Eng. Prog. 2022, 41, 418–426. [Google Scholar]
- Entezari, M.; Godini, H.; Sheikhmohammadi, A.; Esrafili, A. Enhanced degradation of polychlorinated biphenyls with simultaneous usage of reductive and oxidative agents over UV/sulfite/TiO2 process as a new approach of advanced oxidation/reduction processes. J. Water Process Eng. 2019, 32, 100983. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Rozalen, M.; Rivera-Utrilla, J.; Polo, A.M.S.; Mota, A.J. Comparative Study of the Oxidative Degradation of Different 4-Aminobenzene Sulfonamides in Aqueous Solution by Sulfite Activation in the Presence of Fe(0), Fe(II), Fe(III) or Fe(VI). Water 2019, 11, 2332. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, L.; Chen, J.; Ding, J.; Wan, Y.; Wang, S.; Wang, Z.; Zhou, A.; Ma, J. Enhanced degradation of organic contaminants by zero-valent iron/sulfite process under simulated sunlight irradiation. Water Res. 2019, 149, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Yirsaw, B.D.; Megharaj, M.; Chen, Z.; Naidu, R. Environmental application and ecological significance of nano-zero valent iron. J. Environ. Sci. 2016, 44, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, X.; Chen, Y. Preparation and application in wastewater treatment of supported metal catalysts. China Synth. Resin Plast. 2023, 40, 57–64. [Google Scholar]
- Mushajiang, Z.; Zhao, J.; Xiao, P. Application Progress of Metal Nanomaterials in Persulfate-based Advanced Oxidation Process. J. Mater. Rep. 2023, 37, 194–201. [Google Scholar]
- Wang, X.; Chen, J.; Yao, G.; Hao, Z.; Zhang, Y.; Zhou, X. Advancements in nanoconfined catalytic membranes within the field of advanced oxidation. Mod. Chem. Ind. 2024, 44, 1–6. [Google Scholar]
- Quan, H.; Niu, L.; Shi, D.; Wang, X.; Liang, W.; Zhao, X. Preparation of lron-Carbon Materials Loaded with Nano Zero-Valent lron andTheir Performance of Degrading Antibiotics. Res. Environ. Sci. 2022, 35, 2732–2747. [Google Scholar]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Xu, L.; Xu, G.; Yan, J.; Hu, F. Activation of peroxymonosulfate by single-atom Fe-g-C3N4 catalysts for high efficiency degradation of tetracycline via nonradical pathways: Role of high-valent iron-oxo species and Fe–Nx sites. Chem. Eng. J. 2022, 427, 130803. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Guo, Q.; Wang, Z.; Wei, X.; Han, B.; Luo, X.; Pan, H.; Jiang, J. Bisulfite activated permanganate for oxidative water decontamination. Water Res. 2022, 216, 118331. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Dong, H.; He, D.; Rao, D.; Guan, X. Modeling the Kinetics of Contaminants Oxidation and the Generation of Manganese(III) in the Permanganate/Bisulfite Process. Environ. Sci. Technol. 2016, 50, 1473–1482. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Peng, Y.; Zhao, L.; Ma, J. Development and research progress of permanganate pre-oxidation in water purification. Acta Sci. Circumstantiae 2023, 43, 1–10. [Google Scholar]
- Zhang, J.; Zhang, H.; Wang, D.; Cui, F. Generation and utilization of radicals during enhanced permanganate oxidation: A review. Environ. Chem. 2021, 40, 487–496. [Google Scholar]
- Yang, M.; Li, K.; Wang, T.; Liu, R.; Hu, C. Al and Mn speciation changes during the pre-oxidation with potassium permanganate and coagulation removing natural organic matter and its membrane fouling behavior. Chemosphere 2023, 346, 140641. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Zi, H.; Tang, Y.; Zheng, X.; Wang, P.; Huang, J.; Wu, H.; Song, P.; Wu, L.; et al. Investigation of the redox behavior of biochar-based bipolar electrochemistry in porous media. Chem. Eng. J. 2023, 470, 144384. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Fu, M.; Ma, J.; Ning, P. Novel sequential process for enhanced dye synergistic degradation based on nano zero-valent iron and potassium permanganate. Chemosphere 2016, 155, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Lin, Y.; Zhang, T.; Hu, C.; Pan, Y.; Pan, R.; Tang, Y.; Xu, B.; Gao, N. Enhanced coagulation and oxidation by the Mn(VII)-Fe(III)/peroxymonosulfate process: Performance and mechanisms. Water Res. 2022, 226, 119200. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, Q.; Chen, B.-Y.; Hong, J. Oxidation of bisphenol A by persulfate via Fe3O4-α-MnO2 nanoflower-like catalyst: Mechanism and efficiency. Chem. Eng. J. 2019, 357, 337–347. [Google Scholar] [CrossRef]
- Huang, G.X.; Wang, C.Y.; Yang, C.W.; Guo, P.C.; Yu, H.Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef]
- Qiao, L.; Shi, Y.; Cheng, Q.; Liu, B.; Liu, J. The removal efficiencies and mechanism of aniline degradation by peroxydisulfate activated with magnetic Fe-Mn oxides composite. J. Water Reuse Desalin. 2021, 11, 212–223. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Wei, Y.; Xun, G.; Hu, F. Activation of peroxymonosulfate by single atom Co-N-C catalysts for high-efficient removal of chloroquine phosphate via non-radical pathways: Electron-transfer mechanism. Chem. Eng. J. 2022, 429, 132245. [Google Scholar] [CrossRef]
- Zhang, B.Y. Active Site Regulation of Fe Composite CN Catalyst and Its Non-Free Radical Catalytic Mechanism for PMS. Master’s Thesis, Wuhan Textile University, Wuhan, China, 2020. [Google Scholar]
- Huang, J.; Zhang, H. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review. Environ. Int. 2019, 133, 105141. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Yun, J.; Peng, W.; Zuo, M.; Ding, Z.; Hu, J. Degradation of Rhodamine B using potassium peroxymonosulfate catalyzed by Mn3O4-MnOOH binary composites. Ind. Water Treat. 2023, 43, 124–131. [Google Scholar]
- Li, Y.; Wang, Y.; Liao, X. Degradation Effect of Phenol in Simulated Wastewater by CuO/Ac Catalyzed Persulfate. Res. Environ. Sci. 2018, 31, 1949–1956. [Google Scholar]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129475. [Google Scholar] [CrossRef]
- Li, F.; Su, L.; Su, F.; Lin, J. Preparation and Properties of WO3/g-C3N4 Composite Photocatalyst. J. Nanjing Norm. Univ. (Eng. Technol. Ed.) 2023, 23, 40–46. [Google Scholar]
- Koskin, A.P.; Larichev, Y.V.; Stepanenko, S.A.; Dubinin, Y.V.; Ayupov, A.B.; Saraev, A.A.; Suprun, E.A.; Yeletsky, P.M. Carbonized Melamine Cyanurate as a Palladium Catalyst Support for the Dehydrogenation of N-heterocyclic Compounds in LOHC Technology. J. Carbon Res. 2023, 9, 83. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C. Handbook of x-ray photoelectron spectroscopy: A reference book of standard spectra for identification and interpretation of XPS data. Chem. Phys. Lett. 1992, 220, 7–10. [Google Scholar]
- Wang, J.Y.; Sun, D.D.; Sun, Y.C.; He, Q.; Ma, C.; Wang, G.; Zhang, X.; Hao, J.; Wu, X. Using g-C3N4 supported iron cobalt bimetal for activating peroxymonosulfate and degrading methylene blue. Ind. Water Treat. 2018, 11, 4. [Google Scholar]
- Peng, X.; Wu, J.; Dai, H.; Guo, C.; Li, C.; Xu, L.; Xu, G.; Hu, F. Degradation mechanism of methylene blue by Fe-g-C3N4(600) catalyst activation peroxymonosulfate. Acta Sci. Circumstantiae 2022, 42, 225–236. [Google Scholar]
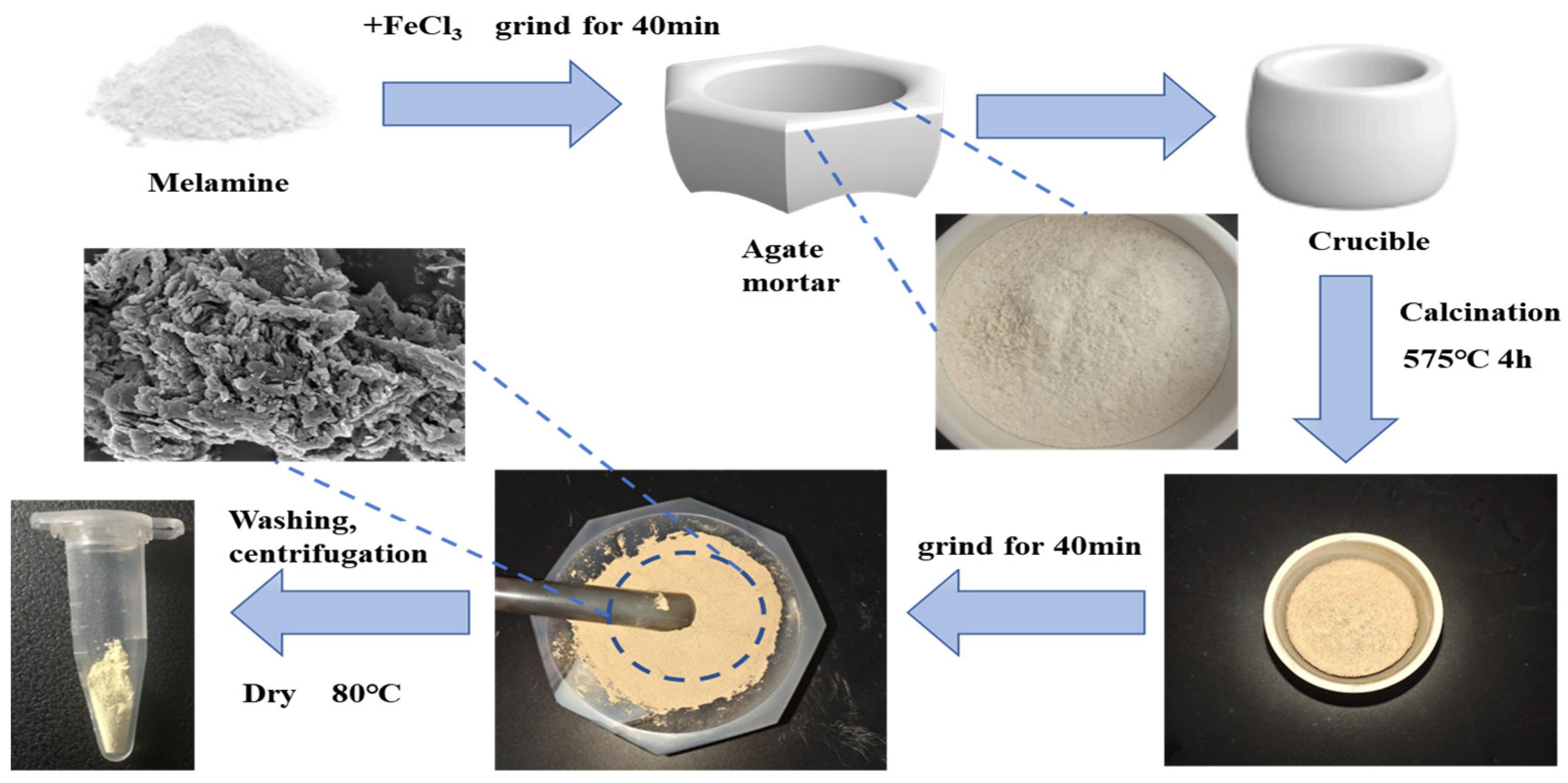

| Reagent | Specification | Manufacturer |
|---|---|---|
| melamine | Analytical pure AR | Shandong Yusuo Chemical Technology Co., Ltd, Heze, China |
| anhydrous ferric chloride | Analytical pure AR | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China |
| Fe(III)-g-C3N4 | 4% | self-made |
| potassium permanganate | Analytical pure AR | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China |
| potassium persulfate | Analytical pure AR | Xilong Science Co., Ltd., Shantou, China |
| methylene blue(MB) | Analytical pure AR | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China |
| Methyl Orange | Analytical pure AR | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China |
| rhodamine b | Analytical pure AR | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China |
| Instrument | Specification/Model | Manufacturer |
|---|---|---|
| muffle furnace | SX2-5-12 | YuanDong Electrical Furnace Factory, Changsha, China |
| analytical balance | ME104E/02 | Mettler-Toledo Instruments (Shanghai) Co., Ltd., Shanghai, China |
| high speed centrifuge | TG16-WS | Hunan Xiangyi Instrument Development Co., Ltd., Changsha, China |
| electric blast drying oven | DHG-9070A | Tianjin Kenuoyi Electronic Technology Co., Ltd., Tianjin, China |
| multi-head magnetic stirring apparatus | HJ-4A | Bonsee Technology (Shanghai) Co., Ltd., Shanghai, China |
| X-ray energy spectrometer | Thermo Scientific ESCALAB Xi+ | Thermo Fisher Scientific, Waltham, MA, USA |
| X-ray diffractometer | Bruker D8 advance | Bruker (Beijing) Technology Co., Ltd., Beijing, China |
| scanning electron microscop | ZEISS Sigma 300 | Carl Zeiss AG, Oberkochen, German |
| ultraviolet-visible spectrophotometer | UV-1800 | Shanghai Meipuda Instrument Co., Ltd., Shanghai, China |
| electron paramagnetic resonance | Bruker EMXplus | Bruker (Beijing) Technology Co., Ltd., Beijing, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Gu, H.; Wang, Q.; Chen, M.; Ma, W.; Zhang, H. Synthesis of Fe(III)-g-C3N4 and Applications of Synergistic Catalyzed PMS with Mn(VII) for Methylene Blue Degradation. Sustainability 2024, 16, 2364. https://doi.org/10.3390/su16062364
Li L, Gu H, Wang Q, Chen M, Ma W, Zhang H. Synthesis of Fe(III)-g-C3N4 and Applications of Synergistic Catalyzed PMS with Mn(VII) for Methylene Blue Degradation. Sustainability. 2024; 16(6):2364. https://doi.org/10.3390/su16062364
Chicago/Turabian StyleLi, Lin, Huangling Gu, Qiong Wang, Meiyin Chen, Wenjing Ma, and Hongwei Zhang. 2024. "Synthesis of Fe(III)-g-C3N4 and Applications of Synergistic Catalyzed PMS with Mn(VII) for Methylene Blue Degradation" Sustainability 16, no. 6: 2364. https://doi.org/10.3390/su16062364
APA StyleLi, L., Gu, H., Wang, Q., Chen, M., Ma, W., & Zhang, H. (2024). Synthesis of Fe(III)-g-C3N4 and Applications of Synergistic Catalyzed PMS with Mn(VII) for Methylene Blue Degradation. Sustainability, 16(6), 2364. https://doi.org/10.3390/su16062364






