Climate Change and Jump Dispersal Drive Invasion of the Rosy Wolfsnail (Euglandina rosea) in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Presence and Occurrence Records
Local Species Data Collection and Recording
2.2. Species Presence in the Contiguous United States
2.2.1. Collecting North American Data on E. rosea
2.2.2. Data Processing
2.2.3. Model Calibration
2.2.4. Model Validation
3. Results
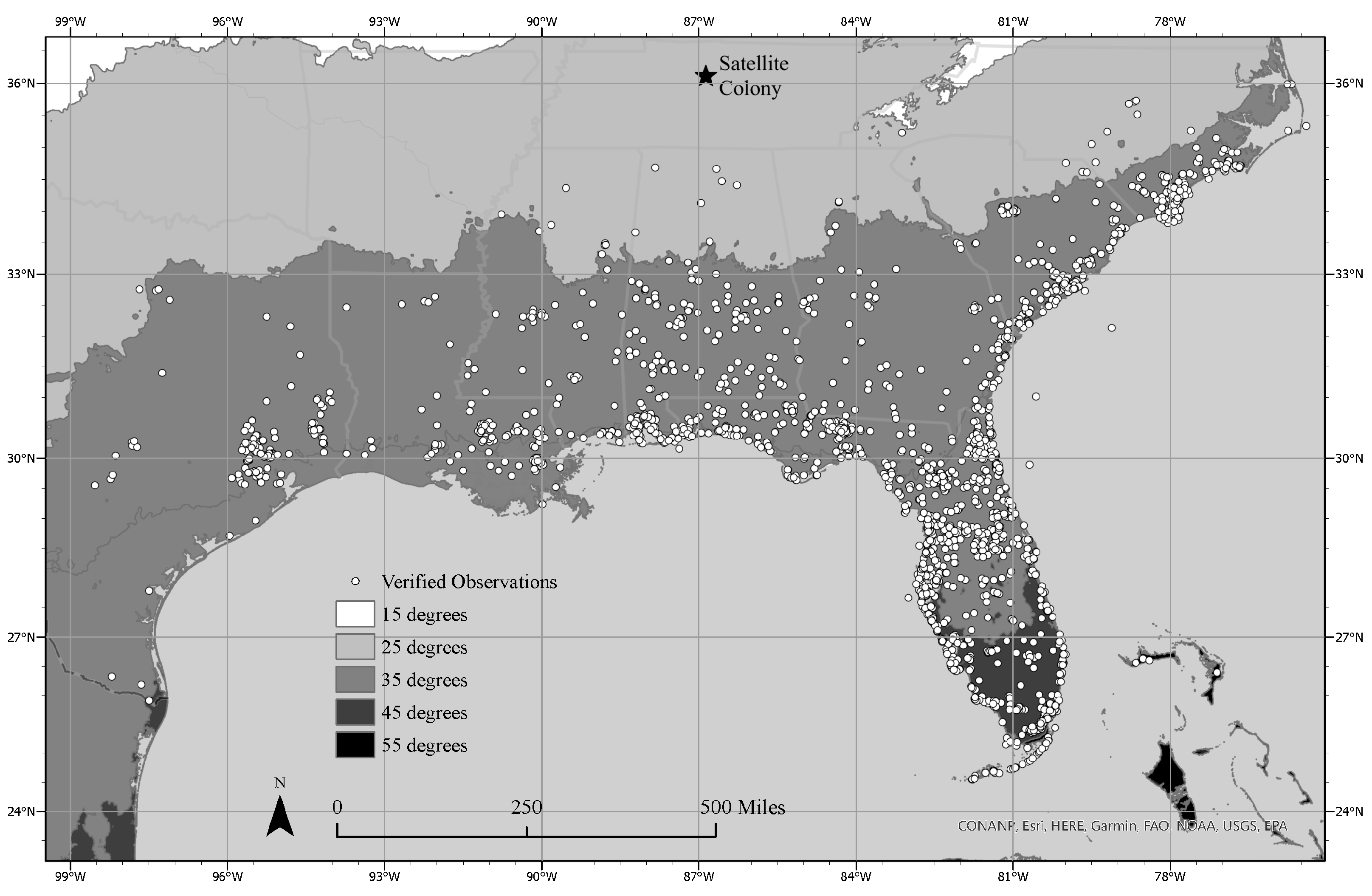
3.1. Current Geographical Range
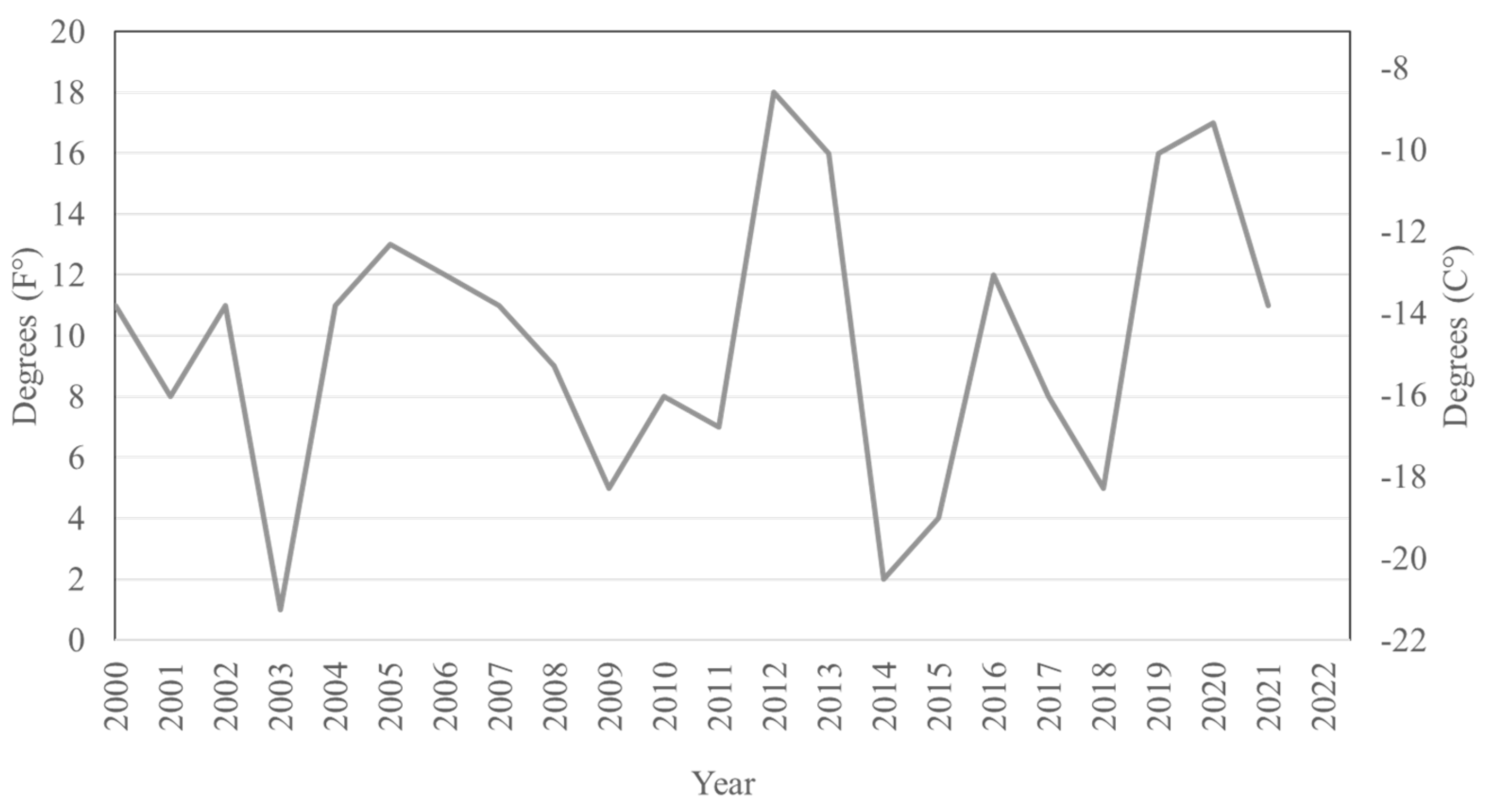
3.2. Satellite Population Persistence
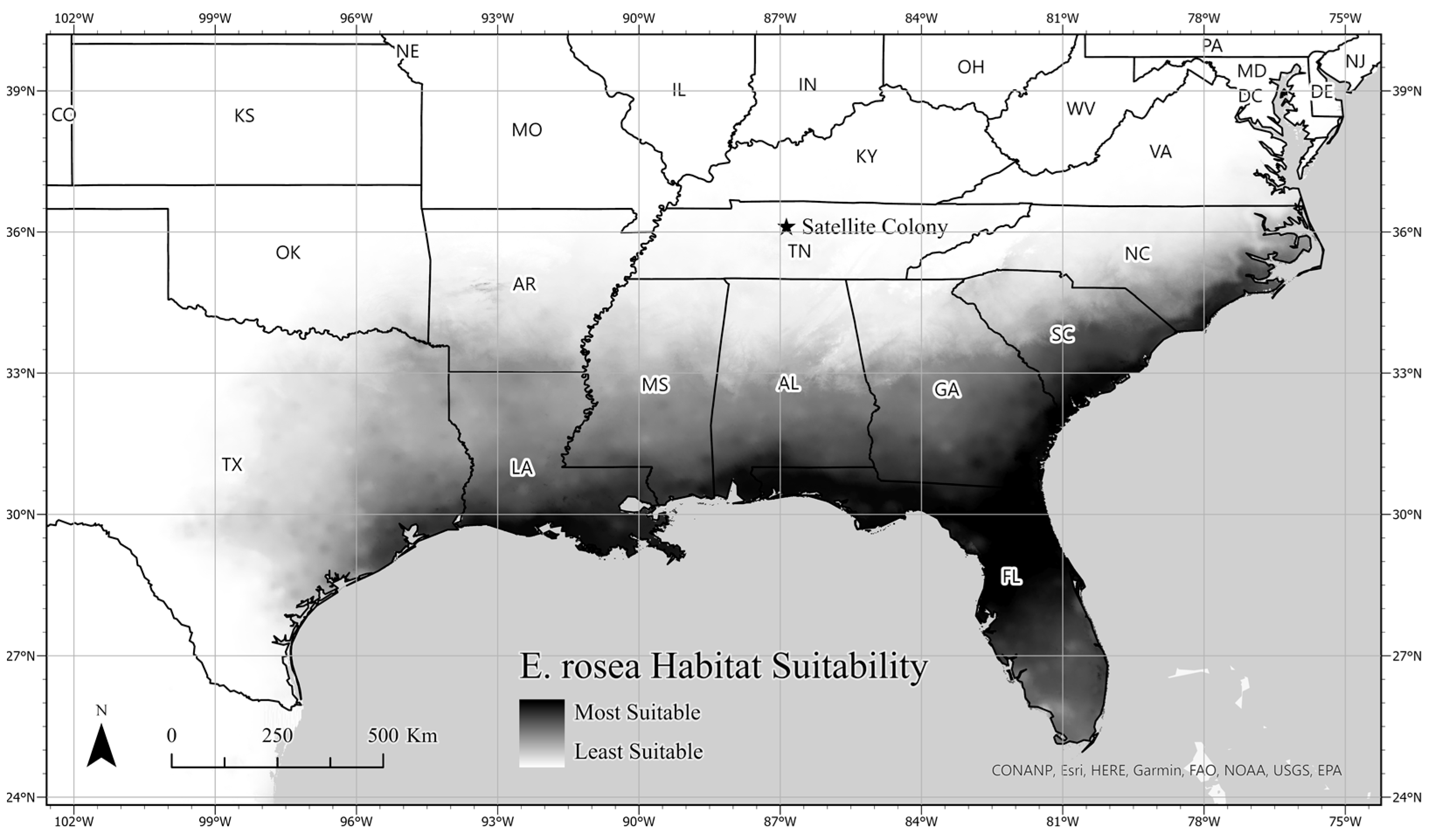
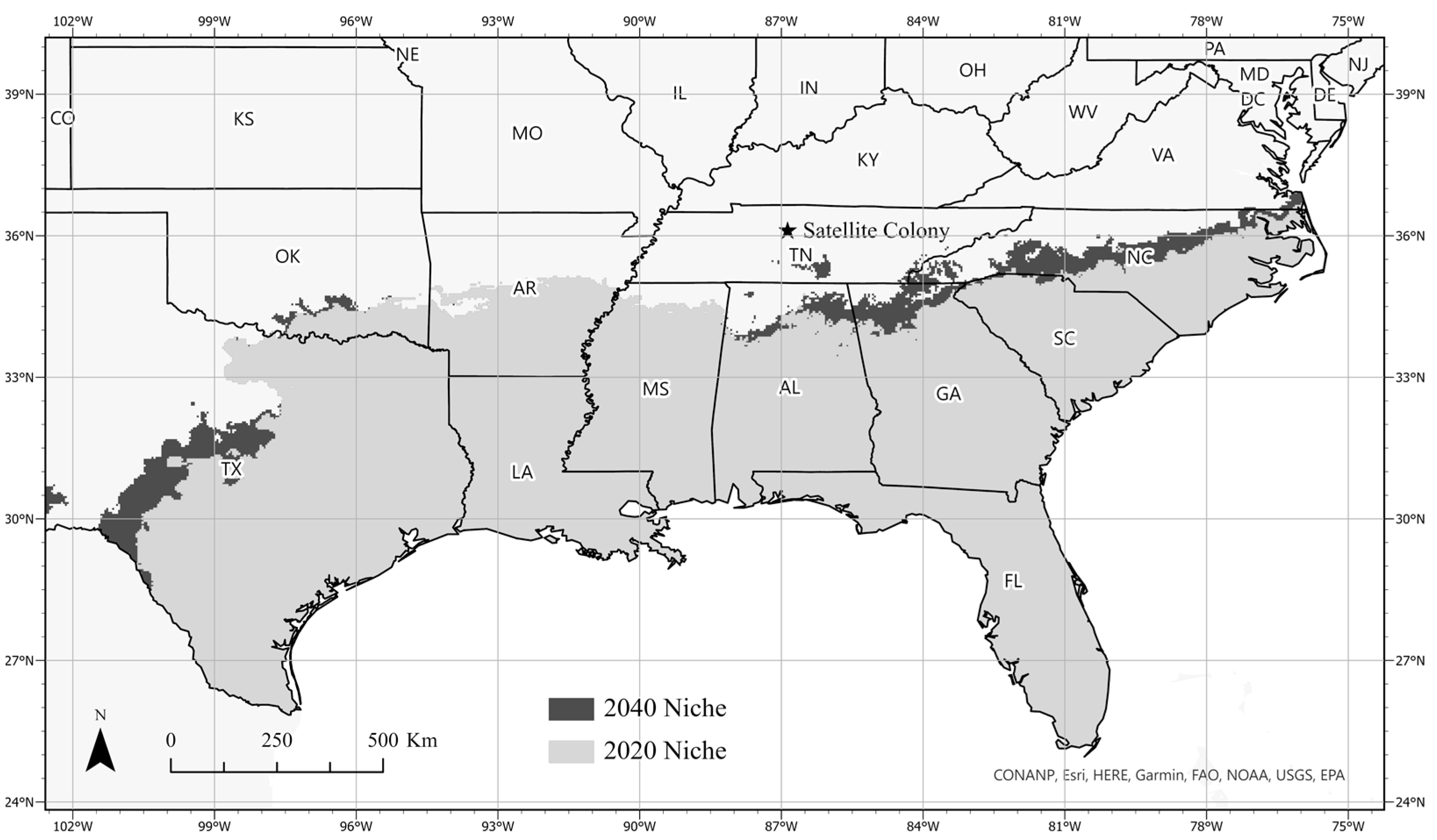
3.3. Ecological Niche Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burrows, M.T.; Schoeman, D.S.; Buckley, L.B.; Moore, P.; Poloczanska, E.S.; Brander, K.M.; Brown, C.; Bruno, J.F.; Duarte, C.M.; Halpern, B.S.; et al. The Pace of Shifting Climate in Marine and Terrestrial Ecosystems. Science 2011, 334, 652–655. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-Related Range Shifts—A Global Multidimensional Synthesis and New Research Directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G.; Andrus, J.E. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A. Direct Impacts of Recent Climate Warming on Insect Populations. Integr. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef]
- Pauchard, A.; Milbau, A.; Albihn, A.; Alexander, J.; Burgess, T.; Daehler, C.; Englund, G.; Essl, F.; Evengård, B.; Greenwood, G.B.; et al. Non-Native and Native Organisms Moving into High Elevation and High Latitude Ecosystems in an Era of Climate Change: New Challenges for Ecology and Conservation. Biol. Invasions 2016, 18, 345–353. [Google Scholar] [CrossRef]
- Gerlach, J.; Barker, G.M.; Bick, C.S.; Bouchet, P.; Brodie, G.; Christensen, C.C.; Collins, T.; Coote, T.; Cowie, R.H.; Fiedler, G.C.; et al. Negative Impacts of Invasive Predators Used as Biological Control Agents against the Pest Snail Lissachatina Fulica: The Snail Euglandina ‘Rosea’ and the Flatworm Platydemus Manokwari. Biol. Invasions 2021, 23, 997–1031. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boujelas, S.; De Poorter, M. 100 of the World’s Invasive Species; ISSG: Auckland, New Zealand, 2004. [Google Scholar]
- Cook, A. Factors Affecting Prey Choice and Feeding Technique in the Carnivorous Snail Euglandina rosea Ferussac. J. Molluscan Stud. 1989, 55, 469–477. [Google Scholar] [CrossRef]
- Gerlach, J. The Ecology of the Carnivorous Snail Euglandina rosea. Ph.D. Thesis, Wadham College, Oxford, UK, 1994. [Google Scholar]
- Davis, C.J.; Butler, G.D. Introduced Enemies of the Giant African Snail, Achatina Fulica Bowdich, in Hawaii (Pulmonata: Achatinidae). Proc. Hawaii. Entomol. Soc. 1964, XVIII, 377–390. [Google Scholar]
- Lv, S.; Zhang, Y.; Steinmann, P.; Zhou, X.-N. Emerging Angiostrongyliasis in Mainland China. Emerg. Infect. Dis. 2008, 14, 161–164. [Google Scholar] [CrossRef] [PubMed]
- USDA. Lissachatina Fulica. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/gas/lissachatina-fulica.pdf (accessed on 26 March 2023).
- Ezzell, C. Strangers in Paradise: Alien Species Disrupt the Ecology of Hawaii. Sci. News 1992, 149, 314–316. [Google Scholar] [CrossRef]
- Solem, A. How Many Hawaiian Land Snail Species Are Left? And What We Can Do for Them. Bish. Mus. Occas. Pap. 1990, 30, 27–40. [Google Scholar]
- Hadfield, M.G.; Miller, S.E.; Carwile, A.H. The Decimation of Endemic Hawaiian Tree Snails by Alien Predators’. Am. Zool. 1993, 33, 610–622. [Google Scholar] [CrossRef][Green Version]
- Meyer, W.M.; Cowie, R.H. Feeding Preferences of Two Predatory Snails Introduced to Hawaii and Their Conservation Implications. Malacologia 2010, 53, 135–144. [Google Scholar] [CrossRef]
- Holland, B.S.; Chock, T.; Lee, A.; Sugiura, S. Tracking Behavior in the Snail Euglandina rosea: First Evidence of Preference for Endemic vs. Biocontrol Target Pest Species in Hawaii. Am. Malacol. Bull. 2012, 30, 153–157. [Google Scholar] [CrossRef]
- Hadfield, M.G. Extinction in Hawaiian Achatinelline Snails. Malacologia 1986, 27, 67–81. [Google Scholar]
- Holland, B.S.; Hadfield, M.G. Origin and Diversification of the Endemic Hawaiian Tree Snails (Achatinellidae: Achatinellinae) Based on Molecular Evidence. Mol. Phylogenet. Evol. 2004, 32, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.H. Variation in Species Diversity and Shell Shape in Hawaiian Land Snails: In Situ Speciation and Ecological Relationships. Evolution 1995, 49, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. Global Decline of Nonmarine Mollusks. Bioscience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Simberloff, D.; Stiling, P. How Risky Is Biological Control? Ecology 1996, 77, 1965–1974. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Irwin, K.L.; McKinney, M.L.; Womble, S.; Irwin, K.L. First Reported Occurrence of the Invasive Land Snail Euglandia Rosea in Tennessee: Implications for Global Warming. Tenn. Acad. Sci. 2016, 91, 49–51. [Google Scholar]
- Auffenberg, K.; Stange, L.A. Snail-Eating Snails of Florida, Gastropoda; University of Florida: Gainsville, FL, USA, 2021. [Google Scholar]
- GBIF.org (12 November 2023) GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0056190-231002084531237 (accessed on 12 November 2023).
- NOAA Global Summary of the Year. Available online: https://www.ncei.noaa.gov/data/gsoy/ (accessed on 24 May 2023).
- Ivanova, N.V.; Shashkov, M.P. The Possibilities of GBIF Data Use in Ecological Research. Russ. J. Ecol. 2021, 52, 1–8. [Google Scholar] [CrossRef]
- iNaturalist Observations of Euglandina rosea from the United States. Available online: https://www.inaturalist.org (accessed on 7 May 2023).
- Cox, G. What Is a ‘Verifiable Observation’ and How Does It Reach ‘Research Grade’? Available online: https://www.inaturalist.org/posts/26549-what-is-a-verifiable-observation-and-how-does-it-reach-research-grade (accessed on 11 November 2023).
- Maldonado, C.; Molina, C.I.; Zizka, A.; Persson, C.; Taylor, C.M.; Albán, J.; Chilquillo, E.; Rønsted, N.; Antonelli, A. Estimating Species Diversity and Distribution in the Era of Big Data: To What Extent Can We Trust Public Databases? Glob. Ecol. Biogeogr. 2015, 24, 973–984. [Google Scholar] [CrossRef] [PubMed]
- AdaptWest Project. Gridded Current and Projected Climate Data for North America at 1km Resolution, Generated Using the ClimateNA v7.30 Software (T. Wang et al., 2022). Available online: https://adaptwest.databasin.org (accessed on 11 November 2023).
- Mahony, C.R.; Wang, T.; Hamann, A.; Cannon, A.J. A Global Climate Model Ensemble for Downscaled Monthly Climate Normals over North America. Int. J. Climatol. 2022, 42, 5871–5891. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papeş, M. A Checklist for Maximizing Reproducibility of Ecological Niche Models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Modell. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for Customizable and Reproducible Modeling of Species’ Niches and Distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Root, T. Environmental Factors Associated with Avian Distributional Boundaries. J. Biogeogr. 1988, 15, 489–505. [Google Scholar] [CrossRef]
- Phillips, S.B.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. [Internet] Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 19 February 2024).
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K.A. Evaluating Resource Selection Functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.M.; Jiménez-valverde, A.; Real, R. AUC: A Misleading Measure of the Performance of Predictive Distribution Models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the Ability of Habitat Suitability Models to Predict Species Presences. Ecol. Modell. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-444-33364-0. [Google Scholar]
- Simberloff, D. Invasive Species; Oxford University Press: Oxford, UK, 2013; ISBN 9780199922017. [Google Scholar]
- Ansart, A.; Vernon, P.; Daguzan, J. Elements of Cold Hardiness in a Littoral Population of the Land Snail Helix Aspersa (Gastropoda: Pulmonata). J. Comp. Physiol. B 2002, 172, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Slotsbo, S.; Hansen, L.M.; Holmstrup, M. Low Temperature Survival in Different Life Stages of the Iberian Slug, Arion lusitanicus. Cryobiology 2010, 62, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Miranda, N.A.F.; Cumming, G.S. The Role of Waterbirds in the Dispersal of Aquatic Alien and Invasive Species. Divers. Distrib. 2015, 21, 744–754. [Google Scholar] [CrossRef]
- Planchuelo, G.; Catalán, P.; Delgado, J.A. Gone with the Wind and the Stream: Dispersal in the Invasive Species Ailanthus Altissima. Acta Oecologica 2016, 73, 31–37. [Google Scholar] [CrossRef]
- Buck, J.H.; Marshall, J.M. Hitchhiking as a Secondary Dispersal Pathway for Adult Emerald Ash Borer, Agrilus Planipennis. Great Lakes Entomol. 2009, 41, 197–199. [Google Scholar]
- Perkins, A.T.; Phillips, B.L.; Baskett, M.L.; Hastings, A. Evolution of Dispersal and Life History Interact to Drive Accelerating Spread of an Invasive Species. Ecol. Lett. 2013, 16, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Coulson, R.N.; Witter, J.A. Forest Entomology: Ecology and Management; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1984; ISBN 9780471025733. [Google Scholar]
- Bergey, E.A.; Figueroa, L.L.; Mather, C.M.; Martin, R.J.; Ray, E.J.; Kurien, J.T.; Westrop, D.R.; Suriyawong, P. Trading in Snails: Plant Nurseries as Transport Hubs for Non-Native Species. Biol. Invasions 2014, 16, 1441–1451. [Google Scholar] [CrossRef]
- Dyer, A.R.; Cochran, J.E.; Phillips, J.M.; Layne, K.I.; Berry, M.E.; Kule, A.K. Bagged Commercial Soils Are an Avenue for Regional Dispersal of Weedy Plant Species. Am. Midl. Nat. 2017, 178, 275–283. [Google Scholar] [CrossRef]
- Bergey, E.A.; Figueroa, L.L. Residential Yards as Designer Ecosystems: Effects of Yard Management on Land Snail Species Composition. Ecol. Appl. 2016, 26, 2536–2545. [Google Scholar] [CrossRef]
- Bergey, E.A. Dispersal of a Non-Native Land Snail across a Residential Area Is Modified by Yard Management and Movement Barriers. Urban. Ecosyst. 2019, 22, 325–334. [Google Scholar] [CrossRef]
- Dinkins, B.J.; Dinkins, G.R. An Inventory of the Land Snails and Slugs (Gastropoda: Caenogastropoda and Pulmonata) of Knox County, Tennessee. Am. Malacol. Bull. 2018, 36, 1–22. [Google Scholar] [CrossRef]
- Borden, J.B.; Flory, S.L. Urban Evolution of Invasive Species. Front. Ecol. Environ. 2021, 19, 184–191. [Google Scholar] [CrossRef]
- Gallo, K.P.; Tarpley, J.D.; Mcnab, A.L.; Karl, T.R. Assessment of Urban Heat Islands: A Satellite Perspective. Atmos. Res. 1995, 37, 37–43. [Google Scholar] [CrossRef]
- Yang, L.; Qian, F.; Song, D.X.; Zheng, K.J. Research on Urban Heat-Island Effect. Proc. Eng. 2016, 169, 11–18. [Google Scholar] [CrossRef]
- Frank, S.D.; Just, M.G. Can Cities Activate Sleeper Species and Predict Future Forest Pests? A Case Study of Scale Insects. Insects 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Greenlees, M.J.; Harris, S.; White, A.W.; Shine, R. The Establishment and Eradication of an Extra-Limital Population of Invasive Cane Toads. Biol. Invasions 2018, 20, 2077–2089. [Google Scholar] [CrossRef]
- Jevrejeva, S.; Jackson, L.P.; Riva, R.E.M.; Grinsted, A.; Moore, J.C. Coastal Sea Level Rise with Warming above 2 °C. Proc. Natl. Acad. Sci. USA 2016, 113, 13342–13347. [Google Scholar] [CrossRef] [PubMed]
| Variable Abbreviation | Description |
|---|---|
| MAT | Mean annual temperature (°C) |
| MWMT | Mean temperature of the warmest month (°C) |
| MCMT | Mean temperature of the coldest month (°C) |
| TD | Difference between MCMT and MWMT (°C) |
| MAP | Mean annual precipitation (mm) |
| MSP | Mean summer (May to Sep) precipitation (mm) |
| AHM | Annual heat moisture index, calculated as (MAT + 10)/(MAP/1000) |
| SHM | Summer heat moisture index, calculated as MWMT/(MSP/1000) |
| DD_0 | Degree-days below 0 °C (chilling degree days) |
| DD5 | Degree-days above 5 °C (growing degree days) |
| DD_18 | Degree-days below 18 °C |
| DD18 | Degree-days above 18 °C |
| NFFD | Number of frost-free days |
| FFP | Frost-free period |
| bFFP | Julian date on which the frost-free period begins |
| eFFP | Julian date on which the frost-free period ends |
| PAS | Precipitation as snow (mm) |
| EMT | Extreme minimum temperature over 30 years |
| EXT | Extreme maximum temperature over 30 years |
| Eref | Hargreave’s reference evaporation |
| CMD | Hargreave’s climatic moisture index |
| MAR | Mean annual solar radiation (MJ m−2 d−1) |
| RH | Mean annual relative humidity (%) |
| CMI | Hogg’s climate moisture index (mm) |
| DD1040 | (10 < DD < 40) degree-days above 10 °C and below 40 °C |
| Tave_wt | Winter (December to February) mean temperature (°C) |
| Tave_sp | Spring (March to May) mean temperature (°C) |
| Tave_sm | Summer (June to August) mean temperature (°C) |
| Tave_at | Autumn (September to November) mean temperature (°C) |
| PPT_wt | Winter (December to February) precipitation (mm) |
| PPT_sp | Spring (March to May) precipitation (mm) |
| PPT_sm | Summer (June to August) precipitation (mm) |
| PPT_at | Autumn (September to November) precipitation (mm) |
| Relative Age Category | Approximate Age | Shell Length |
|---|---|---|
| Hatchling | 0–41 days | <10 mm |
| Juvenile | 42–311 days | 10–30 mm |
| Subadult | 312–460 days | 31–40 mm |
| Adult | >460 days | >40 mm |
| Description | Percent Contribution |
|---|---|
| Mean winter temperature (C°) (December–February) | 49.0% |
| Winter precipitation (mm) (December–February) | 26.1% |
| Summer precipitation (mm) (June–August) | 25.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mills, D.H.; McKinney, M.L. Climate Change and Jump Dispersal Drive Invasion of the Rosy Wolfsnail (Euglandina rosea) in the United States. Sustainability 2024, 16, 1929. https://doi.org/10.3390/su16051929
Mills DH, McKinney ML. Climate Change and Jump Dispersal Drive Invasion of the Rosy Wolfsnail (Euglandina rosea) in the United States. Sustainability. 2024; 16(5):1929. https://doi.org/10.3390/su16051929
Chicago/Turabian StyleMills, Dana H., and Michael L. McKinney. 2024. "Climate Change and Jump Dispersal Drive Invasion of the Rosy Wolfsnail (Euglandina rosea) in the United States" Sustainability 16, no. 5: 1929. https://doi.org/10.3390/su16051929
APA StyleMills, D. H., & McKinney, M. L. (2024). Climate Change and Jump Dispersal Drive Invasion of the Rosy Wolfsnail (Euglandina rosea) in the United States. Sustainability, 16(5), 1929. https://doi.org/10.3390/su16051929





