Deodorisation of Ventilated Air from a Fat-Processing Plant Using Different Types of Biofilter Fillings and Membranes
Abstract
1. Introduction
1.1. Odours in Food Processing
1.2. Biofiltration Methods of Deodorisation
2. Material and Methods
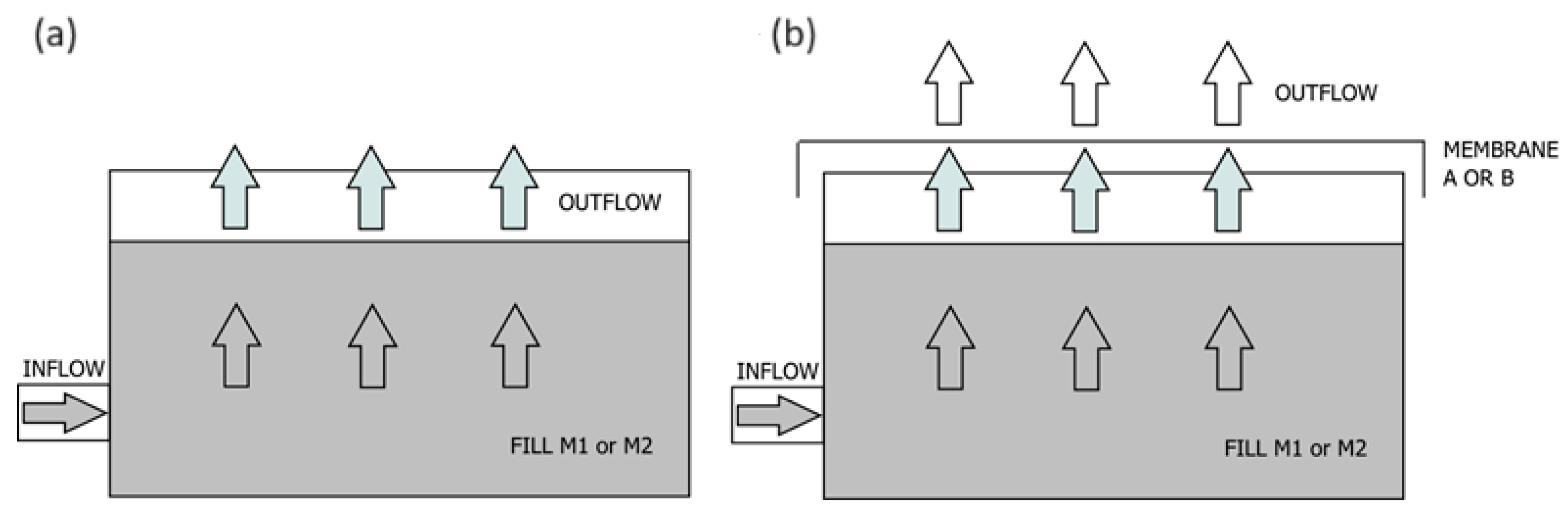
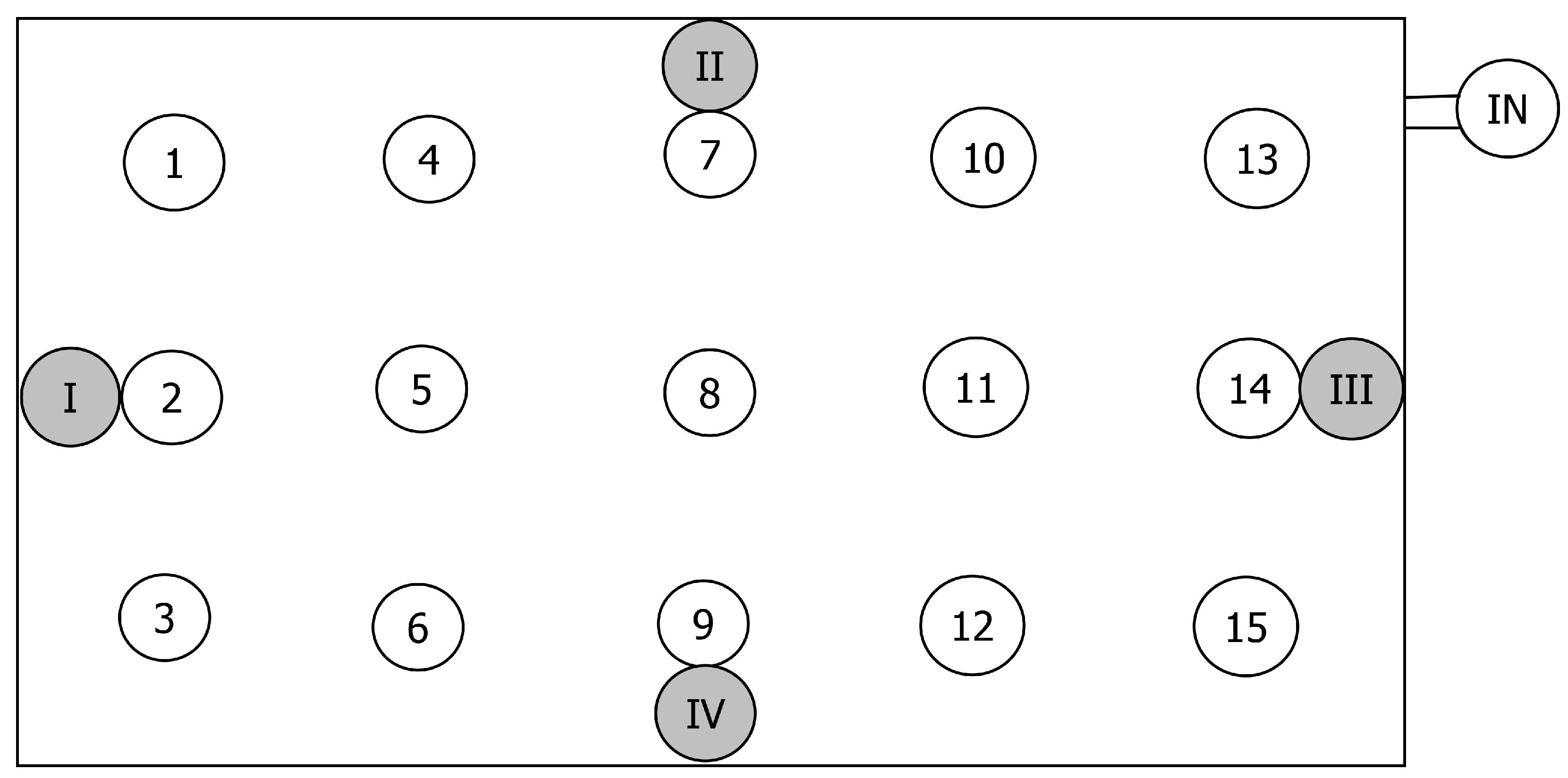
2.1. Biofilter
2.2. Research Variants
- 10 series for M1 filling: without membrane—4 series; membrane A—3 series; membrane B—3 series;
- 10 series for M2 filling: without membrane—4 series; membrane A—3 series; membrane B—3 series.
2.3. Gas Sampling
2.4. Odour Measurement
2.5. Statistical Methods
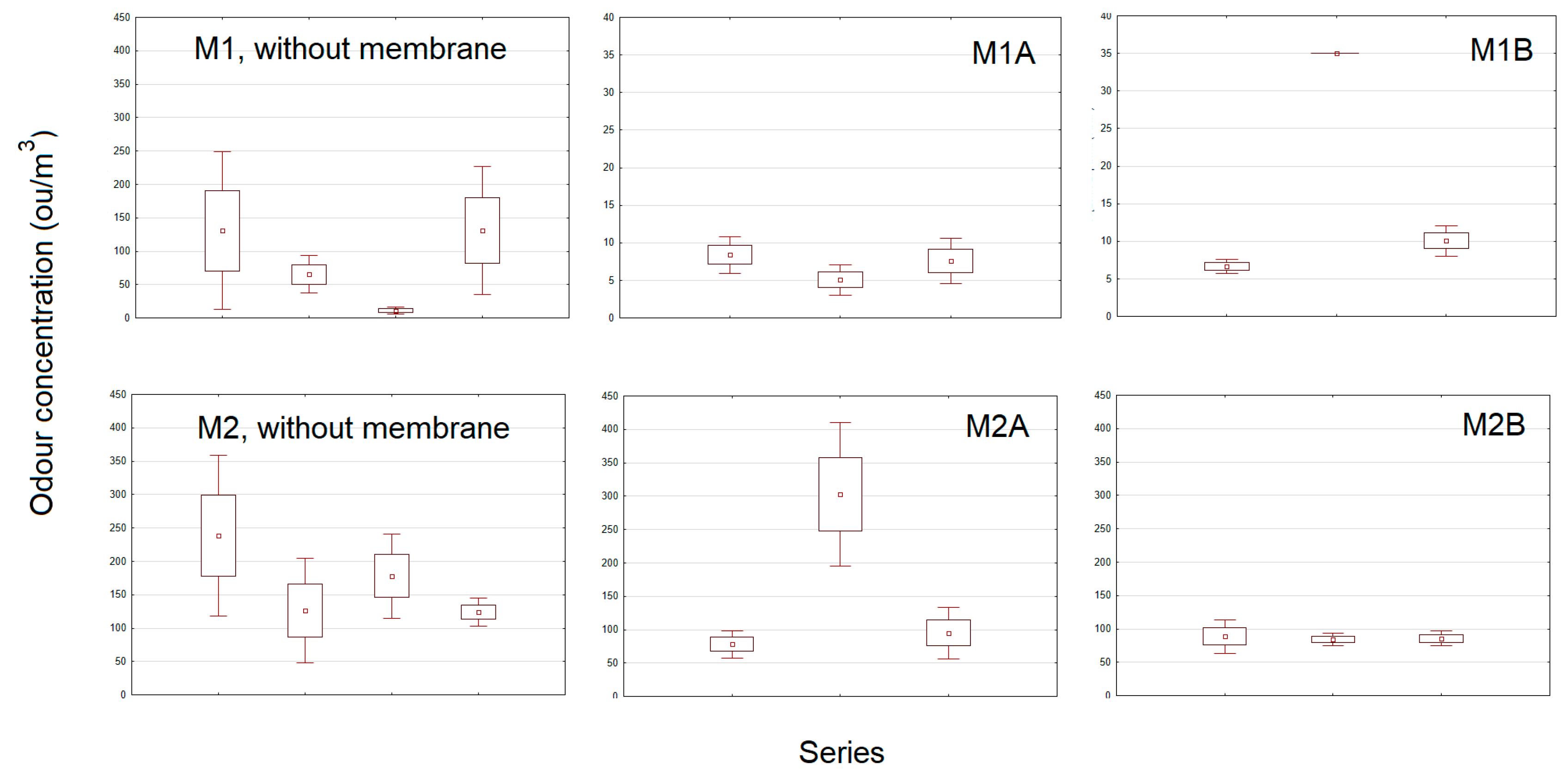
3. Results and Discussion
- M1, without membrane—the difference between tests from 5.01 and 12.01, 5.01 and 26.01, and 12.01 and 2.02 was statistically significant (p < 0.05). The difference between tests from 5.01 and 2.02 and 12.01 and 26.01 was not statistically significant.
- M1A—the difference between the tests on 6.02 and 13.02 and between the tests on 13.02 and 27.02 was statistically significant, while it was not statistically significant (p > 0.05) between the tests on 6.02 and 27.02.
- M2, without membrane—a statistically significant (p < 0.05) difference was found between the tests of 17.04 and 24.04, 17.04 and 22.05, 24.04 and 15.05, and 15.05 and 22.05. A statistically insignificant difference was found between the tests of 17.04 and 15.05 and 24.04 and 22.05.
- M2A—the difference between the tests of 5.06 and 15.06 and 15.06 and 26.06 was statistically significant, and the difference between the tests of 5.06 and 26.06 was statistically insignificant.
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feilberg, A.; Liu, D.; Adamsen, A.P.S.; Hansen, M.J.; Jonassen, K.E.N. Odorant Emissions from Intensive Pig Production Measured by Online Proton-Transfer-Reaction Mass Spectrometry. Environ. Sci. Technol. 2010, 44, 5894–5900. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Szyłak-Szydłowski, M. The Application of In Situ Methods to Monitor VOC Concentrations in Urban Areas—A Bibliometric Analysis and Measuring Solution Review. Sustainability 2022, 14, 8815. [Google Scholar] [CrossRef]
- Grzelka, A.; Romanik, E.; Miller, U. Odour Nuisance Assessment of the Food Industry Wastewater Treatment Plant. E3S Web Conf. 2019, 100, 00024. [Google Scholar] [CrossRef]
- Anupoju, G.R.; Potumarthi, R.; Jetty, A. Operation of Biofilter with Mixed Agricultural Residue as Filter Material: Effects of Humidification and Inlet Hydrogen Sulfide Volume Fraction on the Performance. Chem. Biochem. Eng. Q. 2006, 20, 189–196. [Google Scholar]
- Hartikainen, T.; Ruuskanen, J.; Vanhatalo, M.; Martikainen, P.J. Removal of Ammonia from Air by a Peat Biofilter. Environ. Technol. 1996, 17, 45–53. [Google Scholar] [CrossRef]
- Kim, N.J.; Hirai, M.; Shoda, M. Comparison of Organic and Inorganic Carriers in Removal of Hydrogen Sulfide in Biofilters. Environ. Technol. 1998, 19, 1233–1241. [Google Scholar] [CrossRef]
- Gandu, B.; Palanivel, S.; Juntupally, S.; Arelli, V.; Begum, S.; Anupoju, G.R. Removal of NH3 and H2S from Odor Causing Tannery Emissions Using Biological Filters: Impact of Operational Strategy on the Performance of a Pilot-Scale Bio-Filter. J. Environ. Sci. Health Part A 2021, 56, 625–634. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, F. The Oil Palm Wastes in Malaysia; Books on Demand: Paris, France, 2013; pp. 75–93. ISBN 978-953-51-1105-4. [Google Scholar]
- Zaman, N.Q.; Yusup, Y.; Yaacof, N. Verification of Receptor Exposure to Palm Oil Mill Odor Using In-Field Olfactometer with Odor Characteristic. Chem. Eng. Trans. 2016, 54, 271–276. [Google Scholar] [CrossRef]
- Yaacof, N.; Zaman, N.; Yusop, Y. Odour Intensity Assessment at Different Area of a Palm Oil Mill Using Olfactometry Method. Appl. Mech. Mater. 2015, 802, 472–477. [Google Scholar] [CrossRef]
- Psillakis, E.; Gekas, V. Odor Problems in the Food Industry. In Odors in the Food Industry; Springer: New York, NY, USA, 2006; pp. 1–13. ISBN 978-0-387-33510-0. [Google Scholar]
- Skinner, J.A.; Lewis, K.A.; Bardon, K.S.; Tucker, P.; Catt, J.A.; Chambers, B.J. An Overview of the Environmental Impact of Agriculture in the U.K. J. Environ. Manag. 1997, 50, 111–128. [Google Scholar] [CrossRef]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological Methods for Odor Treatment—A Review. J. Clean. Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Gandu, B.; Sandhya, K.; Gangagni Rao, A.; Swamy, Y.V. Gas Phase Bio-Filter for the Removal of Triethylamine (TEA) from Air: Microbial Diversity Analysis with Reference to Design Parameters. Bioresour. Technol. 2013, 139, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.A.; Chandrashekhar, B. Physicochemical and Biochemical Approaches for Treatment of Gaseous Emissions Containing NOx. Crit. Rev. Environ. Sci. Technol. 2014, 44, 34–96. [Google Scholar] [CrossRef]
- Searcy, E.M.L.; Zhang, Q.; Cicek, N. The Use of a Biofilter for Reducing Off-Gas Odour from an Industrial Fermentation Process. Environ. Technol. 2005, 26, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Delhoménie, M.-C.; Heitz, M. Biofiltration of Air: A Review. Crit. Rev. Biotechnol. 2005, 25, 53–72. [Google Scholar] [CrossRef]
- Han, M.-F.; Wang, C.; Yang, N.-Y.; Hu, X.-R.; Wang, Y.-C.; Duan, E.-H.; Ren, H.-W.; Hsi, H.-C.; Deng, J.-G. Performance Enhancement of a Biofilter with pH Buffering and Filter Bed Supporting Material in Removal of Chlorobenzene. Chemosphere 2020, 251, 126358. [Google Scholar] [CrossRef]
- Liu, J.; Yue, P.; Zang, N.; Lu, C.; Chen, X. Removal of Odors and VOCs in Municipal Solid Waste Comprehensive Treatment Plants Using a Novel Three-Stage Integrated Biofilter: Performance and Bioaerosol Emissions. Front. Environ. Sci. Eng. 2021, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Wéry, N. Bioaerosols from Composting Facilities—A Review. Front. Cell. Infect. Microbiol. 2014, 4, 42. [Google Scholar] [CrossRef]
- Affek, K.; Tabernacka, A.; Załęska-Radziwiłł, M.; Doskocz, N.; Muszyński, A. Bioaerosol Emission from Biofilters: Impact of Bed Material Type and Waste Gas Origin. Atmosphere 2021, 12, 1574. [Google Scholar] [CrossRef]
- Sercu, B.; Peixoto, J.; Demeestere, K.; Van Elst, T.; Van Langenhove, H. Odors Treatment: Biological Technologies. In Odors in the Food Industry; Springer: New York, NY, USA, 2006; pp. 125–158. ISBN 978-0-387-33510-0. [Google Scholar]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. Volatile Organic Compounds, Ammonia and Hydrogen Sulphide Removal Using a Two-Stage Membrane Biofiltration Process. Chem. Eng. Res. Des. 2021, 165, 69–80. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Szulczyński, B.; Gębicki, J.; Hupka, J. Treatment of Malodorous Air in Biotrickling Filters: A Review. Biochem. Eng. J. 2019, 141, 146–162. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, R.; Jiang, H.; Bai, M.; Lin, K.; Zhang, M.; Ren, L. Removal of Hydrogen Sulfide and Ammonia Using a Biotrickling Filter Packed with Modified Composite Filler. Processes 2022, 10, 2016. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, C.; Huang, K. Biofiltration Performance and Characteristics of High-Temperature Gaseous Benzene, Hexane and Toluene. Chem. Eng. J. 2015, 279, 689–695. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, Measurement, and Control of Odor in Livestock Farms: A Review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Tung, T.N.; Chiemchaisri, W.; Chiemchaisri, C.; Sanguanpak, S. Removal of greenhouse gas in biofilter using organic and inorganic media. Vietnam. J. Sci. Technol. Eng. 2021, 63, 83–89. [Google Scholar] [CrossRef]
- Zhang, S.; You, J.; Kennes, C.; Cheng, Z.; Ye, J.; Chen, D.; Chen, J.; Wang, L. Current Advances of VOCs Degradation by Bioelectrochemical Systems: A Review. Chem. Eng. J. 2018, 334, 2625–2637. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lv, Y.-H.; Wang, C.; Jiang, G.-Y.; Han, M.-F.; Deng, J.-G.; Hsi, H.-C. Microbial Community Evolution and Functional Trade-Offs of Biofilm in Odor Treatment Biofilters. Water Res. 2023, 235, 119917. [Google Scholar] [CrossRef] [PubMed]
- Re, A.; Schiavon, M.; Torretta, V.; Polvara, E.; Invernizzi, M.; Sironi, S.; Caruson, P. Application of Different Packing Media for the Biofiltration of Gaseous Effluents from Waste Composting. Environ. Technol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Sheoran, K.; Siwal, S.S.; Kapoor, D.; Singh, N.; Saini, A.K.; Alsanie, W.F.; Thakur, V.K. Air Pollutants Removal Using Biofiltration Technique: A Challenge at the Frontiers of Sustainable Environment. ACS Eng. Au 2022, 2, 378–396. [Google Scholar] [CrossRef]
- Estrada, J.M.; Quijano, G.; Lebrero, R.; Muñoz, R. Step-Feed Biofiltration: A Low Cost Alternative Configuration for off-Gas Treatment. Water Res. 2013, 47, 4312–4321. [Google Scholar] [CrossRef]
- Su, Q.; Dai, D.; Liao, Y.; Han, H.; Wu, J.; Ren, Z. Synthetic Microbial Consortia to Enhance the Biodegradation of Compost Odor by Biotrickling Filter. Bioresour. Technol. 2023, 387, 129698. [Google Scholar] [CrossRef] [PubMed]
- Lebrero, R.; Rangel, M.G.L.; Munoz, R. Characterization and biofiltration of a real odorous emission from wastewater treatment plant sludge. J. Environ. Manag. 2013, 116, 50–57. [Google Scholar] [CrossRef]
- Lelicińska-Serafin, K.; Rolewicz-Kalińska, A.; Manczarski, P. VOC Removal Performance of a Joint Process Coupling Biofiltration and Membrane-Filtration Treating Food Industry Waste Gas. Int. J. Environ. Res. Public Health 2019, 16, 3009. [Google Scholar] [CrossRef]
- Muszyński, A.; Tabernacka, A.; Załęska-Radziwiłł, M. How to Reduce the Emission of Microorganisms from a Biofilter Used to Treat Waste Gas from a Food Industry Plant. Atmosphere 2021, 12, 673. [Google Scholar] [CrossRef]
- PN-EN 13725:2022; Stationary Source Emissions—Determination of Odour Concentration by Dynamic Olfactometry and Odour Emission Rate. The Polish Committee for Standardization: Warsaw, Poland, 2022.
- Kulig, A.; Szyłak-Szydłowski, M.; Wiśniewska, M. Application of Field Olfactometry to Monitor the Odour Impact of a Municipal Sewage System. Energies 2022, 15, 4015. [Google Scholar] [CrossRef]
- Kulig, A.; Szyłak-Szydłowski, M. Assessment of the Effects of Wastewater Treatment Plant Modernization by Means of the Field Olfactometry Method. Water 2019, 11, 2367. [Google Scholar] [CrossRef]
- Szyłak-Szydłowski, M. Validation of Odor Concentration from Mechanical-Biological Treatment Piles Using Static Chamber and Wind Tunnel with Different Wind Speed Values. J. Air Waste Manag. Assoc. 2017, 67, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Szyłak-Szydłowski, M. Odour Samples Degradation During Detention in Tedlar® Bags. Water Air Soil. Pollut. 2015, 226, 227. [Google Scholar] [CrossRef][Green Version]
- ISO 13301:2002; General Guidance for Measuring Odour, Flavour and Taste Detection Thresholds by a Three-Alternative Forced-choice (3-AFC) Procedure. ISO: Geneva, Switzerland, 2002.
- Chen, L.; Hoff, S.; Hoff, S. Mitigating Odors from Agricultural Facilities: A Review of Literature Concerning Biofilters. Appl. Eng. Agric. 2009, 25, 751–766. [Google Scholar] [CrossRef]
- Janni, K.A.; Maier, W.J.; Kuehn, T.; Yang, C.-H.; Bridges, B.; Vesley, D.; Nellis, M.A. Evaluation of Biofiltration of Air—An Innovative Air Pollution Control Technology. ASHRAE Trans. 2001, 107, 198–214. [Google Scholar]
- DeBruyn, J.C.; Mann, D.D.; Zhang, Q. Comparison of the Odour Levels of Biofiltered Air and Ambient Farmyard Air. Can. Biosyst. Eng. 2001, 43, 6. [Google Scholar]
- Mann, D.D.; DeBruyn, J.C.; Zhang, Q. Design and Evaluation of an Open Biofilter for Treatment of Odour from Swine Barns during Sub-Zero Ambient Temperatures. Can. Biosyst. Eng. 2002, 44, 21–26. [Google Scholar]
- Lau, A.; Cheng, K. Removal of Odor Using Biofilter from Duck Confinement Buildings. J. Environ. Sci. Health Part A 2007, 42, 955–959. [Google Scholar] [CrossRef]
- Nicolai, R.; Janni, K. Determining Pressure Drop through Compost-Wood Chip Biofilter Media. In Proceedings of the 2001 ASAE Annual Meeting, Philadelphia, PA, USA, 4–7 August 2001. [Google Scholar] [CrossRef]
- Liu, T.; Dong, H.; Zhu, Z.; Shang, B.; Yin, F.; Zhang, W.; Zhou, T. Effects of Biofilter Media Depth and Moisture Content on Removal of Gases from a Swine Barn. J. Air Waste Manag. Assoc. 2017, 67, 1288–1297. [Google Scholar] [CrossRef]
- Sheridan, B.A.; Curran, T.P.; Dodd, V.A. Assessment of the Influence of Media Particle Size on the Biofiltration of Odorous Exhaust Ventilation Air from a Piggery Facility. Bioresour. Technol. 2002, 84, 129–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.; Hoff, S.J.; Koziel, J.A.; Cai, L.; Zelle, B.; Sun, G. Performance Evaluation of a Wood-Chip Based Biofilter Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectroscopy-Olfactometry. Bioresour. Technol. 2008, 99, 7767–7780. [Google Scholar] [CrossRef]
- Martinec, M.; Hartung, E.; Jungbluth, T.; Schneider, F.; Wieser, P.H. Reduction of gas, odor and dust emissions from swine operations with biofilters. In Proceedings of the 2001 ASAE Annual Meeting, Philadelphia, PA, USA, 4–7 August 2001; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA. [Google Scholar] [CrossRef]
- Chen, L.; Hoff, S. A Two-Stage Wood Chip-Based Biofilter System to Mitigate Odors from a Deep-Pit Swine Building. Appl. Eng. Agric. 2012, 28, 893–901. [Google Scholar] [CrossRef]
- Luo, J.; Van Oostrom, A. Biofilters for Controlling Animal Rendering Odour—A Pilot-Scale Study. Pure Appl. Chem. 1997, 69, 2403–2410. [Google Scholar] [CrossRef]
| Parameter | Stumpwood Chips and Pine Bark | Stumpwood Chips, Pine Bark, and Compost from Green Waste |
|---|---|---|
| M1 | M2 | |
| Total organic matter [% d.m.] | 86.0 (85.0–87.5) | 45.0 (40.6–47.8) |
| Total moisture content [%] | 63.4 (60.6–66.3) | 46.8 (42.7–50.5) |
| pH | 6.77 (6.75–6.79) | 7.44 (7.27–7.70) |
| Specific surface [m2/g] | 0.55 (0.37–0.67) | 1.67 (1.53–1.80) |
| Substitute diameter [mm] | 37.1 (34.8–39.6) | 8.7 (6.7–9.9) |
| Parameter | Membrane A | Membrane B |
|---|---|---|
| More Permeable, Thinner | Less Permeable, Thicker | |
| Average area weight (g/m2) | 400 ± 1 | 474 ± 3 |
| Average air permeability (mm/s) | 17.8 | 3.9 |
| Average water tightness (cm H2O) | 199 | >2000 |
| Filling | Membrane | Date | Odour Concentration (ou/m3) | |||
|---|---|---|---|---|---|---|
| Minimum | Median | Average | Maximum | |||
| M1 | - | 5.01. | 82 | 109 | 130.8 | 328 |
| 12.01. | 44 | 66 | 65.4 | 94 | ||
| 26.01. | 4 | 11 | 10.9 | 14 | ||
| 2.02. | 82 | 109 | 131.1 | 219 | ||
| M1A | 6.02. | 6 | 9 | 8.4 | 9 | |
| 13.02. | 4 | 6 | 5.1 | 6 | ||
| 27.02. | 6 | 9 | 7.6 | 9 | ||
| M1B | 9.03. | 6 | 7 | 6.7 | 7 | |
| 23.03. | 35 | 35 | 35 | 35 | ||
| 27.03. | 9 | 11 | 10.1 | 11 | ||
| M2 | - | 17.04. | 131 | 238 | 238.5 | 328 |
| 24.04. | 73 | 126 | 126.6 | 219 | ||
| 15.05. | 131 | 178 | 177.9 | 219 | ||
| 22.05. | 109 | 124 | 123.7 | 131 | ||
| M2A | 5.06. | 66 | 75 | 78.3 | 94 | |
| 15.06. | 219 | 328 | 303.1 | 390 | ||
| 26.06. | 73 | 94 | 95.2 | 131 | ||
| M2B | 3.07. | 82 | 131 | 88.5 | 131 | |
| 10.07. | 82 | 94 | 84.4 | 94 | ||
| 24.07. | 82 | 94 | 86 | 94 | ||
| Odour Concentration (ou/m3) | ||||
|---|---|---|---|---|
| Min. | Median | Average | Max. | |
| Inflow | 1201 | 3300 | 3248 | 6000 |
| Variants: | - | |||
| M1 without membrane | 4 | 82 | 84.6 | 328 |
| M2 without membrane | 73 | 164 | 166.7 | 328 |
| M1A | 4 | 6 | 7.0 | 9 |
| M1B | 6 | 11 | 17.2 | 35 |
| M2A | 66 | 94 | 158.8 | 390 |
| M2B | 82 | 82 | 86.3 | 131 |
| Fillings | |||||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | ||||||
| Date | 12.01 | 26.01 | 02.02 | 17.04 | 24.04 | 15.05 | 22.05 |
| Point No. | Removal efficiency of odorous compounds in the filter bed (%) | ||||||
| 1 | 98.7 | 99.6 | 91.2 | 92.7 | 97.6 | 94.5 | 96.7 |
| 2 | 98.0 | 99.7 | 95.6 | 92.7 | 97.3 | 92.7 | 96.7 |
| 3 | 98.3 | 99.7 | 96.7 | 94.5 | 97.3 | 94.5 | 96.0 |
| 4 | 98.3 | 99.6 | 91.2 | 92.7 | 96.9 | 94.5 | 96.0 |
| 5 | 97.5 | 99.6 | 95.6 | 95.6 | 96.4 | 95.6 | 96.0 |
| 6 | 98.3 | 99.6 | 95.6 | 89.1 | 95.6 | 94.5 | 96.0 |
| 7 | 98.3 | 99.7 | 96.7 | 92.7 | 96.9 | 94.5 | 96.7 |
| 8 | 98.5 | 99.6 | 95.6 | 92.7 | 95.6 | 95.6 | 96.0 |
| 9 | 97.8 | 99.8 | 95.6 | 92.7 | 94.5 | 94.5 | 96.0 |
| 10 | 97.8 | 99.7 | 95.6 | 92.7 | 92.7 | 92.7 | 96.0 |
| 11 | 97.8 | 99.7 | 95.6 | 92.7 | 95.6 | 92.7 | 96.7 |
| 12 | 97.2 | 99.6 | 95.6 | 92.7 | 94.5 | 94.5 | 96.7 |
| 13 | 97.5 | 99.9 | 93.4 | 89.1 | 94.5 | 94.5 | 96.0 |
| 14 | 97.8 | 99.7 | 95.6 | 89.1 | 95.6 | 92.7 | 96.0 |
| 15 | 98.5 | 99.7 | 91.2 | 89.1 | 95.6 | 92.7 | 96.0 |
| Technological Variant | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1A | M1B | M2A | M2B | |||||||||
| Date | 6.02 | 13.02 | 27.02 | 09.03 | 23.03 | 27.03 | 5.06 | 15.06 | 26.06 | 3.07 | 10.07 | 24.07 |
| Point No. | Removal efficiency of odorous compounds with biofilter membranes (%) | |||||||||||
| 1 | 91.7 | 91.8 | 82.9 | 80.0 | 97.6 | 98.8 | 54.3 | 69.6 | 62.6 | 75.0 | 62.6 | 65.1 |
| 2 | 91.7 | 91.8 | 82.9 | 82.9 | 97.6 | 98.5 | 59.8 | 54.5 | 62.6 | 75.0 | 62.6 | 60.0 |
| 3 | 94.5 | 94.5 | 74.3 | 80.0 | 97.6 | 98.5 | 59.8 | 54.5 | 40.2 | 75.0 | 62.6 | 60.0 |
| 4 | 91.7 | 91.8 | 82.9 | 80.0 | 97.6 | 98.5 | 54.3 | 54.5 | 62.6 | 75.0 | 62.6 | 65.1 |
| 5 | 91.7 | 94.5 | 74.3 | 80.0 | 97.6 | 98.5 | 54.3 | 69.6 | 57.1 | 71.3 | 62.6 | 65.1 |
| 6 | 91.7 | 94.5 | 82.9 | 82.9 | 97.6 | 98.8 | 54.3 | 45.9 | 57.1 | 71.3 | 57.1 | 65.1 |
| 7 | 91.7 | 91.8 | 82.9 | 80.0 | 97.6 | 98.8 | 59.8 | 54.5 | 62.6 | 75.0 | 57.1 | 65.1 |
| 8 | 94.5 | 91.8 | 74.3 | 80.0 | 97.6 | 98.8 | 42.7 | 69.6 | 62.6 | 60.1 | 57.1 | 60.0 |
| 9 | 94.5 | 94.5 | 74.3 | 80.0 | 97.6 | 98.8 | 54.3 | 54.5 | 62.6 | 71.3 | 62.6 | 60.0 |
| 10 | 91.7 | 94.5 | 82.9 | 80.0 | 97.6 | 98.8 | 54.3 | 54.5 | 40.2 | 75.0 | 62.6 | 65.1 |
| 11 | 91.7 | 91.8 | 74.3 | 82.9 | 97.6 | 98.8 | 42.7 | 54.5 | 57.1 | 75.0 | 62.6 | 65.1 |
| 12 | 91.7 | 91.8 | 74.3 | 82.9 | 97.6 | 98.5 | 42.7 | 69.6 | 57.1 | 75.0 | 62.6 | 65.1 |
| 13 | 91.7 | 94.5 | 74.3 | 82.9 | 97.6 | 98.5 | 42.7 | 54.5 | 57.1 | 75.0 | 62.6 | 65.1 |
| 14 | 91.7 | 91.8 | 74.3 | 80.0 | 97.6 | 98.5 | 54.3 | 54.5 | 40.2 | 71.3 | 62.6 | 65.1 |
| 15 | 91.7 | 94.5 | 82.9 | 80.0 | 97.6 | 98.5 | 54.3 | 54.5 | 66.7 | 75.0 | 62.6 | 60.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyłak-Szydłowski, M.; Kulig, A. Deodorisation of Ventilated Air from a Fat-Processing Plant Using Different Types of Biofilter Fillings and Membranes. Sustainability 2024, 16, 1939. https://doi.org/10.3390/su16051939
Szyłak-Szydłowski M, Kulig A. Deodorisation of Ventilated Air from a Fat-Processing Plant Using Different Types of Biofilter Fillings and Membranes. Sustainability. 2024; 16(5):1939. https://doi.org/10.3390/su16051939
Chicago/Turabian StyleSzyłak-Szydłowski, Mirosław, and Andrzej Kulig. 2024. "Deodorisation of Ventilated Air from a Fat-Processing Plant Using Different Types of Biofilter Fillings and Membranes" Sustainability 16, no. 5: 1939. https://doi.org/10.3390/su16051939
APA StyleSzyłak-Szydłowski, M., & Kulig, A. (2024). Deodorisation of Ventilated Air from a Fat-Processing Plant Using Different Types of Biofilter Fillings and Membranes. Sustainability, 16(5), 1939. https://doi.org/10.3390/su16051939





