1. Introduction
Packaging is crucial in preserving the quality, safety, and security of various food products. As described by Robertson, its primary functions include protection, containment, convenience, and communication [
1,
2,
3]. However, besides these functions, there is a growing emphasis on recycling packaging to address end-of-life challenges. Packaging recycling, especially plastic packaging, is currently low, with an average of 42% throughout the European Union in 2018 [
4]. To address this issue, political initiatives at the European level demand an incremental increase in packaging recycling rates [
5]. Recycling rates create pressure on packaging solutions that are not easily recyclable. In fact, by 2030, the European Union aims for all plastic packaging to be reusable or recyclable [
6]. Achieving this goal will require significant investments in most countries’ collection, sorting, and recycling infrastructure. Furthermore, recycling design principles must be widely implemented [
7,
8,
9]. Investing in infrastructure and adopting packaging designs that facilitate recycling is essential to meet the EU’s target and improve recycling rates. This includes considering factors such as material selection, the number of layers and components, the ease of separation, and compatibility with existing recycling systems. By promoting a comprehensive design for recycling principles and improving recycling infrastructure, the EU and its member countries can work toward a more sustainable and circular approach to packaging. The transformation towards more sustainable packaging is supported by guidelines provided by both industry and academia. These guidelines offer material selection, packaging design, packaging aid, and decoration recommendations, considering different regions or countries’ collection, sorting, and recycling infrastructure [
10,
11,
12].
Multilayer food packaging, combining polymers, paper, aluminum, and coatings, poses recycling challenges despite its environmental efficiency shown in life-cycle assessments [
13,
14,
15]. Its complex composition limits recyclability within current waste management systems, notably in Europe, where mechanical recycling through granulation is prevalent. To overcome these challenges, efforts are focused on enhancing the recyclability of packaging materials and their compatibility with existing recycling infrastructure, aiming for more sustainable recycling practices. In Europe, the predominant recycling approach is mechanical recycling during granulation processes, which involves the processing of materials together [
3,
13,
16,
17,
18]. Efforts to improve packaging material recyclability and compatibility with current recycling systems are underway to address multilayer packaging challenges and promote sustainable recycling practices [
13,
15,
16].
MLPW recycling presents challenges for the recycling industry due to difficulties in identifying, sorting, and separating the diverse layers using current standard technologies [
19]. As a result, MLPW is often categorized as mixed plastic waste in post-consumer streams and is frequently incinerated with energy recovery in European countries rather than being prioritized for recycling as part of a circular economy practice [
13]. In low-income countries, MLPW is often disposed of in landfills, and a portion of it also ends up in the environment due to its lightweight nature and short lifespan [
20].
There are two main advanced methods for recycling MLPW to address these challenges. The first method involves separating the distinct components of the packaging for further treatment of the materials individually. The second method is processing all layers together, which prepares the packaging for subsequent recycling techniques [
13,
21]. Delamination or selective dissolution can treat the separated structure, while compatibilization allows the recycling of MLPW as a single stream [
22]. In addition, emerging recycling routes, such as chemical processes such as pyrolysis, which produce feedstock, offer alternatives that do not require layer separation [
23].
Continued studies and the development of new technologies aim to improve plastic recycling, and there is the potential for new treatment methods to emerge in the coming years [
23,
24,
25,
26,
27]. For example, recent research has explored the possibility of enzymes secreted by microorganisms to facilitate depolymerization processes and degradation of PET into its monomers [
28,
29]. In general, MLPW recycling is a complex process. Still, ongoing research and technological advancements promise to improve recycling rates and find innovative solutions for the sustainable management of these packaging materials. In recent developments, a dissolution technique has been used successfully to recover all layers of multilayer composite waste, including waste printed circuit boards and packaging waste. This technique involves using various solvents to achieve a recycling rate exceeding 98% [
30,
31]. The process can be summarized as separating composite layers by dissolving the adhesive polymer and breaking the adhesive bonding between the layers of waste packaging. Solvents such as xylene, toluene, hexane, ethanol, and acetone have been used [
14,
27,
32]. Although the results of this chemical treatment approach have shown promise regarding high recycling rates and the quality of the recovered materials, particular challenges remain. After the dissolution process, an additional step, such as evaporation or the addition of an antisolvent, is required to extract the dissolved polymer. Unfortunately, these subsequent steps consume significant power and produce high CO
2-equivalent emissions [
33]. The high energy consumption and emissions associated with the evaporation process, or the addition of antisolvents, are limitations that need to be addressed to enhance the environmental sustainability of the dissolution technique. More research and innovation are required to develop more energy-efficient and environmentally friendly methods to extract the dissolved polymer and minimize the carbon footprint of the overall recycling process [
34].
However, using the dissolution technique to recover all layers of multilayer composite waste represents a significant advance in recycling these complex materials. It demonstrates the potential to achieve high recycling rates. Continued efforts to optimize the process and address its environmental impact will contribute to developing more sustainable recycling practices for multilayer packaging and other similar waste streams [
31].
Nitric acid can be used as a selective solvent to dissolve specific components of multilayer packaging, facilitating the separation of layers. Ultrasonic treatment is used to enhance the efficiency of the separation process by applying high-frequency sound waves that induce vibrations and mechanical forces, which aid in the disintegration and detachment of layers [
35].
Therefore, this study aimed to investigate the mechanical properties of polymers extracted from multilayer plastic waste (MLPW) through a process that involves separation using nitric acid and ultrasonic treatment. This process is designed to separate the different layers of the multilayer packaging and extract the polymers for further analysis. Understanding these physical factors and their influence on the mechanical properties of polymers is crucial for material design, selection, and application in various industries, including packaging, automotive, aerospace, and others [
36,
37,
38].
This research continues from a previous publication on the delamination of multilayer food packaging waste. Examination of the mechanical properties of the polymers obtained from MLPW after separation using nitric acid and ultrasonic treatment contributes [
35]. Understanding the recycling potential of multilayer plastic waste and developing sustainable recycling strategies for these complex materials is widely considered essential.
Mechanical properties encompass a broad range of characteristics that dictate how materials respond to mechanical forces or stresses. This includes crucial attributes such as strength, elasticity, and durability. Analyzing these properties sheds light on the recycled polymers’ behavior and provides invaluable insights into their suitability for a diverse array of applications.
Through a comprehensive study of the mechanical properties, this research aims to determine the optimal utilization of these recycled polymers in various industries. Potential applications span critical sectors such as packaging, construction, and manufacturing. This effort aligns with the broader objective of promoting environmentally conscious approaches to MLPW management, thus contributing to a more sustainable and circular economy. Studying the mechanical properties of polymers recovered from multilayer food packaging by nitric acid is integral to advancing sustainable waste management practices, reducing environmental impact, and promoting a circular economy. It could revolutionize how we handle and repurpose complex packaging materials.
Considering the above, this study contributes to recycling, offering a promising avenue to repurpose multilayer food packaging waste effectively. By bridging the gap between theory and practical application, this research addresses an environmental concern and paves the way for innovative solutions in waste management and sustainable material usage.
2. Materials and Methods
This study performed delamination experiments using potato chip packages obtained from post-consumer waste. Packaging waste consists of a mixture of different types of packaging materials. However, this study selected a specific kind of multilayer potato chip packaging, which featured polymer, aluminum, and a visually appealing print. Packaging materials from a single company were used for all experiments to ensure precision and consistency. The packages were cut into strips with different widths of 0.5 cm, 1.0 cm, and 1.5 cm while maintaining a consistent length of 12.0 cm. The choice of polymer length for the test was made according to the stipulated technical specifications. The distance between the clamps was set at 10 cm, with an additional 1 cm extension provided at both ends to facilitate the secure anchoring of the polymer within the clamps.
The delamination process involved using an aqueous solution containing nitric acid at three different concentrations: 20%, 25% and 30%. Nitric acid was diluted with distilled water. The choice of nonconcentrated nitric acid was considered appropriate, as it facilitated the delamination of the packaging layers and dissolved the aluminum layer.
Nitric acid plays a pivotal role in the surface modification of polymers by oxidizing their surfaces and introducing polar functional groups. This modification enhances adhesion properties, making these polymers more suitable for coatings, printing, or bonding with other materials, unlike sulfuric or hydrochloric acid, which may not efficiently introduce functional groups without an oxidizing agent and are more likely to cause corrosion or degradation. Regarding environmental stress cracking resistance, nitric acid’s oxidative interaction can modify surface properties without compromising the bulk properties of polymers, maintaining resistance to environmental stress cracking. In contrast, exposure to other inorganic acids under high concentrations or temperatures could accelerate stress cracking. In cleaning and degreasing applications, nitric acid’s oxidizing nature effectively removes contaminants from polymer surfaces, which is helpful for further processing or high-cleanliness applications. While capable of cleaning surfaces, other inorganic acids, such as sulfuric or hydrochloric, may not remove organic contaminants or oxidize impurities as effectively as nitric acid. Lastly, waste management benefits from nitric acid use, as its reaction by-products, primarily nitrates, are more soluble and easier to manage than the sulfates or chlorides from sulfuric or hydrochloric acid reactions, impacting environmental and waste treatment processes favorably [
39,
40,
41].
Temperature played a critical role in the experiments because of the presence of polymers with distinct melting points. To prevent adverse effects on the edges of the strips, the packaging strips were initially placed in the solution at room temperature before gradually increasing the temperature. A thermoregulated ultrasonic bath was used to control and monitor the temperature of the acid solution in the flask. The temperature was increased at 2–3 degrees per minute, and experimental temperatures of 55 °C, 65 °C, and 75 °C were used.
The delamination process involves immersing the packing strips in a nitric acid solution in a flask in an ultrasonic bath filled with room-temperature water. The flask is then stirred and subjected to ultrasound while the temperature gradually increases until the aluminum layer melts and the polymer layers separate. The process is complete when the aluminum has wholly dissolved, and the polymer layers have separated. As the polymer layers augment the sample volume, choosing an appropriately sized container and leaving enough space is essential. Laboratory-scale reactors were used in the study [
35].
The standard “ASTM D882-18 standard test method for Tensile Properties of thin plastic sheeting” was used to analyze the mechanical properties of polymers [
42].
The tensile test was executed with meticulous attention to the controlled stretching of the polymer specimen while safeguarding its structural integrity and securing fixation within a specialized clamping apparatus. These clamps are purposefully designed to orchestrate a uniform application of force throughout the longitudinal expanse of the specimen. The termination of the test protocol was precipitated by the moment the polymer undergoes fracturation, typically resulting in the specimen cleaving into two discrete segments.
Polymers derived from a series of 10 different experiments conducted under varying conditions (
Table 1) were subjected to testing. In each experimental run, three distinct polymers were recovered, corresponding to the number of polymer layers within the packaging structure. Furthermore, each polymer was subjected to a rigorous testing regimen conducted in triplicate.
Investigating the mechanical properties of the polymers obtained from the delamination process is crucial to understanding their suitability for potential recycling applications or other purposes. This provides valuable information on the potential performance and applicability of these polymers in various industries and contributes to the development of sustainable solutions for multilayer packaging waste.
3. Results and Discussion
3.1. Packaging Structure
Samples weighing approximately 1 g were taken during the delamination process of the multilayer packaging. The polymers recovered after the delamination process corresponded, on average, to 93.38% of the initial sample mass. The composition of the recovered polymers was quantified, with polymer 1 accounting for 45.35%, polymer 2 for 13.55%, and polymer 3 for 34.49% of the recovered mass. The 6.62% weight variation was attributed to the packaging components that dissolved during the process, including adhesives, paints, aluminum, and any polymer particles that had separated.
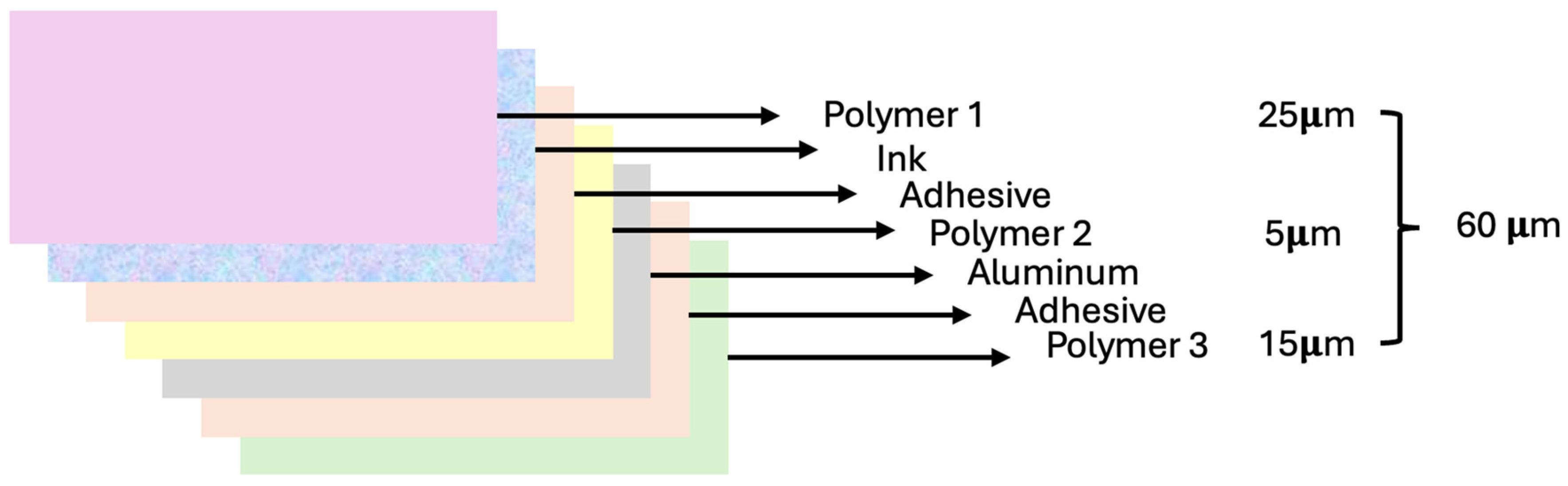
A precision thickness gauge was used to accurately quantify the thickness of each polymer within the packaging material. This instrument provided precise measurements of the recovered polymers. The packaging material under investigation comprised three distinct polymers, each serving a unique role in its composition.
The aggregate thickness of the packaging material was measured before any delamination as 60 μm. The outer layer, denoted as Polymer 1 (P1), had a thickness of 25 μm. Subsequently, for polymer 1, additional layers were identified, comprising adhesive and colored ink resin. Polymer 2 (P2), placed within the packaging, exhibited a remarkably slender thickness of only 5 μm. This layer demonstrated exceptional elasticity, allowing it to be easily charged with electricity and stretch to filament-like dimensions. Succeeding layers were constructed from a combination of aluminum and adhesive material [
43]. Ultimately, polymer 3 (P3), with a thickness of 15 μm, was the innermost polymer layer of the packaging material that directly encountered food. After accounting for the thicknesses of the respective polymers, the remaining layer, comprising residue, adhesive, and aluminum components, is 15 μm in thickness.
Figure 1 visually outlines the structure of the analyzed packaging, providing a theoretical representation based on the results of our research [
35,
44,
45,
46,
47].
3.2. The Surface of Recovered Polymers
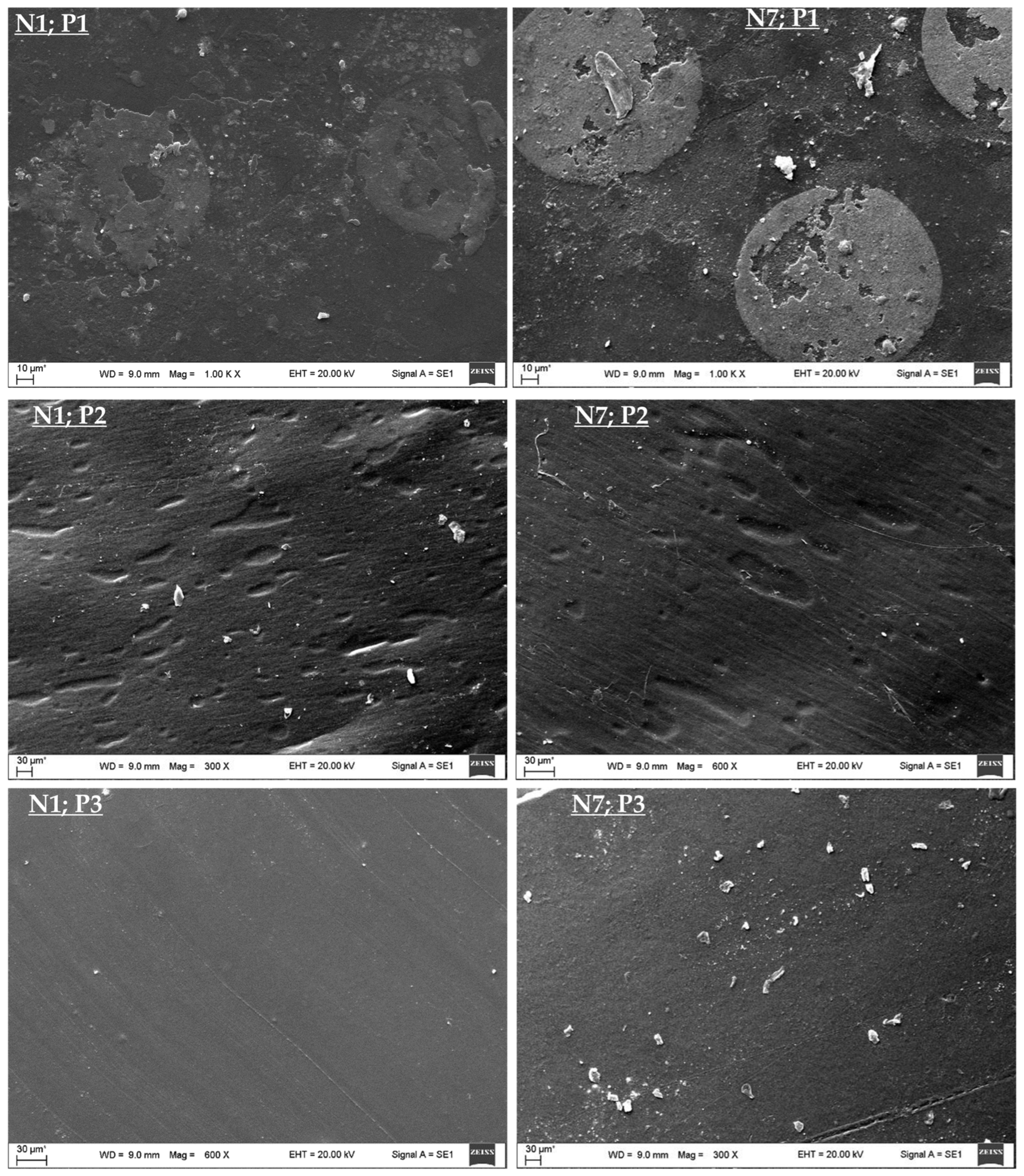
Polymer 1 is in close proximity to the paint layer, resulting in a very intricate surface to clean. The visible remnants of material adhesion in the surface photographs support this observation.
Upon examining the SEM photographs, it becomes apparent that the applied torque led to the detachment of microplastic particulates and the emergence of pores within the flexible structure of polymer 2. One possible explanation is the effect of stress; applying mechanical stress, such as torque or stretching, might cause the polymer chains to reorient or separate, resulting in the formation of voids or pores on the surface [
48]. Additionally, degradation or a chemical reaction could occur if the polymer was exposed to chemicals that react with it, like a solvent or an acid, leading to pore formation [
39]. The manufacturing process itself could also play a significant role in influencing the surface texture of the polymer sheets. Factors such as cooling rates, pressure during molding, or impurities might contribute to the development of pores. Moreover, environmental factors like temperature fluctuations, UV light exposure, or moisture could cause the pores to form due to the material’s expansion and contraction or the breakdown of polymer chains [
49].
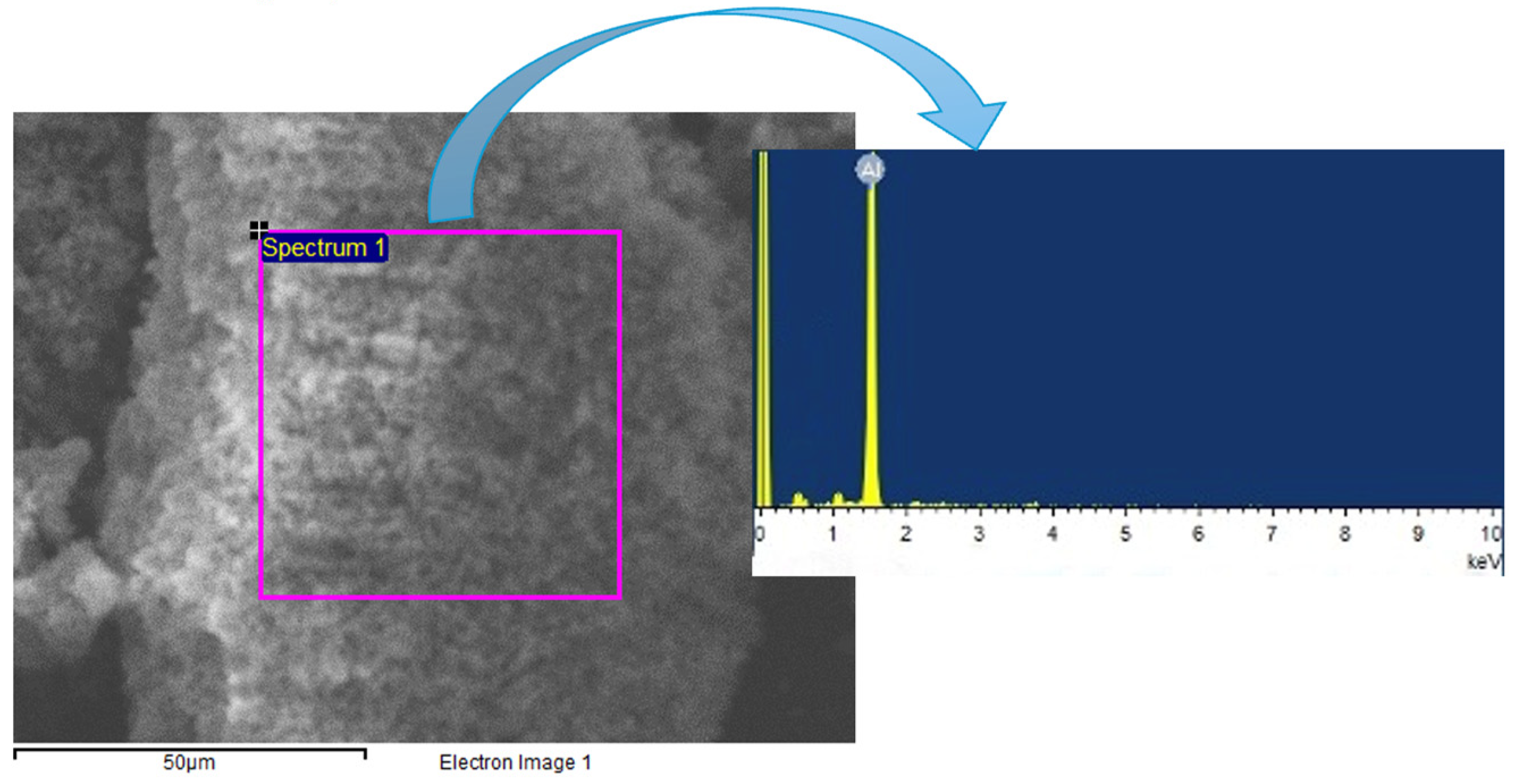
In stark contrast, polymer 3 interfaces with the aluminum layer. Although residual traces of aluminum are visible in the photographs, these traces are separated from the polymer. It should be noted that a subsequent iterative cleaning process could effectively remove any remaining aluminum remnants.
Figure 2 shows the EDS analysis of residues on the surface of polymer 3.
Figure 3 presents SEM photographs of the N1 and N7 samples, revealing details of the three layers.
Figure 4 provides SEM photographs of the N4 and N9 samples, providing insight into their respective layers.
3.3. Maximum Strength Required for Polymer Fracture under Identical Parameters
To understand the mechanical responses of polymers after recovery from multilayer food packaging with nitric acid, we assessed the maximum strength required for polymer fracture under identical experimental parameters. The findings revealed nuanced behaviors distinct from those of each polymer type.
For Polymer 1 (P1), a notable observation pertained to the variability in fracture behavior. In trial N1, polymer 1 displayed robustness with a maximum strength of 5.11 MPa. However, in trial N7, there was a substantial drop to 3.55 MPa, indicating reduced strength. Trials N2, N4, N8, and N9 exhibited closely aligned strength values, ranging between 4.52 and 4.67 MPa, suggesting relatively consistent performance under these conditions.
Unlike P1, polymer 2 (P2) exhibited minimal fluctuations in the strength required for the fracture. The maximum strength in N1, N2, and N9 was consistently 0.09 MPa. However, Trial N4 recorded a slightly lower strength of 0.06 MPa, while Trials N7 and N8 demanded a marginally higher strength at 0.07 MPa, presenting a stable trend overall.
The most significant variations were observed in the case of polymer 3 (P3). Maximum strength (Fmax) fluctuated considerably, ranging from 1.79 to 3.19 MPa. Trial N2 exhibited the highest strength at 3.19 MPa, while the lowest was observed in trial N9 at 1.79 MPa. Trials N4 and N7 showed comparable results, with strengths of 2.25 MPa and 2.27 MPa, respectively, highlighting the complexity of the response of P3.
Figure 5 adds a visual layer to our analysis, comparing the maximum strength required to break the recovered polymers. This visual representation illustrates the influence of ultrasound on fracture behavior, with trials N1, N2, and N4 involving ultrasound, while Trials N7, N8, and N9 were conducted without ultrasound. Polymers are denoted as P1 for polymer 1, P2 for polymer 2, and P3 for Polymer 3.
Beyond mechanical characteristics, the impact of nitric acid concentration on polymer behavior emerges as a pivotal factor. Trials N2 and N8, with lower concentrations of nitric acid (c = 20%), contrast to Trials N1, N4, N7 and N9, where a higher concentration prevailed (c = 30%). Trial N1, despite the use of elevated nitric acid concentrations, exhibited a brief duration of exposure (45 min), yet it did not impart any discernible alterations in polymer properties.
A salient illustration of the influence is found in Trials N7 and N8, particularly regarding polymer 1. Despite nearly identical conditions, the reduction in acid concentration was correlated with an increase in the experimental process (tN7 = 780 min and tN8 = 1350 min), resulting in a prolonged interaction between the polymer sample and the nitric acid medium. It is conceivable that the prolonged exposure to nitric acid, coupled with mechanical agitation, contributed to the weakening of the polymer’s structure.
3.4. Tensile Strength of Separated Polymers
The maximum strength of a polymer represents the force it can endure before fracturing under consistent testing conditions. In contrast, the tensile strength of recovered polymers refers to the maximum stress during stretching or pulling tests after a prior deformation and recovery process. Both metrics are crucial to understanding the mechanical resistance of polymers in various applications.
Tensile strength, an essential property in engineering, has profound implications in material science, mechanical engineering, and structural engineering. Often interchangeably referred to as fracture strength or rupture, it is a crucial metric shaping material in various structural contexts.
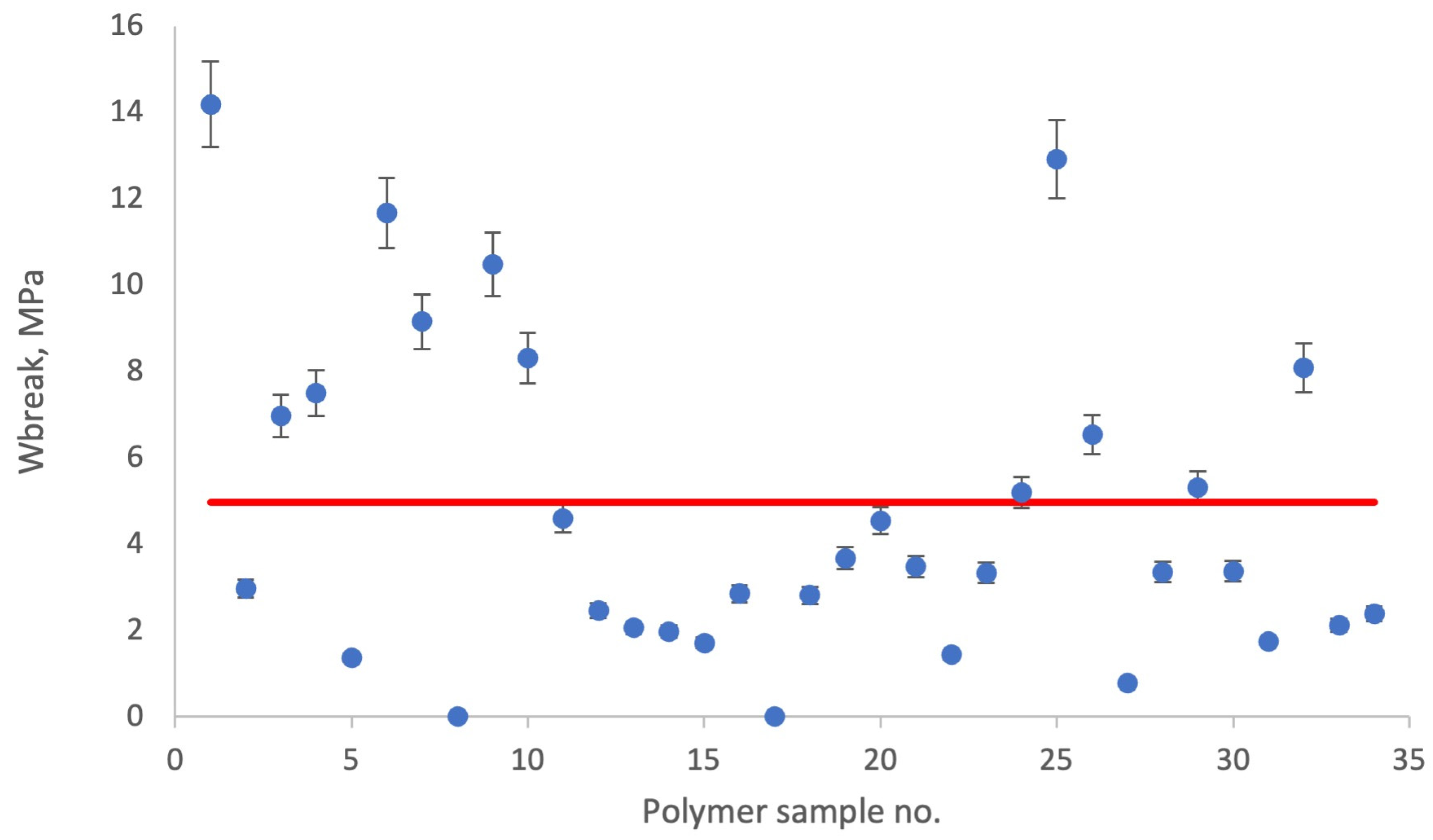
Polymer 1 (P1): Transitioning to tensile strength,
Figure 6 provides a detailed scatter plot showing the tensile strength distribution (W
break, MPa) for polymer 1. The calculated mean value of W
break was 4.99 MPa, and the skewness coefficient of 1.12 adds a statistical dimension, signaling the departure from a symmetrical distribution.
Polymer 3 (P3): In
Figure 7, the exploration of Polymer 3’s tensile strength measurements unfolds. The calculated mean value of W
break was recorded at 3.17 MPa, accompanied by a skewness coefficient of 0.91, contributing insight into the distribution characteristics of P3’s tensile strength.
As we navigate the intricacies of polymer 2 (P2), the results of the conducted tests yield inconclusive findings. This ambiguity arises from the inherent softness of the polymer, hindering the precise capture of the exact breaking moment by the utilized software. This acknowledgment emphasizes the intrinsic challenges of characterizing softer materials and underscores the need for nuanced testing methodologies in such scenarios.
3.5. Polymer Elasticity
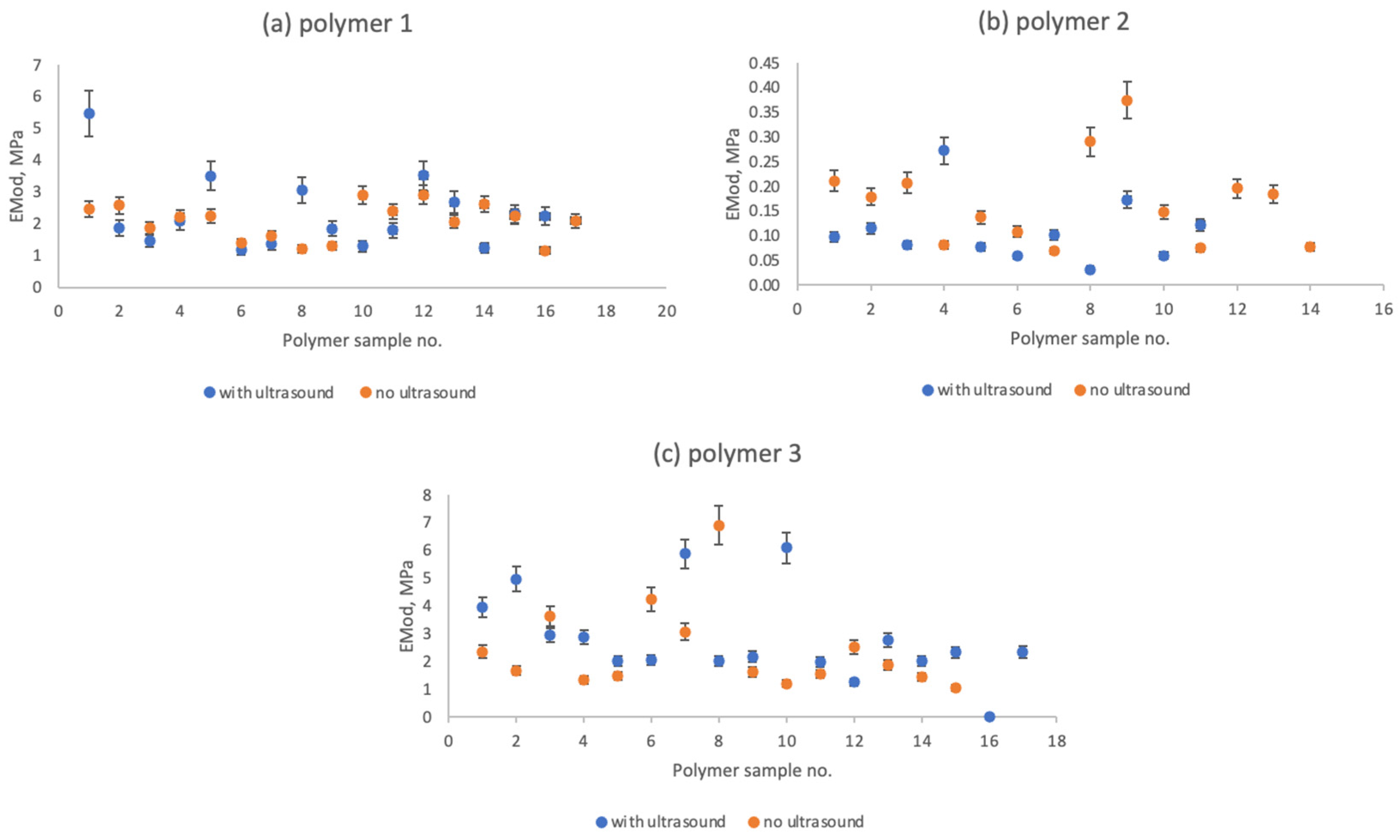
The interaction with nitric acid significantly impacts a given polymer’s elasticity (Emod, MPa). Nitric acid, known for its potent oxidizing nature, interacts with polymers, altering their chemical composition and physical attributes. Exposure to nitric acid can induce chain scission, cross-linking, and other chemical modifications within the polymer’s structural framework. These chemical changes result in a reduction in elasticity. Oxidative processes and chemical reactions have the potential to cleave polymer chains, thereby weakening the overall structural integrity of the material. Consequently, the polymer becomes more susceptible to brittleness and this reduces its ability to return to its original configuration after deformation.
The findings arising from evaluating the elasticity of polymers serve as a basis for assessing the influence of ultrasound on the elastic properties (
Figure 8).
Given that ultrasound is most prominently influential in dictating the duration of the procedural sequence [
35], the opportunity arose to investigate the implications of extended and abbreviated interactions with nitric acid on polymer elasticity. This investigation sheds light on the various effects stemming from varying periods of exposure to nitric acid, which subsequently affected the polymer’s elastic characteristics of the polymer.
3.6. FTIR Spectroscopy Analysis
An FTIR analysis is necessary to know the chemical structure of the polymers. Infrared spectroscopy is a crucial technique for characterizing the molecular structure of polymers and provides information about the functional groups present in the material. The analysis focuses on identifying specific absorption bands within the IR spectrum that indicate different functional groups, such as O-H, N-H, C-H, and C=O stretches alongside N-H bending and C=C stretching vibrations.
Figure 9 shows the FTIR spectrum of polymer recovered from multilayer packaging.
Broad peaks around 3300 cm
−1 typically indicate O-H stretching from alcohols or N-H stretching from amines. Sharp peaks around 3000 cm
−1 can indicate C-H stretching in alkanes. Peaks between 2850 and 2960 cm
−1 are characteristic of C-H stretching vibrations in methylene (-CH2-) groups, which are common in polymers. Peaks around 1700 cm
−1 are a common area for C=O stretching vibrations, suggesting the presence of carbonyl groups, which are found in many polymers like polyesters and polyamides. Peaks between 1600 and 1500 cm
−1 in this region often show N-H bending in amines or C=C stretching in alkenes. Peaks between 1450 and 1350 cm
−1 can correspond to C-H bending in methylene groups. Peaks between 1300 and 1000 cm
−1 are complex regions but can include C-O stretching in ethers, esters, and alcohols. The region below 1000 cm
−1 is known as the fingerprint region and is very specific to individual compounds, often used to differentiate between isomers [
45,
50,
51,
52].
Polymer 1 shows strong absorption in the carbonyl and fingerprint regions, which could indicate a polypropylene type of polymer. Polymer 3 shows a very flat baseline with few features, which could suggest it either does not contain many IR-active functional groups. C-H stretching vibrations around 2950 cm
−1 for the asymmetric and symmetric stretching of CH3 and CH2 group C-H bending vibrations near 1450 cm
−1 for the bending modes of CH3 groups, and around 1375 cm
−1 for CH2 bending, methyl rocking around 1150 cm
−1 to 1000 cm
−1 suggest polymers 1 and 3 are polypropylene. Although polypropylene typically does not show strong absorption bands near 1700 cm
−1 since it does not contain any carbonyl (C=O) groups, the assumption can be made that additional peaks could be residues from dyes in the prints layer. Polymer 2 has less pronounced peaks and lacks a strong carbonyl peak, which might suggest a different type of polymer, potentially with fewer functional groups that absorb in the IR range or a highly crystalline or oriented sample that reflects IR light. C-H Stretching absorption bands around 2950–2850 cm
−1 are due to the asymmetric and symmetric stretching vibrations of the CH2 groups that are abundant in polyethylene. C-H Bending has a moderate to strong absorption band around 1460 cm
−1 and one around 1375 cm
−1 due to the bending vibrations of the CH2 and CH3 groups, respectively. Similar FTIR spectrums characterize low-density polyethylene (LDPE) [
53,
54].
3.7. Recovered Polymer Comparison with Neat Polymer and Problem-Solving Solution
For comparison, the mechanical properties of neat low-density polyethylene (LDPE) encompass a tensile strength range of 10.3 to 18.0 MPa and a modulus of elasticity spanning from 0.150 to 0.520 GPa. Neat polypropylene, on the other hand, is characterized by a Young’s modulus of 1325 MPa, a tensile strength of 34 MPa, and a bending strength of 41 MPa [
55,
56]. These benchmarks provide context for assessing the relative performance and mechanical integrity of the polymers studied.
The analysis shows that the mechanical properties of the polymers have undergone significant degradation due to the delamination process. This degradation manifests itself in a reduction in the polymer’s inherent strength, elasticity, and durability, which are crucial parameters in assessing the functional viability of polymers for various applications. However, it is crucial to emphasize that while this change in mechanical properties impairs the intended primary functions of the polymers, it does not categorically preclude their potential for repurposing or alternative applications. Further investigation into the extent of mechanical degradation and exploration of potential routes for the utilization of these materials is warranted. This approach not only promotes a sustainable perspective towards material use but also opens up opportunities for innovative applications of polymers that have undergone such structural changes. There are also methods to improve the mechanical properties of polymers polypropylene and low-density polyethylene, which are widely used thermoplastic materials with a wide range of applications. To counteract the deterioration of mechanical properties due to aging, processing, or environmental influences, various strategies can be used to restore and improve them. These strategies are applicable to both PP and LDPE, with specific approaches tailored to the unique properties of each polymer.
One common method is to reprocess the polymers with the addition of compatibilizers and stabilizers to mitigate the effects of degradation and improve material compatibility [
57,
58]. This approach can significantly improve the mechanical properties and durability of the materials. Another important technique is controlled thermal treatment, which aims to relieve internal stresses in the polymer matrix and improve crystallinity [
59]. Such thermal treatment can lead to improved mechanical strength and thermal resistance. Blending the degraded polymer with another polymer is also a viable strategy [
46]. This method utilizes the mechanical and physical properties of both materials to produce a composite with superior properties. In the case of LDPE, known for its elasticity and flexibility, adding plasticizers can improve pliability and extend the range of applications. In the case of polypropylene, reinforcement with fillers is particularly beneficial. The incorporation of glass fibers or carbon fibers into the PP matrix can significantly increase stiffness, strength, and thermal stability, making the material suitable for more demanding applications [
60].
Selecting a particular restoration or enhancement method depends on the type of degradation, the desired mechanical properties, and the intended use of the polymer. Tailoring the restoration approach to the application’s specific requirements ensures effective rejuvenation of these widely used thermoplastics.
3.8. Microplastics from Recovered Polymers
Scanning electron microscopy (SEM) was instrumental in comprehensively examining the recovered film’s surface characteristics.
The discernment of microplastic constituents on the surface of the recuperated polymer following the delamination process constitutes a pivotal facet necessitating thorough scrutiny within the recycling endeavor. This occurrence warrants considerable attention because of its potential ramifications, necessitating a robust foundation for further inquiry and enhancement initiatives. Microplastics’ presence is significant for the quality of reclaimed materials and broader ecological considerations associated with recycling.
The factors contributing to the emergence of microplastics on polymer surfaces are inherently multifaceted and can be ascribed to an amalgamation of influences. These include, but are not confined to, the deployment of polymers in adhesive capacities during the production phase and the conceivable degradation of polymers during thermal and spinning procedures. The cumulative effect of these influences is reflected in the introduction of trace additives and microplastic entities within the reclaimed polymer samples.
The visual representation of these phenomena is eloquently depicted in the SEM photographs, presented in
Figure 10. These photographs emphasize and accentuate the conspicuous presence of microplastics separated from the film’s surface. This empirical evidence accentuates the pressing need for a deep understanding of the implications of nitric acid on the compositional constitution and structural soundness of the reclaimed film.
Sustained research efforts in this domain are indispensable, constituting an instrumental step toward devising methodologies for improving microplastic incidence and optimizing recycling protocols pertinent to multilayer packaging waste. Furthermore, exploring potential strategies for mitigating or eradicating microplastics from reclaimed materials emerges as a promising avenue for prospective investigation, which deserves focused scholarly attention.
4. Conclusions
In summary, this study has contributed valuable insight into the multifaceted factors that profoundly influence the properties of polymers during the delamination process in the context of multilayer packaging waste recycling. Comprehensive investigation encompassing critical parameters, including separation conditions (namely, temperature and acid concentration), sample dimensions, application of ultrasound, and the separation duration, has elucidated facets governing the recyclability and conceivable applications of the reclaimed polymers.
Polymer 1 (P1) exhibited variability in its fracture behavior, with a notable range in maximum strength required for fracture from 3.55 MPa to 5.11 MPa. This variability suggests a significant impact of experimental conditions on P1’s mechanical strength, indicating a potential decrease in performance stability under varying conditions. On the other hand, polymer 3 (P3) demonstrated even more considerable fluctuations in maximum strength, ranging from 1.79 MPa to 3.19 MPa. These fluctuations reflect P3’s complex response to the experimental conditions, underscoring the nuanced impact of treatment processes on its mechanical properties.
The analysis further delved into tensile strength and elasticity, providing insights into the materials’ resilience and flexibility post-recovery. For P1, a mean tensile strength value of 4.99 MPa with a skewness coefficient of 1.12 suggests a non-symmetrical distribution, indicating variability in its tensile strength. P3 exhibited a mean tensile strength of 3.17 MPa, with a skewness coefficient of 0.91, highlighting differences in mechanical robustness and distribution characteristics between the polymers.
Our research shows that higher temperatures improve the delamination of polymers, which is key for preserving their qualities. We found that the size of packaging strips slightly affects the mechanical properties but is crucial for scalability in industrial uses. Using ultrasound can shorten delamination times, enhancing energy and time efficiency, but it must be optimized to avoid damaging the polymers. Despite the potential of these polymers for industrial use, further research is needed to refine the delamination process and adjust the polymers’ properties. Additionally, the possibility of recycling these polymers into new materials offers a sustainable waste management solution. This study highlights the importance of continued research and innovation in recycling multilayer packaging waste, addressing the challenges of modern packaging materials for sustainable recycling practices.