Combination of Biochar and Advanced Oxidation Processes for the Sustainable Elimination of Pharmaceuticals in Water
Abstract
1. Introduction
2. Pharmaceuticals: Concept, Classification, and Physicochemical Characteristics
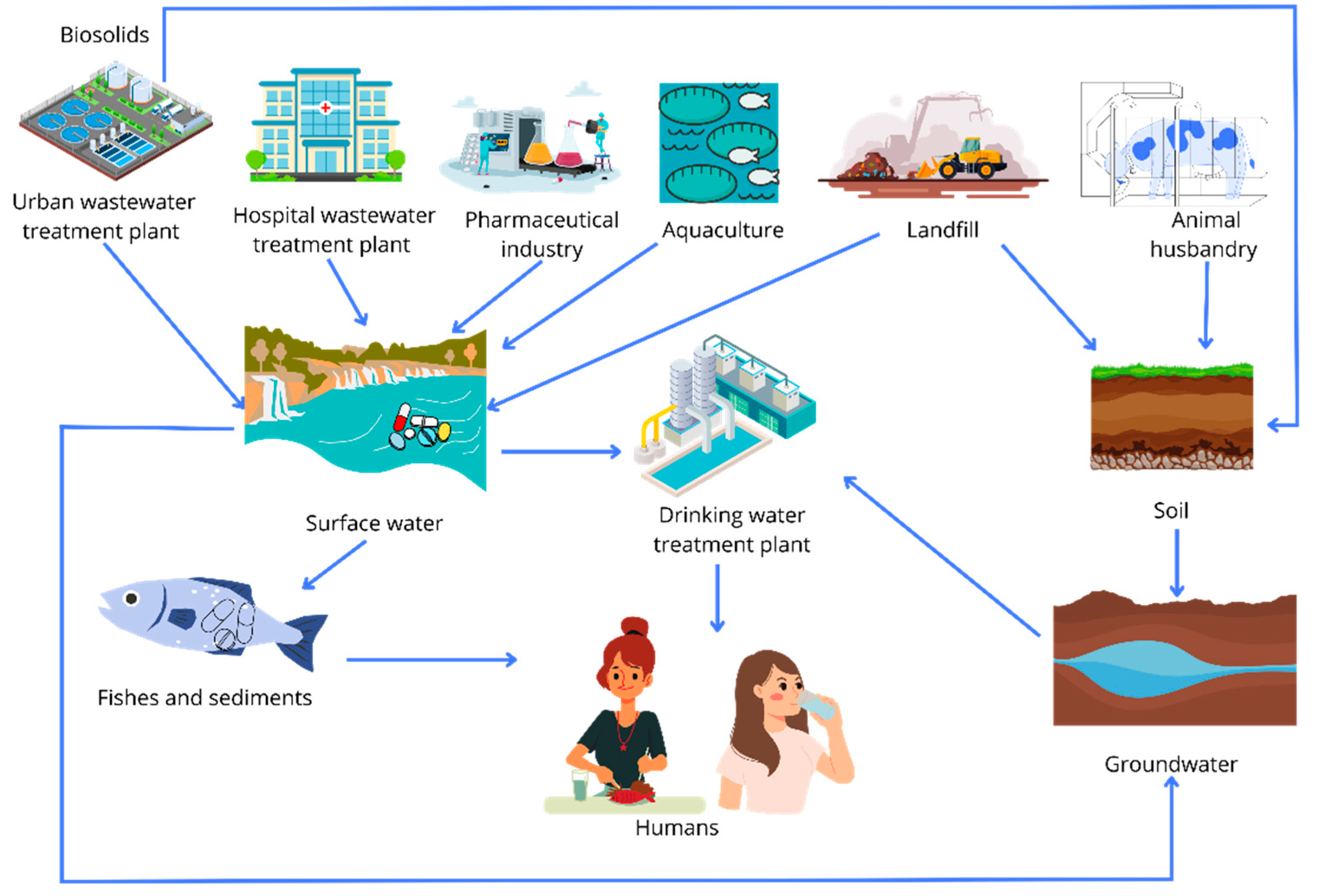
3. Environmental Impacts Associated with the Presence of Pharmaceuticals in Water
3.1. Occurrence and Distribution of Pharmaceuticals in Aquatic Environments
3.2. Environmental Impacts of Pharmaceuticals
4. Advanced Oxidation Processes Applied in the Treatment of Pharmaceuticals
5. Coupling of AOPs and Biochar in the Treatment of Pharmaceuticals
5.1. Degradation Pathways of Pharmaceuticals by Coupling Biochar with AOPs
5.2. Biochar Modifications to Be Used in AOPs
5.2.1. Iron (Fe) Impregnation
5.2.2. Acid–Base Modification
5.2.3. Biochar as a Supporting Material in the Photocatalytic Process
5.2.4. Heteroatom Doping
5.2.5. Biochar as a Cathode in the Electro-Fenton Process
| Pharmaceutical | AOP | Biochar Type and Generation Method | Operational Conditions | Efficiency and Radical Species Involved in the Process | Ref. |
|---|---|---|---|---|---|
| Triclosan (TCS) | Peroxyacetic acid (PAA) process | Hydrolyze rice straw Pyrolysis at 700 °C with N2 flow | [TCS] = 10 mg/L [PAA] = 1.3 mmol/L [Biochar] = 2.9 g/L t = 120 min |
| [129] |
| SFM | Sulfate-based process | Coconut shell Pyrolysis at 700 °C with N2 flow | [SFM] = 0.126 mg/L pH = 5 [HSO5−] = 0.5 mmol/L [Biochar] = 150 mg/L |
| [132] |
| Rifadin (RF) | Sonophotocatalytic process | NiCr–biochar | [RF] = 15 mg/L pH = 8 [NiCr–biochar] = 1 g/L Light = 50 W LED US = 150 W t = 90 min |
| [130] |
| Acetaminophen (ACP) | Sulfate-based process | Cyanobacteria biomass treated with H3PO4 Pyrolys at 500 °C with N2 flow P–biochar | [ACP] = 7.56 mg/L [S2O82−] = 2 mmol/L [P–biochar] = 0.1 g/L [Thiosulfate] = 2 mmol/L t = 90 min |
| [125] |
| Sulfadiazine (SZ) | Sulfate-based process | Red mud Pyrolysis at 800 °C with N2 flow | [SZ] = 20 mg/L [Biochar] = 0.2 g/L [S2O82−] = 2 mmol/L pH = 3 t = 20 min |
| [133] |
| Sulfapyridine (SF) | Sulfate-based process | Maize cob modified with Fe Pyrolysis at 600 °C | [SF] = 10 mg/L T = 22 °C [HSO5−] = 1 mmol/L [Biochar] = 0.1 g/L pH = 8.2 t = 30 min |
| [134] |
| Tetracycline (TC) | Sulfate-based process | Sewage sludge Pyrolysis under a N2 flow | [TC] = 100 mg/L [S2O82−] = 4.2 mmol/L [Biochar] = 0.2 g/L pH = 2.17 T = 25 °C |
| [135] |
| Ciprofloxacin (CIP) | Photocatalytic process | Calotropis gigantea leaves Pyrolysis at 520 °C Biochar modified with ZnO | [CIP] = 100 mg/L [ZnO–biochar] = 1 g/L pH = 7 Light = 3 W LED in the range of 385–750 nm t = 240 min |
| [123] |
| SFM | Sulfate-based process | Red mud and sewage sludge Pyrolysis at 700 °C with N2 flow | [Biochar] = 1.6 g/L [HSO5−] = 0.15 mmol/L [SFM] = 5.07 mg/L T = 25 °C t = 50 min |
| [102] |
6. Biochar Regeneration Techniques
7. Future Perspectives of Coupling AOPs with Biochar in the Elimination of Pharmaceuticals
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Carvalho, A.C.C.; da Silva Paganini, W.; de Almeida Piai, K.; Bocchiglieri, M.M. The Presence of Pharmaceuticals and Caffeine in Water, as Well as the Methods Used to Eliminate Them. Curr. Opin. Environ. Sci. Health 2024, 39, 100550. [Google Scholar] [CrossRef]
- de Sousa Cordeiro, E.; Scaratti, G.; de Souza, D.C.S.; Nickel, C.D.M.; José, H.J.; de Fátima Peralta Muniz Moreira, R.; De Noni, A. Red Mud as Catalyst for the Treatment of Pharmaceuticals Compounds by Advanced Oxidation Processes—A Review. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100938. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Nowrouzi, M.; Madadi Avargani, V.; Sayadi, M.H.; Zendehboudi, S. Design and Optimization of TiO2-Based Photocatalysts for Efficient Removal of Pharmaceutical Pollutants in Water: Recent Developments and Challenges. J. Water Process Eng. 2024, 57, 104597. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, R.; Kumar, A.; Shankar, R.; Khan, N.A.; Gupta, K.N.; Ram, M.; Arya, R.K. Pharmaceutical Waste-Water Treatment via Advanced Oxidation Based Integrated Processes: An Engineering and Economic Perspective. J. Water Process Eng. 2023, 54, 103977. [Google Scholar] [CrossRef]
- Adeoye, J.B.; Tan, Y.H.; Lau, S.Y.; Tan, Y.Y.; Chiong, T.; Mubarak, N.M.; Khalid, M. Advanced Oxidation and Biological Integrated Processes for Pharmaceutical Wastewater Treatment: A Review. J. Environ. Manag. 2024, 353, 120170. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Liu, H.; He, Z.; Show, P.L.; Vasseghian, Y.; Wang, C. Biochar/Layered Double Hydroxides Composites as Catalysts for Treatment of Organic Wastewater by Advanced Oxidation Processes: A Review. Environ. Res. 2023, 234, 116534. [Google Scholar] [CrossRef]
- Lapchuk, I.; Shyichuk, A.; Tatarchuk, T. 8-Application of Hybrid Advanced Oxidation and Adsorption Processes for Pharmaceutical Wastewater Treatment. In The Treatment of Pharmaceutical Wastewater; Khan, A.H., Khan, N.A., Naushad, M., Aziz, H.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 247–275. [Google Scholar] [CrossRef]
- Yang, H.; Lee, C.-G.; Lee, J. Utilizing Animal Manure-Derived Biochar in Catalytic Advanced Oxidation Processes: A Review. J. Water Process Eng. 2023, 56, 104545. [Google Scholar] [CrossRef]
- Jazić, J.; Gross, A.; Glaser, B.; Agbaba, J.; Simetić, T.; Nikić, J.; Maletić, S. Boosting Advanced Oxidation Processes by Biochar-Based Catalysts to Mitigate Pesticides and Their Metabolites in Water Treatment: A Meta-Analysis. J. Environ. Chem. Eng. 2024, 12, 114260. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Hassan, M.A.; Omer, K.M.; Hama, S. Biochar as Green Adsorbents for Pharmaceutical Pollution in Aquatic Environments: A Review. Desalination 2024, 583, 117725. [Google Scholar] [CrossRef]
- Singh, S.; Khan, N.A.; Shehata, N.; Singh, J.; Ramamurthy, P.C. Insight into Biochar as Sustainable Biomass: Production Methods, Characteristics, and Environmental Remediation. J. Clean. Prod. 2024, 475, 143645. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Praveen Akre, A.; Sreedharan, V.; Suresh Kumar, M. Potential Role of Biochar in Advanced Oxidation Processes: A Sustainable Approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Rubio-Clemente, A.; Pérez, J.F. Effect of Main Solid Biomass Commodities of Patula Pine on Biochar Properties Produced under Gasification Conditions. Ind. Crops Prod. 2021, 160, 113123. [Google Scholar] [CrossRef]
- Rubio-Clemente, A.; Gutiérrez, J.; Henao, H.; Melo, A.M.; Pérez, J.F.; Chica, E. Adsorption Capacity of the Biochar Obtained from Pinus Patula Wood Micro-Gasification for the Treatment of Polluted Water Containing Malachite Green Dye. J. King Saud Univ. Eng. Sci. 2023, 35, 431–441. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Fraiha, O.; Hadoudi, N.; Zaki, N.; Salhi, A.; Amhamdi, H.; Akichouh, E.H.; Mourabit, F.; Ahari, M. Comprehensive Review on the Adsorption of Pharmaceutical Products from Wastewater by Clay Materials. Desalin. Water Treat. 2024, 317, 100114. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of Emerging Micropollutants Originating from Pharmaceuticals and Personal Care Products (PPCPs) in Water and Wastewater by Advanced Oxidation Processes: A Review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Kayode-Afolayan, S.D.; Ahuekwe, E.F.; Nwinyi, O.C. Impacts of Pharmaceutical Effluents on Aquatic Ecosystems. Sci. Afr. 2022, 17, e01288. [Google Scholar] [CrossRef]
- Sharma, K.; Thakur, I.S.; Kaushik, G. Occurrence and Distribution of Pharmaceutical Compounds and Their Environmental Impacts: A Review. Bioresour. Technol. Rep. 2021, 16, 100841. [Google Scholar] [CrossRef]
- Moermond, C.T.A.; Berg, C.; Bergstrom, U.; Bielská, L.; Evandri, M.G.; Franceschin, M.; Gildemeister, D.; Montforts, M.H.M.M. Proposal for Regulatory Risk Mitigation Measures for Human Pharmaceutical Residues in the Environment. Regul. Toxicol. Pharmacol. 2023, 143, 105443. [Google Scholar] [CrossRef]
- Ganthavee, V.; Trzcinski, A.P. Removal of Pharmaceutically Active Compounds from Wastewater Using Adsorption Coupled with Electrochemical Oxidation Technology: A Critical Review. J. Ind. Eng. Chem. 2023, 126, 20–35. [Google Scholar] [CrossRef]
- Delgado, N.; Orozco, J.; Zambrano, S.; Casas-Zapata, J.C.; Marino, D. Veterinary Pharmaceutical as Emerging Contaminants in Wastewater and Surface Water: An Overview. J. Hazard. Mater. 2023, 460, 132431. [Google Scholar] [CrossRef]
- Silori, R.; Shrivastava, V.; Singh, A.; Sharma, P.; Aouad, M.; Mahlknecht, J.; Kumar, M. Global Groundwater Vulnerability for Pharmaceutical and Personal Care Products (PPCPs): The Scenario of Second Decade of 21st Century. J. Environ. Manag. 2022, 320, 115703. [Google Scholar] [CrossRef]
- Ngqwala, N.P.; Muchesa, P. Occurrence of Pharmaceuticals in Aquatic Environments: A Review and Potential Impacts in South Africa. S. Afr. J. Sci. 2020, 116, 1–7. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, Z.; Liu, H.; Dong, S.; Nghiem, L.D.; Gao, L.; Chaves, A.V.; Zamyadi, A.; Li, X.; Wang, Q. A Review on Microalgae-Mediated Biotechnology for Removing Pharmaceutical Contaminants in Aqueous Environments: Occurrence, Fate, and Removal Mechanism. J. Hazard. Mater. 2023, 443, 130213. [Google Scholar] [CrossRef]
- Riikonen, S.; Timonen, J.; Sikanen, T. Environmental Considerations along the Life Cycle of Pharmaceuticals: Interview Study on Views Regarding Environmental Challenges, Concerns, Strategies, and Prospects within the Pharmaceutical Industry. Eur. J. Pharm. Sci. 2024, 196, 106743. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Paun, I.; Iancu, V.-I.; Pirvu, F.; Dinu, C.; Niculescu, M.; Petre, V.A. Fate of Pharmaceutical Residue in Two Romanian Rivers Receiving Treated Water: Occurrence, Distribution and Risk Assessment. Sci. Total Environ. 2024, 923, 171359. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S. Health Effects and Risks Associated with the Occurrence of Pharmaceuticals and Their Metabolites in Marine Organisms and Seafood. Sci. Total Environ. 2022, 837, 155780. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Yang, Q.; Jiang, L.; Li, G. Occurrence and Distribution of Pharmaceuticals and Personal Care Products (PPCPs) in Wastewater Related Riverbank Groundwater. Sci. Total Environ. 2022, 821, 153372. [Google Scholar] [CrossRef]
- Nguyen, M.-K.; Lin, C.; Bui, X.-T.; Rakib, M.R.J.; Nguyen, H.-L.; Truong, Q.-M.; Hoang, H.-G.; Tran, H.-T.; Malafaia, G.; Idris, A.M. Occurrence and Fate of Pharmaceutical Pollutants in Wastewater: Insights on Ecotoxicity, Health Risk, and State–of–the-Art Removal. Chemosphere 2024, 354, 141678. [Google Scholar] [CrossRef]
- Spilsbury, F.D.; Inostroza, P.A.; Svedberg, P.; Cannata, C.; Ragas, A.M.J.; Backhaus, T. Defining the Data Gap: What Do We Know about Environmental Exposure, Hazards and Risks of Pharmaceuticals in the European Aquatic Environment? Water Res. 2024, 251, 121002. [Google Scholar] [CrossRef]
- Bavumiragira, J.P.; Ge, J.; Yin, H. Fate and Transport of Pharmaceuticals in Water Systems: A Processes Review. Sci. Total Environ. 2022, 823, 153635. [Google Scholar] [CrossRef]
- Ruziwa, D.T.; Oluwalana, A.E.; Mupa, M.; Meili, L.; Selvasembian, R.; Nindi, M.M.; Sillanpaa, M.; Gwenzi, W.; Chaukura, N. Pharmaceuticals in Wastewater and Their Photocatalytic Degradation Using Nano-Enabled Photocatalysts. J. Water Process Eng. 2023, 54, 103880. [Google Scholar] [CrossRef]
- Kock, A.; Glanville, H.C.; Law, A.C.; Stanton, T.; Carter, L.J.; Taylor, J.C. Emerging Challenges of the Impacts of Pharmaceuticals on Aquatic Ecosystems: A Diatom Perspective. Sci. Total Environ. 2023, 878, 162939. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Masís-Mora, M.; Beita-Sandí, W.; Montiel-Mora, J.R.; Fernández-Fernández, E.; Méndez-Rivera, M.; Arias-Mora, V.; Leiva-Salas, A.; Brenes-Alfaro, L.; Rodríguez-Rodríguez, C.E. Pharmaceuticals in Farms and Surrounding Surface Water Bodies: Hazard and Ecotoxicity in a Swine Production Area in Costa Rica. Chemosphere 2021, 272, 129574. [Google Scholar] [CrossRef]
- Campanha, M.B.; Awan, A.T.; de Sousa, D.N.R.; Grosseli, G.M.; Mozeto, A.A.; Fadini, P.S. A 3-Year Study on Occurrence of Emerging Contaminants in an Urban Stream of São Paulo State of Southeast Brazil. Env. Sci. Pollut. Res. 2015, 22, 7936–7947. [Google Scholar] [CrossRef]
- Botero-Coy, A.M.; Martínez-Pachón, D.; Boix, C.; Rincón, R.J.; Castillo, N.; Arias-Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.; Moncayo-Lasso, A.; Hernández, F. An Investigation into the Occurrence and Removal of Pharmaceuticals in Colombian Wastewater. Sci. Total Environ. 2018, 642, 842–853. [Google Scholar] [CrossRef]
- Salgado Costa, C.; Bahl, F.; Natale, G.S.; Mac Loughlin, T.M.; Marino, D.J.G.; Venturino, A.; Rodriguez-Mozaz, S.; Santos, L.H.M.L.M. First Evidence of Environmental Bioaccumulation of Pharmaceuticals on Adult Native Anurans (Rhinella arenarum) from Argentina. Environ. Pollut. 2023, 334, 122231. [Google Scholar] [CrossRef]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, Fate and Removal of Pharmaceutically Active Compounds (PhACs) in Water and Wastewater Treatment Plants—A Review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- O’Rourke, K.; Virgiliou, C.; Theodoridis, G.; Gika, H.; Grintzalis, K. The Impact of Pharmaceutical Pollutants on Daphnids—A Metabolomic Approach. Environ. Toxicol. Pharmacol. 2023, 100, 104157. [Google Scholar] [CrossRef]
- Hawash, H.B.; Moneer, A.A.; Galhoum, A.A.; Elgarahy, A.M.; Mohamed, W.A.A.; Samy, M.; El-Seedi, H.R.; Gaballah, M.S.; Mubarak, M.F.; Attia, N.F. Occurrence and Spatial Distribution of Pharmaceuticals and Personal Care Products (PPCPs) in the Aquatic Environment, Their Characteristics, and Adopted Legislations. J. Water Process Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- Waleng, N.J.; Nomngongo, P.N. Occurrence of Pharmaceuticals in the Environmental Waters: African and Asian Perspectives. Environ. Chem. Ecotoxicol. 2022, 4, 50–66. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Sharkawy, R.M.; Allam, E.A.; Nabil, G.M.; Louka, F.R.; Salam, M.A.; Elsayed, S.M. Recent Progress in Water Decontamination from Dyes, Pharmaceuticals, and Other Miscellaneous Nonmetallic Pollutants by Layered Double Hydroxide Materials. J. Water Process Eng. 2024, 57, 104625. [Google Scholar] [CrossRef]
- Grabicová, K.; Duchet, C.; Švecová, H.; Randák, T.; Boukal, D.S.; Grabic, R. The Effect of Warming and Seasonality on Bioaccumulation of Selected Pharmaceuticals in Freshwater Invertebrates. Water Res. 2024, 254, 121360. [Google Scholar] [CrossRef]
- Patsialou, S.; Katapodis, D.; Antonopoulou, G.; Charalampous, N.; Qun, Y.; Dailianis, S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Bioremediation and Toxic Removal Efficiency of Raw Pharmaceutical Wastewaters Treated with a Cyanobacteria-Based System Coupled with Valuable Biomass. J. Water Process Eng. 2024, 58, 104895. [Google Scholar] [CrossRef]
- Drzymała, J.; Kalka, J. Ecotoxic Interactions Between Pharmaceuticals in Mixtures: Diclofenac and Sulfamethoxazole. Chemosphere 2020, 259, 127407. [Google Scholar] [CrossRef]
- Nieto, E.; Hampel, M.; González-Ortegón, E.; Drake, P.; Blasco, J. Influence of Temperature on Toxicity of Single Pharmaceuticals and Mixtures, in the Crustacean A. Desmarestii. J. Hazard. Mater. 2016, 313, 159–169. [Google Scholar] [CrossRef]
- Santos, J.; Barreto, A.; Coelho, T.; Carvalho, E.; Pereira, D.; Calisto, V.; Maria, V.L. Amitriptyline Ecotoxicity in Danio rerio (Hamilton, 1822) Embryos—Similar Toxicity Profile in the Presence of Nanoplastics. Environ. Toxicol. Pharmacol. 2024, 106, 104372. [Google Scholar] [CrossRef]
- Mennillo, E.; Arukwe, A.; Monni, G.; Meucci, V.; Intorre, L.; Pretti, C. Ecotoxicological Properties of Ketoprofen and the S(+)-Enantiomer (Dexketoprofen): Bioassays in Freshwater Model Species and Biomarkers in Fish PLHC-1 Cell Line. Env. Toxicol. Chem. 2018, 37, 201–212. [Google Scholar] [CrossRef]
- Siciliano, A.; Guida, M.; Iesce, M.R.; Libralato, G.; Temussi, F.; Galdiero, E.; Carraturo, F.; Cermola, F.; DellaGreca, M. Ecotoxicity and Photodegradation of Montelukast (a Drug to Treat Asthma) in Water. Environ. Res. 2021, 202, 111680. [Google Scholar] [CrossRef]
- Reque, R.; Carneiro, R.D.; Yamamoto, F.Y.; Ramsdorf, W.A.; Martins, L.R.; Guiloski, I.C.; de Freitas, A.M. Ecotoxicity of Losartan Potassium in Aquatic Organisms of Different Trophic Levels. Environ. Toxicol. Pharmacol. 2021, 87, 103727. [Google Scholar] [CrossRef]
- Carlsson, G.; Patring, J.; Kreuger, J.; Norrgren, L.; Oskarsson, A. Toxicity of 15 Veterinary Pharmaceuticals in Zebrafish (Danio rerio) Embryos. Aquat. Toxicol. 2013, 126, 30–41. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Wu, G.-Z.; Zhang, J.-P.; Hu, C.-Q. The Comparative Analysis of Gastrointestinal Toxicity of Azithromycin and 3′-Decladinosyl Azithromycin on Zebrafish Larvae. Toxicol. Appl. Pharmacol. 2023, 469, 116529. [Google Scholar] [CrossRef]
- Almeida, A.C.; Gomes, T.; Lomba, J.A.B.; Lillicrap, A. Specific Toxicity of Azithromycin to the Freshwater Microalga Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2021, 222, 112553. [Google Scholar] [CrossRef]
- Marugán, J.; Rodríguez-Chueca, J.; Esplugas, S.; Sans, C.; Malato, S. Removal of Pharmaceutically Active Compounds (PhACs) in Wastewater by Ozone and Advanced Oxidation Processes. In Removal and Degradation of Pharmaceutically Active Compounds in Wastewater Treatment; Rodriguez-Mozaz, S., Blánquez Cano, P., Sarrà Adroguer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 269–298. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Achak, M.; Sillanpää, M.; Barka, N. Comparative Overview of Advanced Oxidation Processes and Biological Approaches for the Removal Pharmaceuticals. J. Environ. Manag. 2021, 288, 112404. [Google Scholar] [CrossRef]
- Shanmugavel, S.P.; Kumar, G.; Gunasekaran, M. Recent Progress in Mineralization of Emerging Contaminants by Advanced Oxidation Process: A Review. Environ. Pollut. 2024, 341, 122842. [Google Scholar] [CrossRef]
- Kulišťáková, A. Removal of Pharmaceutical Micropollutants from Real Wastewater Matrices by Means of Photochemical Advanced Oxidation Processes—A Review. J. Water Process Eng. 2023, 53, 103727. [Google Scholar] [CrossRef]
- Correa-Sanchez, S.; Peñuela, G.A. Peracetic Acid-Based Advanced Oxidation Processes for the Degradation of Emerging Pollutants: A Critical Review. J. Water Process Eng. 2022, 49, 102986. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Brillas, E. Progress of Antibiotics Removal from Synthetic and Real Waters and Wastewaters by Persulfate-Based Advanced Oxidation Processes. J. Environ. Chem. Eng. 2023, 11, 111303. [Google Scholar] [CrossRef]
- Serna-Carrizales, J.C.; Zárate-Guzmán, A.I.; Flores-Ramírez, R.; Díaz de León-Martínez, L.; Aguilar-Aguilar, A.; Warren- Vega, W.M.; Bailón-García, E.; Ocampo-Pérez, R. Application of Artificial Intelligence for the Optimization of Advanced Oxidation Processes to Improve the Water Quality Polluted with Pharmaceutical Compounds. Chemosphere 2024, 351, 141216. [Google Scholar] [CrossRef]
- Papac, J.; Ballesteros, S.G.; Tonkovic, S.; Kovacic, M.; Tomic, A.; Cvetnić, M.; Kusic, H.; Senta, I.; Terzić, S.; Ahel, M.; et al. Degradation of Pharmaceutical Memantine by Photo-Based Advanced Oxidation Processes: Kinetics, Pathways and Environmental Aspects. J. Environ. Chem. Eng. 2023, 11, 109334. [Google Scholar] [CrossRef]
- Scaria, J.; Nidheesh, P.V. Pre-Treatment of Real Pharmaceutical Wastewater by Heterogeneous Fenton and Persulfate Oxidation Processes. Environ. Res. 2023, 217, 114786. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ma, X.; He, F.; Lu, M.; Zhang, J.; Wang, S.; Dong, P.; Zhao, C. In Situ Generated Iron Oxide Nanosheet on Iron Foam Electrode for Enhanced Electro-Fenton Performance toward Pharmaceutical Wastewater Treatment. J. Hazard. Mater. 2024, 465, 133193. [Google Scholar] [CrossRef]
- Mohamed Isa, E.D.; Shameli, K.; Ch’ng, H.J.; Che Jusoh, N.W.; Hazan, R. Photocatalytic Degradation of Selected Pharmaceuticals Using Green Fabricated Zinc Oxide Nanoparticles. Adv. Powder Technol. 2021, 32, 2398–2409. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The Potential Role of Biochar in the Removal of Organic and Microbial Contaminants from Potable and Reuse Water: A Review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, W.; Yang, Y.; Zhang, X.; Bao, S.; Zhang, X.; Zhang, T.; Leung, K.M.Y. UV-Based Advanced Oxidation Processes for Antibiotic Resistance Control: Efficiency, Influencing Factors, and Energy Consumption. Engineering 2024, 37, 27–39. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Ali Khan, J.; Naushad, M.; Boczkaj, G.; Jamil, F.; Khan, S.; Li, L.; Murtaza, B.; Han, C. Pharmaceuticals Wastewater Treatment via Different Advanced Oxidation Processes: Reaction Mechanism, Operational Factors, Toxicities, and Cost Evaluation—A Review. Sep. Purif. Technol. 2024, 347, 127458. [Google Scholar] [CrossRef]
- Atalay, S.; Ersöz, G. Chapter 7—Hybrid Application of Advanced Oxidation Processes to Dyes′ Removal. In Green Chemistry and Water Remediation: Research and Applications; Sharma, S.K., Ed.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 209–238. [Google Scholar] [CrossRef]
- Rashid Ahmed, H.; Kayani, K.F. A Comparative Review of Fenton-like Processes and Advanced Oxidation Processes for Methylene Blue Degradation. Inorg. Chem. Commun. 2024, 170, 113467. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Gul, N.S.; Sabahat, S.; Sun, J.; Tahir, K.; Shah, N.S.; Muhammad, N.; Rahim, A.; Imran, M.; Iqbal, J.; et al. Removal of Organic Pollutants through Hydroxyl Radical-Based Advanced Oxidation Processes. Ecotoxicol. Environ. Saf. 2023, 267, 115564. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Nam, S.-N.; Choi, J.S.; Park, C.M.; Jang, M.; Lee, J.Y.; Jun, B.-M.; Yoon, Y. Ultrasonic Treatment of Endocrine Disrupting Compounds, Pharmaceuticals, and Personal Care Products in Water: An Updated Review. J. Hazard. Mater. 2024, 474, 134852. [Google Scholar] [CrossRef] [PubMed]
- Parra-Enciso, C.; Avila, B.S.; Rubio-Clemente, A.; Peñuela, G.A. Degradation of Diclofenac through Ultrasonic-Based Advanced Oxidation Processes at Low Frequency. J. Environ. Chem. Eng. 2022, 10, 108296. [Google Scholar] [CrossRef]
- Dhamorikar, R.S.; Lade, V.G.; Kewalramani, P.V.; Bindwal, A.B. Review on Integrated Advanced Oxidation Processes for Water and Wastewater Treatment. J. Ind. Eng. Chem. 2024, 138, 104–122. [Google Scholar] [CrossRef]
- Zorzi, V.; Bertini, A.; Robertson, A.; Berardinelli, A.; Palmisano, L.; Parrino, F. The Application of Advanced Oxidation Processes Including Photocatalysis-Based Ones for the off-Flavours Removal (GSM and MIB) in Recirculating Aquaculture Systems. Mol. Catal. 2023, 551, 113616. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X. Quantitative Analysis of Research Literature on Degradation of Pollutants by Fenton and Fenton-like AOPs Based on CiteSpace. Desalination Water Treat. 2024, 320, 100894. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Q.; Mai, Z.; Wang, M.; Zhao, H.; Xu, K. Strategies for Improving Performance of Iron-Based Catalysts in Activating Heterogeneous Fenton-like Oxidation in Pollutants Degradation: From the Perspective of Materials Structure Design. Process Saf. Environ. Prot. 2024, 190, 794–820. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, D.; Bai, B.; Ma, Z.; Zong, S. Insight into Antibiotic Removal by Advanced Oxidation Processes (AOPs): Performance, Mechanism, Degradation Pathways, and Ecotoxicity Assessment. Chem. Eng. J. 2024, 500, 157134. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. Advanced oxidation process: A Remediation Technique for Organic and Non-Biodegradable Pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Redesigning Electrochemical-Based Fenton Processes: An Updated Review Exploring Advances and Innovative Strategies Using Phenol Degradation as Key Performance Indicator. Appl. Catal. O Open 2024, 194, 206980. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Trellu, C.; Vargas, H.O.; Mousset, E.; Ganiyu, S.O.; Oturan, M.A. Electro-Fenton Process in Combination with Other Advanced Oxidation Processes: Challenges and Opportunities. Curr. Opin. Electrochem. 2023, 37, 101171. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, B.; Liu, J.; Sillanpää, M.; Kim, Y. The Oxidation Treatment of Pharmaceutical Wastewater in H2O2 and PMS System by Iron-Containing Biochar Originated from Excess Sludge. J. Water Process Eng. 2024, 58, 104833. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Kamali, M.; Zhang, X.; Feijoo, S.; Al-Salem, S.M.; Dewil, R.; Appels, L. Biochar in Hydroxyl Radical-Based Electrochemical Advanced Oxidation Processes (eAOPs)—Mechanisms and Prospects. Chem. Eng. J. 2023, 467, 143291. [Google Scholar] [CrossRef]
- Yang, X.; Nguyen, X.C.; Tran, Q.B.; Huyen Nguyen, T.T.; Ge, S.; Nguyen, D.D.; Nguyen, V.-T.; Le, P.-C.; Rene, E.R.; Singh, P.; et al. Machine Learning-Assisted Evaluation of Potential Biochars for Pharmaceutical Removal from Water. Environ. Res. 2022, 214, 113953. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cui, K.; Li, C.-X.; Chen, Y.; Wang, Q.; Yuan, X.; Chen, Y.; Liu, J.; Zhang, Q. Efficient Peroxymonosulfate Activation by Biochar-Based Nanohybrids for the Degradation of Pharmaceutical and Personal Care Products in Aquatic Environments. Chemosphere 2023, 311, 137084. [Google Scholar] [CrossRef] [PubMed]
- Godvin Sharmila, V.; Kumar Tyagi, V.; Varjani, S.; Rajesh Banu, J. A Review on the Lignocellulosic Derived Biochar-Based Catalyst in Wastewater Remediation: Advanced Treatment Technologies and Machine Learning Tools. Bioresour. Technol. 2023, 387, 129587. [Google Scholar] [CrossRef]
- Ahmad, A.; Priyadarshini, M.; Yadav, S.; Ghangrekar, M.M.; Surampalli, R.Y. The Potential of Biochar-Based Catalysts in Advanced Treatment Technologies for Efficacious Removal of Persistent Organic Pollutants from Wastewater: A Review. Chem. Eng. Res. Des. 2022, 187, 470–496. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Burgeoning Prospects of Biochar and Its Composite in Persulfate-Advanced Oxidation Process. J. Hazard. Mater. 2021, 409, 124893. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, B.; Gao, B.; Cheng, N.; Feng, Q.; Chen, M.; Wang, S. Degradation of Organic Pollutants from Water by Biochar-Assisted Advanced Oxidation Processes: Mechanisms and Applications. J. Hazard. Mater. 2023, 442, 130075. [Google Scholar] [CrossRef]
- Li, R.; Lu, X.; Yan, B.; Li, N.; Chen, G.; Cheng, Z.; Hou, L.; Wang, S.; Duan, X. Sludge-Derived Biochar toward Sustainable Peroxymonosulfate Activation: Regulation of Active Sites and Synergistic Production of Reaction Oxygen Species. Chem. Eng. J. 2022, 440, 135897. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-Doping Positively Whilst Sulfur-Doping Negatively Affect the Catalytic Activity of Biochar for the Degradation of Organic Contaminant. Appl. Catal. B Environ. 2020, 263, 118348. [Google Scholar] [CrossRef]
- Wang, J.; Cai, J.; Wang, S.; Zhou, X.; Ding, X.; Ali, J.; Zheng, L.; Wang, S.; Yang, L.; Xi, S.; et al. Biochar-Based Activation of Peroxide: Multivariate-Controlled Performance, Modulatory Surface Reactive Sites and Tunable Oxidative Species. Chem. Eng. J. 2022, 428, 131233. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, C.; Zhao, Y.; Song, C.; Lai, J.; Song, M. Singlet Oxygen in Biochar-Based Catalysts-Activated Persulfate Process: From Generation to Detection and Selectivity Removing Emerging Contaminants. Chem. Eng. J. 2024, 485, 149724. [Google Scholar] [CrossRef]
- Satpati, G.G.; Devi, A.; Kundu, D.; Dikshit, P.K.; Saravanabhupathy, S.; Rajlakshmi; Banerjee, R.; Chandra Rajak, R.; Kamli, M.R.; Lee, S.-Y.; et al. Synthesis, Delineation and Technological Advancements of Algae Biochar for Sustainable Remediation of the Emerging Pollutants from Wastewater-a Review. Environ. Res. 2024, 258, 119408. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Coupling of Advanced Oxidation Technologies and Biochar for the Removal of Dyes in Water. Water 2022, 14, 2531. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, J.; Tan, W.; Tang, H.; Li, H.; Li, Y. Enhanced Degradation of Rhodamine B through Activating Peroxydisulfate by Humic Acid-Fe Incorporated Biochar: Kinetics and Mechanism Studies. J. Mol. Liq. 2024, 396, 123962. [Google Scholar] [CrossRef]
- Tang, C.; Xu, C.; Zhong, G.; Cen, Z.; Ni, Z.; Yao, Z.; Fang, Y.; Qiu, R.; Zhang, S. Unveiling Activation Mechanism of Persulfate by Homologous Hemp-Derived Biochar Catalysts for Enhanced Tetracycline Wastewater Remediation. Bioresour. Technol. 2024, 400, 130684. [Google Scholar] [CrossRef]
- Wang, J.; Shen, M.; Wang, H.; Du, Y.; Zhou, X.; Liao, Z.; Wang, H.; Chen, Z. Red Mud Modified Sludge Biochar for the Activation of Peroxymonosulfate: Singlet Oxygen Dominated Mechanism and Toxicity Prediction. Sci. Total Environ. 2020, 740, 140388. [Google Scholar] [CrossRef]
- Bu, Y.; Li, H.; Yu, W.; Pan, Y.; Li, L.; Wang, Y.; Pu, L.; Ding, J.; Gao, G.; Pan, B. Peroxydisulfate Activation and Singlet Oxygen Generation by Oxygen Vacancy for Degradation of Contaminants. Environ. Sci. Technol. 2021, 55, 2110–2120. [Google Scholar] [CrossRef]
- Zeng, Z.; Umeh, A.; Iyengar, G.A.; Qi, F.; Naidu, R. A Critical Review of Different Types of Biochar-Based Catalysts and Mechanisms in Advanced Oxidation Processes for Organic Contaminants Removal. J. Environ. Chem. Eng. 2024, 12, 114262. [Google Scholar] [CrossRef]
- da Silva Medeiros, D.C.C.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.-H.; Naeth, M.A.; Ok, Y.S.; Chang, S.X.; Gamal El-Din, M. Pristine and Engineered Biochar for the Removal of Contaminants Co-Existing in Several Types of Industrial Wastewaters: A Critical Review. Sci. Total Environ. 2022, 809, 151120. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C.W. Biochar-Supported Nanoscale Zero-Valent Iron as an Efficient Catalyst for Organic Degradation in Groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of Biochar and Biochar Composites and Their Application in a Fenton-like Process for Wastewater Decontamination: A Review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Zhou, P.; Xiong, Z.; He, C.; Liu, Y.; Zhang, H.; Yao, G.; Lai, B. Strategies Based on Electron Donors to Accelerate Fe(III)/Fe(II) Cycle in Fenton or Fenton-like Processes. Chem. Eng. J. 2023, 454, 140096. [Google Scholar] [CrossRef]
- Xue, H.; Deng, L.; Kang, D.; Zhao, Y.; Zhang, X.; Liu, Y.; Chen, H.; Ngo, H.H.; Guo, W. Advanced Biochar-Based Materials for Specific Antibiotics Removal from Hospital Wastewater via Adsorption and Oxidative Degradation. J. Environ. Chem. Eng. 2024, 12, 114275. [Google Scholar] [CrossRef]
- Luo, J.; Yi, Y.; Fang, Z. Effect of Mn-Based Magnetic Biochar /PS Reaction System on Oxidation of Metronidazole. Chemosphere 2023, 332, 138747. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Q.; Di, L.; Zhou, Y.; Zhou, Y. Iron-Based Biochar as Efficient Persulfate Activation Catalyst for Emerging Pollutants Removal: A Review. Chin. Chem. Lett. 2023, 34, 108357. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, S.; Gong, W.; Wang, G.; Qiu, J.; Chen, H. Synthesis, Characterization and Acid Catalysis of Solid Acid from Peanut Shell. Appl. Catal. A Gen. 2014, 469, 284–289. [Google Scholar] [CrossRef]
- Cao, B.; Li, M.; Zhang, T.; Gong, T.; Yang, T.; Xi, B.; Lu, H.; Wang, Z. Dynamics and Mechanisms of Atrazine Adsorption on Biogas-Residue Biochar with Citric Acid Modification. Sep. Purif. Technol. 2024, 337, 126151. [Google Scholar] [CrossRef]
- Kahkeci, J.; Gamal El-Din, M. Biochar-Supported Photocatalysts: Performance Optimization and Applications in Emerging Contaminant Removal from Wastewater. Chem. Eng. J. 2023, 476, 146530. [Google Scholar] [CrossRef]
- Chandra, S.; Jagdale, P.; Medha, I.; Tiwari, A.K.; Bartoli, M.; Nino, A.D.; Olivito, F. Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water—A Review. Toxics 2021, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, X.; Li, J.; Cheng, S.; Zhang, J.; Ma, Y. Recent Progress in TiO2–Biochar-Based Photocatalysts for Water Contaminants Treatment: Strategies to Improve Photocatalytic Performance. RSC Adv. 2024, 14, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.N.; Wu, Z.; Goh, P.S.; Zhou, S. The State-of-the-Art Development of Biochar Based Photocatalyst for Removal of Various Organic Pollutants in Wastewater. J. Clean. Prod. 2023, 429, 139487. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; de Lima Brombilla, V.; Silvestri, S.; Foletto, E.L. Conversion of Spent Coffee Grounds to Biochar as Promising TiO2 Support for Effective Degradation of Diclofenac in Water. Appl. Organomet. Chem. 2020, 34, e6001. [Google Scholar] [CrossRef]
- Qu, K.; Huang, L.; Hu, S.; Liu, C.; Yang, Q.; Liu, L.; Li, K.; Zhao, Z.; Wang, Z. TiO2 Supported on Rice Straw Biochar as an Adsorptive and Photocatalytic Composite for the Efficient Removal of Ciprofloxacin in Aqueous Matrices. J. Environ. Chem. Eng. 2023, 11, 109430. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Bedia, J.; Rodriguez, J.J.; Belver, C. Effect of Activating Agent on the Properties of TiO2/Activated Carbon Heterostructures for Solar Photocatalytic Degradation of Acetaminophen. Materials 2019, 12, 378. [Google Scholar] [CrossRef]
- Manzoor, Q.; Sajid, A.; Ali, Z.; Nazir, A.; Sajid, A.; Imtiaz, F.; Iqbal, S.; Younas, U.; Arif, H.; Iqbal, M. Toxicity Spectrum and Detrimental Effects of Titanium Dioxide Nanoparticles as an Emerging Pollutant: A Review. Desalination Water Treat. 2024, 317, 100025. [Google Scholar] [CrossRef]
- Leite, C.; Coppola, F.; Monteiro, R.; Russo, T.; Polese, G.; Silva, M.R.F.; Lourenço, M.A.O.; Ferreira, P.; Soares, A.M.V.M.; Pereira, E.; et al. Toxic Impacts of Rutile Titanium Dioxide in Mytilus galloprovincialis Exposed to Warming Conditions. Chemosphere 2020, 252, 126563. [Google Scholar] [CrossRef]
- Amir, M.; Fazal, T.; Iqbal, J.; Din, A.A.; Ahmed, A.; Ali, A.; Razzaq, A.; Ali, Z.; Rehman, M.S.U.; Park, Y.-K. Integrated Adsorptive and Photocatalytic Degradation of Pharmaceutical Micropollutant, Ciprofloxacin Employing Biochar-ZnO Composite Photocatalysts. J. Ind. Eng. Chem. 2022, 115, 171–182. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc Oxide-Based Photocatalytic Degradation of Persistent Pesticides: A Comprehensive Review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Shi, C.; Hu, K.; Nie, L.; Wang, H.; Ma, L.; Du, Q.; Wang, G. Degradation of Acetaminophen Using Persulfate Activated with P-Doped Biochar and Thiosulfate. Inorg. Chem. Commun. 2022, 146, 110160. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, S.; He, R.; Zhao, J.; Lv, J.; Kang, W.; Zhang, J. Enhanced Adsorption and Degradation of Antibiotics by Doping Corncob Biochar/PMS with Heteroatoms at Different Preparation Temperatures: Mechanism, Pathway, and Relative Contribution of Reactive Oxygen Species. J. Water Process Eng. 2022, 46, 102626. [Google Scholar] [CrossRef]
- Raj, R.; Tripathi, A.; Das, S.; Ghangrekar, M.M. Is Waste-Derived Catalyst Mediated Electro-Fenton a Sustainable Option for Mitigating Emerging Contaminants from Wastewater? Curr. Opin. Environ. Sci. Health 2024, 37, 100523. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Jin, T.; Li, Z.; Tsang, Y.F.; Liu, B. Efficient H2O2 Generation and Bisphenol A Degradation in Electro-Fenton of O-Doped Porous Biochar Cathode Derived from Spirit-Based Distiller’s Grains. Process Saf. Environ. Prot. 2022, 166, 99–107. [Google Scholar] [CrossRef]
- Tong, W.K.; Dai, C.; Jia, C.; Hu, J.; Gao, M.-T.; Li, J.; Zhang, J.B.; Tang, H.; Liang, Y.; Teng, W.; et al. Eco-Friendly and Stable Triclosan Removal from Groundwater Using Peroxyacetic Acid Activated with Biochar Produced from Saccharification Residues. Chem. Eng. J. 2024, 481, 148422. [Google Scholar] [CrossRef]
- Sadeghi Rad, T.; Yazici, E.S.; Khataee, A.; Gengec, E.; Kobya, M. Ultrasound-Assisted Photocatalytic Decomposition of Rifadin with Biochar and CNT-Based NiCr Layered Double Hydroxides. Surf. Interfaces 2023, 36, 102628. [Google Scholar] [CrossRef]
- Liu, T.; Yao, B.; Luo, Z.; Li, W.; Li, C.; Ye, Z.; Gong, X.; Yang, J.; Zhou, Y. Applications and Influencing Factors of the Biochar-Persulfate Based Advanced Oxidation Processes for the Remediation of Groundwater and Soil Contaminated with Organic Compounds. Sci. Total Environ. 2022, 836, 155421. [Google Scholar] [CrossRef]
- Hung, C.-M.; Chen, C.-W.; Huang, C.-P.; Shiung Lam, S.; Dong, C.-D. Peroxymonosulfate Activation by a Metal-Free Biochar for Sulfonamide Antibiotic Removal in Water and Associated Bacterial Community Composition. Bioresour. Technol. 2022, 343, 126082. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J.; Feng, K.; Liu, B.; Xie, G.; Xing, D. A Green Strategy from Waste Red Mud to Fe0-Based Biochar for Sulfadiazine Treatment by Peroxydisulfate Activation. Chem. Eng. J. 2022, 446, 136944. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C.W. Comparing Biochar- and Bentonite-Supported Fe-Based Catalysts for Selective Degradation of Antibiotics: Mechanisms and Pathway. Environ. Res. 2020, 183, 109156. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic Nitrogen-Doped Sludge-Derived Biochar Catalysts for Persulfate Activation: Internal Electron Transfer Mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Geng, M.; Chen, D.; Lin, H.; Zhang, H. Catalytic Oxidation of Clofibric Acid by Peroxydisulfate Activated with Wood-Based Biochar: Effect of Biochar Pyrolysis Temperature, Performance and Mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Zhang, X.; Bhattacharya, T.; Wang, C.; Kumar, A.; Nidheesh, P.V. Straw-Derived Biochar for the Removal of Antibiotics from Water: Adsorption and Degradation Mechanisms, Recent Advancements and Challenges. Environ. Res. 2023, 237, 116998. [Google Scholar] [CrossRef]
| Type of Pharmaceutical | Example Name and Chemical Formula | Molecular Structure |
|---|---|---|
| Anti-hyperglycemic | Meglitinide C17H16ClNO4 | 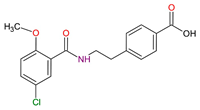 |
| Antidepressants | Fluoxetine C17H18F3NO | 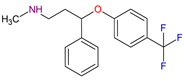 |
| Cytostatic drugs | Docetaxel C43H53NO14 | 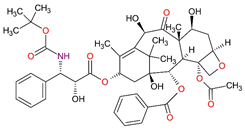 |
| Beta-blockers | Atenolol C14H22N2O3 | 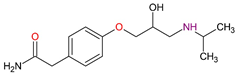 |
| Analgesics | Acetaminophen C8H9NO2 | 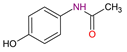 |
| Sedatives | Alprazolam C17H13ClN4 | 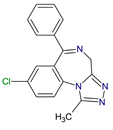 |
| Antivirals | Zanamivir C12H20N4O7 | 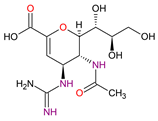 |
| Antibiotics | Azithromycin C38H72N2O12 | 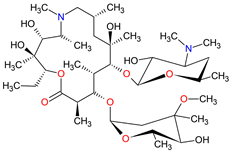 |
| Hormones | Ethinylestradiol C20H24O2 | 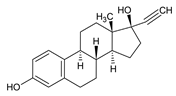 |
| Antiepileptics | Carbamazepine C15H12N2O | 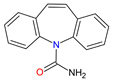 |
| Non-steroidal anti-inflammatory drugs | Ibuprofen C13H18O2 | 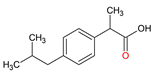 |
| Pharmaceutical | Water Matrix | Concentration (µg/L) | Country | Ref. |
|---|---|---|---|---|
| Non-steroidal anti-inflammatory compounds | ||||
| Ketoprofen | Pig production farm wastewater treatment plant effluent | 14.7 | Costa Rica | [37] |
| Naproxen | Pig production farm wastewater treatment plant effluent, surface water, and urban wastewater effluent | 0.0544–2.8 | Costa Rica, Brazil, and Colombia | [37,38,39] |
| Ibuprofen | Surface water | 0.22–6.95 | Brazil and Argentina | [38,40] |
| Diclofenac | Hospital wastewater | 3.04 | Colombia | [39] |
| Analgesics | ||||
| Acetaminophen | Pig production farm wastewater treatment plant effluent, surface water, urban wastewater effluent, and hospital wastewater | 0.12–50.9 | Costa Rica, Argentina, and Colombia | [37,39,40] |
| Paracetamol | Surface water | 3.67 | Brazil | [38] |
| Antiepileptics | ||||
| Carbamazepine | Surface water, urban wastewater effluent, and hospital wastewater | 0.10–1.39 | Brazil, Argentina, and Colombia | [38,39,40] |
| 2-hydroxy-Carbamazepine | Surface water | 0.079 | Argentina | [40] |
| Epoxy-Carbamazepine | 0.103 | |||
| Beta-blockers | ||||
| Atenolol | Surface water | 0.98 | Brazil | [38] |
| Propranolol | 0.023 | |||
| Hormones | ||||
| 17-β-estradiol | Surface water | 0.0035 | Brazil | [38] |
| Estrone | 0.0099 | |||
| Antihypertensives | ||||
| Valsartan | Surface water | 2.50 | Argentina | [40] |
| Losartan | Urban and hospital wastewater effluents | 1–1.19 | Colombia | [39] |
| Antibiotics | ||||
| Metronidazole | Surface water, urban and hospital wastewater effluents | 0.108–3.54 | Argentina and Colombia | [39,40] |
| Sulfamethoxazole | Surface water and urban wastewater effluent | 0.088–0.35 | ||
| N-Acetyl-Sulfamethoxazole | Surface water | 0.811 | Argentina | [40] |
| Azithromycin | Urban and hospital wastewater effluents | 3.88–6.93 | Colombia | [39] |
| Ciprofloxacin | 0.62–5.56 | |||
| Metronidazole | 0.26–3.54 | |||
| Doxycycline | Urban wastewater effluent | 0.078 | ||
| Clindamycin | Hospital wastewater | 8.34 | ||
| Pharmaceutical | Sentinel Organism | Experimental Conditions | LC50, EC50, LOEC, or NOEC | Observed Effects | Ref. |
|---|---|---|---|---|---|
| Pharmaceutical wastewater (PWW) | Sinapis alba | t = 72 h T = 25 °C The soil in which the seeds were planted was hydrated with PWW | Not reported | PWW inhibited 79.42% of the plant root growth, affecting the plant root length and nutrient intake. PWW exhibited high phytotoxicity (20% of the seed germination was inhibited). | [47] |
| Diclofenac (DF) | Daphnia manga | t = 24, 48 h [Pharmaceutical] = 0.01–1000 mg/L | EC50 = 9.80 mg/L | The pharmaceutical caused negative impacts. The toxicity of pharmaceuticals was intensified when they were mixed. | [48] |
| Sulfamethoxazole (SFM) | EC50 = 43.97 mg/L | ||||
| DF + SFM | EC50 = 13.59 mg/L | ||||
| DF | Atyaephyra desmarestii | t = 96 h T = 20 and 25 °C [DF]20°C = 1.4–7.2 mg/L [DF]25°C = 0.5–8.9 mg/L | LC50 = 6.3 and 6.4 mg/L for 20 and 25 °C, respectively | A decreased in the respiration rate was observed when in contact with the pharmaceutical and an increase in temperature. DF and CBZ even at low concentrations produced respiratory deficiencies in shrimp. | [49] |
| Ibuprofen (IB) | [IB]20°C = 1.9–30.2 mg/L [IB]25°C = 0.9–35.2 mg/L | LC50 = 13.3 and 10.1 mg/L for 20 and 25 °C, respectively | |||
| Carbamazepine (CBZ) | [CBZ]20°C = 57.7–176.3 mg/L [CBZ]25°C = 9–164 mg/L | LC50 = 94.3 and 66.4 mg/L for 20 and 25 °C, respectively | |||
| Pig farm wastewater | Lactuca sativa | t = 6 d T = 20 °C | Not reported | Observed >98% germination inhibition. | [37] |
| Pig farm wastewater treatment plant effluent | Observed >90% germination inhibition. The use of treated wastewater for irrigation led to a potential risk due to the recirculation of antibiotic-resistant bacteria and antibiotic-resistance genes in the surrounding ecosystem. | ||||
| Amitriptyline (AMI) | Danio rerio embryos | [AMI] = 0, 0.003, 0.03, 0.3, 3, and 10 mg/L | Not reported |
| [50] |
| Ketoprofen (KP) | Pseudokirchneriella subcapitata | [KP, DKP] = 1.95–2000 µg/L t = 96 h | NOEC = 7.8 µg/L LOEC = 15.6 µg/L EC50 = 240.2 µg/L | The presence of the substance inhibited the growth of the microalgae. | [51] |
| Dexketoprophen (DKP) | NOEC = 3.9 µg/L LOEC = 7.8 µg/L EC50 = 65.6 µg/L | ||||
| Montelukast (MTL) | Daphnia magna | [MTL] = 1–200 mg/L t = 48 h | EC50 = 16.4 mg/L | Daphnids showed morphological changes. Bioaccumulation of MTL in the gut. | [52] |
| Losartan potassium (LOS) | Desmodesmus subspicatus | [LOS] = 0–38 mg/L t = 72 h T = 23 °C Photoperiod = 24 h | EC50 = 27.93 mg/L | LOS was classified from moderate to low aquatic toxicity. | [53] |
| Daphnia magna | [LOS] = 0–380 mg/L t = 48 h T = 20 °C Comet assay [LOS] = 0.25, 2.5, 200 mg/L t = 48 h | EC50 = 303.7 mg/L | LOS induced genotoxic effects. | ||
| Albendazole (AB) | Danio rerio embryos | [FB] = 0.0022–0.22 mg/L t = 48 h | NOEC = 0.022 mg/L | Exposure to AB increased individual heart rate. | [54] |
| Fenbendazole (FB) | [FB] = 0.0022–0.22 mg/L | NOEC = 0.020 mg/L | FM caused the underdevelopment of the head and eyes. and the tail curve. | ||
| Oxfendazole (OD) | [OD] = 0.10–10 mg/L | NOEC = 4.6 mg/L | |||
| Flumethrin (FM) | [FM] = 0.10–1.0 mg/L | NOEC = 0.022 mg/L | |||
| Azithromycin (AZ) 3′-Decladinosyl azithromycin (AZ3) | Danio rerio larvae and GES-1 human cells | For Danio rerio [AZ, AZ3] = 0.1, 0.5, and 0.8 mmol/L; t = 6 h For GES-1 cells [AZ, AZ3] = 600 µmol/L; t = 24 h | Not reported | AZ3 (AZ metabolite) exhibits a higher gastrointestinal toxicity. AZ and AZ3 induced cytotoxic effects in GES-1 cells. | [55] |
| AZ | Raphidocelis subcapitata | t = 72 h T = 22 °C | EC50 = 0.051 mg/L NOEC = 0.010 mg/L LOEC = 0.033 mg/L | AZ was classified as very toxic for aquatic environments. AZ affected microalgal photosynthetic capacity and electron transfer chain. AZ induced genotoxic effects. | [56] |
| Type of AOP | Characteristics | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| UV/H2O2 system |
|
|
| [60,70,71] |
| Persulfate-based process |
|
|
| [60,71] |
| UV/O3 system |
|
|
| [60,71,72] |
| (Photo-)Fenton and (photo-)Fenton-like processes |
|
|
| [73,74] |
| Ultrasonication |
|
|
| [75,76,77,78] |
| Heterogeneous photocatalysis process |
|
|
| [60,78] |
| Heterogeneous (photo-)Fenton process |
|
|
| [79,80] |
| Electro-(photo-)Fenton process |
|
|
| [81,82,83,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Combination of Biochar and Advanced Oxidation Processes for the Sustainable Elimination of Pharmaceuticals in Water. Sustainability 2024, 16, 10761. https://doi.org/10.3390/su162310761
Gallego-Ramírez C, Chica E, Rubio-Clemente A. Combination of Biochar and Advanced Oxidation Processes for the Sustainable Elimination of Pharmaceuticals in Water. Sustainability. 2024; 16(23):10761. https://doi.org/10.3390/su162310761
Chicago/Turabian StyleGallego-Ramírez, Carolina, Edwin Chica, and Ainhoa Rubio-Clemente. 2024. "Combination of Biochar and Advanced Oxidation Processes for the Sustainable Elimination of Pharmaceuticals in Water" Sustainability 16, no. 23: 10761. https://doi.org/10.3390/su162310761
APA StyleGallego-Ramírez, C., Chica, E., & Rubio-Clemente, A. (2024). Combination of Biochar and Advanced Oxidation Processes for the Sustainable Elimination of Pharmaceuticals in Water. Sustainability, 16(23), 10761. https://doi.org/10.3390/su162310761








