Sequestration of Methylene Blue Dye in a Fixed-Bed Column Using Activated Carbon-Infused Polyurethane Composite Adsorbent Derived from Coconut Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Bio-Based Polyol
2.4. Preparation of MB Solution
2.5. Preparation of ACIP Composite Foam
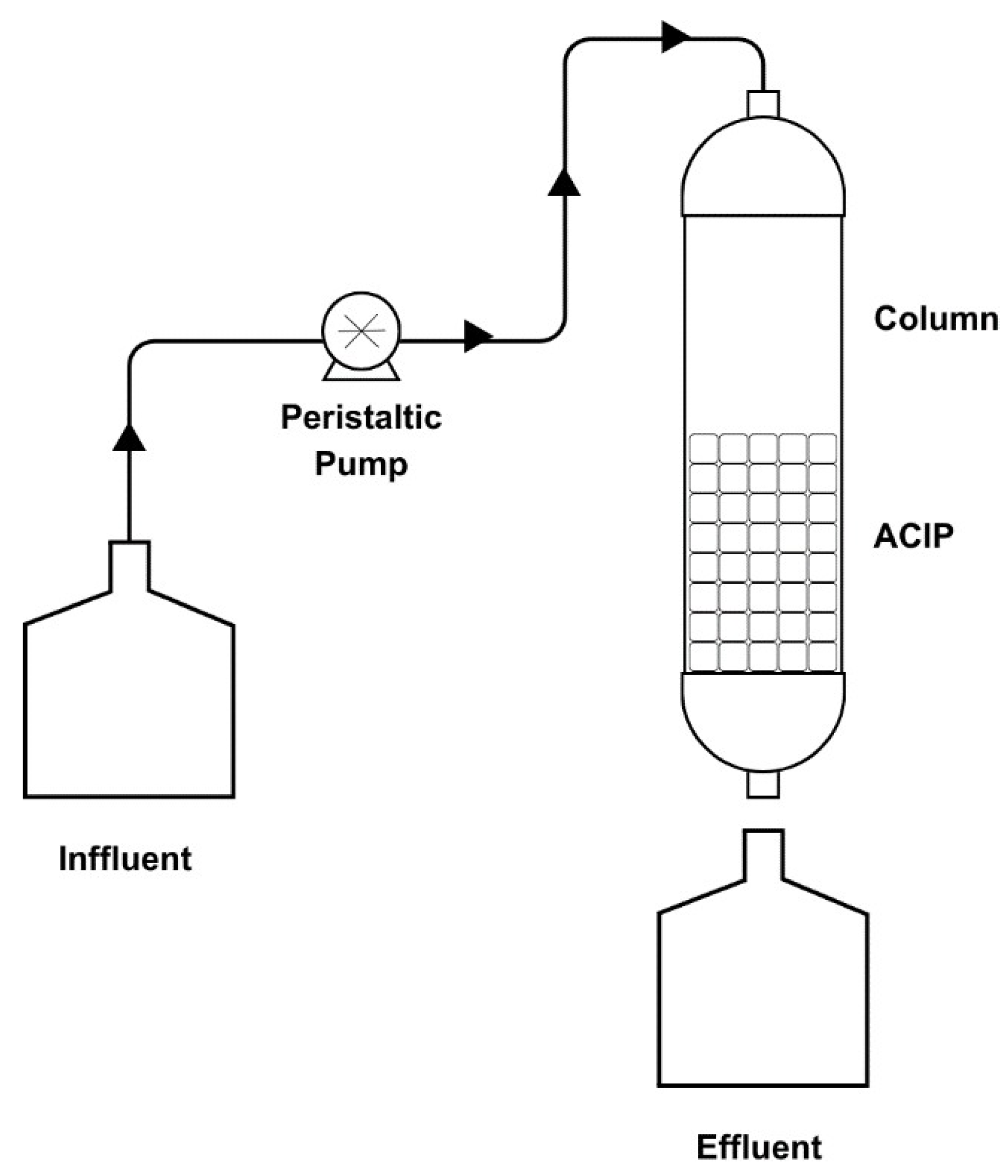
2.6. The Fixed-Bed Column Experiment
2.7. Column Studies
2.8. Dynamic Models
2.9. Desorption of ACIP
3. Results and Discussion
3.1. Adsorbent Characterization
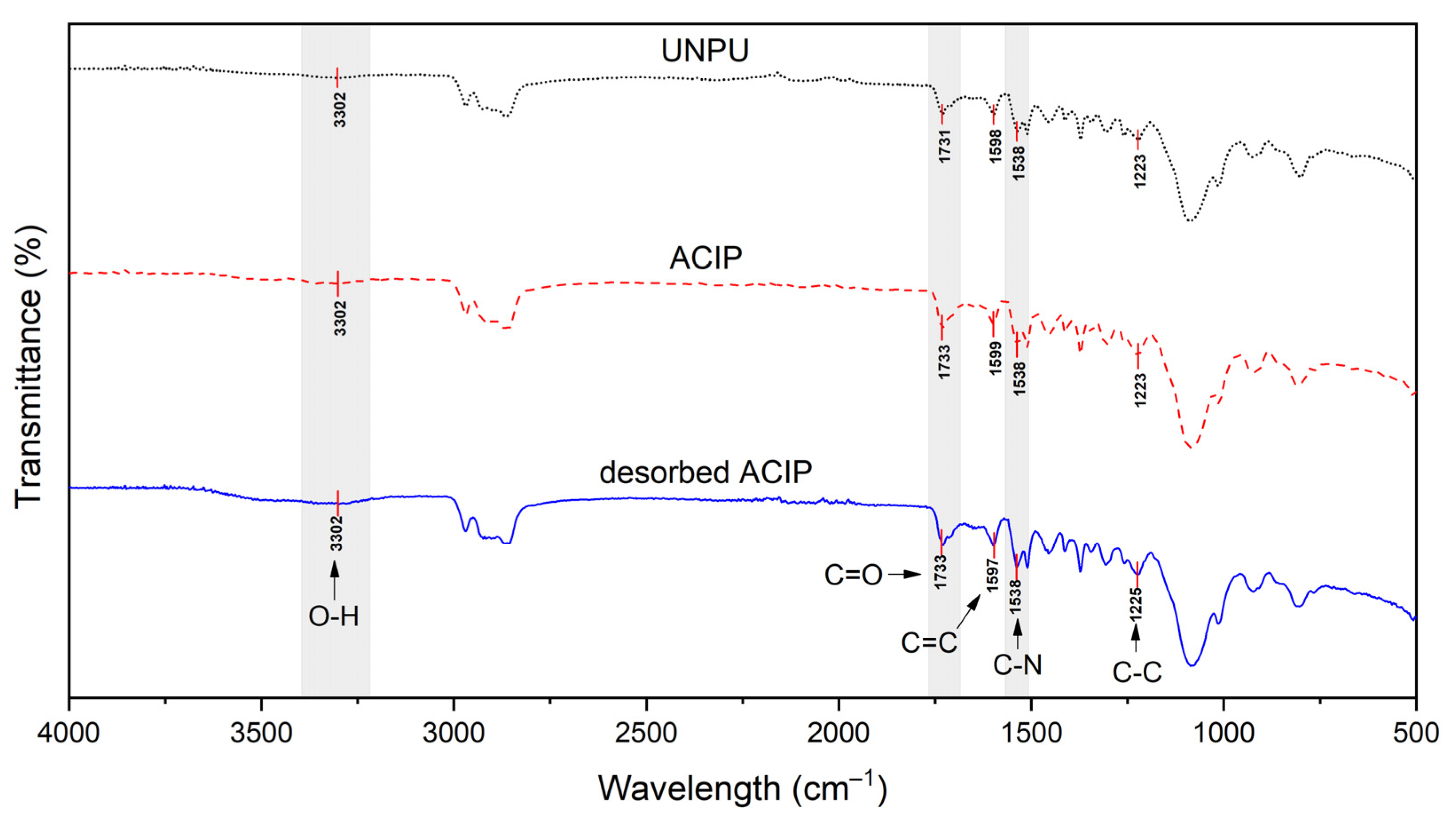
3.1.1. Fourier Transform Infrared Analysis
3.1.2. Scanning Electron Microscope and Pycnometer Analysis
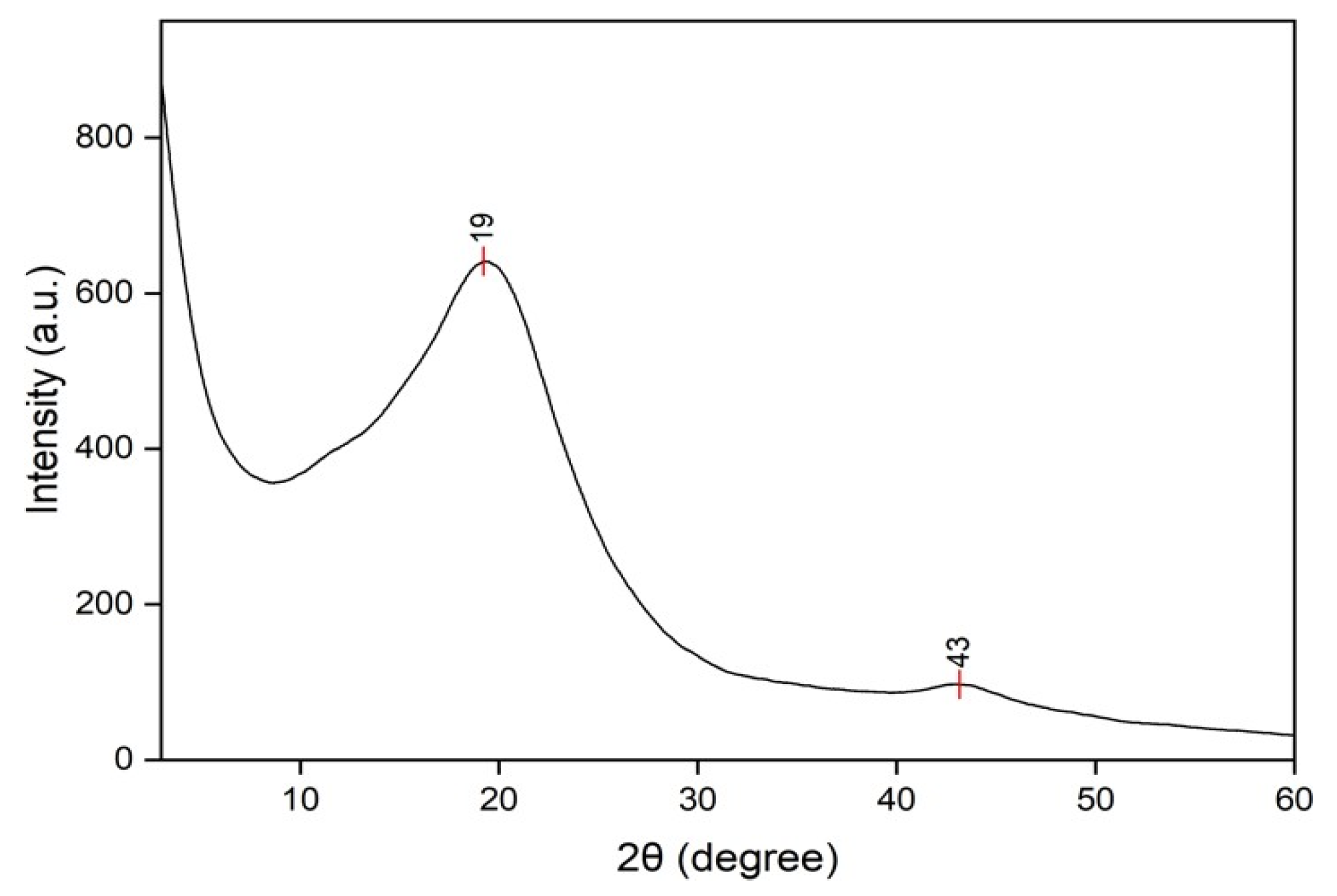
3.1.3. X-Ray Diffraction Analysis
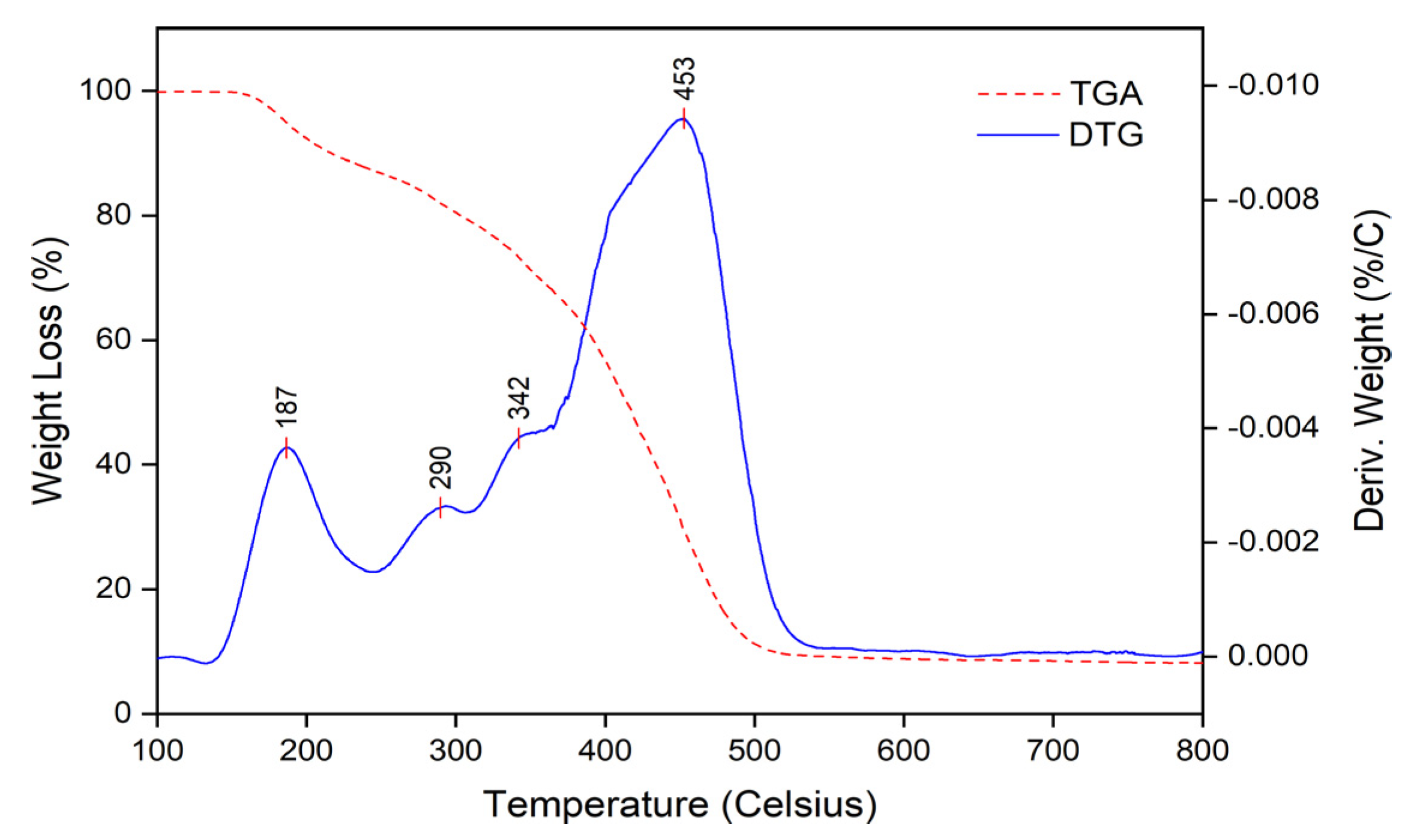
3.1.4. Thermogravimetric Analysis
3.2. The Column Analysis
3.2.1. Effect of Initial MB Concentration
3.2.2. Effect of ACIP Composite Bed Height
3.2.3. Effect of Solution MB Flow Rate
3.2.4. Effect of the Initial MB Solution pH
3.3. The Model Analysis
3.3.1. The Adams–Bohart Model
3.3.2. The Thomas Model
3.3.3. The Yoon–Nelson Model
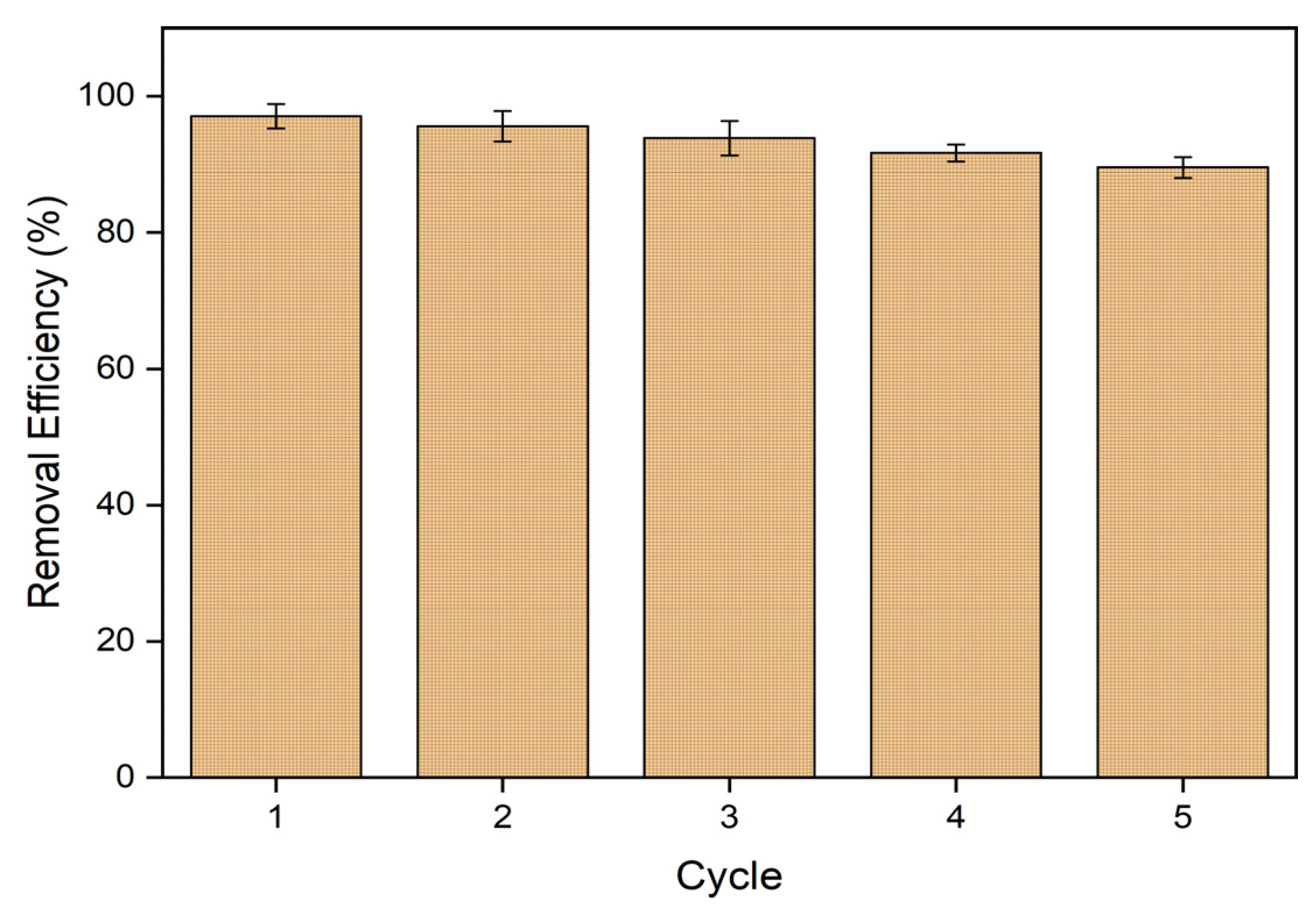
3.3.4. Desorption of MB
3.3.5. Other Adsorbents
3.3.6. Proposed Adsorption Synergism Between ACIP and MB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fritzke, W.; Salla, E.G.; Bagatini, M.D.; da Silva Rosa Bonadiman, B.; Skoronski, E.; Moroni, L.S.; Kempka, A.P. Peroxidase of Cedrela fissilis leaves: Biochemical characterization and toxicity of enzymatically decolored solution of textile dye Brilliant Sky-Blue G. Biocatal. Agric. Biotechnol. 2020, 24, 101553. [Google Scholar] [CrossRef]
- Sen, S.K.; Patra, P.; Das, C.R.; Raut, S.; Raut, S. Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: An impact on its use in irrigation of wheat crop. Water Resour. Ind. 2019, 21, 100106. [Google Scholar] [CrossRef]
- Kusumlata; Ambade, B.; Kumar, A.; Gautam, S. Sustainable Solutions: Reviewing the Future of Textile Dye Contaminant Removal with Emerging Biological Treatments. Limnol. Rev. 2024, 24, 126–149. [Google Scholar] [CrossRef]
- Ma, J.; Jia, Y.; Jing, Y.; Yao, Y.; Sun, J. Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dyes Pigments 2012, 93, 1441–1446. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Sornaly, H.H.; Ahmed, S.; Titin, K.F.; Islam, M.N.; Parvin, A.; Islam, M.A.; Faruquee, H.M.; Biswas, K.K.; Islam, R.; Paul, D.K.; et al. The utility of bioremediation approach over physicochemical methods to detoxify dyes discharges from textile effluents: A comprehensive review study. Sustain. Chem. Pharm. 2024, 39, 101538. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Tian, F.; Wang, Y.; Guo, G.; Ding, K.; Yang, F.; Wang, H.; Cao, Y.; Liu, C. Enhanced azo dye biodegradation at high salinity by a halophilic bacterial consortium. Bioresour. Technol. 2021, 326, 124749. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 04, 22–26. [Google Scholar] [CrossRef]
- Leal Filho, W.; Perry, P.; Heim, H.; Dinis, M.A.P.; Moda, H.; Ebhuoma, E.; Paço, A. An overview of the contribution of the textiles sector to climate change. Front. Environ. Sci. 2022, 10, 973102. [Google Scholar] [CrossRef]
- Ghaly, A. Production, Characterization and Treatment of Textile Effluents: A Critical Review. J. Chem. Eng. Process Technol. 2013, 5, 1–19. [Google Scholar] [CrossRef]
- Laing, I.G. The impact of effluent regulations on the dyeing industry. Rev. Prog. Color. Relat. Top. 1991, 21, 56–71. [Google Scholar] [CrossRef]
- Makrigianni, V.; Giannakas, A.; Hela, D.; Papadaki, M.; Konstantinou, I. Adsorption of methylene blue dye by pyrolytic tire char in fixed-bed column. Desalin. Water Treat. 2017, 65, 346–358. [Google Scholar] [CrossRef]
- Sarkar Phyllis, A.K.; Tortora, G.; Johnson, I. The Fairchild Books Dictionary of Textiles; Bloomsbury Publishing: London, UK, 2021. [Google Scholar]
- Baldez, E.E.; Robaina, N.F.; Cassella, R.J. Employment of polyurethane foam for the adsorption of Methylene Blue in aqueous medium. J. Hazard. Mater. 2008, 159, 580–586. [Google Scholar] [CrossRef]
- Oh, H.-J.; Choung, Y.-K.; Lee, S.; Choi, J.-S.; Hwang, T.-M.; Kim, J.H. Adsorption of methylene blue by phoenix tree leaf powder in a fixed-bed column: Experiments and prediction of breakthrough curves. Desalination 2009, 245, 284–297. [Google Scholar]
- Ghaedi, M.; Zeinali, N.; Ghaedi, A.M.; Teimuori, M.; Tashkhourian, J. Artificial neural network-genetic algorithm based optimization for the adsorption of methylene blue and brilliant green from aqueous solution by graphite oxide nanoparticle. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2014, 125, 264–277. [Google Scholar] [CrossRef]
- Belen, M.A.; Jacinto, J.L.; Go, N.; Santos, L.T.; Zagala, A.J.C.; Santos, R.A.; Apodaca, D.C. Assessment of the impact of open waste dumpsite on groundwater quality: A case study of the Payatas dumpsite in Quezon City, Philippines. IOP Conf. Ser. Earth Environ. Sci. 2019, 344, 012050. [Google Scholar] [CrossRef]
- Hessel, C.; Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007, 83, 171–180. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and carcinogenesis studies of methylene blue trihydrate (Cas No. 7220-79-3) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2008, 540, 1–224. [Google Scholar]
- Xu, J.Z.; Dai, L.; Wu, B.; Ding, T.; Zhu, J.J.; Lin, H.; Chen, H.L.; Shen, C.Y.; Jiang, Y. Determination of methylene blue residues in aquatic products by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2009, 32, 4193–4199. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Gong, J.L.; Zhang, Y.L.; Jiang, Y.; Zeng, G.M.; Cui, Z.H.; Liu, K.; Deng, C.H.; Niu, Q.Y.; Deng, J.H.; Huan, S.Y. Continuous adsorption of Pb(II) and methylene blue by engineered graphite oxide coated sand in fixed-bed column. Appl. Surf. Sci. 2015, 330, 148–157. [Google Scholar] [CrossRef]
- Boucherdoud, A.; Kherroub, D.E.; Bestani, B.; Benderdouche, N.; Douinat, O.; History, A. Fixed-bed adsorption dynamics of methylene blue from aqueous solution using alginate-activated carbon composites adsorbents ARTICLE INFO ABSTRACT/RESUME. Alger. J. Environ. Sci. Technol. Mon. Ed. 2021, 8, 2329–2337. [Google Scholar]
- Hussain, A.; Madan, S.; Madan, R. Removal of Heavy Metals from Wastewater by Adsorption. In Heavy Metals—Their Environmental Impacts and Mitigation; Books on Demand: Norderstedt, Germany, 2021. [Google Scholar]
- Maulina, S.; Iriansyah, M. Characteristics of activated carbon resulted from pyrolysis of the oil palm fronds powder. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012072. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, X.; Zhang, Y.; Tang, Q.; Yuan, Z.; De Hoop, C.F.; Cao, J.; Hao, S.; Liang, T.; Li, F.; et al. Bamboo-Based Biofoam Adsorbents for the Adsorption of Cationic Pollutants in Wastewater: Methylene Blue and Cu(II). ACS Omega 2021, 6, 23447–23459. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wang, Q.; Di, J.; Miao, S.; Yu, J. Amino-Functionalized Porous Nanofibrous Membranes for Simultaneous Removal of Oil and Heavy-Metal Ions from Wastewater. ACS Appl. Mater. Interfaces 2019, 11, 1672–1679. [Google Scholar] [CrossRef]
- Braun, T.; Navratil, J.D.; Farag, A.B. Polyurethane Foam Sorbents in Separation Science; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ahmad, A.; Siti Nurul, S.N.A.; Choong, T.S.Y.; Abdullah, A.H.; Mastuli, M.S.; Othman, N.; Jiman, N.N. Green flexible polyurethane foam as a potent support for Fe-Si adsorbent. Polymers 2019, 11, 2011. [Google Scholar] [CrossRef]
- Hong, H.J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated cellulose nanofibrils(CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef]
- Salunkhe, B.; Schuman, T.P. Super-Adsorbent Hydrogels for Removal of Methylene Blue from Aqueous Solution: Dye Adsorption Isotherms, Kinetics, and Thermodynamic Properties. Macromol 2021, 1, 256–275. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High adsorption capacity of phenol and methylene blue using activated carbon derived from lignocellulosic agriculture wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Kang, P.; Fang, Z.; Hao, J.; Wu, H.; Zhu, Y.; Guo, K. Flow Reactor Synthesis of Bio-Based Polyol from Soybean Oil for the Production of Rigid Polyurethane Foam. Ind. Eng. Chem. Res. 2020, 59, 17513–17519. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Soybean-oil-based waterborne polyurethane dispersions: Effects of polyol functionality and hard segment content on properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Li, C.; Cai, X.; Yang, Z.; Zhang, F.; Liu, Y.; Yang, G.; Wang, Q.; Fang, G.; Zhang, X. A study on coconut fatty acid diethanolamide-based polyurethane foams. RSC Adv. 2022, 12, 13548–13556. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, S. The Preparation and Characterization of Polyurethane Foam with Coconut Oil Polyol and Rapeseed Oil Polyol. J. Polym. Environ. 2021, 29, 2421–2434. [Google Scholar] [CrossRef]
- Tomon, T.R.B.; Estrada, R.J.R.; Fernandez, R.M.D.; Capangpangan, R.Y.; Lubguban, A.A.; Dumancas, G.G.; Alguno, A.C.; Malaluan, R.M.; Bacosa, H.P.; Lubguban, A.A. Coconut power: A sustainable approach for the removal of Cr6+ ions using a new coconut-based polyurethane foam/activated carbon composite in a fixed-bed column. RSC Adv. 2023, 13, 20941–20950. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics I. A Theoretical Model for Respirator Cartridge Service Life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Baral, S.S.; Das, N.; Ramulu, T.S.; Sahoo, S.K.; Das, S.N.; Chaudhury, G.R. Removal of Cr(VI) by thermally activated weed Salvinia cucullata in a fixed-bed column. J. Hazard. Mater. 2009, 161, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fang, L.; Qin, Y.; Wu, W.; Mao, C.; Zhu, H. Oil sorbents with high sorption capacity, oil/water selectivity and reusability for oil spill cleanup. Mar. Pollut. Bull. 2014, 84, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.; Zilouei, H.; Abdolmaleki, A.; Asadinezhad, A.; Nikkhah, A.A. Impregnation of polyurethane foam with activated carbon for enhancing oil removal from water. Int. J. Environ. Sci. Technol. 2016, 13, 699–710. [Google Scholar] [CrossRef]
- Nam, G.; Choi, S.; Byun, H.; Rhym, Y.M.; Shim, S.E. Preparation of macroporous carbon foams using a polyurethane foam template replica method without curing step. Macromol. Res. 2013, 21, 958–964. [Google Scholar] [CrossRef]
- El Malti, W.; Hamieh, M.; Noaman, A.; El-Dine, R.N.; Hijazi, A.; Al-Khatib, W. Polyurethane Loaded with Vegetable Activated Carbon for Heavy Metals Removal from Water. J. Ecol. Eng. 2021, 22, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Trifol, J.; Plackett, D.; Szabo, P.; Daugaard, A.E.; Giacinti Baschetti, M. Effect of Crystallinity on Water Vapor Sorption, Diffusion, and Permeation of PLA-Based Nanocomposites. ACS Omega 2020, 5, 15362–15369. [Google Scholar] [CrossRef]
- Dingcong, R.G.; Malaluan, R.M.; Alguno, A.C.; Estrada, D.J.E.; Lubguban, A.A.; Resurreccion, E.P.; Dumancas, G.G.; Al-Moameri, H.H.; Lubguban, A.A. A novel reaction mechanism for the synthesis of coconut oil-derived biopolyol for rigid poly(urethane-urea) hybrid foam application. RSC Adv. 2023, 13, 1985–1994. [Google Scholar] [CrossRef]
- Udayakumar, M.; El Mrabate, B.; Koós, T.; Szemmelveisz, K.; Kristály, F.; Leskó, M.; Filep, Á.; Géber, R.; Schabikowski, M.; Baumli, P.; et al. Synthesis of activated carbon foams with high specific surface area using polyurethane elastomer templates for effective removal of methylene blue. Arab. J. Chem. 2021, 14, 103214. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Z.; Feng, C.; Li, M.; Chen, R.; Sugiura, N. Investigations on the batch and fixed-bed column performance of fluoride adsorption by Kanuma mud. Desalination 2011, 268, 76–82. [Google Scholar] [CrossRef]
- Tamez Uddin, M.; Rukanuzzaman, M.; Maksudur Rahman Khan, M.; Akhtarul Islam, M. Adsorption of methylene blue from aqueous solution by jackfruit (Artocarpus heteropyllus) leaf powder: A fixed-bed column study. J. Environ. Manag. 2009, 90, 3443–3450. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Malkoc, E.; Nuhoglu, Y.; Dundar, M. Adsorption of chromium(VI) on pomace-An olive oil industry waste: Batch and column studies. J. Hazard. Mater. 2006, 138, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; dos Santos, J.M.N.; Rosa, R.; Pinto, L.A.A.; Pavan, F.A.; Lima, E.C. Fixed bed adsorption of Methylene Blue by ultrasonic surface modified chitin supported on sand. Chem. Eng. Res. Des. 2015, 100, 302–310. [Google Scholar] [CrossRef]
- Ko, D.C.K.; Porter, J.F.; McKay, G. Optimized correlations for the fixed-bed adsorption of metal ions on bone char. Chem. Eng. Sci. 2000, 55, 5819–5829. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. Removal of nickel(II) ions from aqueous solution using crab shell particles in a packed bed up-flow column. J. Hazard. Mater. 2004, 113, 223–230. [Google Scholar] [CrossRef]
- Zulfadhly, Z.; Mashitah, M.D.; Bhatia, S. Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ. Pollut. 2001, 112, 463–470. [Google Scholar] [CrossRef]
- Han, R.; Zou, L.; Zhao, X.; Xu, Y.; Xu, F.; Li, Y.; Wang, Y. Characterization and properties of iron oxide-coated zeolite as adsorbent for removal of copper(II) from solution in fixed bed column. Chem. Eng. J. 2009, 149, 123–131. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Zhang, Z.; Shi, J.; Yang, J. Removal of copper(II) and lead(II) from aqueous solution by manganese oxide coated sand. I. Characterization and kinetic study. J. Hazard. Mater. 2006, 137, 384–395. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ahamad, T. Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: Kinetics, isotherm and mechanism. J. Environ. Manag. 2018, 223, 29–36. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2003, 39, 599–613. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X.; Fu, K. Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: A fixed-bed column study. Bioresour. Technol. 2012, 113, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O.; Turan, M.; Turan, A.Z.; Faki, A.; Engin, A.B. Feasibility analysis of color removal from textile dyeing wastewater in a fixed-bed column system by surfactant-modified zeolite (SMZ). J. Hazard. Mater. 2009, 166, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Mashkoor, F.; Nasar, A. Polyaniline/Tectona grandis sawdust: A novel composite for efficient decontamination of synthetically polluted water containing crystal violet dye. Groundw. Sustain. Dev. 2019, 8, 390–401. [Google Scholar] [CrossRef]
- Elkholy, A.S.; Yahia, M.S.; Elnwawy, M.A.; Gomaa, H.A.; Elzaref, A.S. Synthesis of activated carbon composited with Egyptian black sand for enhanced adsorption performance toward methylene blue dye. Sci. Rep. 2023, 13, 4209. [Google Scholar] [CrossRef] [PubMed]
- Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Lau, K.L.; Alias, N.H. Simultaneous adsorption of malachite green and methylene blue dyes in a fixed-bed column using poly (acrylonitrile-co-acrylic acid) modified with thiourea. Molecules 2020, 25, 2650. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Jeyanthi, J. Simultaneous removal of binary dye from textile effluent using cobalt ferrite-alginate nanocomposite: Performance and mechanism. Microchem. J. 2018, 145, 791–800. [Google Scholar] [CrossRef]
- Taweekarn, T.; Wongniramaikul, W.; Sriprom, W.; Limsakul, W.; Choodum, A. Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column. Polymers 2023, 15, 3989. [Google Scholar] [CrossRef]
- Li, W.; Yue, Q.; Tu, P.; Ma, Z.; Gao, B.; Li, J.; Xu, X. Adsorption characteristics of dyes in columns of activated carbon prepared from paper mill sewage sludge. Chem. Eng. J. 2011, 178, 197–203. [Google Scholar] [CrossRef]
- Mahamadi, C.; Mawere, E. Continuous flow biosorptive removal of methylene blue and crystal violet dyes using alginate–water hyacinth beads. Cogent Environ. Sci. 2019, 5, 15493. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosan-clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Yhon, J.; Mendoza, J.; Osorio, E.; Domínguez, M.P. Continuous Removal of Dyes from Wastewater Using Banana-Peel Bioadsorbent: A Low-Cost Alternative for Wastewater Treatment. Sustainability 2023, 15, 9870. [Google Scholar] [CrossRef]
- Li, X.; Han, D.; Zhang, M.; Li, B.; Wang, Z.; Gong, Z.; Liu, P.; Zhang, Y.; Yang, X. Removal of toxic dyes from aqueous solution using new activated carbon materials developed from oil sludge waste. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123505. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Statistical modeling of methylene blue dye adsorption by high surface area mesoporous activated carbon from bamboo chip using KOH-assisted thermal activation. Energy Ecol. Environ. 2020, 5, 456–469. [Google Scholar] [CrossRef]
- Tran, H.N.; Wang, Y.F.; You, S.J.; Chao, H.P. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of Π–Π interactions. Process Saf. Environ. Prot. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Mesoporous Crosslinked Chitosan-Activated Charcoal Composite for the Removal of Thionine Cationic Dye: Comprehensive Adsorption and Mechanism Study. J. Polym. Environ. 2020, 28, 1095–1105. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Cai, J.; Wilson, K.; Lee, A.F. Bio/hydrochar Sorbents for Environmental Remediation. Energy Environ. Mater. 2020, 3, 453–468. [Google Scholar] [CrossRef]

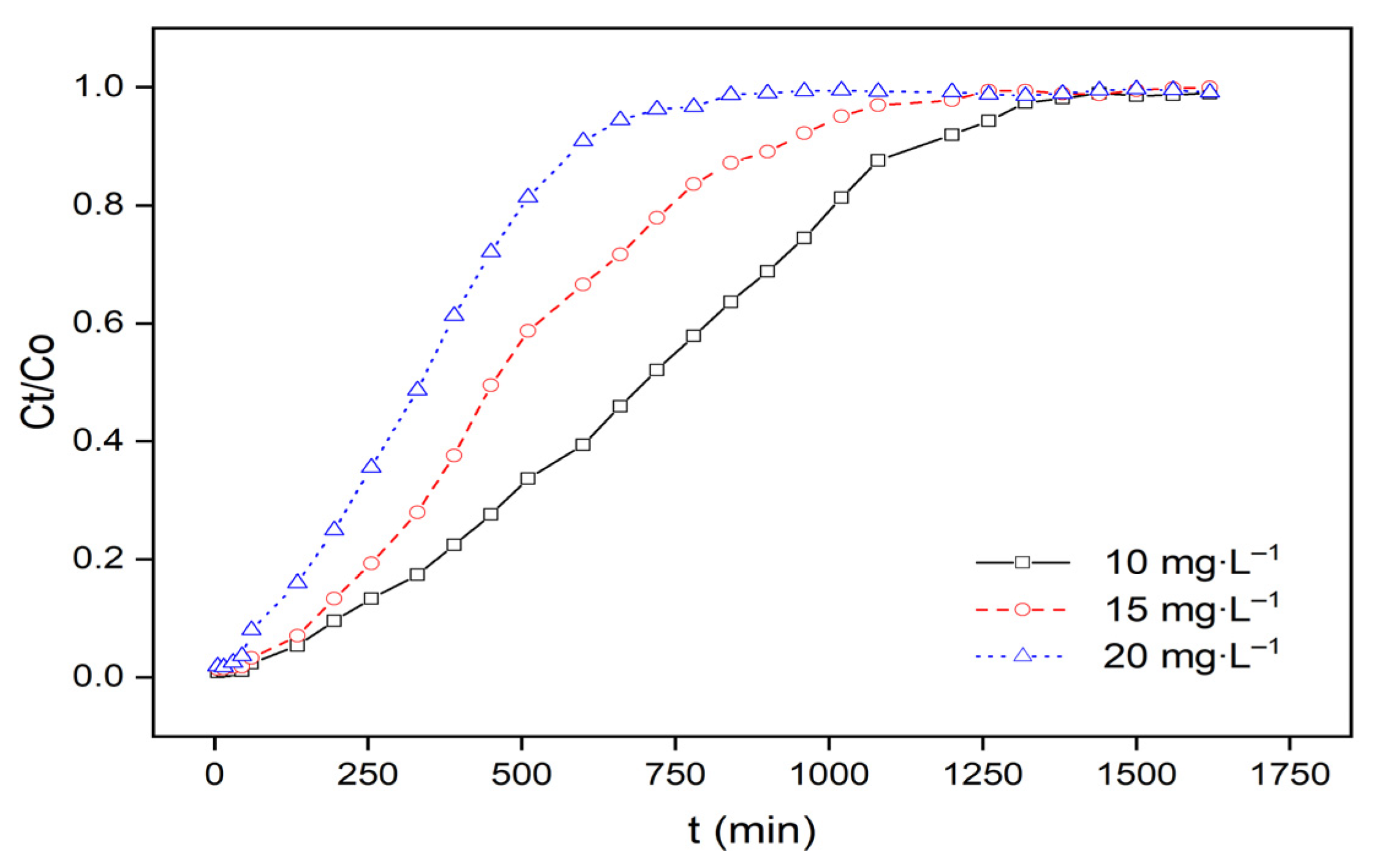
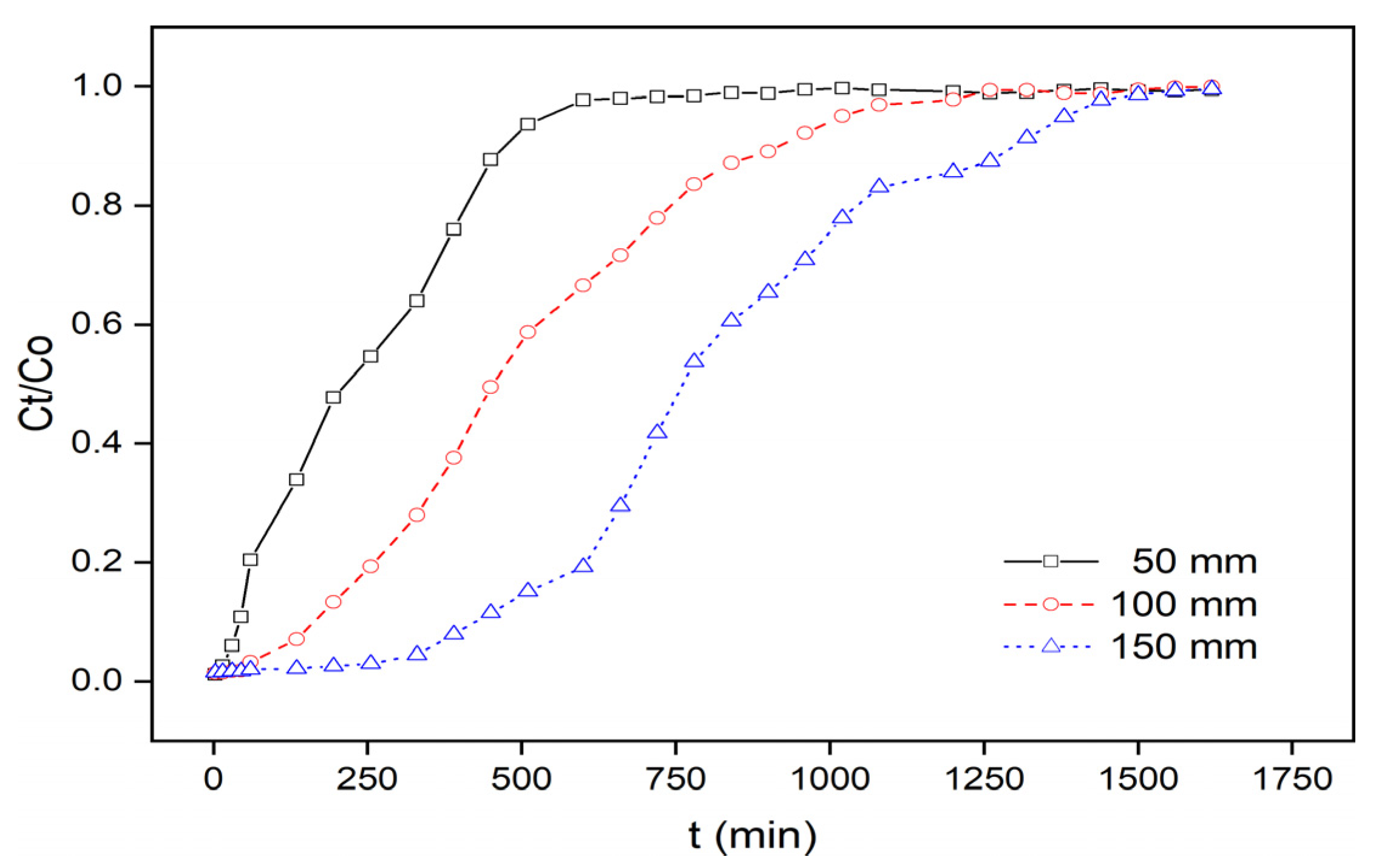

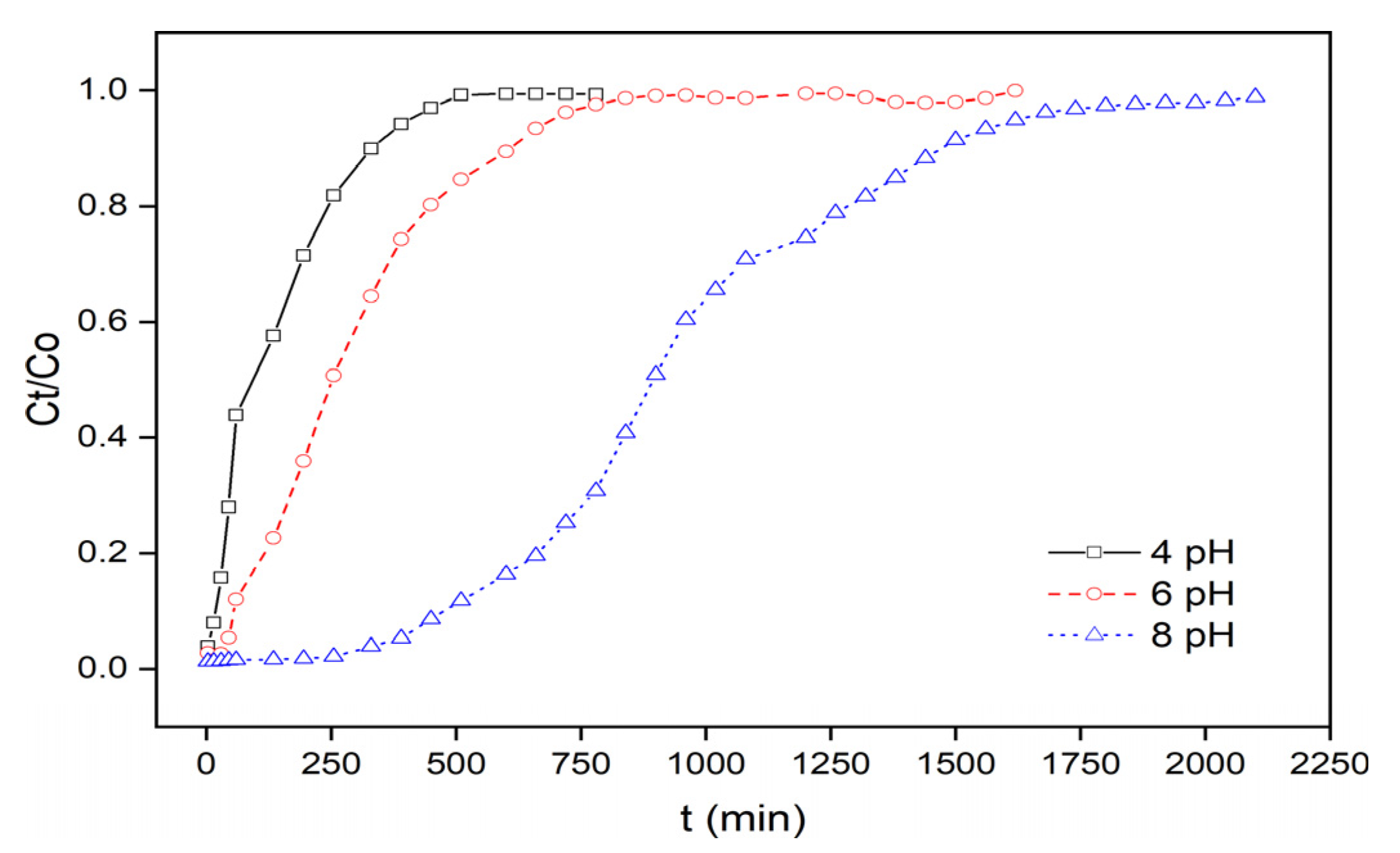

| Inlet Concentration (mg·L−1) | ACIP Bed Height (mm) | Flow Rate (mL·min−1) | pH | qtotal (mg) | qeq (mg·g−1) | veff (mL) | ttotal (min) |
|---|---|---|---|---|---|---|---|
| 10 | 100 | 8 | 6 | 54.89 | 10.98 | 11,040 | 1380 |
| 15 | 100 | 8 | 6 | 59.78 | 11.96 | 9600 | 1200 |
| 20 | 100 | 8 | 6 | 63.87 | 12.77 | 7200 | 900 |
| 15 | 50 | 8 | 6 | 28.82 | 11.53 | 5280 | 660 |
| 15 | 150 | 8 | 6 | 96.47 | 12.86 | 12,000 | 1500 |
| 15 | 100 | 6 | 6 | 77.09 | 15.42 | 9360 | 1560 |
| 15 | 100 | 10 | 6 | 52.31 | 10.46 | 9000 | 900 |
| 15 | 100 | 10 | 4 | 20.25 | 4.05 | 5400 | 540 |
| 15 | 100 | 10 | 8 | 141.27 | 28.25 | 20,400 | 2040 |
| Feed Concentration (mg·L−1) | ACIP Bed Height (mm) | Inlet Flow Rate (mL·min−1) | pH | kAB (L-mg−1 min−1) × 10−4 | N0 (mg L−1) | R2 |
|---|---|---|---|---|---|---|
| 10 | 100 | 8 | 6 | 3.2612 | 694.1377 | 0.8140 |
| 15 | 100 | 8 | 6 | 2.4456 | 857.9272 | 0.7974 |
| 20 | 100 | 8 | 6 | 2.4417 | 900.3202 | 0.8376 |
| 15 | 50 | 8 | 6 | 3.5092 | 970.7570 | 0.6899 |
| 15 | 150 | 8 | 6 | 2.1195 | 827.9164 | 0.9146 |
| 15 | 100 | 6 | 6 | 2.1181 | 894.1829 | 0.9089 |
| 15 | 100 | 10 | 6 | 2.9093 | 830.5218 | 0.7970 |
| 15 | 100 | 10 | 4 | 3.1910 | 463.9978 | 0.6470 |
| 15 | 100 | 10 | 8 | 1.5781 | 1893.7349 | 0.854 |
| Feed Concentration (mg·L−1) | ACIP Bed Height (mm) | Inlet Flow Rate (mL·min−1) | pH | kTh (L-mg−1 min−1) × 10−4 | qTh (mg L−1) | R2 |
|---|---|---|---|---|---|---|
| 10 | 100 | 8 | 6 | 5.6381 | 11.3896 | 0.9706 |
| 15 | 100 | 8 | 6 | 4.4688 | 13.1442 | 0.9711 |
| 20 | 100 | 8 | 6 | 4.6365 | 13.5278 | 0.9879 |
| 15 | 50 | 8 | 6 | 7.3921 | 12.8581 | 0.9383 |
| 15 | 150 | 8 | 6 | 3.6353 | 13.8249 | 0.9893 |
| 15 | 100 | 6 | 6 | 3.2857 | 15.9385 | 0.9843 |
| 15 | 100 | 10 | 6 | 5.8508 | 11.6545 | 0.9771 |
| 15 | 100 | 10 | 4 | 8.6588 | 4.6352 | 0.9417 |
| 15 | 100 | 10 | 8 | 3.0400 | 29.2860 | 0.9941 |
| Feed Concentration (mg·L−1) | ACIP Bed Height (mm) | Inlet Flow Rate (mL·min−1) | pH | KYN (min−1) × 10−3 | τ (min) | R2 |
|---|---|---|---|---|---|---|
| 10 | 100 | 8 | 6 | 5.6381 | 711.8503 | 0.9706 |
| 15 | 100 | 8 | 6 | 6.7032 | 547.6747 | 0.9711 |
| 20 | 100 | 8 | 6 | 9.2729 | 422.7436 | 0.9879 |
| 15 | 50 | 8 | 6 | 11.0880 | 267.8778 | 0.9383 |
| 15 | 150 | 8 | 6 | 5.4529 | 813.2294 | 0.9893 |
| 15 | 100 | 6 | 6 | 4.9285 | 885.4724 | 0.9843 |
| 15 | 100 | 10 | 6 | 8.7761 | 388.4836 | 0.9771 |
| 15 | 100 | 10 | 4 | 12.9880 | 154.5078 | 0.9417 |
| 15 | 100 | 10 | 8 | 4.5600 | 976.1989 | 0.9941 |
| Adsorbent | Cycles | Eluent | Removal Efficiency | Reference |
|---|---|---|---|---|
| Pyrolytic tire char | 3 | 0.1 M HNO3 | 64% | [14] |
| Monolithic starch cryogel | 3 | Ethanol | 62% | [71] |
| Alginate–water hyacinth beads | 3 | 0.1 M HNO3 | 81% | [73] |
| Polyaniline/Tectona grandis sawdust | 4 | 0.1 M HCl | <50% | [67] |
| Chitosan–clay composite | 5 | Distilled water | >50% | [74] |
| ACIP | 5 | 0.1 M HCl | 86% | This study |
| Adsorbents | qeq | Reference |
|---|---|---|
| Banana peel bioadsorbent | 22.11 | [75] |
| Pyrolytic tire char | 3.85 | [14] |
| Zeolite | 4.36 | [61] |
| Coal-based commercial activated carbon | 12.06 | [72] |
| Poly(acrylonitrile-co-acrylic acid)-modified thiourea | 28.51 | [69] |
| Unmodified PUF | 4.00 | [16] |
| ACIP | 25.25 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada, R.J.R.; Tomon, T.R.B.; Fernandez, R.M.D.; Omisol, C.J.M.; Dumancas, G.G.; Alguno, A.C.; Ramos, M.S.K.; Malaluan, R.M.; Lubguban, A.A. Sequestration of Methylene Blue Dye in a Fixed-Bed Column Using Activated Carbon-Infused Polyurethane Composite Adsorbent Derived from Coconut Oil. Sustainability 2024, 16, 10757. https://doi.org/10.3390/su162310757
Estrada RJR, Tomon TRB, Fernandez RMD, Omisol CJM, Dumancas GG, Alguno AC, Ramos MSK, Malaluan RM, Lubguban AA. Sequestration of Methylene Blue Dye in a Fixed-Bed Column Using Activated Carbon-Infused Polyurethane Composite Adsorbent Derived from Coconut Oil. Sustainability. 2024; 16(23):10757. https://doi.org/10.3390/su162310757
Chicago/Turabian StyleEstrada, Renz John R., Tomas Ralph B. Tomon, Rubie Mae D. Fernandez, Christine Joy M. Omisol, Gerard G. Dumancas, Arnold C. Alguno, Maria Sheila K. Ramos, Roberto M. Malaluan, and Arnold A. Lubguban. 2024. "Sequestration of Methylene Blue Dye in a Fixed-Bed Column Using Activated Carbon-Infused Polyurethane Composite Adsorbent Derived from Coconut Oil" Sustainability 16, no. 23: 10757. https://doi.org/10.3390/su162310757
APA StyleEstrada, R. J. R., Tomon, T. R. B., Fernandez, R. M. D., Omisol, C. J. M., Dumancas, G. G., Alguno, A. C., Ramos, M. S. K., Malaluan, R. M., & Lubguban, A. A. (2024). Sequestration of Methylene Blue Dye in a Fixed-Bed Column Using Activated Carbon-Infused Polyurethane Composite Adsorbent Derived from Coconut Oil. Sustainability, 16(23), 10757. https://doi.org/10.3390/su162310757









