Sustainable Management Strategies for Fruit Processing Byproducts for Biorefineries: A Review
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Determinants of Fruit Processing Byproducts
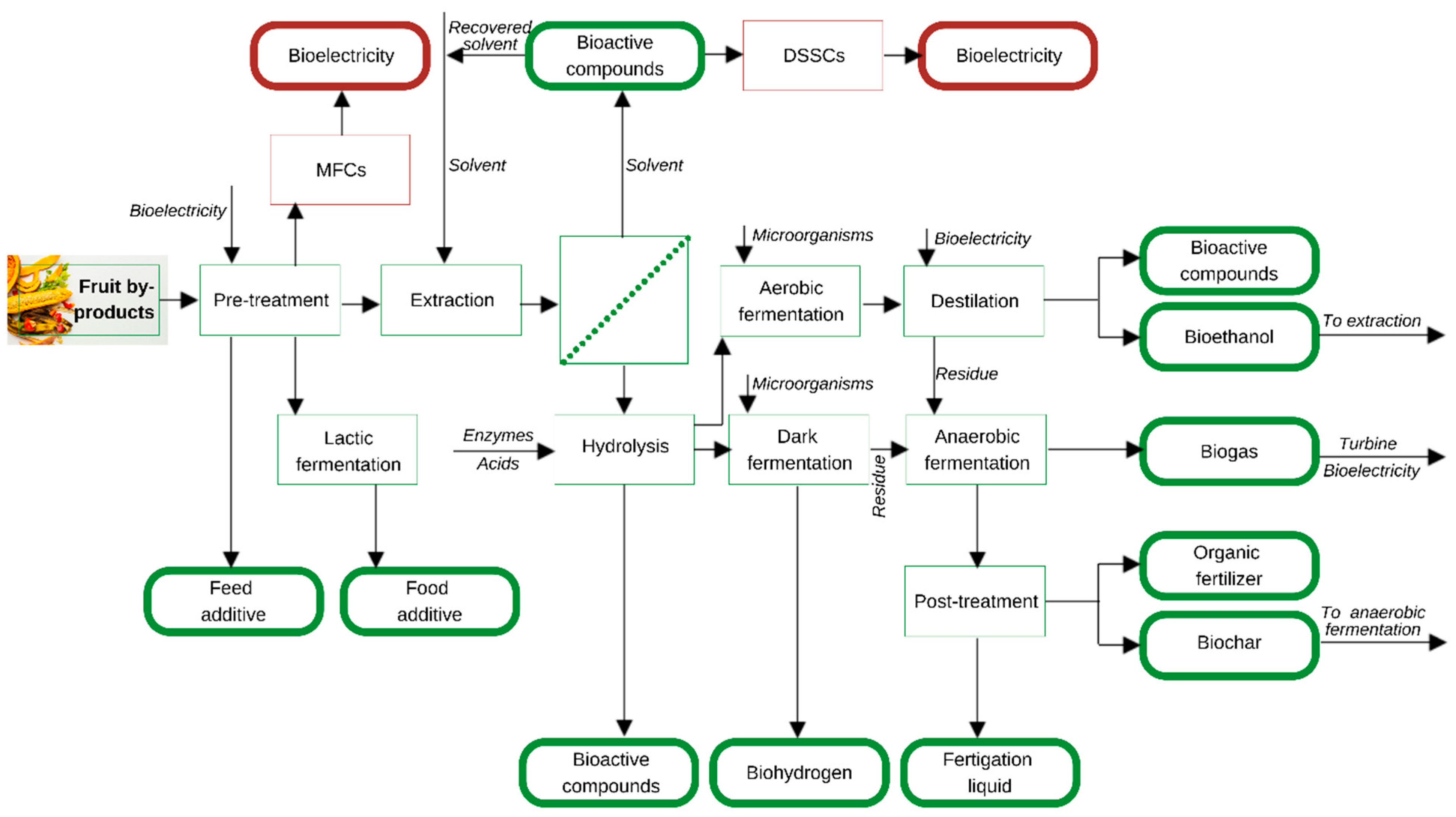
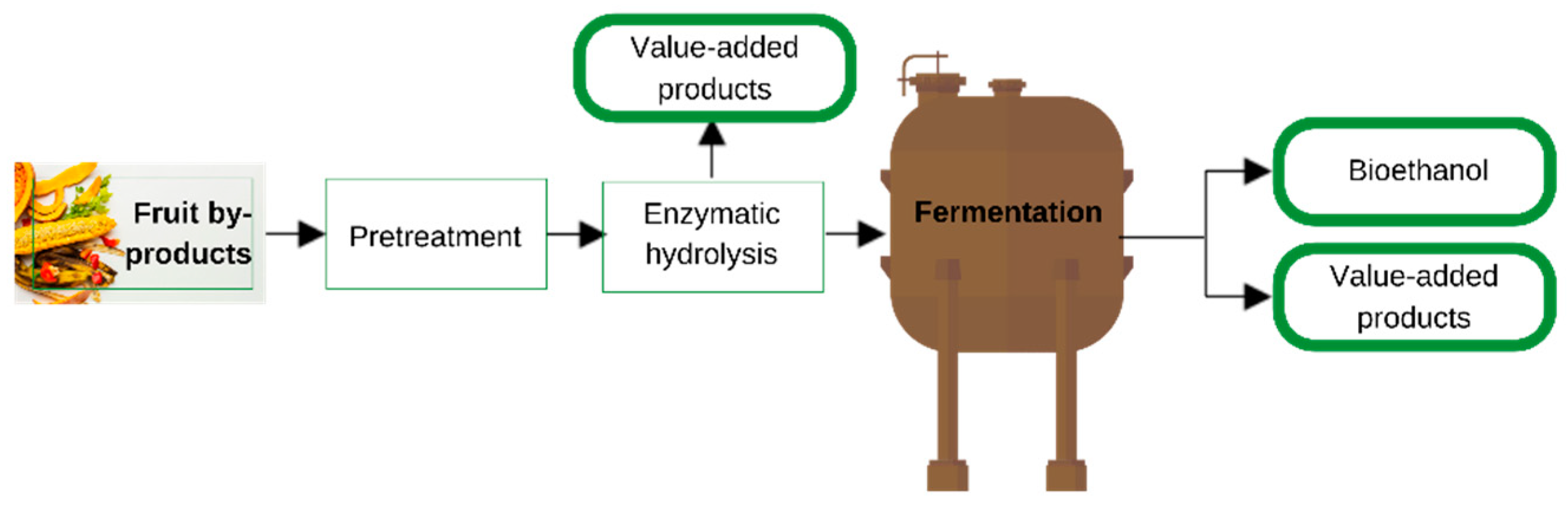
3.2. Integration of Fruit Byproducts in Biorefineries
3.2.1. Pretreatment
3.2.2. Extraction
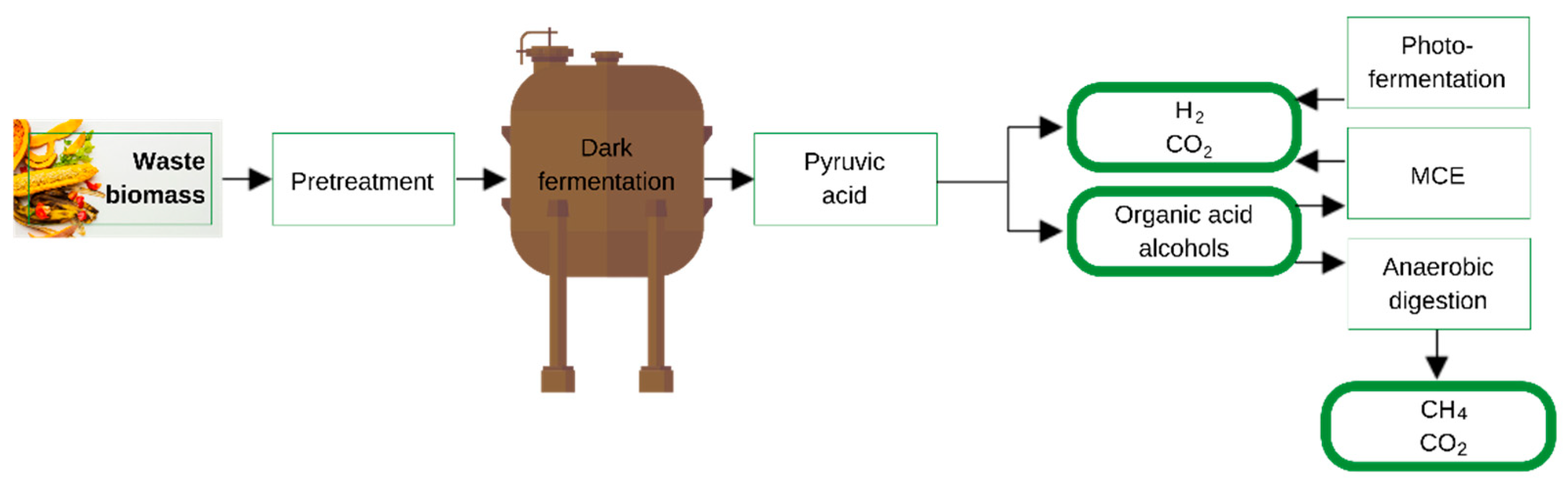
3.2.3. Dark or Aerobic Fermentation
3.2.4. Anaerobic Digestion
3.2.5. Post-Treatment
3.2.6. Alternative Stages in the Biorefining Process
Microbial Fuel Cells (MFCs)
Dye-Sensitized Solar Cells (DSSCs)
| Substrate | Sensitizers | Efficiency [%] | Reference |
|---|---|---|---|
| banana peel | carotenoids/chlorophyll | 0.21 | [139] |
| tangerine peel | flavanone | 0.71 | [140] |
| pineapple peel | chlorophyll/flavonols | 0.002 | [141] |
| black chokeberry pomace | anthocyanins | 0.105 | [131] |
| pomegranate leaf | chlorophyll | 0.597 | [142] |
| mangosteen peel | α-magnostin, anthocyanins | 2.63 | [132] |
4. Conclusions
- ✓
- Increasing the efficiency and economy of the proposed technological stages;
- ✓
- Increasing the level of waste management in the fruit industry;
- ✓
- The development and implementation of new technologies for increasing the management of fruit byproducts;
- ✓
- Developing new products based on fruit byproducts;
- ✓
- Increasing the awareness of products resulting from the proposed technological processes.
Author Contributions
Funding
Conflicts of Interest
References
- Dubey, P.; Yousuf, O. An overview of fruit by-products valorization: A step towards sustainable utilization. Ind. J. Pure Appl. Biosci. 2021, 9, 46–55. [Google Scholar] [CrossRef]
- Redondo-Gómez, C.; Rodríguez Quesada, M.; Vallejo Astúa, S.; Murillo Zamora, J.P.; Lopretti, M.; Vega-Baudrit, J.R. Biorefinery of Biomass of Agro-Industrial Banana Waste to Obtain High-Value Biopolymers. Molecules 2020, 25, 3829. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of vegetable and fruit by-products as functional ingredient and food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef]
- Ding, Z.; Ge, Y.; Sar, T.; Kumar, V.; Harirchi, S.; Binod, P.; Sirohi, R.; Sindhu, R.; Wu, P.; Lin, F.; et al. Valorization of tropical fruits waste for production of commercial biorefinery products—A review. Bioresour. Technol. 2023, 374, 128793. [Google Scholar] [CrossRef]
- Negro, V.; Ruggeri, B.; Fino, D.; Tonini, D. Life cycle assessment of orange peel waste management. Resour. Conserv. Recycl. 2017, 127, 148–158. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabro, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Wastewater Management in Citrus Processing Industries: An Overview of Advantages and Limits. Water 2019, 11, 2481. [Google Scholar] [CrossRef]
- Bayram, B.; Ozkan, G.; Kostka, T.; Capanoglu, E.; Esatbeyoglu, T. Valorization and application of fruit and vegetable wastes and by-products for food packaging materials. Molecules 2021, 26, 4031. [Google Scholar] [CrossRef] [PubMed]
- Pateiro-Moure, M.; Novoa-Munoz, J.C.; Arias-Estevez, M.; Lopez-Periago, E.; Martinez-Carballo, E.; Simal-Gandara, J. Quaternary herbicides retention by the amendment of acid soils with a bentonite-based waste from wineries. J. Hazard. Mater. 2009, 164, 769–775. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Valetti, N.W.; Pastrana-Castro, L.M.; Teixeira, J.A.; Pico, G.A.; Pintado, M.M. Optimization of bromelain isolation from pineapple by-products by polysaccharide complex formation. Food Hydrocoll. 2019, 87, 792–804. [Google Scholar] [CrossRef]
- Jeong, D.; Park, H.; Jang, B.K.; Ju, Y.B.; Shin, M.H.; Oh, E.J.; Lee, E.J.; Kim, S.R. Recent advances in the biological valorization of citrus peel waste into fuels and chemicals. Bioresour. Technol. 2021, 323, 124603. [Google Scholar] [CrossRef]
- Budzianowski, W.M. High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renew. Sustain. Energy Rev. 2017, 70, 793–804. [Google Scholar] [CrossRef]
- Teshome, E.; Teka, T.A.; Nandasiri, R.; Rout, J.R.; Harouna, D.V.; Astatkie, T.; Urugo, M.M. Fruit By-Products and Their Industrial Applications for Nutritional Benefits and Health Promotion: A Comprehensive Review. Sustainability 2023, 15, 7840. [Google Scholar] [CrossRef]
- Jalal, H.; Giammarco, M.; Lanzoni, L.; Akram, M.Z.; Mammi, L.M.E.; Vignola, G.; Chincarini, M.; Formigoni, A.; Fusaro, I. Potential of Fruits and Vegetable By-Products as an Alternative Feed Source for Sustainable Ruminant Nutrition and Production: A Review. Agriculture 2023, 13, 286. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Zoidis, E.; Goliomytis, M.; Simitzis, P. Utilization of Agro-Industrial By-Products for Sustainable Poultry Production. Sustainability 2023, 15, 3679. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, T.; Wang, X.; Lu, X. Apple pomace as a potential valuable resource for full-components utilization: A review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Costa, J.M.; Ampese, L.C.; Ziero, H.D.D.; Sganzerla, W.G.; Forster-Carneiro, T. Apple pomace biorefinery: Integrated approaches for the production of bioenergy, biochemicals, and value-added products—An updated review. J. Environ. Chem. Eng. 2022, 10, 108358. [Google Scholar] [CrossRef]
- Kim, I.J.; Jeong, D.; Kim, S.R. Upstream processes of citrus fruit waste biorefinery for complete valorization. Bioresour. Technol. 2022, 362, 127776. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Costa, J.M.; Forster-Carneiro, T. Valorization of apple pomace by-products from the juice processing industry using pressurized liquid technology. J. Environ. Chem. Eng. 2023, 11, 110907. [Google Scholar] [CrossRef]
- Luo, J.; Ma, Y.; Xu, Y. Valorization of apple pomace using a two-step slightly acidic processing strategy. Renew. Energy 2020, 152, 793–798. [Google Scholar] [CrossRef]
- Santos, J.R.; Viana, G.C.C.; Barbosa, R.S.; de S. Borges, M.; Rambo, M.K.D.; Bertuol, D.A.; Scapin, E. Effect of different pretreatments of Passiflora edulis peel biomass on the conversion process into bioproducts for biorefineries. Sustain. Chem. Environ. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Abraham, R.A.; Joshi, J.T.; Abdullah, S. A comprehensive review of pineapple processing and its by-product valorization in India. Food Chem. Adv. 2023, 3, 100416. [Google Scholar] [CrossRef]
- Santra, S.; Das, M.; Karmakar, S.; Banerjee, R. NADES assisted integrated biorefinery concept for pectin recovery from kinnow (Citrus reticulate) peel and strategic conversion of residual biomass to L(+) lactic acid. Int. J. Biol. Macromol. 2023, 250, 126169. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of apple pomaces for biofuel production: A biorefinery approach. Biomass Bioenergy 2020, 142, 105785. [Google Scholar] [CrossRef]
- Tigunova, O.; Bratishko, V.; Shulga, S. Apple pomace as an alternative substrate for butanol production. AMB Expr. 2023, 13, 138. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An integrated green biorefinery approach towards simultaneous recovery of pectin and polyphenols coupled with bioethanol production from waste pomegranate peels. Bioresour. Technol. 2018, 266, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Borujeni, N.E.; Alavijeh, M.K.; Denayer, J.F.M.; Karimi, K. A novel integrated biorefinery approach for apple pomace valorization with significant socioeconomic benefits. Renew. Energy 2023, 208, 275–286. [Google Scholar] [CrossRef]
- Borujeni, N.E.; Karimi, K.; Denayer, J.F.M.; Kumar, R. Apple pomace biorefinery for ethanol, mycoprotein, and value-added biochemicals production by Mucor indicus. Energy 2022, 240, 122469. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Barroso, T.L.C.T.; Sganzerla, W.G.; Costa, J.M.; Saia, F.T.; Colpini, L.M.S.; Forster-Carneiro, T. Subcritical water hydrolysis of grape pomace as a sustainable pretreatment for anaerobic digestion in a biorefinery concept. Fuel 2024, 363, 130899. [Google Scholar] [CrossRef]
- Arun, K.B.; Madhavan, A.; Anoopkumar, A.N.; Surendhar, A.; Kuriakose, L.L.; Tiwari, A.; Sirohi, R.; Kuddus, M.; Rebello, S.; Awasthi, M.K.; et al. Integrated biorefinery development for pomegranate peel: Prospects for the production of fuel, chemicals and bioactive molecules. Bioresour. Technol. 2022, 362, 127833. [Google Scholar] [CrossRef]
- Villegas-Yarlequé, M.; Tirado-Kulieva, V.A.; Seminario-Sanz, R.S.; Camacho-Orbegoso, E.W.; Calderón-Castillo, B.; Bruno-Coveñas, P. Bibliometric analysis and text mining to reveal research trends on fruit by-products under circular economy strategies. Sustain. Chem. Pharm. 2023, 35, 101232. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sowjanya, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of agro-industrial wastes: Circular bioeconomy and biorefinery process—A sustainable symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Manhongo, T.T.; Chimphango, A.F.A.; Thornley, P.; Röder, M. Current status and opportunities for fruit processing waste biorefineries. Renew. Sustain. Energy Rev. 2022, 155, 111823. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Sirohi, R.; Ahluwalia, V.; Zhou, Y.; Sindhu, R.; Binod, P.; Singhnia, R.R.; Patel, A.K.; Juneja, A.; et al. Sustainable blueberry waste recycling towards biorefinery strategy and circular bioeconomy: A review. Bioresour. Technol. 2021, 332, 125181. [Google Scholar] [CrossRef]
- Sylvester, A.; Tate, M.; Johnstone, D. Beyond synthesis: Re-presenting heterogeneous research literature. Behav. Inf. Technol. 2013, 32, 1199–1215. [Google Scholar] [CrossRef]
- Templier, M.; Paré, G. A framework for guiding and evaluating literature reviews. Commun. Assoc. Inf. Syst. 2015, 37, 112–137. [Google Scholar] [CrossRef]
- Turnbull, D.; Chugh, R.; Luck, J. Systematic-narrative hybrid literature review: A strategy for integrating a concise methodology into a manuscript. Soc. Sci. Humanit. Open 2023, 7, 100381. [Google Scholar] [CrossRef]
- Snyder, H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Baker, J.D. The Purpose, Process, and Methods of Writing a Literature Review. AORN J. 2016, 103, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Dominguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Davila-Avina, J.E.; Gonzalez-Aguilar, G.A. The agro-industro potential of exotic fruit by-products as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Pachołek, B. Development directions for food design research using by-products in the perspective of innovative European economy. In Innovations in Food Quality Development; Dmowski, R., Ed.; Uniwersytet Morski w Gdyni: Gdynia, Poland, 2020; pp. 105–116. [Google Scholar]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Valorisation of banana peel: A biorefinery approach. Rev. Chem. Eng. 2016, 32, 651–666. [Google Scholar] [CrossRef]
- Rivas-Cantu, R.C.; Jones, K.D.; Mills, P.L. A citrus waste-based biorefinery as a source of renewable energy: Technical advances and analysis of engineering challenges. Waste Manag. Res. 2013, 31, 413–420. [Google Scholar] [CrossRef]
- Roda, A.; Lambri, M. Food uses of pineapple waste and by-products: A review. Int. J. Food Sci. Technol. 2019, 54, 1009–1017. [Google Scholar] [CrossRef]
- De Torres, C.; Diaz-Maroto, M.C.; Hermosin-Gutierrez, I.; Perez-Coello, M.S. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot). J. Food Sci. 2012, 77, H192–H201. [Google Scholar] [CrossRef] [PubMed]
- Radojčin, M.; Pavkov, I.; Bursać Kovačević, D.; Putnik, P.; Wiktor, A.; Stamenković, Z.; Kešelj, K.; Gere, A. Effect of selected drying methods and emerging drying intensification technologies on the quality of dried fruit: A review. Processes 2021, 9, 132. [Google Scholar] [CrossRef]
- Vashisth, T.; Singh, R.K.; Pegg, R.B. Effects of drying on the phenolics content and antioxidant activity of muscadine pomace. LWT-Food Sci. Technol. 2011, 44, 1649–1657. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Rodriguez Garcia, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6446–6466. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Garavand, F.; Rahaee, S.; Vahedikia, N.; Jafari, S.M. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends Food. Sci. Technol. 2019, 89, 26–44. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pingret, D.; Fabiano-Tixier, A.S.; Bourvellec, C.L.; Renard, C.M.G.C.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Gadonna-Widehem, P.; Laguerre, J.C. Characterization of microbial inactivation by microwave heating. In Global Food Security and Wellness; Barbosa-Canovas, G.V., Pastore, G.M., Candogan, K., Medina Meza, I.G., da Silva Lannes, S.C., Buckle, K., Yada, R.Y., Rosenthal, A., Eds.; Springer: New York, NY, USA, 2017; pp. 489–517. [Google Scholar]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo-fermentation of lignocellulosic biomass. Biomass Convers. Biorefin. 2020, 13, 8465–8483. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Nanda, S. Biohydrogen production through dark fermentation. Chem. Eng. Technol. 2020, 43, 601–612. [Google Scholar] [CrossRef]
- Basak, B.; Jeon, B.H.; Kim, T.H.; Lee, J.C.; Chatterjee, P.K.; Lim, H. Dark fermentative hydrogen production from pretreated lignocellulosic biomass: Effects of inhibitory by-products and recent trends in mitigation strategies. Renew. Sustain. Energy Rev. 2020, 133, 110338. [Google Scholar] [CrossRef]
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Jahim, J.M.d. Biohydrogen production through photo fermentation or dark fermentation using waste as a substrate: Overview, economics, and future prospects of hydrogen usage. Biofuel Bioprod. Biorefin. 2013, 7, 334–352. [Google Scholar] [CrossRef]
- Saha, R.; Bhattacharya, D.; Mukhopadhyay, M. Enhanced production of biohydrogen from lignocellulosic feedstocks using microorganisms: A comprehensive review. Energy Convers. Manag. X 2021, 13, 100153. [Google Scholar] [CrossRef]
- Singh, T.; Alhazmi, A.; Mohammad, A.; Srivastava, N.; Haque, S.; Sharma, S.; Singh, R.; Yoon, T.; Gupta, V.K. Integrated biohydrogen production via lignocellulosic waste: Opportunity, challenges & future prospects. Bioresour. Technol. 2021, 338, 125511. [Google Scholar] [CrossRef]
- Temudo, M.F.; Kleerebezem, R.; van Loosdrecht, M. Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnol. Bioeng. 2007, 98, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gomez, X.; Fernandez, C.; Fierro, J.; Sanchez, M.E.; Escapa, A.; Moran, A. Hydrogen production: Two stage processes for waste degradation. Bioresour. Technol. 2011, 102, 8621–8627. [Google Scholar] [CrossRef] [PubMed]
- Redwood, M.D.; Paterson-Beedle, M.; Macaskie, L.E. Integrating dark and light biohydrogen production strategies: Towards the hydrogen economy. Rev. Environ. Sci. Biotechnol. 2009, 8, 149–185. [Google Scholar] [CrossRef]
- Yang, H.; Shi, B.; Ma, H.; Guo, L. Enhanced hydrogen production from cornstalk by dark- and photo-fermentation with diluted alkali-cellulase two-step hydrolysis. Int. J. Hydrogen Energy 2015, 40, 12193–12200. [Google Scholar] [CrossRef]
- Sinha, P.; Pandey, A. An evaluative report and challenges for fermentative biohydrogen production. Int. J. Hydrogen Energy 2011, 36, 7460–7478. [Google Scholar] [CrossRef]
- Kothari, R.; Singh, D.P.; Tyagi, V.V.; Tyagi, S.K. Fermentative hydrogen production—An alternative clean energy source. Renew. Sustain. Energy Rev. 2012, 16, 2337–2346. [Google Scholar] [CrossRef]
- Alzate-Gaviria, L.M.; Sebastian, P.J.; Perez-Hernandez, A.; Eapen, D. Comparison of two anaerobic systems for hydrogen production from the organic fraction of municipal solid waste and synthetic wastewater. Int. J. Hydrogen Energy 2007, 32, 3141–3146. [Google Scholar] [CrossRef]
- Cahyari, K.; Hidayat, M.S.; Syamsiah, S.; Sarto, S. Optimization of hydrogen production from fruit waste through mesophilic and thermophilic dark fermentation: Effect of substrate-to-inoculum ratio. Malays. J. Anal. Sci. 2019, 23, 116–123. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Keskin, T.; Yazgin, O.; Gunay, B.; Arslan, K.; Azbar, N. Biohydrogen production from autoclaved fruit and vegetable wastes by dry fermentation under thermophilic condition. Int. J. Hydrogen Energy 2019, 44, 18776–18784. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Y.; Wang, X.; Huang, J. Biohydrogen production from apple pomace by anaerobic fermentation with river sludge. Int. J. Hydrogen Energy 2010, 35, 3058–3064. [Google Scholar] [CrossRef]
- Doi, T.; Matsumoto, H.; Abe, J.; Morita, S. Application of rice rhizosphere microflora for hydrogen production from apple pomace. Int. J. Hydrogen Energy 2010, 35, 7369–7376. [Google Scholar] [CrossRef]
- Camargo, F.P.; Sakamoto, I.K.; Duarte, I.C.S.; Varesche, M.B.A. Influence of alkaline peroxide assisted and hydrothermal pretreatment on biodegradability and bio-hydrogen formation from citrus peel waste. Int. J. Hydrogen Energy 2019, 44, 22888–22903. [Google Scholar] [CrossRef]
- Camargo, F.P.; Sakamoto, I.K.; Silva, E.L.; Duarte, I.C.S.; Varesche, M.B.A. Bioaugmentation with Enterococcus casseliflavus: A hydrogen-producing strain isolated from citrus peel waste. Waste Biomass Valor. 2021, 12, 895–911. [Google Scholar] [CrossRef]
- Nathoa, C.; Sirisukpoca, U.; Pisutpaisal, N. Production of hydrogen and methane from banana peel by two phase anaerobic fermentation. Energy Procedia 2014, 50, 702–710. [Google Scholar] [CrossRef]
- Da Silva Mazareli, R.C.; Sakamoto, I.K.; Silva, E.L.; Varesche, M.B.A. Bacillus sp. isolated from banana waste and analysis of metabolic pathways in acidogenic systems in hydrogen production. J. Environ. Manag. 2019, 247, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Mazareli, R.C.; Montoya, A.C.V.; Delforno, T.P.; Centurion, V.B.; de Oliveira, V.M.; Silva, E.L.; Varesche, M.B.A. Enzymatic routes to hydrogen and organic acids production from banana waste fermentation by autochthonous bacteria: Optimization of pH and temperature. Int. J. Hydrogen Energy 2021, 46, 8454–8468. [Google Scholar] [CrossRef]
- Wang, C.H.; Lin, P.J.; Chang, J.S. Fermentative conversion of sucrose and pineapple waste into hydrogen gas in phosphate-buffered culture seeded with municipal sewage sludge. Process Biochem. 2006, 41, 1353–1358. [Google Scholar] [CrossRef]
- Mahato, R.K.; Kumar, D.; Rajagopalan, G. Biohydrogen production from fruit waste by Clostridium strain BOH3. Renew Energy 2020, 153, 1368–1377. [Google Scholar] [CrossRef]
- Hwang, J.H.; Choi, J.A.; Abou-Shanab, R.A.I.; Min, B.; Song, H.; Kim, Y.; Lee, E.S.; Jeon, B.H. Feasibility of hydrogen production from ripened fruits by a combined two-stage (dark/dark) fermentation system. Bioresour. Technol. 2011, 102, 1051–1058. [Google Scholar] [CrossRef]
- Gökçek, O.B.; Baş, F.; Muratçobanoğlu, H.; Demirel, S. Investigation of the effects of magnetite addition on biohydrogen production from apple pulp waste. Fuel 2023, 339, 127475. [Google Scholar] [CrossRef]
- Martinez-Merino, V.; Gil, M.J.; Cornejo, A. Biomass sources for hydrogen production. In Renewable Hydrogen Technologies; Gandia, L.M., Arzamendi, G., Dieguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 87–110. [Google Scholar]
- Cardona, C.A.; Sanchez, O.J. Fuel ethanol production process design trends and integration opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef]
- Demiray, E.; Kut, A.; Karatay, S.E.; Dönmez, G. Usage of soluble soy protein on enzymatically hydrolysis of apple pomace for cost-efficient bioethanol production. Fuel 2021, 289, 119785. [Google Scholar] [CrossRef]
- Magyar, M.; da Costa Sousa, L.; Jin, M.; Sarks, C.; Balan, V. Conversion of apple pomace waste to ethanol at industrial relevant conditions. Appl. Microbiol. Biotechnol. 2016, 100, 7349–7358. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Lee, I.G.; Khanal, S.K.; Park, B.J.; Bae, H.J. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl. Energy 2015, 140, 65–74. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of food waste biorefinery: A review on valorisation pathways, techno- economic constraints, and environmental assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef] [PubMed]
- Protzko, R.J.; Latimer, L.N.; Ze Martinho de Reus, E.; Seibert, T.; Benz, J.P.; Dueber, J.E. Engineering Saccharomyces cerevisiae for co-utilization of D-galacturonic acid and D-glucose from citrus peel waste. Nat. Commun. 2018, 9, 5059. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, K.; Baldwin, E.A.; Buslig, B.S. Production of ethanol from enzymatically hydrolyzed orange peel by the yeast Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 1994, 45, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.; Fazzino, F.; Sidari, R.; Zema, D.A. Optimization of orange peel waste ensiling for sustainable anaerobic digestion. Renew. Energy 2020, 154, 849–862. [Google Scholar] [CrossRef]
- Widmer, W.W.; Narciso, J.A.; Grohmann, K.; Wilkins, M.R. Simultaneous saccharification and fermentation of orange processing waste to ethanol using Kluyveromyces marxianus. Biol. Eng. Trans. 2009, 2, 17–29. [Google Scholar] [CrossRef]
- Widmer, W.W.; Zhou, W.; Grohmann, K. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresour. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef] [PubMed]
- Boluda-Aguilar, M.; Lopez-Gomez, A. Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. Ind. Crop Prod. 2013, 41, 188–197. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, J.H.; Wi, S.G.; Kim, K.H.; Bae, H.J. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy 2013, 102, 204–210. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of hydrothermal carbonisation with anaerobic digestion. Opportunities for valorisation of digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Vaez, X.; Karimi, K.; Denayer, J.F.M.; Kumar, R. Evaluation of apple pomace biochemical transformation to biofuels and pectin through a sustainable biorefinery. Biomass Bioenergy 2023, 172, 106757. [Google Scholar] [CrossRef]
- Hegde, S.; Lodge, J.S.; Trabold, T.A. Characteristics of food processing wastes and their use in sustainable alcohol production. Renew. Sustain. Energy Rev. 2018, 81, 510–523. [Google Scholar] [CrossRef]
- Davila, J.A.; Rosenberg, M.; Cardona, C.A. Techno-economic and environmental assessment of p-cymene and pectin production from orange peel. Waste Biomass Valor. 2015, 6, 253–261. [Google Scholar] [CrossRef]
- Pourbafrani, M.; Forgacs, G.; Horvath, I.S.; Niklasson, C.; Taherzadeh, M.J. Production of biofuels, limonene and pectin from citrus wastes. Bioresour. Technol. 2010, 101, 4246–4250. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.A.; Silva, T.H.L.; Melo Oliveira, C.R.; Jucá, J.F.T.; Melo Sales Santos, A.F. Silage as a pre-treatment of orange bagasse waste to increase the potential for methane generation. Sci. Total Environ. 2022, 823, 153613. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Sim, Y.B.; Ko, J.; Park, S.Y.; Kim, G.B.; Kim, S.H. Biohydrogen and biomethane production from food waste using a two-stage dynamic membrane bioreactor (DMBR) system. Bioresour. Technol. 2022, 352, 127094. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.A.; Tavares, F.; Alves, M.M.; Cavaleiro, A.J.; Pereira, M.A. Garden and food waste co-fermentation for biohydrogen and biomethane production in a two-step hyperthermophilic-mesophilic process. Bioresour. Technol. 2019, 278, 180–186. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, I.; Ribichini, M.; Tsagarakis, K.P. Biomethane as an energy resource for achieving sustainable production: Economic assessments and policy implications. Sustain. Prod. Consum. 2023, 35, 13–27. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Tampio, E.; Marttinen, S.; Rintala, J. Liquid fertilizer products from anaerobic digestion of food waste: Mass nutrient energy balance of four digestate liquid treatment systems. J. Clean. Prod. 2016, 125, 22–32. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; Scotti, S.; Adani, F. Effects of biodrying process on municipal solid waste properties. Bioresour. Technol. 2011, 102, 7443–7450. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Zhao, W.; Wang, Y.; Cui, P.; Zeng, R.J.; Yu, L.; Zhou, S. Electric field induces electron flow to simultaneously enhance the maturity of aerobic composting and mitigate greenhouse gas emissions. Bioresour. Technol. 2019, 279, 234–242. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K. Attempts to alleviate inhibitory factors of anaerobic digestate for enhanced microalgae cultivation and nutrients removal: A review. J. Environ. Manag. 2022, 304, 114266. [Google Scholar] [CrossRef]
- Du, C.; Abdullah, J.J.; Greetham, D.; Fu, D.; Yu, M.; Ren, L.; Li, S.; Lu, D. Valorisation of food waste into biofertiliser and its field application. J. Clean. Prod. 2018, 187, 273–274. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Cheong, J.C.; Lee, J.T.; Lim, J.W.; Song, S.; Tan, J.K.; Chiam, Z.Y.; Yap, K.Y.; Lim, E.Y.; Zhang, J.; Tan, H.T.; et al. Closing the food waste loop: Food waste anaerobic digestate as fertiliser for the cultivation of the leafy vegetable, xiao bai cai (Brassica rapa). Sci. Total Environ. 2020, 715, 136789. [Google Scholar] [CrossRef] [PubMed]
- Rincon, C.A.; De Guardia, A.; Couvert, A.; Le Roux, S.; Soutrel, I.; Daumoin, M.; Benoist, J.C. Chemical and odor characterisation of gas emissions released during composting of solid wastes and digestates. J. Environ. Manag. 2019, 233, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Manu, M.K.; Li, D.; Liwen, L.; Jun, Z.; Varjani, S.; Wong, J.W. A review on nitrogen dynamics and mitigation strategies of food waste digestate composting. Bioresour. Technol. 2021, 334, 125032. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Manu, M.K.; Li, D.; Wang, C.; Varjani, S.; Ladumor, N.; Michael, L.; Xu, Y.; Wong, J.W.C. Food waste digestate composting: Feedstock optimisation with sawdust and mature compost. Bioresour. Technol. 2021, 341, 125759. [Google Scholar] [CrossRef]
- Ding, Z.; Ge, Y.; Gowd, S.C.; Singh, E.; Kumar, V.; Chaurasia, D.; Kumar, V.; Rajendran, K.; Bhargava, P.C.; Wu, P.; et al. Production of biochar from tropical fruit tree residues and ecofriendly applications—A review. Bioresour. Technol. 2023, 376, 128903. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Han, L.; Yang, Y.; Xia, X.; Yang, Z.; Wu, F.; Li, F.; Feng, Y.; Xing, B. Application of hydrochar altered soil microbial community composition and the molecular structure of native soil organic carbon in a paddy soil. Environ. Sci. Technol. 2020, 54, 2715–2725. [Google Scholar] [CrossRef]
- Akarsu, K.; Duman, G.; Yilmazer, A.; Keskin, T.; Azbar, N.; Yanik, J. Sustainable valorisation of food wastes into solid fuel by hydrothermal carbonisation. Bioresour. Technol. 2019, 292, 121959. [Google Scholar] [CrossRef]
- Cerda, A.; Mejias, L.; Rodriguez, P.; Rodriguez, A.; Artola, A.; Font, X.; Gea, T.; Sanchez, A. Valorisation of digestate from biowaste through solid-state fermentation to obtain value added bioproducts: A first approach. Bioresour. Technol. 2019, 271, 409–416. [Google Scholar] [CrossRef]
- Rodriguez, P.; Cerda, A.; Font, X.; Sanchez, A.; Artola, A. Valorisation of biowaste digestate through solid state fermentation to produce biopesticides from bacillus thuringiensis. Waste Manag. 2019, 93, 63–71. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhar, B.R. A multi-perspective review on microbial electrochemical technologies for food waste valorisation. Bioresour. Technol. 2021, 342, 125950. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Shen, X.; Liu, W.; Yi, W.; Li, Z.; Liu, S. Synchronously electricity generation and degradation of biogas slurry using microbial fuel cell. Renew. Energy 2019, 142, 158–166. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Sun, S.; Ma, F.; Zhou, S.; Lee, J.K.; Yao, H. Power generation in dual chamber microbial fuel cells using dynamic membranes as separators. Energy Convers. Manag. 2018, 165, 488–494. [Google Scholar] [CrossRef]
- Mohan, S.V.; Chandrasekhar, K. Solid phase microbial fuel cell (SMFC) for harnessing bioelectricity from composite food waste fermentation: Influence of electrode assembly and buffering capacity. Bioresour. Technol. 2011, 102, 7077–7085. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Lee, Y.C.; Liao, F.Y. Effect of composting parameters on the power performance of solid microbial fuel cells. Sustainability 2015, 7, 12634–12643. [Google Scholar] [CrossRef]
- Miran, W.; Nawaz, M.; Jang, J.; Lee, D.S. Conversion of orange peel waste biomass to bioelectricity using a mediator-less microbial fuel cell. Sci. Total Environ. 2016, 547, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Liana, E.; Toding, O.S.L.; Virginia, C.; Suhartini, S. Conversion banana and orange peel waste into electricity using microbial fuel cell. IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 012049. [Google Scholar] [CrossRef]
- Ghazali, N.F.; Mahmood, N.A.N.; Abu Bakar, N.F.; Ibrahim, K.A. Temperature dependence of power generation of empty fruit bunch (EFB) based microbial fuel cell. Malays. J. Fundam. Appl. Sci. 2019, 15, 489–491. [Google Scholar] [CrossRef]
- Kebaili, H.; Kameche, M.; Innocent, C.; Ziane, F.Z.; Sabeur, S.A.; Sahraoui, T.; Ouis, M.; Zerrouki, A.; Charef, M.A. Treatment of fruit waste leachate using microbial fuel cell: Preservation of agricultural environment. Acta Ecol. Sin. 2021, 41, 97–105. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Omine, K.; Yasufuku, N.; Bushra, Q.S.; Hyodo, M.; Nakata, Y. Bioelectricity from kitchen and bamboo waste in a microbial fuel cell. Waste Manag. Res. 2014, 32, 124–130. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–739. [Google Scholar] [CrossRef]
- Błaszczyk, A. Strategy to improve the performance of metal-free dye-sensitized solar cells. Dyes Pigment. 2018, 149, 707–718. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Ali, S.R.; Khan, S. Progress in dye sensitized solar cell by incorporating natural photosensitizers. Sol. Energy 2019, 181, 490–509. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Joachimiak-Lechman, K.; Sady, S.; Tański, T.; Szindler, M.; Drygał, A. Environmental performance of dye-sensitized solar cells based on natural dyes. Sol. Energy 2021, 215, 346–355. [Google Scholar] [CrossRef]
- Maiaugree, W.; Lowpa, S.; Towannang, M.; Rutphonsan, P.; Tangtrakarn, A.; Pimanpang, S.; Maiaugree, P.; Ratchapolthavisin, N.; Sang-Aroon, W.; Jarernboon, W.; et al. A dye sensitized solar cell using natural counter electrode and natural dye derived from mangosteen peel waste. Sci. Rep. 2015, 13, 15230. [Google Scholar] [CrossRef]
- Eka, C.P.; Yuliarto, B.; Suyatman, S. Performance of natural carotenoids from Musa aromatica and Citrus medica var lemon as photosensitizers for dye-sensitized solar cells with TiO2 nanoparticle. Adv. Mater. Res. 2013, 789, 167–170. [Google Scholar] [CrossRef]
- Prima, E.C.; Hidayat, N.N.; Yuliarto, B.; Suyatman Dipojono, H.K. A combined spectroscopic and TDDFT study of natural dyes extracted from fruit peels of Citrus reticulata and Musa acuminata for dye-sensitized solar cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 15, 112–125. [Google Scholar] [CrossRef]
- Adedokun, O.; Adedeji, O.L.; Bello, I.T.; Awodele, M.K.; Awodugba, A.O. Fruit peels pigment extracts as a photosensitizer in ZnO-based dye-sensitized solar cells. Chem. Phys. Impact 2021, 3, 100039. [Google Scholar] [CrossRef]
- Chang, H.; Lo, Y.J. Pomegranate leaves and mulberry fruit as natural sensitizers for dye-sensitized solar cells. Sol. Energy 2010, 84, 1833–1837. [Google Scholar] [CrossRef]


| Fruit | Global Production (106 t) According to FAOSTAT Database | Typical Losses and Waste (%) | Ref. | Potential Byproduct Amounts (106 t) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |||||
| banana |  | 114.95 | 112.11 | 113.29 | 116.65 | 117.52 | 119.83 | 30 | [1,2] | 33.67–35.95 |
| apple |  | 82.37 | 85.09 | 83.12 | 85.91 | 87.48 | 86.44 | 25 | [3] | 20.59–21.61 |
| grape |  | 76.52 | 74.43 | 73.51 | 80.04 | 77.00 | 78.03 | 20 | [4] | 14.70–16.01 |
| orange |  | 75.58 | 72.99 | 73.39 | 73.45 | 75.99 | 75.46 | 50–60 | [5,6] | 43.79–45.59 |
| mango |  | 46.79 | 47.07 | 52.00 | 53.51 | 55.03 | 54.83 | 60 | [7] | 28.07–33.02 |
| tangerine |  | 33.16 | 32.24 | 32.65 | 34.16 | 38.56 | 38.6 | 50 | [8] | 16.12–19.30 |
| melon |  | 25.71 | 26.62 | 26.70 | 27.1 | 27.01 | 28.47 | 30 | [9] | 7.71–8.13 |
| pineapple |  | 25.81 | 25.95 | 27.39 | 28.33 | 28.21 | 27.82 | 30–60 | [1] | 15.94–17.00 |
| lemon |  | 16.99 | 17.08 | 17.67 | 19.66 | 20.11 | 21.35 | 50 | [8] | 8.50–10.67 |
| grapefruit |  | 8.88 | 8.99 | 8.66 | 9.04 | 9.26 | 9.34 | 50 | [8] | 4.33–4.67 |
| Substrate | Microorganisms | Pretreatment | Temperature [°C] | Bio-H2 Production | Reference |
|---|---|---|---|---|---|
| apple peel | Microbial consortium | not applied H2SO4 solution NH3 liquor | 37 | 41.28 mL/g TS a 76.68 mL/g TS a 101.08 mL/g TS a | [71] |
| apple pomace | Rize rhizosphere microflora | not applied | 35 | 2.28 mol H2/mol hexose | [76] |
| citrus peel | Microbial consortium | not applied Alkali solution hydrothermolysis | 30 | 13.55 mmol/L 7.27 mmol/L 8.19 mmol/L | [77] |
| citrus peel | Enterococcus casseliflavus | not applied | 37 | 13.9 mmol/L 1.09 mmol/h | [78] |
| banana peel | Anaerobic sludge | not applied | 37 | 352.8 mL 2.0 mL/h | [79] |
| banana waste | Bacillus sp. | not applied | 37 | 71 mL/g 6.1 mL H2/h | [80] |
| banana waste | Autochthonous bacteria consortium | not applied | 37 | 70.19 mL/g 12.43 mL H2/h | [81] |
| pineapple waste | Municipal sewage sludge | HCl solution | 37 | 5920 mmol H2/g COD b 745 mL/h/L | [82] |
| fruit waste | Clostridium strain BOH3 | microwave moist heat | 37 | 359.97 mL/g | [83] |
| fully ripened fruits: grape, apple, pear | Sewage sludge | heat | 35 | 2.2 mol H2/mol glucose | [84] |
| apple pulp waste | Sporolactobacillus, Clostridium, Coprothermobacter | not applied | 37 | 73.59 mL/g VS c | [85] |
| Substrate | Pretreatment | Enzymes | Fermentation Process | Microorganism | Ethanol Productivity [g/L/h] | Reference |
|---|---|---|---|---|---|---|
| orange peel | milling | pectinase, cellulase, glucosidase | SHF | S. cerevisiae | 4.7 | [93] |
| orange peel | acidic steam explosion | pectinase, cellulase, glucosidase | SSF | S. cerevisiae | 2.7 | [94,95] |
| orange peel | steam explosion | pectinase, cellulase, glucosidase | SSF | Kluyveromyces marxianus | 3.45 | [96] |
| lemon peel | steam explosion | pectinase, cellulase, glucosidase | SSF | S. cerevisiae | 67.8 a | [97] |
| tangerine peel | steam explosion | pectinase, cellulase, glucosidase | SSF | S. cerevisiae | 59.3 a | [85] |
| tangerine peel | popping | pectinase, cellulase, glucosidase | SHEF | S. cerevisiae | 46.2 a | [85] |
| tangerine peel | - | in-house enzymes | SSF | S. cerevisiae | 3.28 | [98] |
| grapefruit peel | - | in-house enzymes | SSF | S. cerevisiae | 2.40 | [93] |
| lemon peel | - | in-house enzymes | SSF | S. cerevisiae | 2.18 | [93] |
| apple pomace | acidic heating | cellulase | SHF | S. cerevisiae | 1.10 | [83] |
| apple pomace | alkali heating | pectinase, cellulase, hemicellulase | SSF | S. cerevisiae | 1.5 | [84] |
| apple pomace | acidic treatment | pectinase, cellulase, hemicellulase | SSF | S. cerevisiae | 190 g/kg DM b | [99] |
| apple pomace | acidic treatment | pectinase, cellulase, hemicellulase | SHF | S. cerevisiae | 136,3 g/kg DM | [100] |
| apple pomace | ethanol treatment | pectinase, cellulase, hemicellulase | SHF | S. cerevisiae | 173,3 g/kg DM | [28] |
| Substrate | MFC | Microorganisms | Maximum Voltage [V] | Current Density [mA/cm2] | Power Density [mW/cm2] | Reference |
|---|---|---|---|---|---|---|
| banana peel | dual-chamber | indigenous microorganisms | 0.492 | - | - | [130] |
| grape waste | single-chamber | no data | 0.5 | - | 825 | [131] |
| orange peel | dual-chamber | Anaerobic sludge: Enterococcus, Paludibacter, Pseudomonas | 0.59 | 847 | 358.8 | [123] |
| fruit waste | dual-chamber | fermentative bacteria | 0.26 | 1.0 | 24.2 | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczyk, A.; Sady, S.; Pachołek, B.; Jakubowska, D.; Grzybowska-Brzezińska, M.; Krzywonos, M.; Popek, S. Sustainable Management Strategies for Fruit Processing Byproducts for Biorefineries: A Review. Sustainability 2024, 16, 1717. https://doi.org/10.3390/su16051717
Błaszczyk A, Sady S, Pachołek B, Jakubowska D, Grzybowska-Brzezińska M, Krzywonos M, Popek S. Sustainable Management Strategies for Fruit Processing Byproducts for Biorefineries: A Review. Sustainability. 2024; 16(5):1717. https://doi.org/10.3390/su16051717
Chicago/Turabian StyleBłaszczyk, Alfred, Sylwia Sady, Bogdan Pachołek, Dominika Jakubowska, Mariola Grzybowska-Brzezińska, Małgorzata Krzywonos, and Stanisław Popek. 2024. "Sustainable Management Strategies for Fruit Processing Byproducts for Biorefineries: A Review" Sustainability 16, no. 5: 1717. https://doi.org/10.3390/su16051717
APA StyleBłaszczyk, A., Sady, S., Pachołek, B., Jakubowska, D., Grzybowska-Brzezińska, M., Krzywonos, M., & Popek, S. (2024). Sustainable Management Strategies for Fruit Processing Byproducts for Biorefineries: A Review. Sustainability, 16(5), 1717. https://doi.org/10.3390/su16051717







