Hydrogen Gas Adsorption of the Triassic Chang 7 Shale Member in the Ordos Basin, China
Abstract
1. Introduction
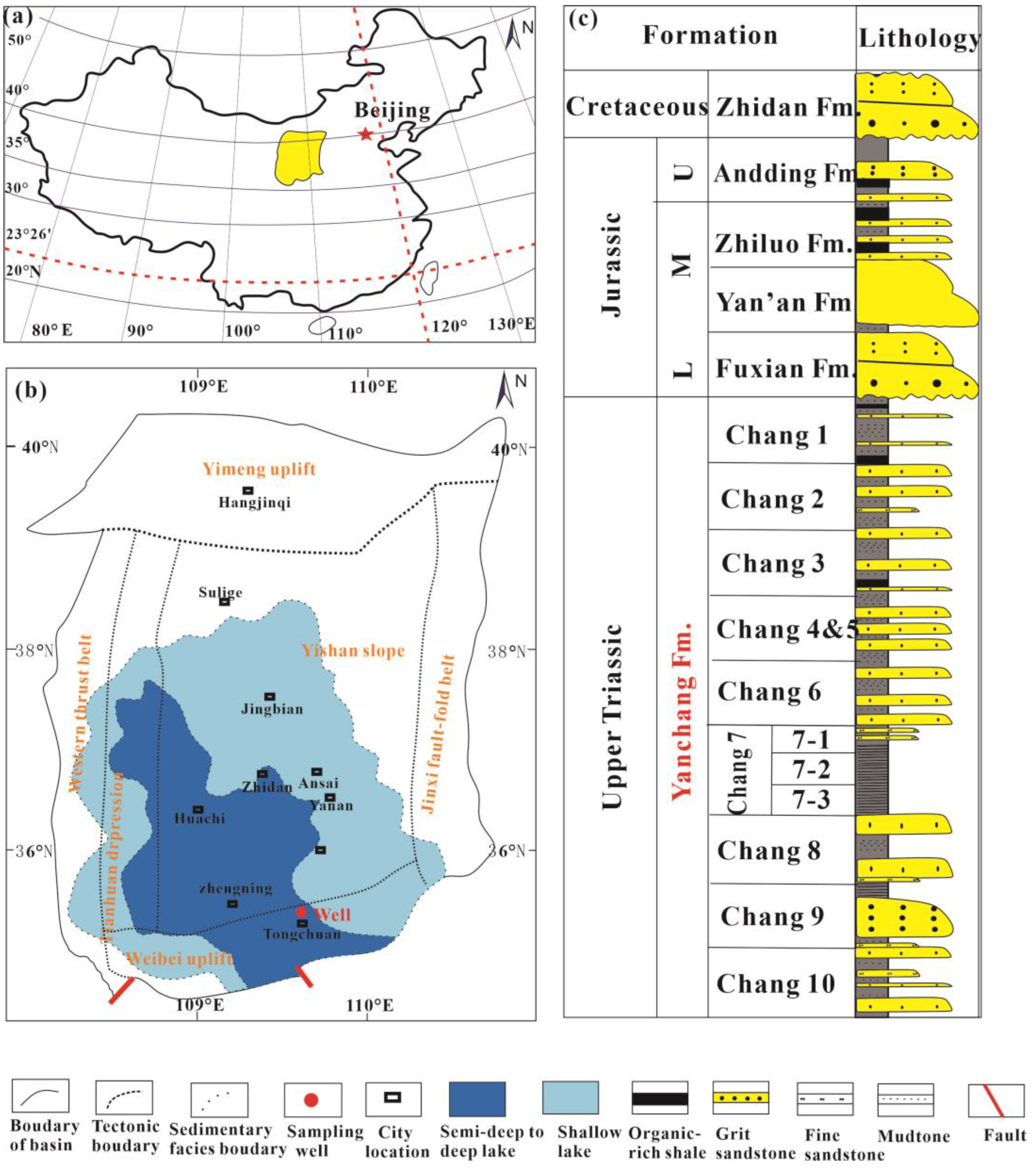
2. Geological Background
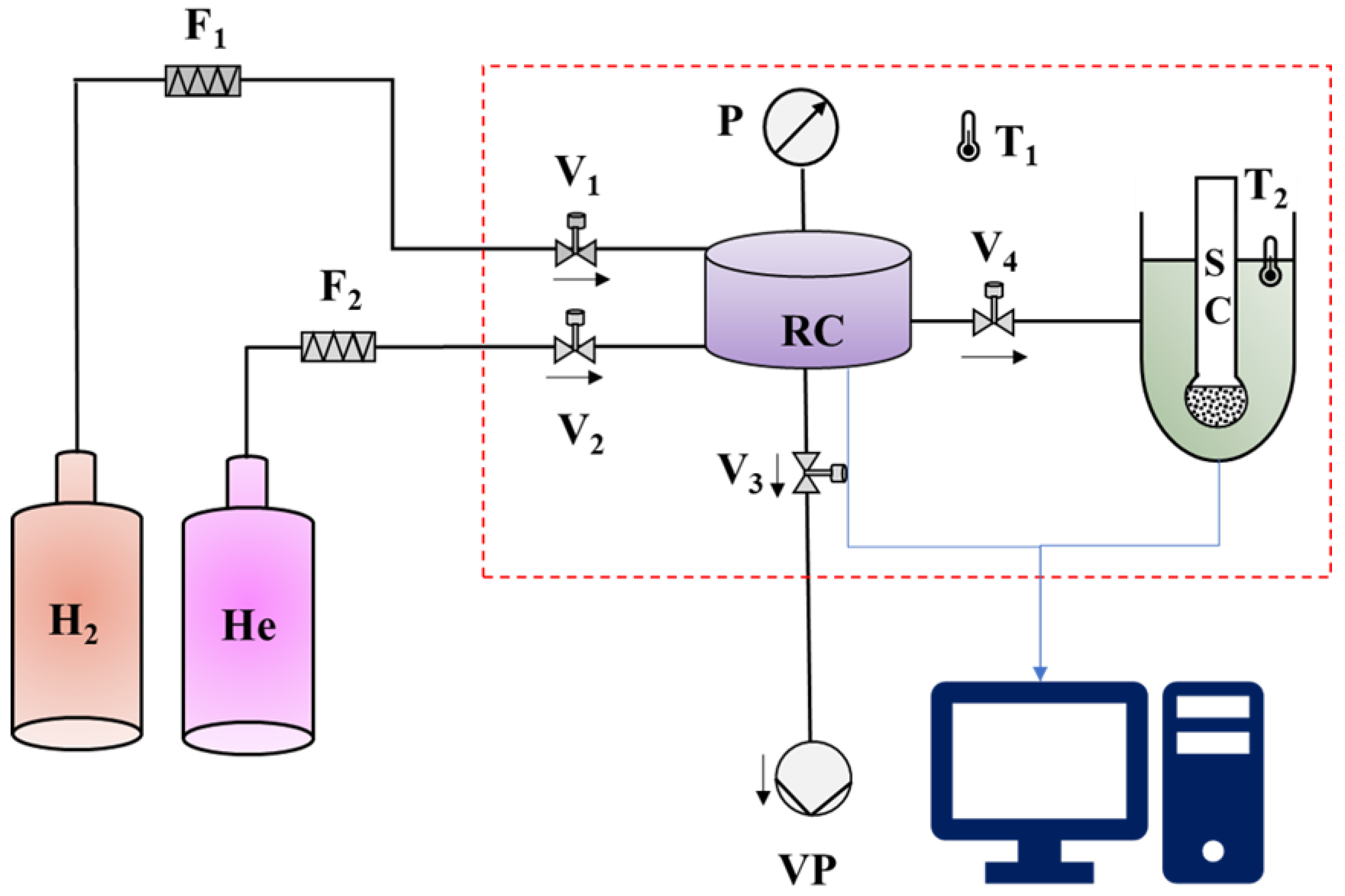
3. Materials and Methods
4. Results
4.1. Characteristics of the Chang 7 Shale Member Samples
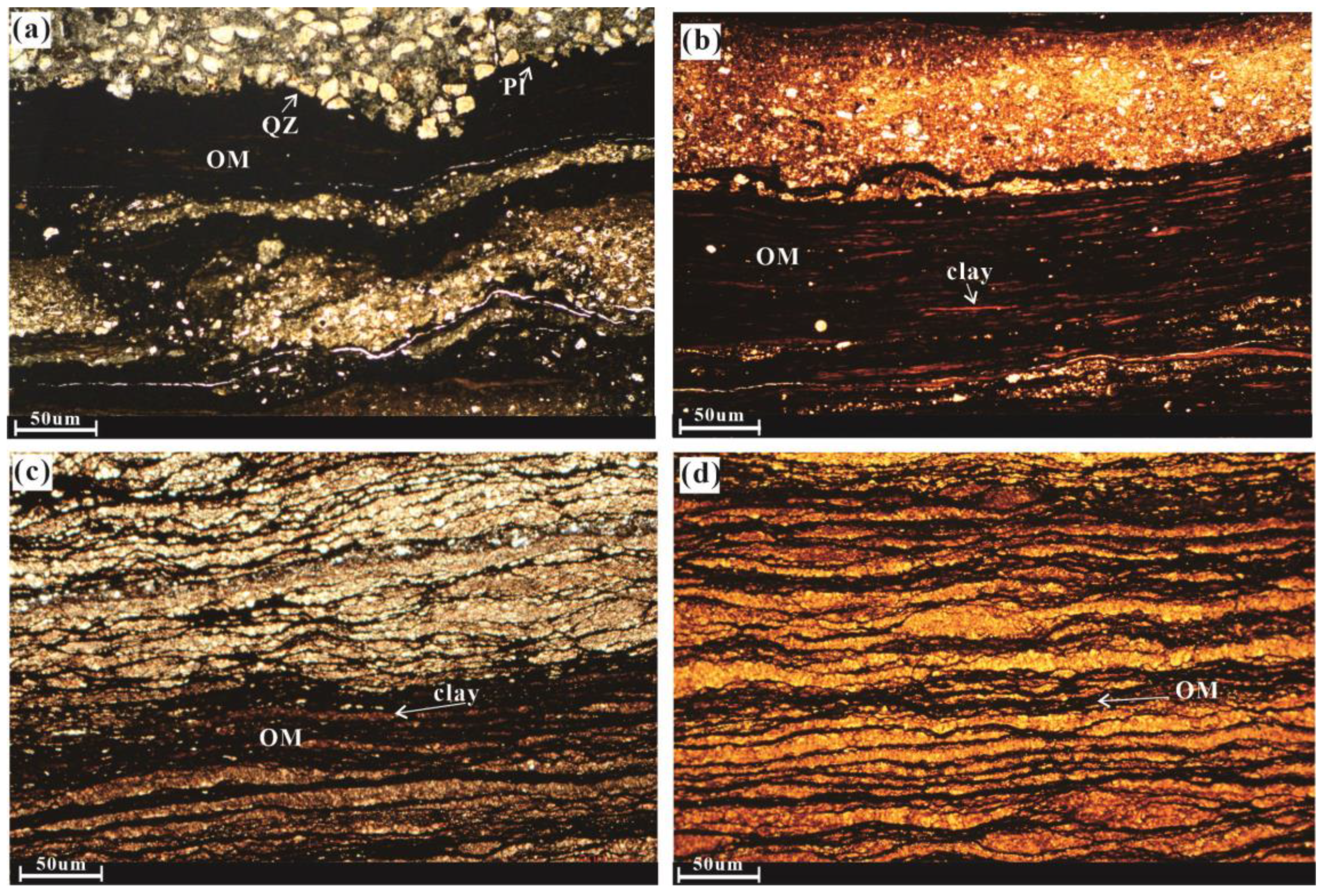
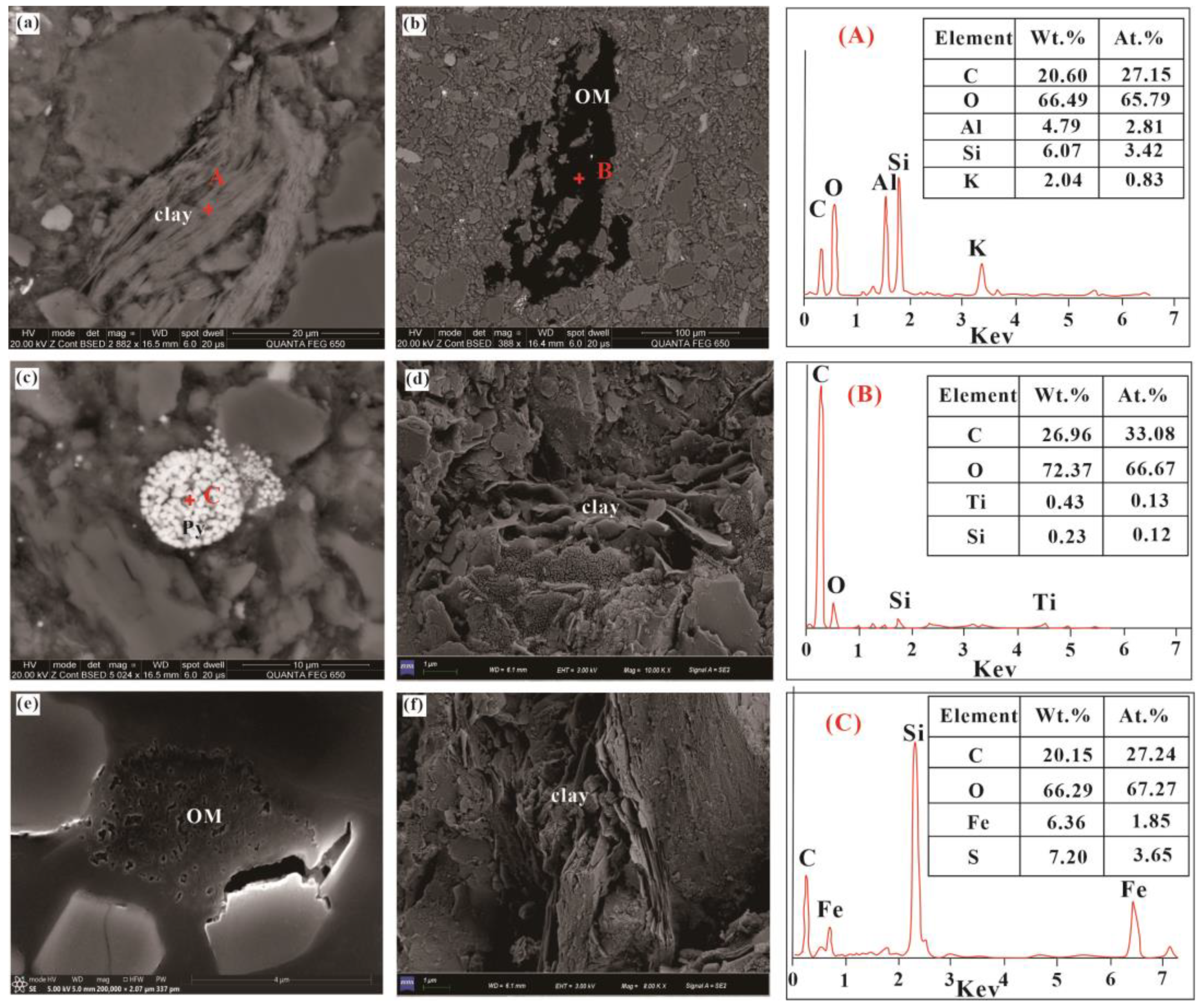
4.2. Microscopic Characteristics of the Chang 7 Shale Member
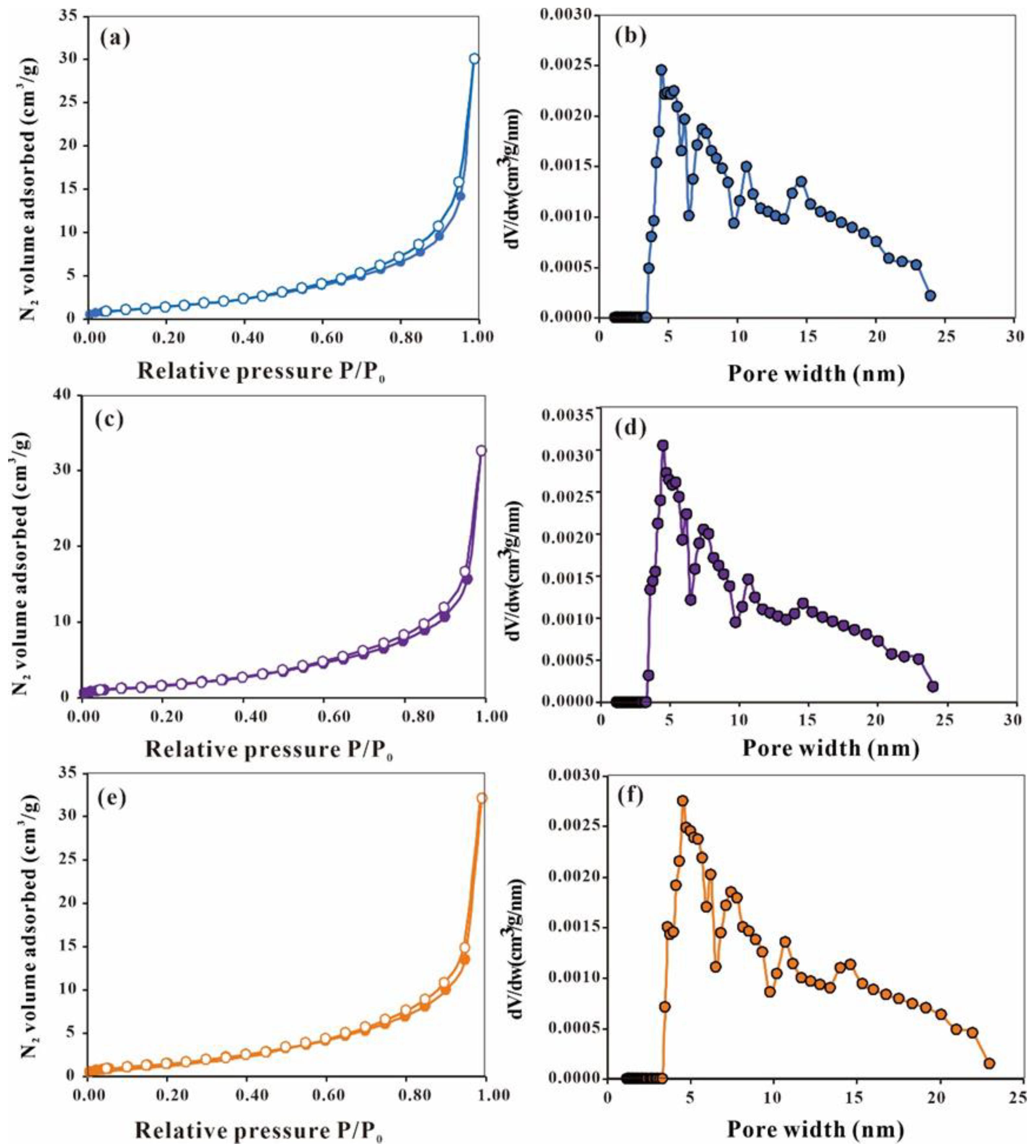
4.3. Pore Characteristics of the Chang 7 Shale Member
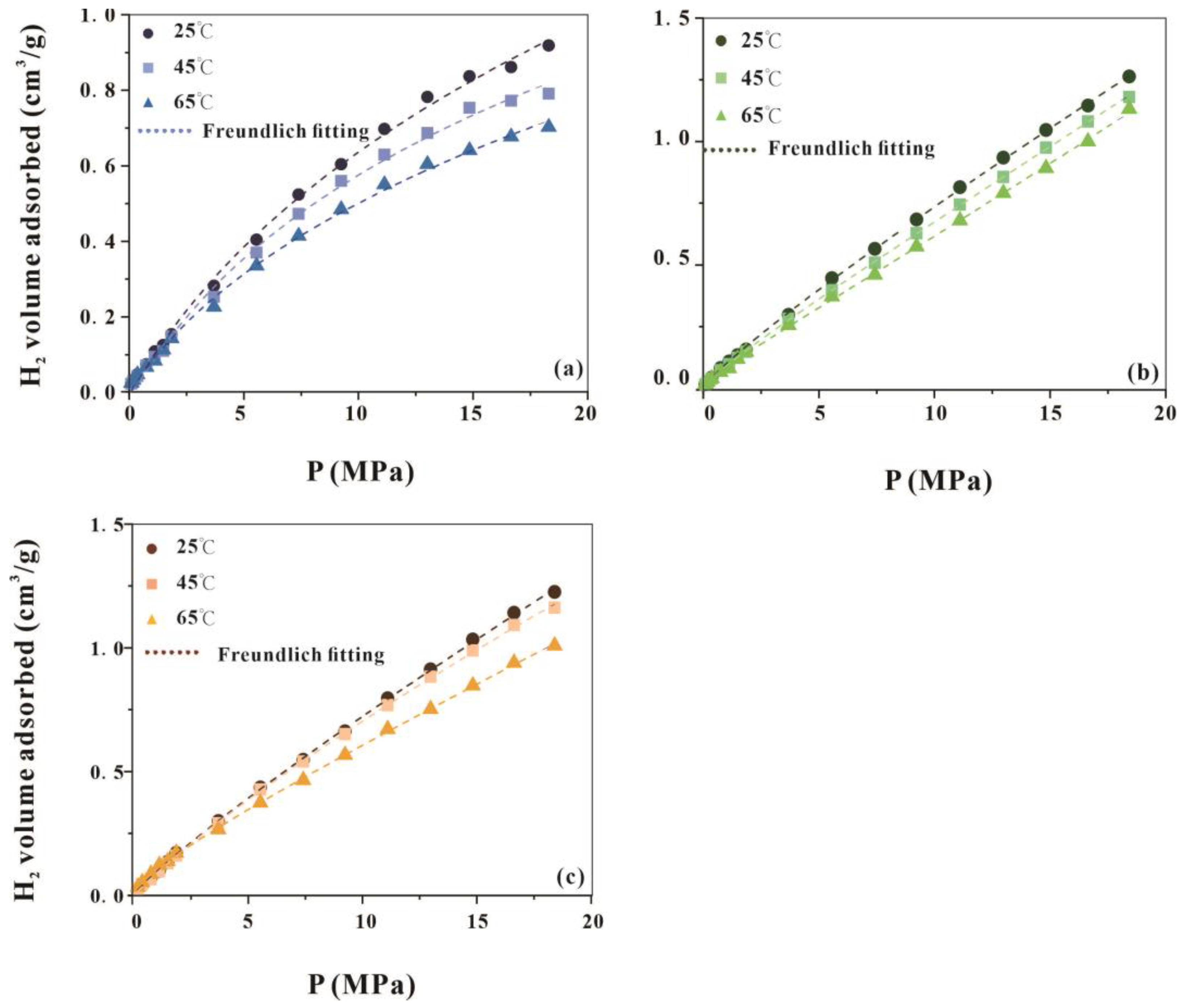
4.4. High-Pressure Hydrogen Adsorption Capacity of the Chang 7 Shale Member
5. Discussion
6. Conclusions
- The primary components of Chang 7 Shale Member samples are organic matter (kerogen) and clay minerals (exceeding 30%) primarily composed of I/S and illite. Microscopic observations of the sample bedding revealed well-developed layers.
- The Chang 7 Shale Member exhibits diverse pore types, including intergranular, intragranular, and intercrystalline types. Nitrogen adsorption isotherm curves are V-shaped and exhibit H3-type hysteresis loops, indicating the prevalence of slit-shaped pores, cracks, and wedge-shaped structures.
- The adsorption of hydrogen by the Chang 7 Shale Member decreases with increasing temperature and increases with increasing pressure. Organic matter (kerogen) and clay minerals exert considerable influence on hydrogen adsorption.
- The adsorption behaviour conforms to the Freundlich and Langmuir equation models. And the Freundlich equation is a better fit, suggesting multilayer adsorption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Zhao, Q.; Zhang, G.; Xiong, B. Energy revolution: From a fossil energy era to a new energy era. Nat. Gas Ind. B 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.; Sun, Y. Enhanced storage of hydrogen at the temperature of liquid nitrogen. Int. J. Hydrogen Energy 2004, 29, 319–322. [Google Scholar] [CrossRef]
- Urbaniec, K.; Bakker, R.R. Biomass residues as raw material for dark hydrogen fermentation—A review. Int. J. Hydrogen Energy 2015, 40, 3648–3658. [Google Scholar] [CrossRef]
- Nowotny, J.; Veziroglu, T.N. Impact of hydrogen on the environment. Int. J. Hydrogen Energy 2011, 36, 13218–13224. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, C.; Tian, W.; Zhang, D.; Su, G.; Qiu, S. Study on high-temperature hydrogen dissociation for nuclear thermal propulsion reactor. Nucl. Eng. Des. 2022, 392, 111753. [Google Scholar] [CrossRef]
- Yang, N.; Deng, J.; Wang, C.; Bai, Z.; Qu, J. High pressure hydrogen leakage diffusion: Research progress. Int. J. Hydrogen Energy 2024, 50, 1029–1046. [Google Scholar] [CrossRef]
- Heesterman, A.R.G. Renewable energy supply and carbon capture: Capturing all the carbon dioxide at zero cost. Clean Technol. Environ. Policy 2019, 21, 1177–1191. [Google Scholar] [CrossRef]
- Ahmad, T.; Zhang, D.; Huang, C.; Zhang, H.; Dai, N.; Song, Y.; Chen, H. Artificial intelligence in sustainable energy industry: Status Quo, challenges and opportunities. J. Clean. Prod. 2021, 289, 125834. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development-A technological and geopolitical overview. Int. J. Hydrogen Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, W.; Su, A.; Zhang, S.; Li, M.; Dang, Y.; Xu, F.; Zhou, R.; Zhang, D.; Xu, Z.; et al. Geochemistry and origin of the giant Quaternary shallow gas accumulations in the eastern Qaidam Basin, NW China. Org. Geochem. 2005, 36, 1636–1649. [Google Scholar] [CrossRef]
- Han, S.B.; Xiang, C.H.; Du, X.; Xie, L.F.; Huang, J.; Wang, C.S. Geochemistry and origins of hydrogen-containing natural gases in deep Songliao basin, China: Insights from continental scientific drilling. Pet. Sci. 2023, in press. [Google Scholar] [CrossRef]
- Huang, S.; Fang, X.; Liu, D.; Fang, C.; Huang, T. Natural gas genesis and sources in the Zizhou gas field, Ordos Basin, China. Int. J. Coal Geol. 2015, 152, 132–143. [Google Scholar] [CrossRef]
- Zhijun, J.; Lu, W. Does Hydrogen Reservoir Exist in Nature. Earth Sci. 2022, 47, 3858–3859. [Google Scholar]
- Wang, L.; Cheng, J.; Jin, Z.; Sun, Q.; Zou, R.; Meng, Q.; Liu, K.; Su, Y.; Zhang, Q. High-pressure hydrogen adsorption in clay minerals: Insights on natural hydrogen exploration. Fuel 2023, 344, 127919. [Google Scholar] [CrossRef]
- Weaver, C.E. A Discussion on the Origin of Clay Minerals in Sedimentary Rocks. Clays Clay Miner. 1956, 5, 159–173. [Google Scholar] [CrossRef]
- Rafiei, M.; Lӧhr, S.; Baldermann, A.; Webster, R.; Kong, C. Quantitative petrographic differentiation of detrital vs diagenetic clay minerals in marine sedimentary sequences: Implications for the rise of biotic soils. Precambrian Res. 2020, 350, 105948. [Google Scholar] [CrossRef]
- Howell, A.L.; Bentley Sr, S.J.; Xu, K.; Ferrell, R.E., Jr.; Muhammad, Z.; Septama, E. Fine sediment mineralogy as a tracer of latest Quaternary sediment delivery to a dynamic continental margin: Pandora Trough, Gulf of Papua, Papua New Guinea. Mar. Geol. 2014, 357, 108–122. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Ma, L. Tectonic and stratigraphic controls of hydrocarbon systems in the Ordos basin: A multicycle cratonic basin in central China. AAPG Bull. 2005, 89, 255–269. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Z.; Liu, Q.; Liu, K.; Meng, Q.; Huang, X.; Su, Y.; Zhang, Q. The occurrence pattern of natural hydrogen in the Songliao Basin, P.R. China: Insights on natural hydrogen exploration. Int. J. Hydrogen Energy 2024, 50, 261–275. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.; Wang, X.; Yu, B.; Ding, W.; Xiong, J.; Yang, Y.; Wang, L.; Yang, C. Shale characteristics in the southeastern Ordos Basin, China: Implications for hydrocarbon accumulation conditions and the potential of continental shales. Int. J. Coal Geol. 2014, 128–129, 32–46. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Zhu, R.; Tang, X.; Liu, K.; Jin, Z. Geological controls on the pore system of lacustrine unconventional shale reservoirs: The Triassic Chang 7 member in the Ordos Basin, China. Geoenergy Sci. Eng. 2023, 221, 111139. [Google Scholar] [CrossRef]
- Dai, J.; Zou, C.; Dong, D.; Ni, Y.; Wu, W.; Gong, D.; Wang, Y.; Huang, S.; Huang, J.; Fang, C.; et al. Geochemical characteristics of marine and terrestrial shale gas in China. Mar. Pet. Geol. 2016, 76, 444–463. [Google Scholar] [CrossRef]
- Cui, J.W.; Zhu, R.K.; Luo, Z.; Li, S. Sedimentary and geochemical characteristics of the Triassic Chang 7 member shale in the southeastern Ordos Basin, Central China. Pet. Sci. 2019, 16, 285–297. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; Zhang, Q.; Zhu, R.; Tang, X.; Liu, K.; Dong, L. Effects of clay minerals and organic matter on pore evolution of the early mature lacustrine shale in the Ordos Basin, China. J. Asian Earth Sci. 2023, 246, 105516. [Google Scholar] [CrossRef]
- Hou, L.-H.; Wu, S.-T.; Jing, Z.-H.; Jiang, X.-H.; Yu, Z.-C.; Hua, G.; Su, L.; Yu, C.; Liao, F.-R.; Tian, H. Effects of types and content of clay minerals on reservoir effectiveness for lacustrine organic matter rich shale. Fuel 2022, 327, 125043. [Google Scholar] [CrossRef]
- Hua, Y.; Xinshe, L.I.U. Progress in Paleozoic coal-derived gas exploration in the Ordos Basin, West China. Pet. Explor. Dev. 2014, 41, 144–152. [Google Scholar]
- Li, J.; Zhuang, X.; Querol, X.; Font, O.; Moreno, N. A review on the applications of coal combustion products in China. Int. Geol. Rev. 2018, 60, 671–716. [Google Scholar] [CrossRef]
- Liu, R.; Ren, Z.; Ye, H.; Ren, W.; Wang, K.; Huai, Y.; Qi, K. Potential evaluation of geothermal resources: Exemplifying some municipalities and key strata in Ordos Basin as a study case. Geol. Bull. China 2021, 40, 565–576. [Google Scholar]
- Jiu, B.; Huang, W.; Mu, N. Mineralogy and elemental geochemistry of Permo-Carboniferous Li-enriched coal in the southern Ordos Basin, China: Implications for modes of occurrence, controlling factors and sources of Li in coal. Ore Geol. Rev. 2022, 141, 104686. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.; Shan, Y.; Xiong, J. Upper Paleozoic coal measures and unconventional natural gas systems of the Ordos Basin, China. Geosci. Front. 2012, 3, 863–873. [Google Scholar] [CrossRef]
- Ritts, B.D.; Hanson, A.D.; Darby, B.J.; Nanson, L.; Berry, A. Sedimentary record of Triassic intraplate extension in North China: Evidence from the nonmarine NW Ordos Basin, Helan Shan and Zhuozi Shan. Tectonophysics 2004, 386, 177–202. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.-I.; Jones, D.P.; Wang, X. Prevention of Apoptosis by Bcl-2: Release of Cytochrome c from Mitochondria Blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, X.; Wang, X.; Shi, B.; Luo, X.; Zhang, L.; Lei, Y.; Jiang, C.; Liu, P. Gas geochemical evidences for biodegradation of shale gases in the Upper Triassic Yanchang Formation, Ordos Basin, China. Int. J. Coal Geol. 2017, 179, 139–152. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Chen, G.; He, L.; Xu, Y.; Chen, L.; Chen, D. Petrographic and geochemical characteristics of the lacustrine black shales from the Upper Triassic Yanchang Formation of the Ordos Basin, China: Implications for the organic matter accumulation. Mar. Pet. Geol. 2017, 86, 52–65. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Jiang, Z.; Yin, L.; Chen, M.; Liu, D. Major factors controlling lamina induced fractures in the Upper Triassic Yanchang formation tight oil reservoir, Ordos basin, China. J. Asian Earth Sci. 2018, 166, 107–119. [Google Scholar] [CrossRef]
- Yang, R.; Fan, A.; Han, Z.; van Loon, A. Lithofacies and origin of the Late Triassic muddy gravity-flow deposits in the Ordos Basin, central China. Mar. Pet. Geol. 2017, 85, 194–219. [Google Scholar] [CrossRef]
- Pan, S.; Zou, C.; Li, J.; Yang, Z.; Liu, E.; Han, Y. Unconventional shale systems: A comparative study of the “in-source sweet spot” developed in the lacustrine Chang 7 Shale and the marine Barnett Shale. Mar. Pet. Geol. 2018, 100, 540–550. [Google Scholar] [CrossRef]
- GB/T19145-2003; Determination of Total Organic Carbon in Sedimentary Rock. Chinese Standard: Beijing, China, 2003.
- Dubinin, M.M. Recent Equilibrium and Kinetic Studies of the Adsorption of Gases and Vapours by Microporous Adsorbents. Russ. Chem. Rev. 1977, 46, 1021. [Google Scholar] [CrossRef]
- Al-Ameri, T. Khasib and Tannuma oil sources, East Baghdad oil field, Iraq. Mar. Pet. Geol. 2011, 28, 880–894. [Google Scholar] [CrossRef]
- Tang, S.; Tang, D.; Liu, S.; Li, S.; Tang, J.; Wang, M.; Zhang, A.; Pu, Y. Multiscale pore characterization of coal measure reservoirs and gas storage and transport behavior in Yanchuannan gas field of China. AAPG Bull. 2022, 106, 2387–2415. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Fan, J.; Zhu, Y.B.; Wang, F.; Wu, H. Transport of shale gas in microporous/nanoporous media: Molecular to pore-scale simulations. Energy Fuels 2020, 35, 911–943. [Google Scholar] [CrossRef]
- Jaroniec, M.; Kruk, M.; Olivier, J.P. Standard Nitrogen Adsorption Data for Characterization of Nanoporous Silicas. Langmuir 1999, 15, 5410–5413. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Vishnyakov, A.; Russo, R.; Neimark, A.V. Unified approach to pore size characterization of microporous carbonaceous materials from N2, Ar, and CO2 adsorption isotherms. Langmuir 2000, 16, 2311–2320. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Wu, R.; Bi, J.; Zhang, K. Shale reservoir storage of hydrogen: Adsorption and diffusion on shale. Fuel 2024, 357, 129919. [Google Scholar] [CrossRef]
- Ho, T.A.; Dang, S.T.; Dasgupta, N.; Choudhary, A.; Rai, C.S.; Wang, Y. Nuclear magnetic resonance and molecular simulation study of H2 and CH4 adsorption onto shale and sandstone for hydrogen geological storage. Int. J. Hydrogen Energy 2024, 51, 158–166. [Google Scholar] [CrossRef]
- Kuila, U.; McCarty, D.K.; Derkowski, A.; Fischer, T.B.; Topór, T.; Prasad, M. Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 2014, 135, 359–373. [Google Scholar] [CrossRef]
- Saidian, M.; Godinez, L.J.; Prasad, M. Effect of clay and organic matter on nitrogen adsorption specific surface area and cation exchange capacity in shales (mudrocks). J. Nat. Gas Sci. Eng. 2016, 33, 1095–1106. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Chai, P.; Bian, J.; Wang, X.; Liu, Y.; Yin, X.; Pan, S.; Pan, Z. Pore Characterization of Different Clay Minerals and Its Impact on Methane Adsorption Capacity. Energy Fuels 2020, 34, 12204–12214. [Google Scholar] [CrossRef]

| Sample No. | Quartz | Microcline | Dolomite | Plagioclase | Pyrite | Illite | Kaolinite | Chlorite | Montmorillonite |
|---|---|---|---|---|---|---|---|---|---|
| 412 | 12 | 27 | 3 | 13 | 4 | 17 | 5 | 7 | 12 |
| 413 | 12 | 19 | 2 | 17 | 5 | 17 | 6 | 8 | 14 |
| 427 | 8 | 15 | 15 | 12 | 6 | 15 | 5 | 9 | 15 |
| Sample | Depth (m) | Lithology | Relative Clay Mineral Content (%) | Mixed-Layer Ratio, S (%) | |||
|---|---|---|---|---|---|---|---|
| I/S | I | K | C | I/S | |||
| 412 | 223.4 | Mudstone | 86 | 11 | 1 | 2 | 15 |
| 413 | 223.96 | Mudstone | 88 | 8 | 1 | 3 | 15 |
| 427 | 227.91 | Mudstone | 90 | 9 | n.d. | 1 | 15 |
| Samples | Quartz | Plagioclase | Microcline | Chlorite | Mica | Others |
|---|---|---|---|---|---|---|
| S1 | 26 | 27 | 16 | 7 | 23 | 1 |
| S2 | 8 | 74 | 10 | 2 | 6 | |
| S3 | 19 | 48 | 13 | 5 | 14 | 1 |
| S4 | 22 | 53 | 7 | 5 | 12 | 1 |
| S5 | 21 | 36 | 16 | 5 | 21 | 1 |
| S6 | 22 | 47 | 9 | 5 | 16 | 1 |
| S7 | 17 | 34 | 33 | 5 | 11 | |
| S8 | 21 | 36 | 20 | 8 | 15 | |
| S9 | 12 | 47 | 22 | 6 | 13 | |
| S10 | 15 | 55 | 11 | 5 | 5 | 9 |
| S11 | 17 | 15 | 6 | 6 | 56 |
| Sample No. | Tmax (°C) | S1 (mg/g) | S2 (mg/g) | TOC (wt %) | TS (wt %) |
|---|---|---|---|---|---|
| 412 | 435 | 2.58 | 42.4 | 9.684 | 3.86 |
| 413 | 433 | 2 | 34.69 | 7.992 | 5.19 |
| 427 | 436 | 4.11 | 72.99 | 14.32 | 2.54 |
| Sample No. | SSA (m2/g) | APD (nm) | TPV (cm3/g) |
|---|---|---|---|
| 412 | 5.811 | 32.02 | 0.047 |
| 413 | 6.568 | 30.69 | 0.050 |
| 427 | 6.097 | 32.50 | 0.050 |
| Sample No. | Temperature | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| VL | PL | R2 | n | k | R2 | ||
| 412 | 25 | 2.13 | 23.29 | 0.9918 | 1.2982 | 0.0176 | 0.99785 |
| 45 | 2.11 | 25.76 | 0.9968 | 1.3104 | 0.0163 | 0.9975 | |
| 65 | 1.35 | 16.76 | 0.9975 | 1.4024 | 0.018 | 0.9963 | |
| 413 | 25 | 3.83 | 37.33 | 0.9703 | 1.1588 | 0.0135 | 0.99932 |
| 45 | 3.45 | 38.26 | 0.9997 | 1.1674 | 0.0134 | 0.9989 | |
| 65 | 3.01 | 35.34 | 0.9934 | 1.3365 | 0.0194 | 0.99912 | |
| 427 | 25 | 3.85 | 38.90 | 0.9799 | 1.1674 | 0.0141 | 0.99943 |
| 45 | 3.04 | 30.06 | 0.9866 | 1.1605 | 0.0127 | 0.99954 | |
| 65 | 2.60 | 30.10 | 0.9863 | 1.1831 | 0.0127 | 0.99814 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jin, Z.; Wang, G.; Huang, X.; Su, Y.; Zhang, Q. Hydrogen Gas Adsorption of the Triassic Chang 7 Shale Member in the Ordos Basin, China. Sustainability 2024, 16, 1960. https://doi.org/10.3390/su16051960
Wang L, Jin Z, Wang G, Huang X, Su Y, Zhang Q. Hydrogen Gas Adsorption of the Triassic Chang 7 Shale Member in the Ordos Basin, China. Sustainability. 2024; 16(5):1960. https://doi.org/10.3390/su16051960
Chicago/Turabian StyleWang, Lu, Zhijun Jin, Guanping Wang, Xiaowei Huang, Yutong Su, and Qian Zhang. 2024. "Hydrogen Gas Adsorption of the Triassic Chang 7 Shale Member in the Ordos Basin, China" Sustainability 16, no. 5: 1960. https://doi.org/10.3390/su16051960
APA StyleWang, L., Jin, Z., Wang, G., Huang, X., Su, Y., & Zhang, Q. (2024). Hydrogen Gas Adsorption of the Triassic Chang 7 Shale Member in the Ordos Basin, China. Sustainability, 16(5), 1960. https://doi.org/10.3390/su16051960






