Abstract
Rapid economic development and increased demand for mineral products in China have led to extensive extraction of various ores, resulting in significant environmental challenges associated with the generation of industrial solid waste, particularly iron tailings. Despite being a major mining nation, China faces issues of wasteful practices, with substantial amounts of valuable elements lost during the processing of iron ore. This study addresses the urgent need for sustainable solutions by proposing an innovative approach for the recovery of valuable elements from iron tailings. The proposed process involves a sequential application of acid leaching, chemical precipitation, and Metal-Organic Frameworks (MOFs) ion adsorption. The pre-treated iron tailings were leached in HCl solution with pH 1.5 at 70 °C for 2 h, and the co-leaching efficiency of 98.1% V, 98.2% Mo, 99.3% Fe, and 98.7% Mg was obtained. Chemical precipitation is then employed to isolate Fe, Mg V, and Mo and promote the formation of targeted compounds, ensuring concentration and purity. The integration of MOF ion adsorption, known for its high surface area and tunable pore structures, provides an efficient platform for selectively capturing and recovering target ions. 97.7% V and 96.3% Mo were selectively extracted from Zirconium 1,4-carboxybenzene metal-organic framework (UiO-66) adsorption system with pH 5.0 at 30 °C for 6 h, and 91.7% V and 90.3% Mo were selectively extracted from 2-methylimidazole zinc salt metal-organic framework (ZIF-8) adsorption system with pH 5 at 30 °C for 6.0 h. This three-stage process offers an efficient method for the recovery of valuable elements from iron tailings.
1. Introduction
With the continuous enhancement of Chinese national strength and economic development, the demand for mineral products has surged, leading to an increase in the extraction of various ores [1]. Despite being a major mining nation, China currently faces severe issues related to wasteful practices in the extraction and processing of mineral resources. Statistics reveal that the average grade of selected iron ore in China is only 25.54%, resulting in significant waste production, with 2.5 to 3.0 tons of iron tailings generated per ton of iron ore produced [2]. The environmental impact of industrial solid waste further exacerbates the situation.
As mining activities continue, the depletion of resources from mines has uncovered the potential value of residual solid waste [3]. Current statistics from the “2020 National Annual Report on Environmental Pollution Control of Solid Waste in Large and Medium-sized Cities” indicate that industrial enterprises generated an astonishing 1.03 billion tons of tailings, with only 27.0% effectively utilized via comprehensive methods in 2019 [4]. The accumulation of tailings has reached a staggering 2.07 × 1010 tons by the end of 2018, particularly with iron tailings accounting for a substantial portion [5]. To maximize the sustainable development of various mineral resources, the comprehensive, effective, value-added, and ecological utilization of solid waste generated during the mining, selection, and smelting processes is imperative [6].
Iron tailings (IOTs), a significant component of industrial solid waste, are characterized by low concentrations of valuable elements [7]. As the population continues to grow and living standards rise, the demand for metals is bound to increase. Mining, as a foundational industry, plays a vital role in societal development, but it is also the largest waste-producing industry globally [8]. Following the extraction, processing, and smelting of ores, substantial amounts of solid waste, including waste rock, tailings, and slag, are generated [9]. The chemical and mineral composition of iron tailings depends on the mineralogical properties of processed iron ore, the nature of processing fluids used, processing efficiency, and weathering degree [10]. Iron tailings in China generally exhibit characteristics such as low grade, fine particle size, and susceptibility to mud formation. In the process of extracting valuable minerals or metals from iron ore, the residual material with a grade lower than the extraction grade is formed as iron tailings [11]. These tailings are then mixed with process water and chemical reagents to form a slurry, which is transported through pipelines to nearby tailings storage facilities [12]. Subsequently, using pressure filtration or gravity, iron tailings are separated from process water, allowing the recycled upper layer of process water to be reintroduced into the extraction process [13]. However, as ore grinding continues, the particle size of iron tailings becomes finer, and some clay minerals absorb water, making it challenging to separate the remaining tailings from water [14]. Massive storage of iron tailings in tailings ponds or tailings dams not only harms the environment but also poses a serious threat to human life safety [15]. Additionally, the significant amount of valuable elements contained in iron tailings results in substantial resource wastage due to inadequate utilization [16]. The chemical composition of iron tailings varies based on geological conditions and formation factors of the mining site [17]. According to the 2020 China Environmental Statistics Yearbook, in 2019, China’s production of iron tailings reached 5.36 × 108 tons, but only 1.16 × 108 tons were comprehensively utilized, leading to a utilization rate of less than 30% [18]. Traditional tailings storage involves direct discharge of low-concentration tailings into tailings ponds [19]. However, these tailings ponds are proven to be highly unstable, and their dam failure or leakage could result in catastrophic consequences for human, economic, and environmental well-being [20]. The inadequate management of tailings has led to the accumulation of over 250 billion tons of solid waste from mining activities in China, with approximately 13,000 tailings ponds, exceeding 100 billion tons in total [21]. Furthermore, iron tailings, being composed of fine particles, lack the ability to fix organic substances and are prone to infiltrate groundwater via rainwater runoff [22]. This infiltration can negatively impact the local soil structure and contaminate the groundwater environment [23].
Given the challenges posed by the substantial production of iron tailings and the urgent need for sustainable solutions [24], this study explores a novel approach to the recovery of valuable elements from iron tailings. The proposed process involves a sequence of acid leaching, chemical precipitation, and Metal-Organic Frameworks (MOFs) ion adsorption [25]. In the acid-leaching stage, iron tailings are subjected to a controlled acidic environment to selectively dissolve and extract valuable elements. This step is crucial for enhancing the efficiency of subsequent recovery processes [26]. Following acid leaching, chemical precipitation is employed to isolate specific elements and promote the formation of targeted compounds. This ensures the concentration and purity of the extracted valuable elements, laying the foundation for subsequent utilization [27]. The integration of MOF ion adsorption represents a cutting-edge technique for selectively capturing and recovering target ions from the solution. MOFs, known for their high surface area and tunable pore structures, provide an efficient platform for ion adsorption [28]. The combination of these three stages—acid leaching, chemical precipitation, and MOF ion adsorption—offers a comprehensive and efficient process for the recovery of valuable elements from iron tailings [29]. This approach not only addresses the environmental challenges associated with iron tailings but also aligns with the broader goals of sustainable development and resource conservation outlined in China’s national policies [30]. By implementing this advanced recovery process, we aim to contribute to the eco-friendly and economically viable utilization of iron tailings, thereby reducing the environmental footprint of mining activities [31]. This research endeavors to provide a valuable contribution to the ongoing efforts toward creating a circular economy in the mining industry, where waste is transformed into resources using innovative and sustainable technologies [32].
Therefore, the sustainable management of industrial solid waste, particularly iron tailings, is imperative for achieving the goals of ecological civilization and sustainable development [33]. This study introduces a novel recovery process that integrates acid leaching, chemical precipitation, and MOF ion adsorption, offering a promising avenue for the efficient extraction of valuable elements from iron tailings [34]. The proposed approach aligns with the principles of resource conservation, environmental protection, and circular economy advocated in China’s national policies. Via the implementation of advanced technologies and comprehensive utilization strategies, we aspire to mitigate the environmental impact of mining activities and contribute to the creation of a more sustainable and eco-friendly mining industry [35]. The proposed recovery process provides a theoretical basis for the treatment and disposal of iron tailings.
2. Materials and Methods
2.1. Study Area
The Turpan region is located in the Xinjiang Uygur Autonomous Region in northwestern China (Figure 1). This region, characterized by its arid climate and unique topography, is part of the Tianshan metallogenic belt, known for its rich mineral resources. The iron ore deposits in Turpan are primarily sedimentary-metamorphic and hydrothermal-metamorphic in origin. These deposits are known for their high-grade iron content, with some areas exhibiting ore with iron content as high as 60%. The predominant form of iron ore found in this region is magnetite, often accompanied by smaller amounts of hematite and siderite. The extraction of iron ore in Turpan has been an ongoing activity, contributing significantly to the local economy and the broader iron and steel industry in China. Mining operations in the region employ both open-pit and underground mining methods, considering the depth and nature of the ore bodies. The mining sector in Turpan is supported by well-established infrastructure, including transportation and processing facilities, which aid in the efficient production and distribution of iron ore. While mining activities in Turpan of China have contributed to economic growth, they have also created environmental challenges, such as land degradation, depletion of water resources, and air pollution, which are of concern.
Figure 1.
Study area location map.
2.2. Chemicals and Reagents
Iron tailings were obtained from an iron mine located in Turpan City, Xinjiang Province, China. Impurities such as fallen leaves, stones, and so on are simply removed from the sampled iron tailings. Secondly, the iron tailings are mixed evenly on the kraft paper; the iron tailings with large particles are initially broken and dried in the laboratory fume hood (STFG-01, Shenzheng SUNOLAB, Shenzheng, China). The ventilation time was 7 days, and it was turned and mixed every day. After the drying time was over, the iron tailings were sampled by quartering method and then dried in a 105 °C electric blast (XMTA-6000-L, Hunan Lianzhou electrical appliance, Zhuzhou, China) drying oven for ½ d. The dried iron tailings were ground to the required particle size (100 µm) by a planetary ball mill (QM-3SP2, Nanjing NADA Instrument, Nanjing, China) and stored in a sealed sample box [36]. Two groups of parallel samples were analyzed in the follow-up experiments.
Zirconium 1,4-carboxybenzene metal-organic framework (UiO-66, C₄₈H₂₈O₃₂Zr₆) and 2-methylimidazole zinc salt metal-organic framework (ZIF-8, C4H6N2Zn) were acquired from Aladdin Chemical Co., Ltd. (Shanghai, China), while other chemical reagents were sourced from Sinopharm Chemical Co., Ltd. (Shanghai, China).
2.3. Experimental Procedure
The ground iron tailings, weighing 10 g, were placed into a beaker. Two adsorption reaction systems, UiO-66 and ZIF-8, were prepared to compare their extraction efficiencies of V and Mo. In the UiO-66 adsorption system, UiO-66 with a mass ratio of 0.01 was sequentially added to an HCl solution with a concentration ranging from 0.5 to 2.5 mol/L. In the ZIF-8 adsorption system, ZIF-8 with a mass ratio of 0.01 was simultaneously added to an HCl solution with a concentration ranging from 0.5 to 2.5 mol/L. Subsequently, a specific amount of iron tailings was introduced into the adsorption reaction system, and the leaching process was carried out at temperatures between 10 °C and 50 °C for 1.0 h to 9.0 h at a liquid-solid ratio of 6–14 [37]. The leaching solutions underwent high-speed centrifugation to separate the leachate and residue. The pH of the leachate was adjusted to 10 using HCl and NaOH solutions to precipitate and remove Fe and Mg. After removing Fe and Mg, the leachates were analyzed to determine the concentrations of V and Mo in the solutions. V and Mo leaching efficiencies were evaluated using thiocyanate spectrophotometrically colorimetry using an ultraviolet spectrophotometer [38]. The leaching residues were dried in an oven at 120 °C for 48 h to obtain dried samples for further characterization. All experiments in this study were conducted three times for consistency.
2.4. Response Surface Methodology Simulation
The Response Surface Methodology (RSM) employed a quadratic regression equation with multiple variables to model the functional relationship between factors and response values [39]. Design Expert 13 software and the Box–Behnken model were utilized to optimize factor combinations. Following a review of relevant literature studies and preliminary experiments, pH, temperature, time, and liquid-solid ratio were chosen as the four factors for further analysis. The extraction efficiency of V and Mo served as the response value, and the experimental combinations were designed using the response surface methodology.
3. Results
3.1. V, Mo, Fe, and Mg Leaching Efficiency in HCl Leaching System
The element detection of XRF is shown in Table 1. The results showed that SiO2, Al2O3, and Fe2O3 were the main components of the Iron tailings, and the high content of MgO and V2O5 proves the great recovery potential of the iron tailings [40].

Table 1.
Main composition of iron tailings analyzed using XRF.
The leaching efficiencies of V, Mo, Fe, and Mg in HCl leaching systems were depicted in Figure 2 and Figure 3. In HCl leaching systems, the leaching efficiencies of V, Mo, Fe, and Mg were affected by pH, leaching time, temperature, and liquid-solid ratio. The optimal pH was found to be 2.5, as V, Mo, Fe, and Mg efficiencies initially increased with pH but declined at higher levels. Similarly, the leaching time influenced the leaching efficiencies of V, Mo, Fe, and Mg. V, Mo, Fe, and Mg showed increased efficiency up to 2.0 h, then a decline at 3.0 h, making 3.0 h the optimal duration [41]. Temperature also played an important role. The highest efficiency for all metals was achieved at 70 °C, with declines observed at 90 °C [42]. Additionally, the optimal liquid-solid ratio was determined to be 10, as increasing the ratio beyond this point led to decreased efficiency. A total of 98.1% V, 98.2% Mo, 99.3% Fe, and 98.7% Mg were leached under optimal conditions. Notably, Al did not leach under these conditions due to the formation of a protective Al2O3 layer in the acidic environment. The study’s findings offer a theoretical basis for the recovery and treatment of iron tailings via acid leaching processes.
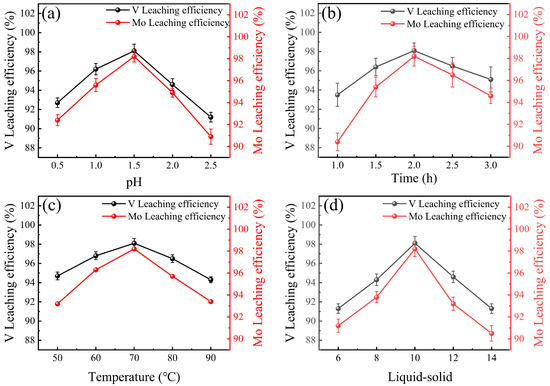
Figure 2.
Influences of (a) pH, (b) time, (c) temperature and (d) liquid-solid ratio on the leaching efficiencies of V and Mo.
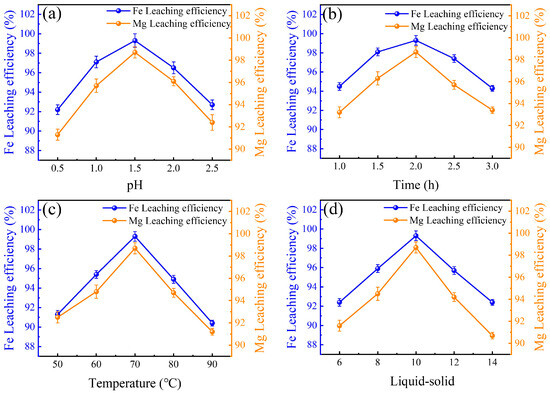
Figure 3.
Influences of (a) pH, (b) time, (c) temperature, and (d) liquid-solid ratio on the leaching efficiencies of Fe and Mg.
3.2. V and Mo Extraction Efficiencies in UiO-66 Adsorption Systems
Before the ion adsorption, NaOH was added to adjust the pH to 10.0, and Fe and Mg generated chemical precipitation to achieve the effect of metal separation. The extraction efficiencies of V and Mo in various adsorption systems are illustrated in Figure 4 and Figure 5. In the UiO-66 adsorption system, the extraction efficiencies of V and Mo varied with pH, extraction time, temperature, and liquid-solid ratio. Optimal extraction of V and Mo occurred at a pH of 5.0, with efficiencies peaking at 97.7% for V and 96.3% for Mo [43]. Both metals reached their highest extraction efficiency within 5 h. Extending time to 9.0 h showed no significant improvement. The optimal extraction temperature was found to be 30 °C, as higher temperatures led to reduced efficiencies and complex decomposition. The optimal liquid-solid ratio was determined to be 10, with higher ratios resulting in decreased efficiencies. These findings suggest that UiO-66, a metal-organic framework, is most effective under specific conditions of pH, time, temperature, and liquid-solid ratio for extracting V and Mo.
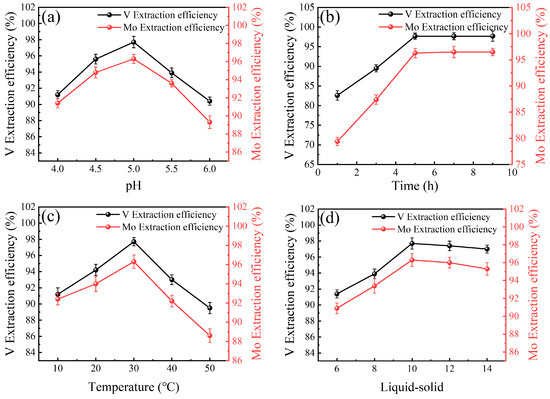
Figure 4.
Influences of (a) pH, (b) time, (c) temperature, and (d) liquid-solid ratio on the extraction efficiencies of V and Mo in the UiO-66 adsorption systems.
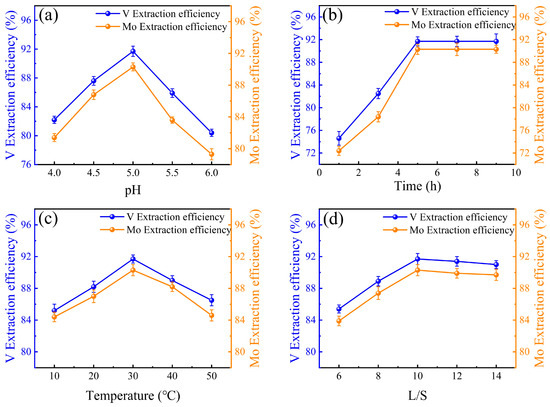
Figure 5.
Influences of (a) pH, (b) time, (c) temperature, and (d) liquid-solid ratio on the extraction efficiencies of V and Mo in the ZIF-8 adsorption systems.
3.3. V and Mo Extraction Efficiencies in ZIF-8 Adsorption Systems
In the ZIF-8 adsorption system, the extraction efficiencies of V and Mo were influenced by pH, extraction time, temperature, and liquid-solid ratio. The optimal pH for both V and Mo extractions was 5.0, as the efficiency increased at this pH but decreased at pH 6.0. ZIF-8, serving as a metal-organic framework, exhibits optimal adsorption under weak acidic or weak alkaline conditions [44]. The extraction efficiency of V rose from 82.2% to 91.7%, and Mo extraction efficiency increased from 81.4% to 90.3% at this optimal pH. The extraction efficiency improved with time, peaking at 5.0 h for both V and Mo. V extraction efficiency increased from 74.6% to 91.7%, and Mo extraction efficiency from 72.4% to 90.3% within this time frame. No significant improvement was observed when the extraction time was extended to 9.0 h. Temperature also played a crucial role, with the highest extraction efficiencies for both V and Mo occurring at 30 °C [45]. Above this temperature, the efficiency decreased, indicating that excessively high temperatures lead to complex decomposition. The liquid-solid ratio impacted extraction efficiency as well. The optimal ratio was determined to be 10.0, as increasing the ratio beyond this point led to a decrease in extraction efficiency for both V and Mo. Overall, the UiO-66 adsorption system was found to be more efficient than ZIF-8 in the extraction of V and Mo. UiO-66 has a wider pore structure and greater adsorption capacity, which is more suitable for the adsorption of metal macromolecular structures. The structural differences between UiO-66 and ZIF-8 affect their ion adsorption capacity, as shown in Figure 6.
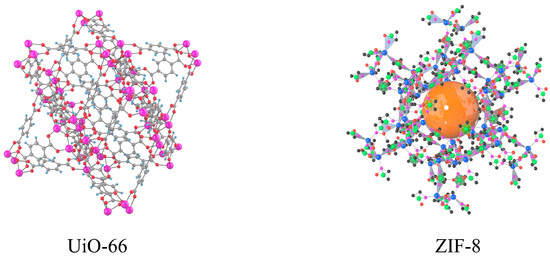
Figure 6.
Structure diagram of UiO-66 and ZIF-8.
4. Discussion
4.1. Box–Behnken Design
The Box–Behnken design is a quadratic design that does not contain any points at the corners of the process space. It’s composed of a central point and the middle points of the edges of the process space. This design is less expensive to run than a full factorial design because it requires fewer experiments for a given number of factors. The data from a Box–Behnken experiment are typically analyzed using multiple regression techniques to fit quadratic models. The analysis aims to find an optimal setting of the independent variables that either maximizes or minimizes the response variable. In conclusion, the Box–Behnken design is a valuable tool for experimenters looking to optimize a process with three or more variables, especially in situations where running a full factorial design is impractical or too costly. This model provides a balance between the amount of information obtained and the resources required to conduct experiments.
4.2. Data Fitting and Model Building
According to these data and results, Box–Behnken model and model building were used in Design Expert 13 for data fitting. According to the single factor experimental results, pH (A), temperature (B), time (C) and liquid-solid ratio (D)were selected as the influencing factors. V and Mo extraction efficiency (y) were the response value, and response surface analysis method was used to optimize the experiment, in order to determine the model and the best experimental conditions [46]. Table 2 showed the experimental design and results of Box–Behnken. The experimental data obtained in Table 2 were fitted by multiple regression, and the oil removal rate (y) was obtained.

Table 2.
Experimental design and results of Box–Behnken.
4.3. Model Analysis
Variance analysis was performed on the regression model, and the results were shown in Table 3, Table 4 and Table 5. R2 of the V extraction efficiency response surface model is 0.9718 in Table 4. It can be seen from Table 3, Table 4 and Table 5 that the F-values of the regression model are 34.41 and 16.63, respectively, p < 0.0001, indicating that the model has high reliability and significance [47]. The missing items were not significant (p > 0.05), indicating a good fit between the model and the experimental values [48]. Table 3 showed that the independent variables C of V extraction efficiency were significant (p < 0.0001), and the interaction term CD was significant (p < 0.05), indicating that extraction time and liquid-solid ratio had significant effects on the extraction efficiency of V [49]. Table 5 showed that the independent variables C of Mo extraction efficiency were significant (p < 0.0001), and the interaction term AC was significant (p < 0.05), indicating that extraction time and liquid-solid ratio had significant effects on the extraction efficiency of Mo [50].

Table 3.
ANOVA for quadratic model of V extraction efficiency.

Table 4.
Fit Statistics of V extraction efficiency.

Table 5.
ANOVA for quadratic model of Mo extraction efficiency.
The interactive influence of various factors on the extraction efficiency of V is shown in Figure 7. The degree of influence of various factors on the response value was related to the slope of the response surface [51]. The steeper the slope of the surface, the more significant the influence of the corresponding response factors [52]. Combined with Figure 7 and Table 3, it can be seen that the influences on the extraction efficiency of V are time, liquid-solid ratio, temperature, and pH. p value of time (C) was less than 0.0001, and the p value of time and liquid-solid ratio interaction term (CD) was 0.0128 [53]. The interaction of surface time and liquid-solid ratio has a great influence on the extraction efficiency of V [54]. The interactive influence of various factors on the extraction efficiency of Mo is shown in Figure 8. Combined with Figure 8 and Table 5, it can be seen that the influences on the extraction efficiency of Mo are time, liquid-solid ratio, temperature, and pH in turn. p value of time (C) was less than 0.0001, and the p value of pH and time interaction term was 0.0446. The interaction of surface pH and time had a great influence on the extraction efficiency of Mo [55]. R2 of the Mo extraction efficiency response surface model is 0.9433 in Table 6. Based on the above data and discussion results, we summarized the corresponding multivariate linear equation, as shown in Equations (1) and (2).
V extraction efficiency = 97.70 − 0.86 × A (pH) − 1.69 × B (Temperature) + 6.73 × C (Time) + 2.07 × D (L/S) + 0.25 × AB − 0.15 × AC + 0.78 × AD + 1.58 × BC + 0.40 × BD + 2.43 × CD − 7.35 × A2 − 7.50 × B2 − 6.49 × C2 − 2.84 × D2
Mo extraction efficiency = 96.30 − 0.58 × A (pH) − 1.83 × B (Temperature) + 8.73 × C (Time) + 2.46 × D (L/S) + 0.93 × AB − 3.20 × AC + 0.28 × AD + 1.18 × BC + 0.10 × BD + 2.10 × CD − 8.21 × A2 − 7.26 × B2 − 7.77 × C2 − 1.98 × D2
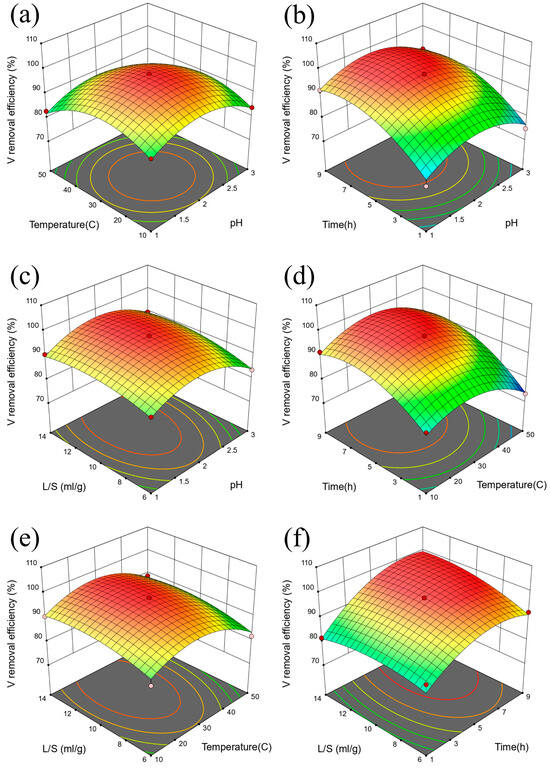
Figure 7.
Response surface diagram of V extraction efficiency for (a) temperature and pH, (b) time and pH, (c) liquid-solid ratio and pH, (d) time and temperature, (e). liquid-solid ratio and temperature, and (f). liquid-solid ratio and time.
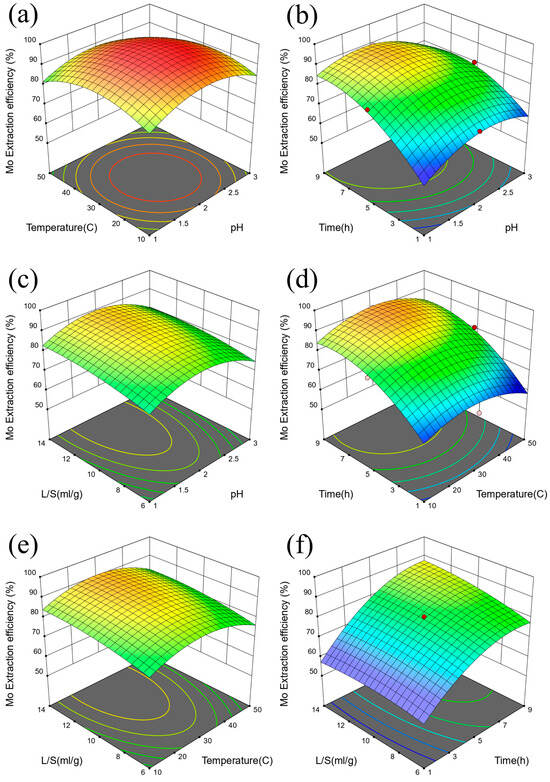
Figure 8.
Response surface diagram of Mo extraction efficiency for (a) temperature and pH, (b) time and pH, (c) liquid-solid ratio and pH, (d) time and temperature, (e). liquid-solid ratio and temperature, and (f). liquid-solid ratio and time.

Table 6.
Fit Statistics of Mo extraction efficiency.
4.4. Iron Tailings Treatment Process Summary
In this study, the method of acid leaching, MOF adsorption, and chemical precipitation was adopted to recover V, Mo, Fe, and Mg from iron tailings (Figure 9) [56]. XRF testing for leaching residue is shown in Table 7. According to the results in Table 7, SiO2 dominated the leaching residue, and the remaining elements were less than 5%. The leached residue was almost non-toxic and could be used as a building material. The whole process had low energy consumption, low equipment requirements, and significant recovery efficiency, which was in line with the national policy of sustainable development of resource exploitation and utilization [57]. It provided a new idea for the follow-up research.
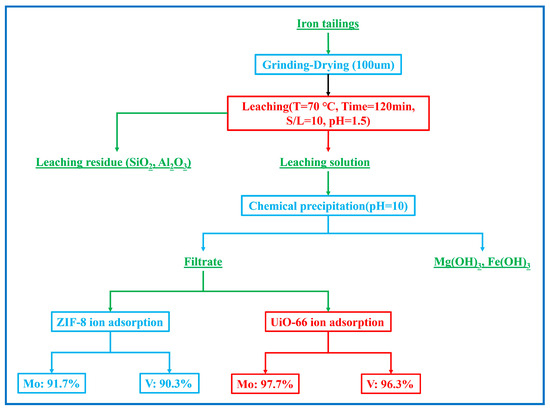
Figure 9.
A high-efficient recovery flow chart for iron tailings.

Table 7.
Main composition of leach residue analyzed by XRF.
4.5. Environmental Impact and Economic Assessment
The process represents a significant advancement in addressing environmental challenges and economic efficiency in China’s mining industry. This innovative approach effectively transforms iron tailings, a prevalent industrial waste, into a valuable source of metals like V, Mo, Fe, and Mg. With co-leaching efficiencies surpassing 98%, this method not only reduces the environmental impact by minimizing waste volume but also mitigates the costs associated with waste management and ecological restoration.
The sequential application of acid leaching, chemical precipitation, and MOF ion adsorption ensures the recovery of metals with high efficiency and purity. MOFs, with their large surface area and customizable pore structures, offer enhanced selectivity in ion adsorption, making the process highly efficient and potentially more profitable. This efficiency is crucial in creating marketable products from waste materials.
From an economic perspective, while the initial investment in this technology might be substantial, the long-term benefits are considerable. Recovering valuable metals from tailings reduces the need for primary mining, lowers operational costs, and creates additional revenue streams. The market demand for these metals, coupled with the sustainable nature of their recovery, adds substantial value to the mining sector.
Environmentally, it significantly reduces waste volume, mitigating pollution and land use for waste disposal while also conserving natural resources by lowering the dependency on virgin ore extraction. The integration of MOFs for selective metal recovery further underscores its alignment with sustainable mining practices, potentially setting new industry standards. Overall, this innovative process demonstrates a promising balance between enhancing economic viability and promoting environmental sustainability, contributing to the principles of a circular economy in the mining sector.
5. Conclusions
5.1. Data Summary
In order to solve the problem of resource waste and environmental impact caused by a large amount of iron tailings, this study systematically explored a new method of high-value recovery of iron tailings from Turpan, Xinjiang, China. First, the co-leaching of V, Mo, Fe, and Mg was obtained by acid leaching method using only hydrochloric acid. Compared with the roasting method, this method was easier to operate and had higher recovery efficiency. MOF material (UiO-66 and ZIF-8) was used to selectively adsorb V and Mo in co-leaching metals. Compared with the traditional chemical precipitation method, this method had higher selectivity and could maximize the separation of magazines and target metals. Specific research results are shown below:
1. The pre-treated iron tailings underwent a meticulous leaching process in an HCl solution with a pH of 1.5, maintained at 70 °C for 2.0 h. Remarkably, this process yielded an impressive co-leaching efficiency, with 98.1% V, 98.2% Mo, 99.3% Fe, and 98.7% Mg leached.
2. 97.7% V and 96.3% Mo were selectively extracted from the UiO-66 adsorption system with pH 5.0 at 30 °C for 6 h, and 91.7% V and 90.3% Mo were selectively extracted from ZIF-8 adsorption system with pH 5.0 at 30 °C for 6.0 h.
3. The Model F-value of 34.41 (ANOVA for Quadratic model of V extraction efficiency) and 16.63 (ANOVA for Quadratic model of Mo extraction efficiency) imply the model of V and Mo are significant. There is only a 0.01% chance that an F-value this large could occur due to noise. The predicted R² of V and Mo extraction efficiency were 0.8373 and 0.6733. The adjusted R² of V and Mo extraction efficiency were 0.9435 and 0.8866. The ratio of 21.158 (V extraction efficiency) and 15.348 (Mo extraction efficiency) indicate an adequate signal. This model can be used to navigate the design space.
4. The three-stage process outlined in this study emerges as a comprehensive and efficient method for recovering valuable elements from iron tailings. By addressing environmental challenges and aligning with sustainable development goals, this innovative approach not only showcases promising extraction efficiencies but also underscores the potential for transformative advancements in the mining industry’s ecological footprint.
5.2. Deficiency and Prospect
In this study, the recycling technology route of HCl leaching and MOF ion adsorption is proposed to recover V, Mo, Fe, and Mg in iron tailings, but due to the limitations of various conditions, it needs further in-depth research and improvement:
(1) This study selects a chemical method for leaching V, Mo, Fe, and Mg, which is limited to laboratory operation and is not suitable for practical application in factories in large quantities.
(2) Adsorption experiment application laboratory preparation of a variety of heavy metal ion wastewater, the actual water quality is relatively complex, the prepared adsorbent should be used for the actual working conditions of water to judge the performance of the adsorbent.
(3) The application effect of the high specific surface area of MOFs as adsorbent and catalyst in the environmental field needs to be verified by further experiments.
Author Contributions
Conceptualization, Z.W. and W.G.; data processing, J.D.; software, T.Z.; validation, Y.Z.; writing—original draft preparation, Y.K.; writing—review and editing, Z.W. and L.F.; visualization, W.G. and X.B.; supervision, X.B. and F.H.; methodology, G.L. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Key Research and Development Project in Turpan, Xinjiang, China (Grant number 2023007). The financial support of Xinjiang Uygur Autonomous Region youth top innovative talent project (Research on Damage and Restoration Mechanism of Gravel Strata under Mining Impact in Desert Mining Area).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Zhongming Wu was employed by the company China Xinjiang Zhongtai Resource Management Co., Ltd. Authors Jianxin Deng, Tuanwei Zhao, Yang Zhou, Yongfu Kang, Xiangxiang Bai, Fei Hong and Longfei Fu were employed by the company Small Open-Pit Coal Mine in Heishan Mining Area, Toksun County, Xinjiang Shengxiong Energy Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tao, L.; Wang, L.; Yang, K.; Wang, X.; Chen, L.; Ning, P. Leaching of iron from copper tailings by sulfuric acid: Behavior, kinetics and mechanism. RSC Adv. 2021, 11, 5741–5752. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Li, J.-W. Zircon and titanite U-Pb dating of the Zhangjiawa iron skarn deposit, Luxi district, North China Craton: Implications for a craton-wide iron skarn mineralization. Ore Geol. Rev. 2017, 89, 309–323. [Google Scholar] [CrossRef]
- Yao, Z.; Qin, B.; Huang, Z.; Ruan, J.; Xu, Z. Green Combined Resource Recycling System for the Recycling of Waste Glass. ACS Sustain. Chem. Eng. 2021, 9, 7361–7368. [Google Scholar] [CrossRef]
- Singh, N.; Tang, Y.; Ogunseitan, O.A. Environmentally Sustainable Management of Used Personal Protective Equipment. Environ. Sci. Technol. 2020, 54, 8500–8502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, M.; He, J.; Liu, C.; Yao, Y.; Xu, J.; Liu, B.; Yin, S.; Xu, X. Recovery of metal fractions from waste printed circuit boards via the vibrated gas-solid fluidized bed. Adv. Powder Technol. 2021, 32, 370–377. [Google Scholar] [CrossRef]
- Bildstein, O.; Claret, F.; Frugier, P. RTM for Waste Repositories. Rev. Mineral. Geochem. 2019, 85, 419–457. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Lu, D.; Gu, X.; Chen, C. The detoxification and utilization of cyanide tailings: A critical review. J. Clean. Prod. 2021, 302, 126946. [Google Scholar] [CrossRef]
- Liu, R.; Huang, F.; Du, R.; Zhao, C.; Li, Y.; Yu, H. Recycling and utilisation of industrial solid waste: An explorative study on gold deposit tailings of ductile shear zone type in China. Waste Manag. Res. J. A Sustain. Circ. Econ. 2015, 33, 570–577. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Zhou, F.-S.; Evelina, L.A.; Liu, J.-L.; Zhou, Y. A review on the industrial solid waste application in pelletizing additives: Composition, mechanism and process characteristics. J. Hazard. Mater. 2022, 423, 127056. [Google Scholar] [CrossRef]
- Rizk, H.E.; Ahmed, I.M.; Metwally, S.S. Selective sorption and separation of molybdenum ion from some fission products by impregnated perlite. Chem. Eng. Process. Process Intensif. 2018, 124, 131–136. [Google Scholar] [CrossRef]
- Smorokov, A.; Kantaev, A.; Bryankin, D.; Miklashevich, A.; Kamarou, M.; Romanovski, V. Low-temperature method for desiliconization of polymetallic slags by ammonium bifluoride solution. Environ. Sci. Pollut. Res. 2022, 30, 30271–30280. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Liu, Y.; Zhou, J.-K.; Liu, M.-D.; Liu, Z.-Z. Recovery of vanadium and molybdenum from spent petrochemical catalyst by microwave-assisted leaching. Int. J. Miner. Metall. Mater. 2019, 26, 33–40. [Google Scholar] [CrossRef]
- Yin, H.; Ma, M.; Bai, J.; Li, Y.; Zhang, S.; Wang, F. Fabrication of foam glass from iron tailings. Mater. Lett. 2016, 185, 511–513. [Google Scholar] [CrossRef]
- Cai, P.-T.; Chen, T.; Chen, B.; Wang, Y.-C.; Ma, Z.-Y.; Yan, J.-H. The impact of pollutant emissions from co-incineration of industrial waste in municipal solid waste incinerators. Fuel 2023, 352, 129027. [Google Scholar] [CrossRef]
- Cai, Y.; Nie, Z.; Ma, L.; Xi, X. Closed-loop recovery of molybdenum and value-added reuse of tungsten from alloy waste in additive manufacturing. J. Environ. Manag. 2023, 348, 119270. [Google Scholar] [CrossRef] [PubMed]
- Michiels, K.; Haesen, A.; Meynen, V.; Spooren, J. Applicability of fine industrial metallic iron-rich waste powders for hydrothermal production of hydrogen gas: The influence of non-ferrous contaminants. J. Clean. Prod. 2018, 195, 674–686. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Deng, Y.; Liu, T.; Li, H. A leaching; solvent extraction; stripping, precipitation and calcination process for the recovery of MoO3 and NiO from spent hydrofining catalysts. Hydrometallurgy 2023, 218, 106046. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Pan, X.; Zhao, X.; Guo, P. Eco-friendly strategy for preparation of high-purity silica from high-silica IOTs using S-HGMS coupling with ultrasound-assisted fluorine-free acid leaching technology. J. Environ. Manag. 2023, 339, 117932. [Google Scholar] [CrossRef]
- Lian, J.; Zhou, F.; Chen, B.; Yang, M.; Wang, S.; Liu, Z.; Niu, S. Enhanced adsorption of molybdenum(VI) onto drinking water treatment residues modified by thermal treatment and acid activation. J. Clean. Prod. 2020, 244, 118719. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, K.; Su, Y.; Shi, Y. An evaluation of iron ore tailings characteristics and iron ore tailings concrete properties. Constr. Build. Mater. 2021, 286, 122968. [Google Scholar] [CrossRef]
- Liu, K.; Wang, M.; Tsang, D.C.W.; Liu, L.; Tan, Q.; Li, J. Facile path for copper recovery from waste printed circuit boards via mechanochemical approach. J. Hazard. Mater. 2022, 440, 129638. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ning, X.-A.; Shen, J.; Ou, W.; Chen, J.; Qiu, G.; Wang, Y.; He, Y. Biomass waste as a clean reductant for iron recovery of iron tailings by magnetization roasting. J. Environ. Manag. 2022, 317, 115435. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Dong, Z.; Jin, Y.; Cui, H. Comparison on micromechanical properties of interfacial transition zone in concrete with iron ore tailings or crushed gravel as aggregate. J. Clean. Prod. 2021, 319, 128737. [Google Scholar] [CrossRef]
- Wang, M.; Wei, C.; Fan, G.; Li, M.; Deng, Z.; Wang, S. Selective extraction of Mo from a Ni–Mo ore using pressure alkaline leaching. Hydrometallurgy 2015, 153, 6–11. [Google Scholar] [CrossRef]
- Ling, S.; Slater, B. Dynamic acidity in defective UiO-66. Chem. Sci. 2016, 7, 4706–4712. [Google Scholar] [CrossRef]
- Tan, H.; Fan, B.; Zheng, S.; Zhang, Y. Recovery and Separation of Vanadium, Nickel, and Molybdenum from the Industrial Waste of a Petroleum Refinery by a Complexation Method. ACS Sustain. Chem. Eng. 2023, 11, 4894–4902. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Zhang, T.; Zhang, G.; Yang, X.; He, Y.; Wang, L.; Duan, C. Metals recovery from dust derived from recycling line of waste printed circuit boards. J. Clean. Prod. 2017, 165, 452–457. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Sun, L.; Liu, Q.; Fei, Z.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; Qiao, X. Pore-expanded UiO-66 pellets for efficient bisphenol A adsorption. Chem. Eng. J. 2023, 455, 140843. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Wu, H.; Mu, L. Persistence and Recovery of ZIF-8 and ZIF-67 Phytotoxicity. Environ. Sci. Technol. 2021, 55, 15301–15312. [Google Scholar] [CrossRef]
- Wei, Z.; Jia, Y.; Wang, S.; Zhou, Z.; Zhang, Z.; Wang, X.; Huang, X.; Gao, Y. Influence of iron tailing filler on rheological behavior of asphalt mastic. Constr. Build. Mater. 2022, 352, 129047. [Google Scholar] [CrossRef]
- Galvão, J.L.B.; Andrade, H.D.; Brigolini, G.J.; Peixoto, R.A.F.; Mendes, J.C. Reuse of iron ore tailings from tailings dams as pigment for sustainable paints. J. Clean. Prod. 2018, 200, 412–422. [Google Scholar] [CrossRef]
- Han, F.; Song, S.; Liu, J.; Huang, S. Properties of steam-cured precast concrete containing iron tailing powder. Powder Technol. 2019, 345, 292–299. [Google Scholar] [CrossRef]
- Bruhn, N.C.P.; Viglioni, M.T.D.; Nunes, R.F.; Calegario, C.L.L. Recyclable waste in Brazilian municipalities: A spatial-temporal analysis before and after the national policy on solid waste. J. Clean. Prod. 2023, 421, 138503. [Google Scholar] [CrossRef]
- Hu, Z.; Li, W.; Duan, H.; Huang, X.; Meng, J.; Yang, L.; Zheng, M. An integrated approach to vivianite recovery from waste activated sludge. Bioresour. Technol. 2023, 371, 128608. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, G.; Luo, K.; Zhang, J.; Li, H.; Li, G.; Zhang, J.; Chen, X.; Xie, F. Synthesis of magnesium-modified ceramsite from iron tailings as efficient adsorbent for phosphorus removal. Sep. Purif. Technol. 2023, 326, 124817. [Google Scholar] [CrossRef]
- Wang, T.; Ren, J.; Ravindra, A.V.; Lv, Y.; Le, T.; Ni, K.O. V and Fe Leaching from a Spent Catalyst in Microwave-Assisted Acid Activation Process. Molecules 2022, 27, 2078. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, G.; Barik, S.P. Process for recovery of metal values from alnico scarps by electro-chemical leaching technique. Sep. Purif. Technol. 2016, 169, 78–82. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Wang, H.; Li, H.; Wu, H. Separation of V (V) and Mo (VI) in roasting-water leaching solution of spent hydrodesulfurization catalyst by co-extraction using P507—N235 extractant. Sep. Purif. Technol. 2020, 248, 117135. [Google Scholar] [CrossRef]
- Majdi, H.; Esfahani, J.A.; Mohebbi, M. Optimization of convective drying by response surface methodology. Comput. Electron. Agric. 2019, 156, 574–584. [Google Scholar] [CrossRef]
- Shi, Z.; Ding, Y.; Ren, J.; He, X.; Zhao, B.; Zhong, J.; Zhu, Y.; Wang, B.; Zhang, S. Oxidation leaching of NiMoV alloy enriched from spent hydrogenation catalysts and solvent extraction of Mo and V. J. Environ. Chem. Eng. 2024, 12, 111714. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, C.; Liu, D.; Li, Y.; Ning, Y.; Yang, S.; Zhang, Y.; Tang, Y.; Tang, Z.; Zhang, W. Investigation of elements (U, V, Sr, Ga, Cs and Rb) leaching and mobility in uranium-enriched coal ash with thermochemical treatment. J. Clean. Prod. 2019, 233, 115–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.-A.; Dreisinger, D.; Lv, C.; Lv, G.; Zhang, W. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J. Hazard. Mater. 2019, 369, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Semivrazhskaya, O.O.; Salionov, D.; Clark, A.H.; Casati, N.P.M.; Nachtegaal, M.; Ranocchiari, M.; Bjelić, S.; Verel, R.; van Bokhoven, J.A.; Sushkevich, V.L. Deciphering the Mechanism of Crystallization of UiO-66 Metal-Organic Framework. Small 2023, 19, 2305771. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of ZIF-8 under oxidative and inert environments: A practical perspective on using ZIF-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Li, Y.; Wang, C.; Yang, S.; Tang, Z.; Liu, D.; Li, Y. Water-leaching characteristic of valuable trace metals (U, V, and Ga) from (NH4)2SO4-treated coal ash: A coprecipitation behavior at high temperature. J. Hazard. Mater. 2020, 388, 122113. [Google Scholar] [CrossRef] [PubMed]
- Macías-Macías, K.Y.; Ceniceros-Gómez, A.E.; Gutiérrez-Ruiz, M.E.; González-Chávez, J.L.; Martínez-Jardines, L.G. Extraction and recovery of the strategic element gallium from an iron mine tailing. J. Environ. Chem. Eng. 2019, 7, 102964. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Luo, S.; Jiang, M.; Liu, C.; Teng, F.; Chen, H.; Shen, H.; Gao, D. Preparation of high performance LiFePO4/C by extracting iron element from iron tailings by concentrated sulfuric acid hot dip method. Ionics 2019, 26, 1645–1655. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Hou, X.; Li, H.; Wang, X.; Hu, W.; Ge, T.; Zhang, J.; Zhu, G.; Xie, H. Extraction of W, and As from spent SCR catalyst by alkali pressure leaching and the pressure leaching mechanism. J. Environ. Manag. 2023, 347, 119107. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Yu, D.; Tian, Q.; Li, D.; Zhang, L. Pyrite as an efficient reductant for magnetization roasting and its efficacy in iron recovery from iron-bearing tailing. Sep. Purif. Technol. 2023, 305, 122511. [Google Scholar] [CrossRef]
- Safari, M.; Abdi, R.; Adl, M.; Kafashan, J. Optimization of biogas productivity in lab-scale by response surface methodology. Renew. Energy 2018, 118, 368–375. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y.; He, Z.; Dong, Z.; Bi, X.; Kong, S.; Wang, L.; He, S.; Hu, H.; Wu, M. Efficient reduction of spent cathode materials via in-situ thermal reduction by defect-rich petroleum coke. Sep. Purif. Technol. 2024, 334, 126029. [Google Scholar] [CrossRef]
- Qiu, G.; Ning, X.; Shen, J.; Wang, Y.; Zhang, D.; Deng, J. Recovery of iron from iron tailings by suspension magnetization roasting with biomass-derived pyrolytic gas. Waste Manag. 2023, 156, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y.; Liu, T.; Hu, P.; Zheng, Q. Optimization of microwave roasting-acid leaching process for vanadium extraction from shale via response surface methodology. J. Clean. Prod. 2019, 234, 494–502. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Khed, V.C.; Nuruddin, M.F. Rubbercrete mixture optimization using response surface methodology. J. Clean. Prod. 2018, 171, 1605–1621. [Google Scholar] [CrossRef]
- Prates, C.D.; Ballotin, F.C.; Limborço, H.; Ardisson, J.D.; Lago, R.M.; Teixeira, A.P.D.C. Heterogeneous acid catalyst based on sulfated iron ore tailings for oleic acid esterification. Appl. Catal. A Gen. 2020, 600, 117624. [Google Scholar] [CrossRef]
- Cui, X.; Geng, Y.; Li, T.; Zhao, R.; Li, X.; Cui, Z. Field application and effect evaluation of different iron tailings soil utilization technologies. Resour. Conserv. Recycl. 2021, 173, 105746. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).