Three-Dimensional Electrochemical Oxidation System with RuO2-IrO2/Ti as the Anode for Ammonia Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
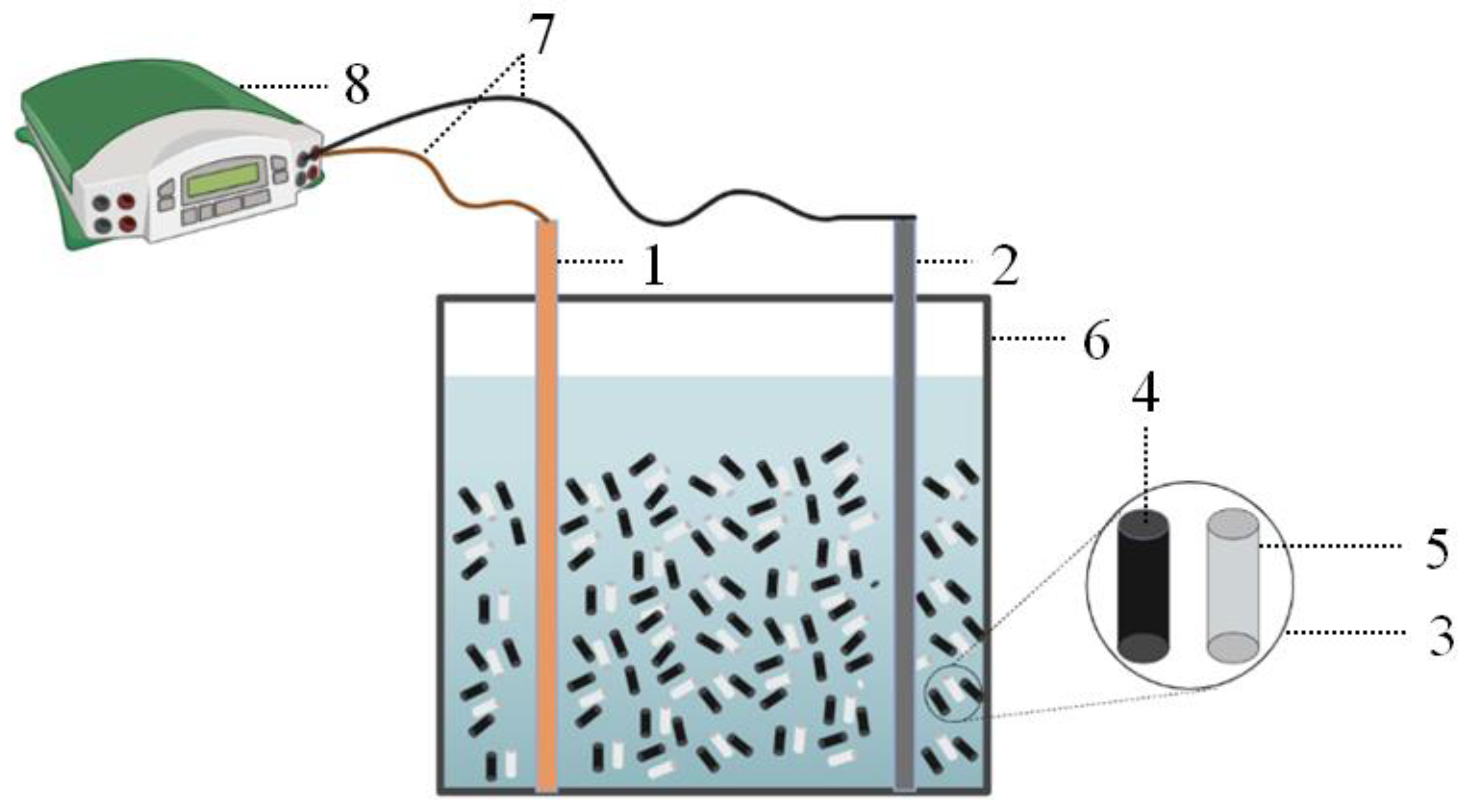
2.2. Experimental Device
2.3. Pretreatment of Filled Particles
2.4. Experimental Design
2.4.1. Optimization of Suitable Single-Factor Conditions
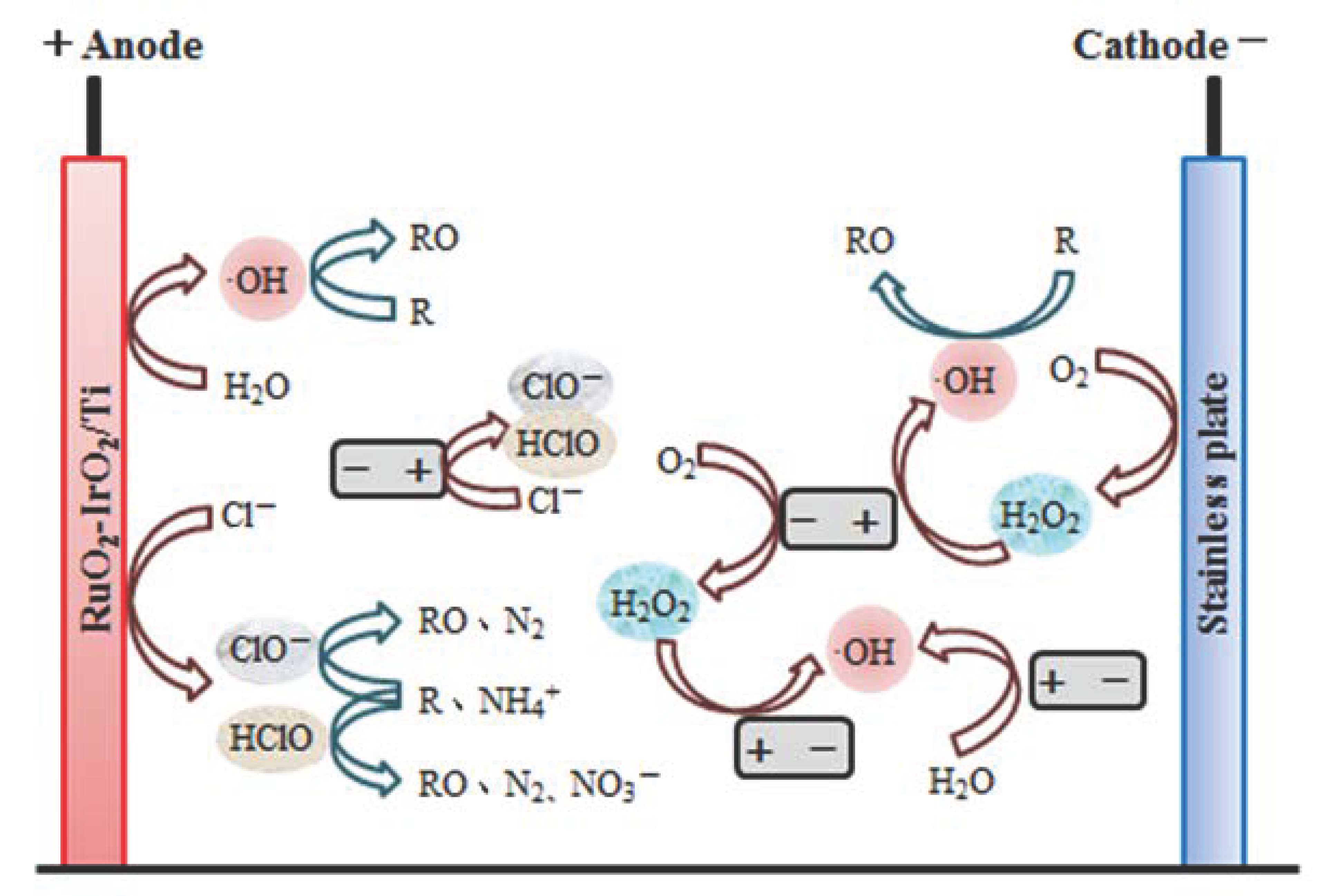
2.4.2. Explore the Removal Pathways of CODCr and NH4+-N
- (1)
- Analysis of direct electrochemical oxidation involving the anode plate surface
- (2)
- Analysis of indirect electrochemical oxidation involving active chlorine
- (3)
- Analysis of indirect electrochemical oxidation involving hydroxyl radicals
2.5. Analytical Items and Measurement Methods
3. Results and Discussion
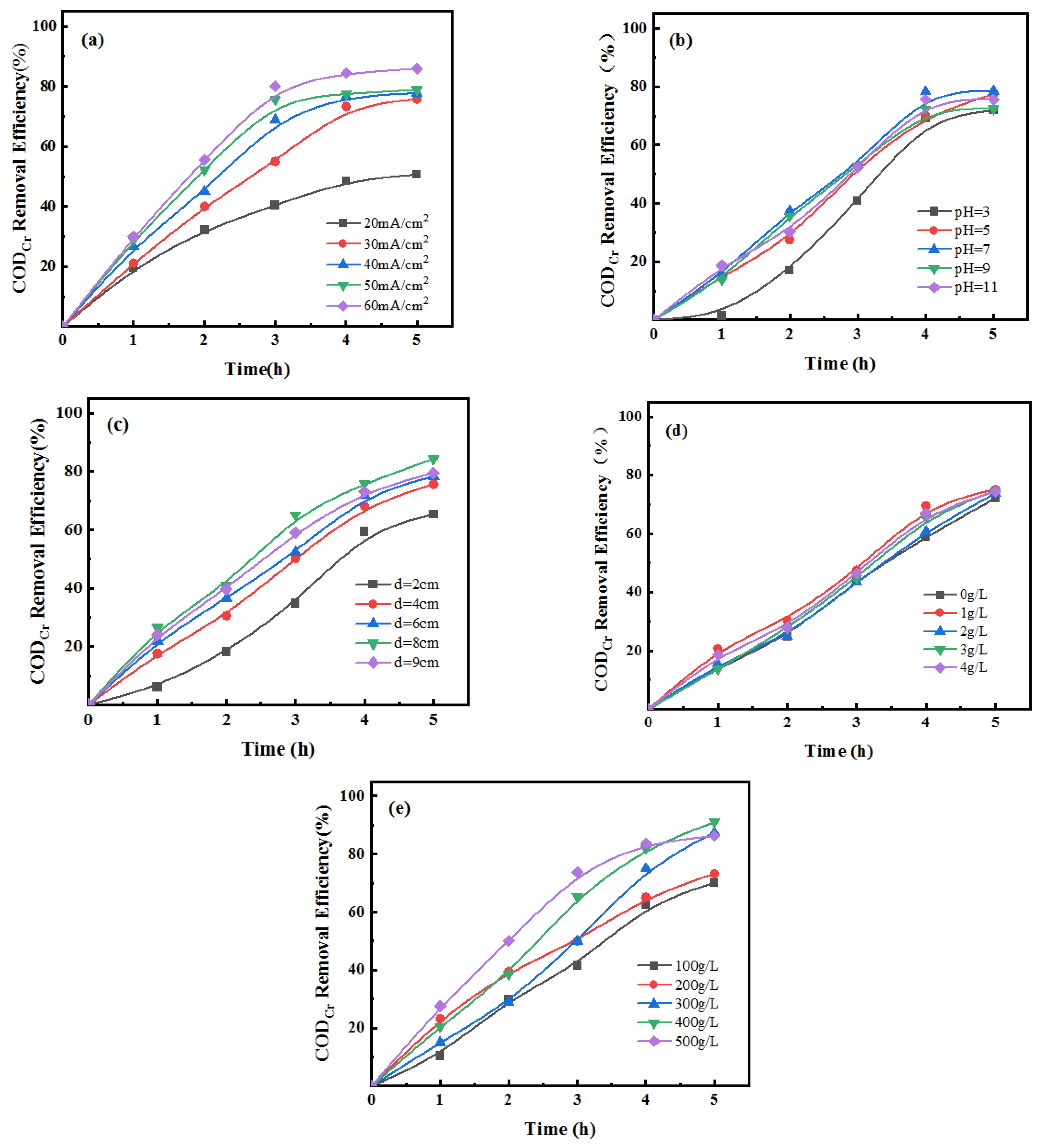
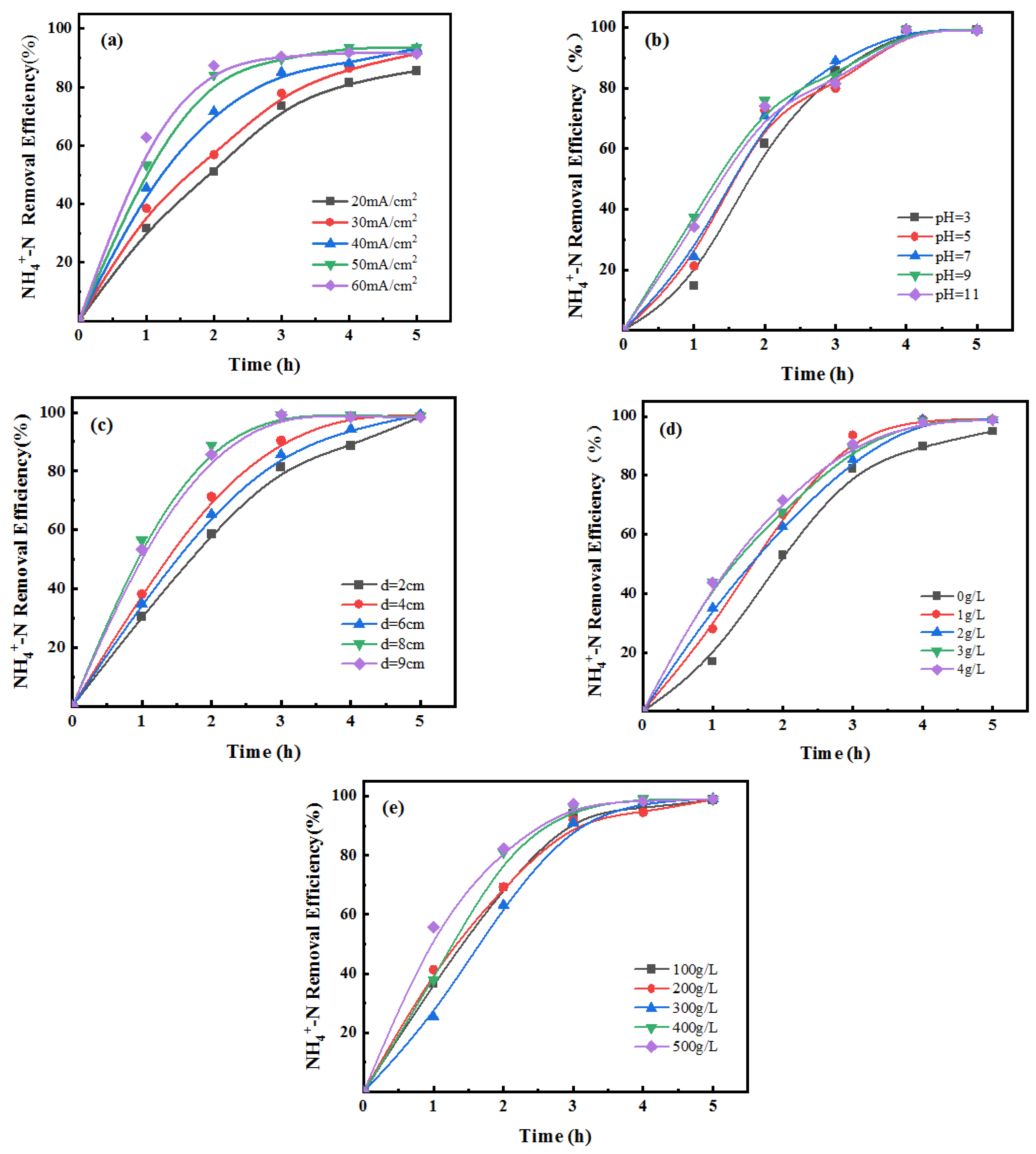
3.1. Optimization of Suitable Single-Factor Conditions
3.1.1. Preferred Current Density
3.1.2. Preferred Initial pH of Wastewater
3.1.3. Preferred Polar Plate Spacing
3.1.4. Preferred Concentration of NaCl
3.1.5. Preferred Particle Filling Amount
3.2. Removal Pathways of CODCr and NH4+-N
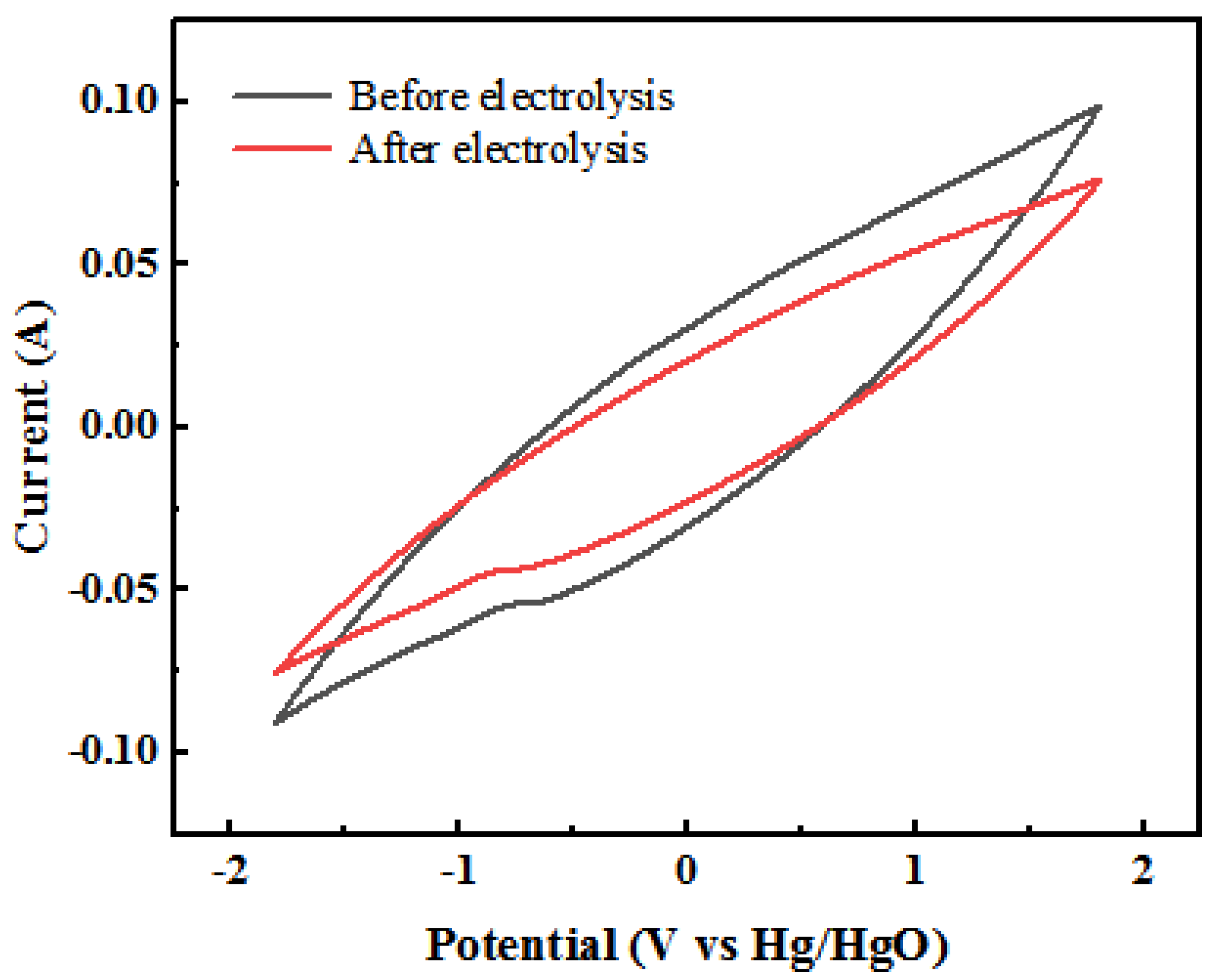
3.2.1. Analysis of Direct Electrochemical Oxidation Involved in the Surface of Anode Plates
- (1)
- Cyclic Voltammetry Curve
- (2)
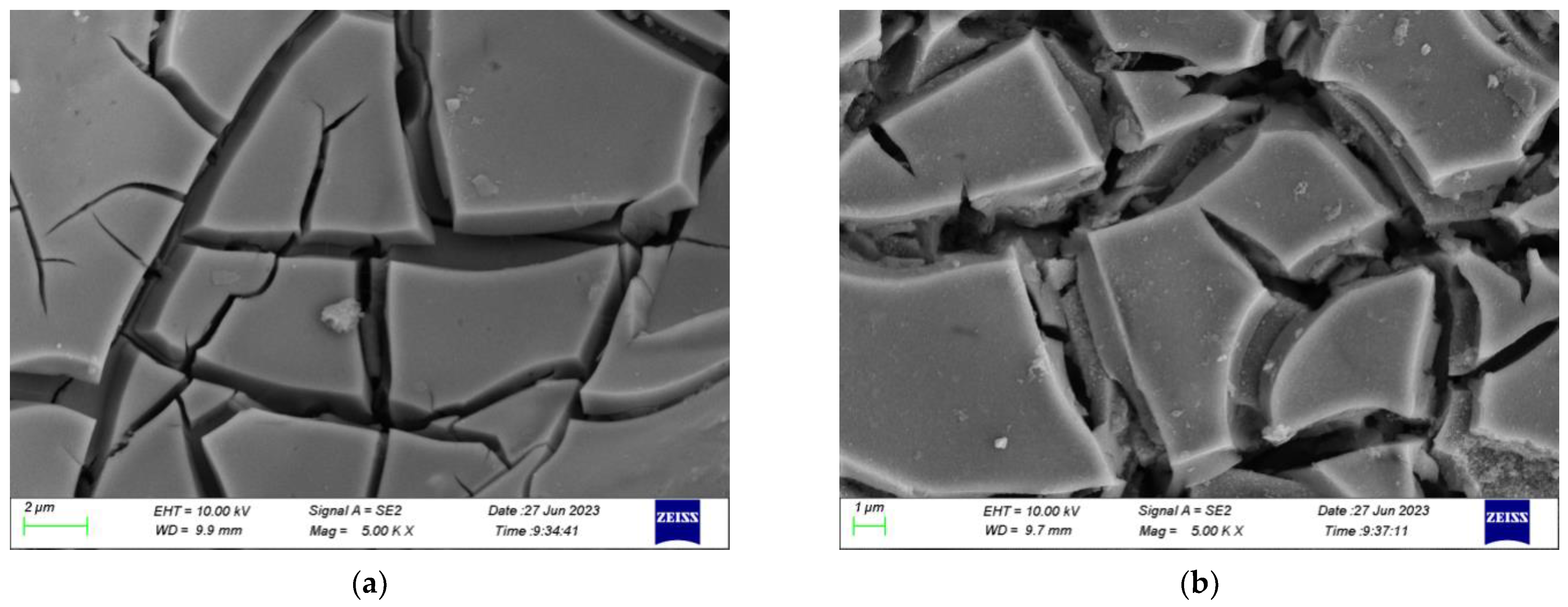
- Scanning Electron Microscope (SEM)
3.2.2. Analysis of Indirect Electrochemical Oxidation Involving Activated Chlorine
3.2.3. Analysis of Indirect Electrochemical Oxidation Involving ·OH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Chen, Y.; Ouyang, K.; Yang, J.; Li, J. Current research situation and prospect of ammonia nitrogen wastewater treatment technology. Ind. Water Treat. 2018, 38, 1–5. [Google Scholar]
- Liu, L. Research status of discharge and treatment of ammonia nitrogen wastewater in China. Sci. Technol. Inf. 2012, 19, 46. [Google Scholar]
- Brennan, B.; Lawler, J.; Regan, F. Recovery of viable ammonia-nitrogen products from agricultural slaughterhouse wastewater by membrane contactors: A review. Environ. Sci.-Water Res. Technol. 2021, 7, 259–273. [Google Scholar] [CrossRef]
- Scherger, L.E.; Zanello, V.; Lexow, C. Impact of Urea and Ammoniacal Nitrogen Wastewaters on Soil: Field Study in a Fertilizer Industry (Bahia Blanca, Argentina). Bull. Environ. Contam. Toxicol. 2021, 107, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, H.; Wang, A. Analysis of ammonia nitrogen, total nitrogen, triple nitrogen conversion and the role of ammonia nitrogen in the evaluation and control of water pollution. Inn. Mong. Water Resour. 2011, 34–36. [Google Scholar]
- Wosiack, P.A.; Lopes, D.D.; Damianovic, M.H.R.Z.; Foresti, E.; Granato, D.; Barana, A.C. Removal of COD and nitrogen from animal food plant wastewater in an intermittently-aerated structured-bed reactor. J. Environ. Manag. 2015, 154, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, L.; Zhang, J. Advance in production process and key manufacture technology for methionine. Chem. Ind. Eng. Prog. 2012, 31, 866–872+888. [Google Scholar]
- Wang, L.; Mou, C.; Wang, L. Treatment Methods and Research Status of High Ammonia-Nitrogen Content Wastewater. Technol. Water Treat. 2021, 47, 1–5+10. [Google Scholar]
- Yao, Q.; Tu, B.; Lu, H.; Lu, Y. Study on the Treatment of Ammonia-nitrogen Wastewater. Leather Manuf. Environ. Technol. 2022, 3, 19–21. [Google Scholar]
- Fu, R.; Zhang, P.-S.; Jiang, Y.-X.; Sun, L.; Sun, X.-H. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Zhao, M.; Dong, H.; Yu, H.; Zhan, S.; Zhang, L. Energy-saving removal of methyl orange in high salinity wastewater by electrochemical oxidation via a novel Ti/SnO2-Sb anode-air diffusion cathode system. Catal. Today 2015, 258, 156–161. [Google Scholar] [CrossRef]
- Shi, H.; Gao, M.; Xu, Z.; Ma, Z.; Ni, J.; Xu, Y.; Wang, Q. Long-term performance of granular activated carbon electrode system for the removal of amoxicillin. J. Water Process. Eng. 2022, 46, 102397. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Wang, C.; Zhao, J.; Zhao, W.; Lu, N.; Qu, J.; Yuan, X.; Zhang, Y.-N. Electrochemical removal of levofloxacin using conductive graphene/polyurethane particle electrodes in a three-dimensional reactor. Environ. Pollut. 2020, 260, 114101. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Liu, Y.; Yang, X.-Y.; Xu, J.; Li, X.-Y. Multiple response optimization for high efficiency energy saving treatment of rhodamine B wastewater in a three-dimensional electrochemical reactor. J. Environ. Manag. 2018, 218, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Viswanathan, K.; Boopathy, R.; Maharaja, P.; Sekaran, G. Three dimensional electro catalytic oxidation of aniline by boron doped mesoporous activated carbon. J. Ind. Eng. Chem. 2015, 21, 942–950. [Google Scholar] [CrossRef]
- Wei, L.; Guo, S.; Yan, G.; Chen, C.; Jiang, X. Electrochemical pretreatment of heavy oil refinery wastewater using a three-dimensional electrode reactor. Electrochim. Acta 2010, 55, 8615–8620. [Google Scholar] [CrossRef]
- Wang, B.; Shu, B.; Ren, H.; Wang, B. Effects of three dimensional electrode filling particles on treatment of MDEA effluent. Chin. J. Environ. Eng. 2017, 11, 205–210. [Google Scholar]
- Zhong, R.; Zhou, D.; Chen, W.; Ji, Q. Investigation of the packing model of particle electrode on the efficiency of threedimensional electrode reactor. Acta Sci. Circumstantiae 2011, 31, 2174–2178. [Google Scholar]
- Baddouh, A.; Bessegato, G.G.; Rguiti, M.M.; El Ibrahimi, B.; Bazzi, L.; Hilali, M.; Zanoni, M.V.B. Electrochemical decolorization of Rhodamine B dye: Influence of anode material, chloride concentration and current density. J. Environ. Chem. Eng. 2018, 6, 2041–2047. [Google Scholar] [CrossRef]
- Sathishkumar, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B-Biol. 2019, 200, 111655. [Google Scholar] [CrossRef]
- Cui, X. Literature Review of DSA Electrode in Wastewater Treatment. Environ. Dev. 2011, 23, 58+63. [Google Scholar]
- Iranpour, F.; Pourzamani, H.; Mengelizadeh, N.; Bahrami, P.; Mohammadi, H. Application of response surface methodology for optimization of reactive black 5 removal by three dimensional electro-Fenton process. J. Environ. Chem. Eng. 2018, 6, 3418–3435. [Google Scholar] [CrossRef]
- Costa, C.R.; Botta, C.M.R.; Espindola, E.L.G.; Olivi, P. Electrochemical treatment of tannery wastewater using DSA® electrodes. J. Hazard. Mater. 2008, 153, 616–627. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, C.; Shin, E.B.; Kirm, S. Effects of Cl-based chemical coagulants on electrochemical oxidation of textile wastewater. Desalination 2003, 155, 59–65. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Hu, C.; Zhou, X. Design, fabrication and high efficient visible-light assisted photoelectric-synergistic performance of 3-D mesoporous DSA electrodes. Mater. Des. 2016, 91, 201–210. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Hu, C.; Zhou, X. Fabrication of a novel Ti/SnO2-Sb-CeO2@TiO2-SnO2 electrode and photoelectrocatalytic application in wastewater treatment. Mater. Sci. Semicond. Process. 2015, 40, 744–751. [Google Scholar] [CrossRef]
- Tran Le, L. Post treatment of ICEAS-biologically landfill leachate using electrochemical oxidation with Ti/BDD and Ti/RuO2 anodes. Environ. Technol. Innov. 2020, 20, 101099. [Google Scholar]
- Chen, X.; Chen, G.; Gao, F.; Yue, P.L. High-performance Ti/BDD electrodes for pollutant oxidation. Environ. Sci. Technol. 2003, 37, 5021–5026. [Google Scholar] [CrossRef] [PubMed]
- Vasilie, S.; Manea, F.; Baciu, A.; Pop, A. Dual use of boron-doped diamond electrode in antibiotics-containing water treatment and process control. Process Saf. Environ. Prot. 2018, 117, 446–453. [Google Scholar] [CrossRef]
- Gao, C.; Chang, M. Ta/BDD Film Electrode for Electrochemical Oxidation Nitrophenol. Acta Physico-Chim. Sin. 2008, 24, 1988–1994. [Google Scholar]
- Montilla, F.; Morallon, E.; Battisti, A.D.; Benedetti, A.; Vazquez, J.L. Preparation and Characterization of Antimony-Doped Tin Dioxide Electrodes. Part 2. XRD and EXAFS Characterization. J. Phys. Chem. 2004, 108, 5044–5050. [Google Scholar] [CrossRef]
- Wang, B.Y.; Shi, Y.Q.; Liu, Z.Z.; Zhang, Y.C.; Wang, Y.L.; Lu, T.X.; Wan, S.-W.; Sun, X.-C. Research Progress of Electrode Materials for Electrochemical Water Treatment. Environ. Sci. Technol. 2022, 45, 197–207. [Google Scholar]
- Perea, L.A.; Palma-Goyes, R.E.; Vazquez-Arenas, J.; Romero-Ibarra, I.; Ostos, C.; Torres-Palma, R.A. Efficient cephalexin degradation using active chlorine produced on ruthenium and iridium oxide anodes: Role of bath composition, analysis of degradation pathways and degradation extent. Sci. Total Environ. 2019, 648, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ukundimana, Z.; Omwene, P.I.; Gengec, E.; Can, O.T.; Kobya, M. Electrooxidation as post treatment of ultrafiltration effluent in a landfill leachate MBR treatment plant: Effects of BDD, Pt and DSA anode types. Electrochim. Acta 2018, 286, 252–263. [Google Scholar] [CrossRef]
- Oliveira, E.M.; Silva, F.R.; Morais, C.C.; Oliveira, T.M.B.; Martínez-Huitle, C.A.; Motheo, A.J.; Albuquerque, C.C.; Castro, S.S.L. Performance of (in)active anodic materials for the electrooxidation of phenolic wastewaters from cashew-nut processing industry. Chemosphere 2018, 201, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Preparation of Titanium Based DSA Electrode and Performance and Mechanism of Electrochemical Oxidation of Aniline Wastewater. Ph.D. Thesis, China University of Geosciences (Peking), Beijing, China, 2020. [Google Scholar]
- Yu, J.; Huang, S.; Li, Y.; Chen, Z. Treatment landfill leachate with three-dimensional electrodes coated by activated carbon. Ind. Water Treat. 2021, 41, 232–237. [Google Scholar]
- Dai, H.; Chen, J.; Miao, X.; Jiang, B.; Gong, X. Effect of alcohols on scavenging efficiencies to hydroxyl radical in UV-Fenton system. China Environ. Sci. 2018, 38, 202–209. [Google Scholar]
- Li, C.; Wang, H.; Cui, X. Treatment of cobalt and manganese containing wastewater by three-dimensional electrode method. Environ. Prot. Chem. Ind. 2021, 41, 43–48. [Google Scholar]
- Jiang, H.; Zhou, D.; Chen, W.; Luo, B.; Fan, Z. Preliminary exploration of electrochemical reaction characteristics in three-dimensional electrode treatment ammonia waste water. China Environ. Sci. 2014, 34, 2551–2555. [Google Scholar]
- Liang, H.; Zhao, L.; Li, L.; Chen, Y.; Peng, H. Catalytic oxidation of chlorine-containing hydroxypropyl guanidine gum simulated wastewater by three-dimensional electrode technology. Ind. Water Treat. 2020, 40, 40–44. [Google Scholar]
- Li, N.; Wang, B.; Yuan, D. Progress on treatment of phenol-containing wastewater by electrochemical technology. Mod. Chem. Ind. 2021, 41, 33–37. [Google Scholar]
- Chen, W.; Li, F.; Mei, P. Mechanism of COD Degradation in Wastewater Using Three Dimension Electrode Method. Environ. Sci. Technol. 2002, 1, 11–12+22–46. [Google Scholar] [CrossRef]
- Wu, N.; Qian, H.; Zhen, L.; Li, Y.; Wang, Y.; Wang, L. Experimental Study on the Treatment of Malachite Green Dyeing Wastewater by Three-Dimensional Electrode Electro-Fenton Process. J. Shenyang Jianzhu Univ. (Nat. Sci.) 2018, 34, 183–192. [Google Scholar]
- Li, Y.; Duan, Y. Treatment of Wastewater Containing High Concentration Ammonia-Nitrogen by Electrochemical Oxidation Process. J. Adv. Mat. Res. 2011, 393–395, 1587–1590. [Google Scholar] [CrossRef]
- Li, Y.; Guo, P. Experiment on Treatment of Nitrobenzene Wastewater by Three-dimensional Electrode Electro-Fenton Method. J. Shenyang Jianzhu Univ. (Nat. Sci.) 2023, 39, 177–184. [Google Scholar]
- Chen, L.; Zhu, J.; Li, J.; Ma, Z.; Yang, Y.; Wang, S. Electrochemical Treatment of DMF Wastewater by TiO2/AC Three-dimensional Electrode. Guangzhou Chem. Ind. 2021, 49, 65–68. [Google Scholar]
- Li, Y.; Xu, S.; Gao, C.; Fu, X. Treatment of reactive brilliant orange X-GN wastewater by three-dimensional electrode-electro-Fenton method. Ind. Water Treat. 2022, 44, 1–15. [Google Scholar] [CrossRef]
- Yuan, J. Research status of ammonia wastewater treatment methods. Sci. Technol. Vis. 2020, 21, 160–161. [Google Scholar] [CrossRef]
- Bu, J.; Deng, Z.; Liu, H.; Li, T.; Yang, Y.; Zhong, S. Bimetallic modified halloysite particle electrode enhanced electrocatalytic oxidation for the degradation of sulfanilamide. J. Environ. Manag. 2022, 312, 114975. [Google Scholar] [CrossRef]
- Hou, Z.; Zhou, J.; Wu, L.; Liang, K.; Song, Y.; Zhang, Q. Study on Synergetic-Photoelectrocatalytic Degradation of Phenol by Nano-TiO2 Three-dimensional Electrode. Nonferrous Met. Eng. 2019, 9, 1–6+28. [Google Scholar]
- Zhang, L.; Duan, A.; Wang, L.; Fu, B.; Liu, X.; Wu, G. Advanced treatment of coking wastewater using three-dimensional fluid bed electrode reactor. Ecol. Environ. Sci. 2012, 21, 370–374. [Google Scholar]
- Singla, J.; Verma, A.; Sangal, V.K. Applications of doped mixed metal oxide anode for the electro-oxidation treatment and mineralization of urine metabolite, uric acid. J. Water Process Eng. 2019, 32, 100944. [Google Scholar] [CrossRef]
- Ozturk, D.; Yilmaz, A.E. Treatment of slaughterhouse wastewater with the electrochemical oxidation process: Role of operating parameters on treatment efficiency and energy consumption. J. Water Process Eng. 2019, 31, 100834. [Google Scholar] [CrossRef]
- Ni, J. Study on the Effect of Loaded γ-MnO2 Particales on the Degradation of Rhodamine B Wastewater by Three-Dimensional Electrode Reactor. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2020. [Google Scholar]
- Niu, X.; Xie, F. Preparation of Ti-base SnRuCo Electrode with Nano-coating. Sci. Technol. Eng. 2007, 11, 2564–2569+2598. [Google Scholar]
- Wang, C.; Huang, Y.-K.; Zhao, Q.; Ji, M. Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects. Chem. Eng. J. 2014, 243, 1–6. [Google Scholar]
- Oliveira, K.S.G.C.; Farinos, R.M.; Veroli, A.B.L.; Ruotolo, A.M. Electrochemical incineration of glyphosate wastewater using three-dimensional electrode. Environ. Technol. 2021, 42, 170–181. [Google Scholar] [CrossRef]
- Su, W.; Yang, J.; Cheng, Z.; Shi, J.; Xu, H. Improvement of a three-dimensional electrochemical reactor for styrene wastewater treatment. Environ. Eng. 2019, 37, 107–110. [Google Scholar]
- Li, X. Development in electrochemical technology for environmental wastewater treatment. Int. J. Electrochem. Sci. 2022, 17, 2212110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Wang, D.; Zhao, Y.; Zhang, Y.; Guo, R. Electrocatalytic Performance of Three-dimensional Electrode System with SnO2/Fe3O4 Particle Electrode. Mater. Rep. 2017, 31, 25–30. [Google Scholar]
- Ding, W.; Li, Q.; Liang, G.; Xiang, X.; Zeng, X.; Su, Q. Characteristics of ammonia removal from water using biochar three-dimensional electrode reactor. J. Harbin Inst. Technol. 2016, 48, 131–135. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Zhao, L.; Zhu, J.; He, D. Three-Dimensional Electrochemical Oxidation System with RuO2-IrO2/Ti as the Anode for Ammonia Wastewater Treatment. Sustainability 2024, 16, 1838. https://doi.org/10.3390/su16051838
Huang Z, Zhao L, Zhu J, He D. Three-Dimensional Electrochemical Oxidation System with RuO2-IrO2/Ti as the Anode for Ammonia Wastewater Treatment. Sustainability. 2024; 16(5):1838. https://doi.org/10.3390/su16051838
Chicago/Turabian StyleHuang, Zhengmin, Li Zhao, Jingping Zhu, and Dongming He. 2024. "Three-Dimensional Electrochemical Oxidation System with RuO2-IrO2/Ti as the Anode for Ammonia Wastewater Treatment" Sustainability 16, no. 5: 1838. https://doi.org/10.3390/su16051838
APA StyleHuang, Z., Zhao, L., Zhu, J., & He, D. (2024). Three-Dimensional Electrochemical Oxidation System with RuO2-IrO2/Ti as the Anode for Ammonia Wastewater Treatment. Sustainability, 16(5), 1838. https://doi.org/10.3390/su16051838




