Flower Strips as an Ecological Tool to Strengthen the Environmental Balance of Fields: Case Study of a National Park Zone in Western Poland
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- A greater species diversity of flowering plants in the flower strip contributes to the increase in the attractiveness of this habitat to a greater extent than the number of flowering plants belonging to one species;
- The most intense flowering period in the flower strip need not coincide with more flowering species, as under favorable conditions, one species can greatly dominate the others. The most intense flowering period in the flower strip occurred in June, when the number of flowering plants was the highest and belonged to one species, T. repens (81.52% of all flowering plants);
- In June, the largest share of beneficial insects were specimens from Hymenoptera/Parasitica species. During the period of intensive flowering of T. repens, this species can be recommended for use by farmers applying the principles of sustainable agriculture;
- The diversity of potentially harmful insects was lower than that of beneficial ones;
- The most numerous occurrence of Thysanoptera/Thripidae was noted in July, when the flower strip was dominated by plant species with white inflorescences (T. repens, D. carota, A. millefolium).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devi, N. Sustainable Agriculture. JASRAE 2022, 19, 106–111. [Google Scholar]
- Malo, M. Sustainable Agriculture: Need of the Hour. Agric. Food E Newsl. 2020, 2, 298–300. [Google Scholar]
- Boix-Fayos, C.; de Vente, J. Challenges and potential pathways towards sustainable agriculture within the European Green Deal. Agric. Syst. 2023, 207, 103634. [Google Scholar] [CrossRef]
- Cahenzli, F.; Sigsgaard, L.; Daniel, C.; Herz, A.; Jamar, L.; Kelderer, M.; Jacobsen, S.K.; Kruczyńska, D.; Matray, S.; Porcel, M.; et al. Perennial flower strips for pest control in organic apple orchards—A pan European study. Agric. Ecosyst. Environ. 2019, 278, 43–53. [Google Scholar] [CrossRef]
- Mateos-Fierro, Z.; Fountain, M.T.; Garratt, M.P.D.; Ashbrook, K.; Westbury, D.B. Active management of wildflower strips in commercial sweet cherry orchards enhances natural enemies and pest regulation services. Agric. Ecosyst. Environ. 2021, 317, 107485. [Google Scholar] [CrossRef]
- Fountain, M.T. Impacts of Wildflower Interventions on Beneficial Insects in Fruit Crops: A Review. Insects 2022, 13, 304. [Google Scholar] [CrossRef]
- Bishop, G.A.; Fijen, T.P.; Desposato, B.N.; Scheper, J.; Kleijn, D. Hedgerows have contrasting effects on pollinators and natural enemies and limited spillover effects on apple production. Agric. Ecosyst. Environ. 2023, 346, 108364. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Müller, J.; Hahn, J. Ensilability of Biomass From Effloresced Flower Strips as Co-substrate in Bioenergy Production. Front. Bioeng. Biotechnol. 2020, 8, 14. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Uyttenbroeck, R.; Hatt, S.; Piqueray, J.; Paul, A.; Bodson, B.; Francis, F.; Monty, A. Creating Perennial Flower Strips: Think Functional! Agric. Agric. Sci. Procedia 2015, 6, 95–101. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Uyttenbroeck, R.; Hatt, S.; Paul, A.; Boeraeve, F.; Piqueray, J.; Francis, F.; Danthine, S.; Frédérich, M.; Dufrêne, M.; Bodson, B.; et al. Pros and cons of flowers strips for farmers. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 225–235. [Google Scholar] [CrossRef]
- Balzan, M.V. Flowering banker plants for the delivery of multiple agroecosystem services. Arthropod Plant Interact. 2017, 11, 743–754. [Google Scholar] [CrossRef]
- Amy, C.; Noël, G.; Hatt, S.; Uyttenbroeck, R.; Van de Meutter, F.; Genoud, D.; Francis, F. Flower Strips in Wheat Intercropping System: Effect on Pollinator Abundance and Diversity in Belgium. Insects 2018, 9, 114. [Google Scholar] [CrossRef]
- Kujawa, K.; Bernacki, Z.; Arczyńska-Chudy, E.; Janku, K.; Karg, J.; Kowalska, J.; Oleszczuk, M.; Sienkiewicz, P.; Sobczyk, D.; Weyssenhoff, D. Kwietne pasy: Rzadko stosowane w Polsce narzędzie wzmacniania integrowanej ochrony roślin uprawnych oraz zwiększania różnorodności biologicznej na terenach rolniczych. J. Prog. Plant Prot. 2018, 58, 115–128. [Google Scholar]
- Buhk, C.; Oppermann, R.; Schanowski, A.; Schanowski, A.; Bleil, R.; Ludemann, J.; Maus, C. Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecol. 2018, 18, 55. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Sienkiewicz, P. Flower Strips and Their Ecological Multifunctionality in Agricultural Fields. Agriculture 2022, 12, 1470. [Google Scholar] [CrossRef]
- Wyss, E.; Pfiffner, L. Biodiversity in Organic Horticulture—An Indicator for Sustainability and a Tool for Pest Management. Acta Hort. 2008, 767, 75–80. [Google Scholar] [CrossRef]
- Szitár, K.; Deák, B.; Halassy, M.; Steffen, C.; Batáry, P. Combination of organic farming and flower strips in agricultural landscapes—A feasible method to maximise functional diversity of plant traits related to pollination. Glob. Ecol. Conserv. 2022, 38, e02229. [Google Scholar] [CrossRef]
- Rozova, Z.; Pástorová, A.; Kuczman, G. Development of flower meadows in an urbanized environment. Ekológia 2023, 42, 218–229. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Tymoszuk, A. Effect of Plant Seed Mixture on Overwintering and Floristic Attractiveness of the Flower Strip in Western Poland. Agriculture 2023, 13, 467. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Wildflower plantings enhance the abundance of natural enemies and their services in adjacent blueberry fields. Biol. Control. 2015, 91, 94–103. [Google Scholar] [CrossRef]
- Hatt, S.; Lopes, T.; Boeraeve, F.; Chen, J.; Francis, F. Pest regulation and support of natural enemies in agriculture: Experimental evidence of within field wildflower strips. Ecol. Eng. 2017, 98, 240–245. [Google Scholar] [CrossRef]
- Pfiffner, L.; Cahenzli, F.; Steinemann, B.; Jamar, L.; Bjørn, M.C.; Porcel, M.; Tasin, M.; Telfser, J.; Kelderer, M.; Lisek, J.; et al. Design, implementation and management of perennial flower strips to promote functional agrobiodiversity in organic apple orchards: A pan—European study. Agric. Ecosyst. Environ. 2019, 278, 61–71. [Google Scholar] [CrossRef]
- Serée, L.; Chiron, F.; Valantin-Morison, M.; Barbottin, A.; Gardarin, A. Flower strips, crop management and landscape composition effects on two aphid species and their natural enemies in faba bean. Agric. Ecosyst. Environ. 2022, 331, 107902. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.-C. Augmenting flower trait diversity in wildflower strips to optimise the conservation of arthropod functional groups for multiple agroecosystem services. J. Insect Conserv. 2014, 18, 713–728. [Google Scholar] [CrossRef]
- Jacobsen, S.K.; Sørensen, H.; Sigsgaard, L. Perennial flower strips in apple orchards promote natural enemies in their proximity. Crop Prot. 2022, 156, 105962. [Google Scholar] [CrossRef]
- Ganser, D.; Knop, E.; Albrecht, M. Sown wildflower strips as overwintering habitat for arthropods: Effective measure or ecological trap? Agric. Ecosyst. Environ. 2019, 275, 123–131. [Google Scholar] [CrossRef]
- Hatt, S.; Francis, F.; Xu, Q.; Wang, S.; Osawa, N. Perennial flowering strips for conservation biological control of insect pests: From picking and mixing flowers to tailored functional diversity. In Integrative Biological Control: Progress in Biological Control; Gao, Y., Hokkanen, H., Menzler-Hokkanen, I., Eds.; Springer: Cham, Switzerland, 2020; pp. 57–71. [Google Scholar]
- Triquet, C.; Roume, A.; Wezel, A.; Tolon, V.; Ferrer, A. In-field cover crop strips support carabid communities and shape the ecological trait repartition in maize fields. Agric. For. Entomol. 2022, 25, 152–163. [Google Scholar] [CrossRef]
- Anjum-Zubair, M.; Schmidt-Entling, M.H.; Querner, P.; Frank, T. Influence of within-field position and adjoining habitat on carabid beetle assemblages in winter wheat. Agric. For. Entomol. 2010, 12, 301–306. [Google Scholar] [CrossRef]
- McCullough, C.; Grab, H.; Angelella, G.; Karpanty, S.; Samtani, J.; Olimpi, E.M.; O’Rourke, M. Diverse landscapes but not wildflower plantings increase marketable crop yield. Agric. Ecosyst. Environ. 2022, 339, 108120. [Google Scholar] [CrossRef]
- Beyer, N.; Gabriel, D.; Westphal, C. Contrasting effects of past and present mass-flowering crop cultivation on bee pollinators shaping yield components in oilseed rape. Agric. Ecosyst. Environ. 2021, 319, 107537. [Google Scholar] [CrossRef]
- Barbir, J.; Azpiazu, C.; Badenes-Pérez, F.R.; Fernandez-Quintanilla, C.; Dorado, J. Functionality of Selected Aromatic Lamiaceae in Attracting Pollinators in Central Spain. J. Econ. Entomol. 2016, 109, 529–536. [Google Scholar] [CrossRef]
- Rollin, O.; Bretagnolle, V.; Decourtye, A.; Aptel, J.; Michel, N.; Vaissiere, B.E.; Mickael, H. Differences of floral resource use between honey bees and wild bees in an intensive farming system. Agric. Ecosyst. Environ. 2013, 179, 78–86. [Google Scholar] [CrossRef]
- Jones, J.; Rader, R. Pollinator nutrition and its role in merging the dual objectives of pollinator health and optimal crop production. Philos. Trans. R. Soc. B 2022, 377, 20210170. [Google Scholar] [CrossRef]
- Carrié, R.J.G.; George, D.R.; Wäckers, F.L. Selection of floral resources to optimise conservation of agriculturally-functional insect groups. J. Insect Conserv. 2012, 16, 635–640. [Google Scholar] [CrossRef]
- Franzén, M.; Nilsson, S. How can we preserve and restore species richness of pollinating insects on agricultural land? Ecography 2008, 31, 698–708. [Google Scholar] [CrossRef]
- Hussain, R.I.; Walcher, R.; Vogel, N.; Krautzer, B.; Rasran, L.; Frank, T. Effectiveness of flowers strips on insect’s restoration in intensive grassland. Agric. Ecosyst. Environ. 2023, 348, 108436. [Google Scholar] [CrossRef]
- Harbo, L.S.; Schulz, G.; Heinemann, H.; Dechow, R.; Poeplau, C. Flower strips as a carbon sequestration measure in temperate croplands. Plant Soil 2023, 482, 647–663. [Google Scholar] [CrossRef]
- Kujawa, K.; Bernacki, Z.; Kowalska, J.; Kujawa, A.; Oleszczuk, M.; Sienkiewicz, P.; Sobczyk, D. Annual Wildflower Strips as a Tool for Enhancing Functional Biodiversity in Rye Fields in an Organic Cultivation System. Agronomy 2020, 10, 1696. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Entling, M.H.; Jacot, K. High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc. R. Soc. B 2015, 282, 20151369. [Google Scholar] [CrossRef]
- Boetzl, F.A.; Krimmer, E.; Krauss, J.; Stean-Dewenter, I. Agri-environmental schemes promote ground-dwelling predators in adjacent oilseed rape fields: Diversity, species traits and distance-decay functions. J. Appl. Ecol. 2019, 56, 10–20. [Google Scholar] [CrossRef]
- Klatt, B.K.; Nilsson, L.; Smith, H.G. Annual flowers strips benefit bumble bee colony growth and reproduction. Biol. Conserv. 2020, 252, 108814. [Google Scholar] [CrossRef]
- Bilski, Z.; Kajdan-Zysnarska, I. Uprawa roślin bobowatych drobnonasiennych. In Centrum Doradztwa Ekologicznego W Brwinowie; Oddział w Poznaniu: Poznań, Poland, 2019. [Google Scholar]
- Schmidt, A.; Kirmer, A.; Kiehl, K.; Tischew, S. Seed mixture strongly affects species-richness and quality of perennial flower strips on fertile soil. Basic Appl. Ecol. 2020, 42, 62–72. [Google Scholar] [CrossRef]
- Wix, N.; Reich, M.; Schaarschmidt, F. Butterfly richness and abundance in flower strips and field margins: The role of local habitat quality and landscape context. Heliyon 2019, 5, e01636. [Google Scholar] [CrossRef]
- Schmied, H.; Getrost, L.; Hamm, A.; Dünzkofer, T. The flower strip dilemma (FSD): An overlooked challenge in nature conservation and a possible first step towards a solution by combining different aged flower strips. Agric. Ecosyst. Environ. 2023, 347, 108375. [Google Scholar] [CrossRef]
- Orlikowska, T. Jak powstają barwy i zapachy kwiatów. Zesz. Probl. Postepow Nauk Roln. 2005, 504, 199–207. [Google Scholar]
- Staab, K.; Yannelli, F.A.A.; Lang, M.; Kollmann, J. Bioengineering effectiveness of seed mixtures for road verges: Functional composition as a predictor of grassland diversity and invasion resistance. Ecol. Eng. 2015, 84, 104–112. [Google Scholar] [CrossRef]
- Ullrich, K. The Influence of Wildflower Strips on Plant and Insect (Hetroptera) Diversity in Anarable Landscape. Ph.D. Thesis, Swiss Federal Institute of Technology (ETH), Zürich, Switzerland, 2001. [Google Scholar]
- Banaszak, J.; Czechowska, W.; Czechowski, W.; Garbarczyk, H.; Sawoniewicz, J.; Wiśniowski, B. Zagrożenia i perspektywy ochrony owadów błonkoskrzydłych (Hymenoptera). Wiad. Entomol. 2000, 18, 177–211. [Google Scholar]
- Sawoniewicz, J. Hymenoptera parasitica (Terebrantes) Błonkówki pasożytnicze (Owadziarki). Czerwona Lista Zwierząt Ginących i Zagrożonych w Polsce; Instytut Ochrony Przyrody Polskiej Akademii Nauk: Kraków, Poland, 2002; pp. 51–53. [Google Scholar]
- Wang, Z.; Tang, P.; Shi, M.; Huang, J.; Chen, X. Flowering plants and entomophagous arthropods in the agricultural landscape: A practise-oriented summary of a complex relationship. Front. Agric. Sci. Eng. 2022, 9, 63–74. [Google Scholar] [CrossRef]
- Jervis, M.A. Functional and evolutionary aspects of mouthpart structure in parasitoid wasps. Biol. J. Linn. Soc. 1998, 63, 461–493. [Google Scholar] [CrossRef]
- Krzyżanowski, R. Wpływ lotnych związków na zachowanie mszyc związane z żerowaniem. Kosmos 2017, 66, 413–420. [Google Scholar]
- Sobral, M.; Losada, M.; Veiga, T.; Guitián, J.; Guitián, J.; Guitián, P. Flower color preferences of insects and livestock: Effects on Gentiana lutea reproductive success. Peer J. 2016, 4, e1636v1. [Google Scholar] [CrossRef]
- Csanády, A.; Oboňa, J.; Zapletalová, L.; Panigaj, L.; Dojčaková, D.; Záleta, B. Hymenopteran color preference using multiple colours of pan traps in Slovakia. Acta Mus. Siles. Sci. Natur. 2021, 70, 33–46. [Google Scholar] [CrossRef]
- Bednar, Z.; Vaupel, A.; Blümel, S.; Herwig, N.; Hommel, B.; Haberlah-Korr, V.; Beule, L. Earthworm and soil microbial communities in flower strip mixtures. Plant Soil 2023, 492, 209–227. [Google Scholar] [CrossRef]
- Dostatny, D.F.; Dajdok, Z. Dzikie gatunki pokrewne roślinom uprawnym występujące w Polsce. In Lista, Zasoby i Zagrożenia; Wydawnictwo Kontekst: Poznań, Poland, 2020. [Google Scholar]
- Lluga-Rizani, K.; Šoljan, D.; Berisha, N.; Kurteshi, K.; Letaj, K. Morphological variability of Trifolium repens L. (Fabaceae). Hacquetia 2021, 20, 281–290. [Google Scholar] [CrossRef]
- Strażyński, P.; Mrówczyński, M. Metodyka Integrowanej Ochrony Koniczyn Dla Doradców; Instytut Ochrony Roślin—Państwowy Instytut Badawczy: Poznań, Poland, 2016; p. 37. [Google Scholar]
- Riley, D.G.; Chitturi, A.; Sparks, A.N. Does natural deposition of pine pollen affect the ovipositional behavior of Frankliniella occidentalis and Frankliniella fusca? Entomol. Exp. Appl. 2007, 124, 133–141. [Google Scholar] [CrossRef]
- Visschers, I.G.S.; Macel, M.; Peters, J.L.; Sergeeva, L.; Bruin, J.; van Dam, N.M. Exploring Thrips Preference and Resistance in Flowers, Leaves, and Whole Plants of Ten Capsicum Accessions. Plants 2023, 12, 825. [Google Scholar] [CrossRef]
- Czyżewska-Suchoń, N. Pasy Kwietne—Rolnictwo Przyjazne Środowisku; Kujawsko-Pomorski Ośrodek Doradztwa Rolniczego w Minikowie: Minikowo, Poland, 2021; pp. 1–24. [Google Scholar]
- Mirab-balou, M.; Chen, X. The Megalurothrips genus-group in Iran (Thysanoptera: Thripidae). Mun. Ent. Zool. 2011, 6, 944–952. [Google Scholar]
- Schütz, L.; Wenzel, B.; Rottstock, T.; Dachbrodt-Saaydeh, S.; Golla, B.; Kehlenbeck, H. How to promote multifunctionality of vegetated strips in arable farming: A qualitative approach for Germany. Ecosphere 2022, 13, e4229. [Google Scholar] [CrossRef]
- Ouvrard, P.; Transon, J.; Jacquemart, A.L. Flower-strip agri-environment schemes provide diverse and valuable summer flower resources for pollinating insects. Biodivers. Conserv. 2018, 27, 2193–2216. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: Aquantitative synthesis. Ecol. Lett. 2021, 23, 1488–1498. [Google Scholar] [CrossRef]
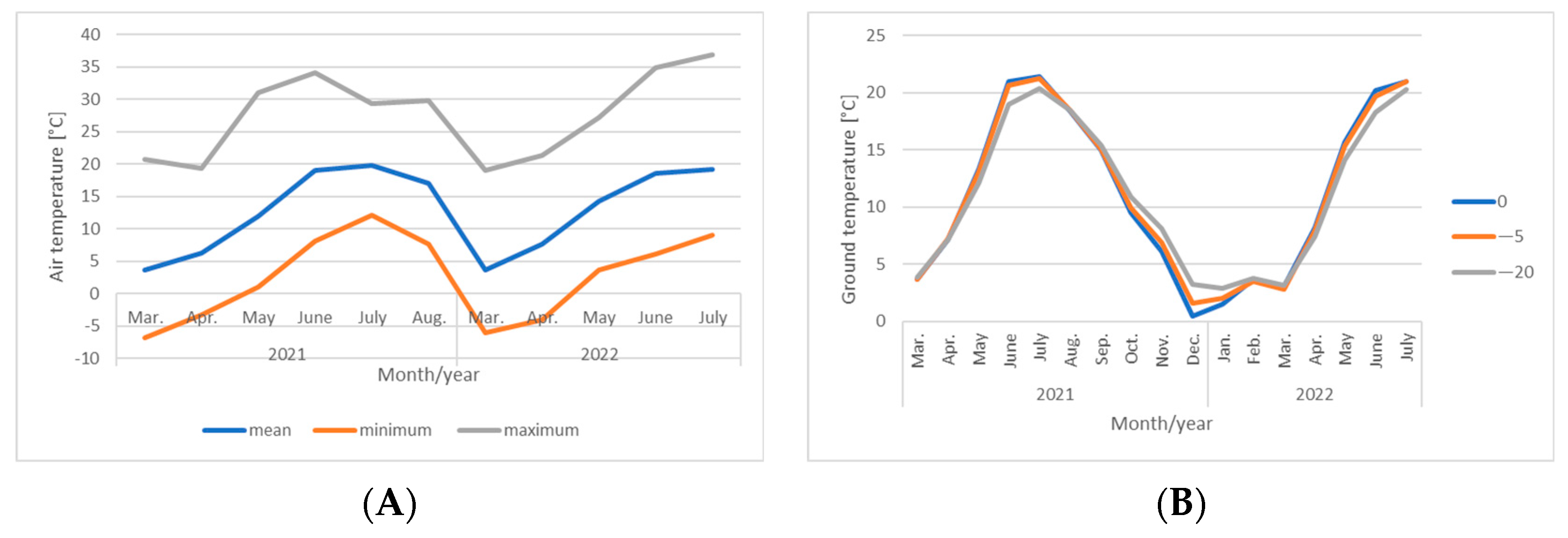
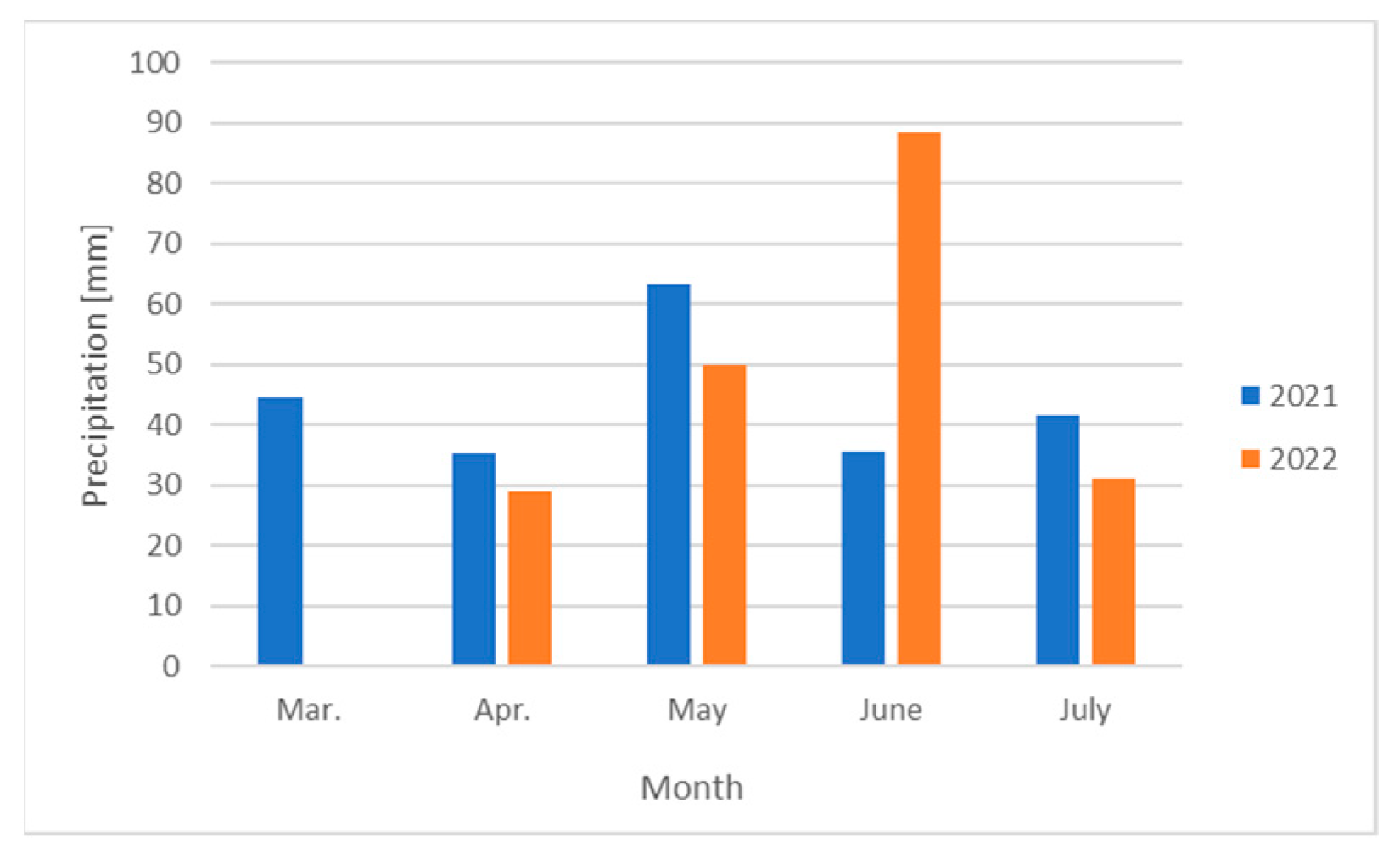
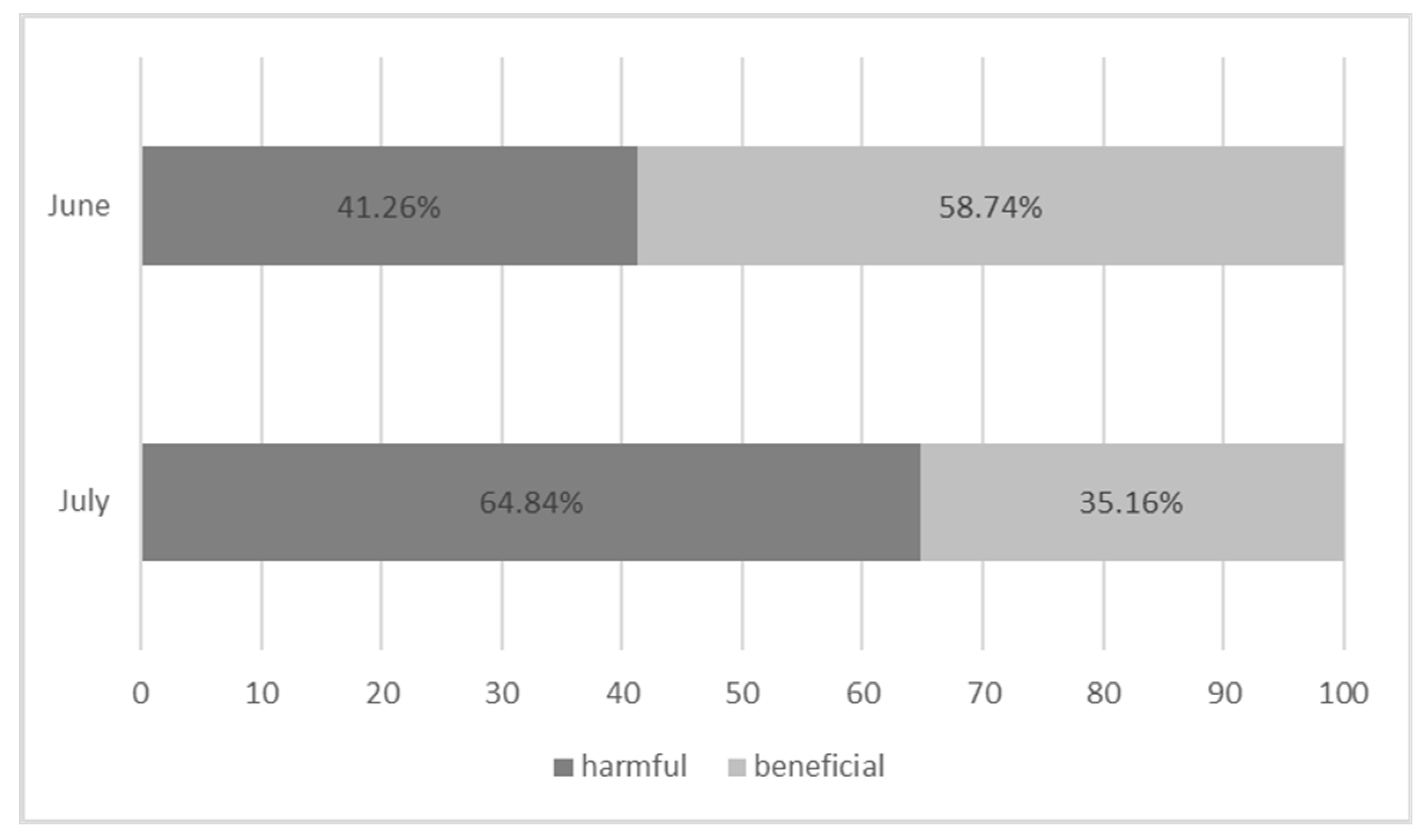
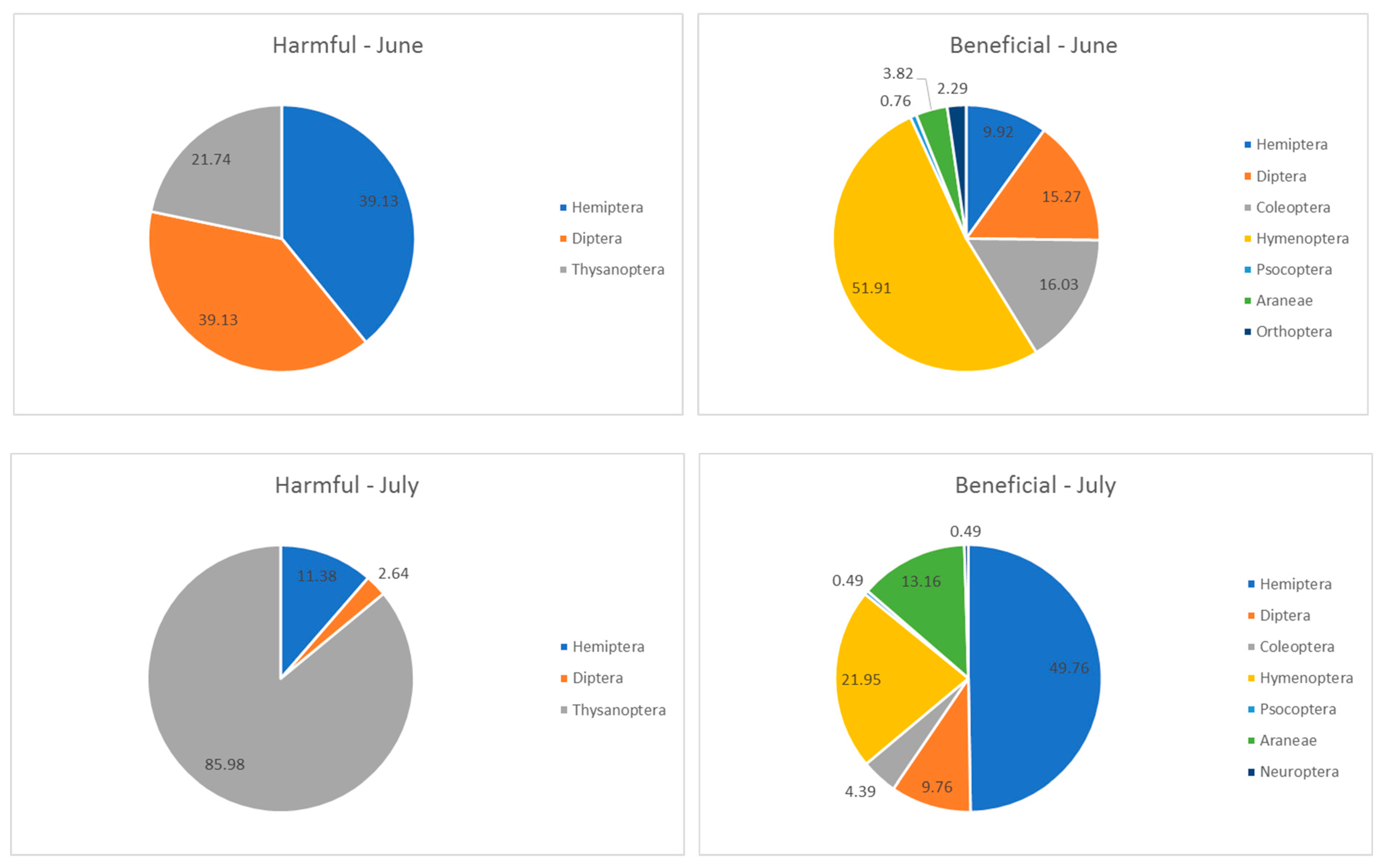
| Plant Origin | Species | Color of Inflorescences | Date of Observations | ||
|---|---|---|---|---|---|
| May | June | July | |||
| Sown seed mixture | Achillea millefolium | white | 0.00 | 7.26 | 17.37 |
| Chrysanthemum leucanthemum | white, yellow | 6.67 | 6.60 | 0.00 | |
| Daucus carota | white | 0.00 | 0.99 | 11.98 | |
| Echium vulgare | violet/blue | 0.00 | 0.66 | 4.19 | |
| Lotus corniculatus | yellow | 0.83 | 0.00 | 4.19 | |
| Trifolium pratense | violet/pink | 5.00 | 2.97 | 6.59 | |
| Trifolium repens | white | 87.50 | 81.52 | 53.29 | |
| Soil seed bank | Malva sp. | violet/pink | 0.00 | 0.00 | 2.40 |
| Date of Observations | Number of | Dry Mass of Arthropod Specimens [g] | ||||
|---|---|---|---|---|---|---|
| Flowering Plants | Flowering Species | Arthropod Specimens | ||||
| Harmful | Beneficial | Total Number | ||||
| June | 60.60 a (±11.88) | 3.20 b (±1.10) | 92 | 131 | 223 | 0.157 |
| July | 33.40 b (±6.37) | 6.20 a (±0.84) | 378 | 205 | 583 | 0.360 |
| June | July | ||||
|---|---|---|---|---|---|
| Order | Suborder/ Superfamily/Family | Share [%] in the Total Number of Arthropods | Order | Suborder/ Superfamily/Family | Share [%] in the Total Number of Arthropods |
| Harmful arthropods | |||||
| Hemiptera phytophagous | Cicadomorpha | 5.38 | Hemiptera | Cicadomorpha | 0.70 |
| Aphididae | 0.90 | Aphididae | 2.57 | ||
| Heteroptera | 9.87 | Heteroptera | 4.12 | ||
| Diptera | Anthomyidae | 0.90 | Diptera | Anthomyidae | 1.37 |
| Agromyziidae | 0.45 | Agromyziidae | 0.34 | ||
| Chloropidae | 14.80 | ||||
| Thysanoptera | Thripidae | 8.97 | Thysanoptera | Thripidae | 55.75 |
| Beneficial arthropods | |||||
| Hemiptera zoophagous | Heteroptera | 5.38 | Hemiptera | Heteroptera | 17.50 |
| Psyllodea | 0.45 | Diptera | Muscidae | 0.51 | |
| Diptera | Muscidae | 0.90 | Syrphidae | 0.17 | |
| other | 8.07 | Pipunculidae | 0.17 | ||
| Coleoptera | Nitidulidae | 3.59 | other | 2.57 | |
| Curculionidae | 4.48 | Coleoptera | Nitidulidae | 0.17 | |
| other | 1.35 | Coccinellidae | 0.34 | ||
| Hymenoptera | Aculeata | 1.35 | other | 1.03 | |
| Parasitica | 29.15 | Hymenoptera | Parasitica | 7.72 | |
| Psocoptera | undefined | 0.45 | Neuroptera | Chrysopidae | 0.17 |
| Araneae | undefined | 2.24 | Psocoptera | undefined | 0.17 |
| Orthoptera | undefined | 1.35 | Araneae | undefined | 4.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antkowiak, M.; Kowalska, J.; Trzciński, P. Flower Strips as an Ecological Tool to Strengthen the Environmental Balance of Fields: Case Study of a National Park Zone in Western Poland. Sustainability 2024, 16, 1251. https://doi.org/10.3390/su16031251
Antkowiak M, Kowalska J, Trzciński P. Flower Strips as an Ecological Tool to Strengthen the Environmental Balance of Fields: Case Study of a National Park Zone in Western Poland. Sustainability. 2024; 16(3):1251. https://doi.org/10.3390/su16031251
Chicago/Turabian StyleAntkowiak, Małgorzata, Jolanta Kowalska, and Paweł Trzciński. 2024. "Flower Strips as an Ecological Tool to Strengthen the Environmental Balance of Fields: Case Study of a National Park Zone in Western Poland" Sustainability 16, no. 3: 1251. https://doi.org/10.3390/su16031251
APA StyleAntkowiak, M., Kowalska, J., & Trzciński, P. (2024). Flower Strips as an Ecological Tool to Strengthen the Environmental Balance of Fields: Case Study of a National Park Zone in Western Poland. Sustainability, 16(3), 1251. https://doi.org/10.3390/su16031251








