Birds as Cultural Ambassadors: Bridging Ecosystem Services and Biodiversity Conservation in Wetland Planning
Abstract
1. Introduction
2. Materials and Methods
2.1. Strategic Approach
2.2. Study Area
2.3. Land Cover Mapping
2.4. Wetland Biodiversity Assesment Through a Bird Survey
2.5. Applied Statistical Procedures on Bird Communities
2.6. Social Survey of Wetland Visitors
- The first section collects general data to outline the visitor’s profile (socio-demographic characteristics, such as gender, age, formal education, occupation, place of residence and awareness of being in a Nature 2000 Network site).
- The second section examines opinions and preferences about visiting the wetland. Respondents were asked to select the best reasons for visiting the wetland from a list of six pre-defined options, with a final option for ‘other reasons’. These options were identified by considering the main purposes of protected areas as defined by the Italian framework law (L. 394/91) [53] that may be relevant for visitors: biodiversity and landscape conservation, integration between man and the natural environment, education, and recreation.
- The third section of the questionnaire explores visitor perceptions of the key ESs provided by the study area. Respondents were asked to indicate their perceptions of the three most important ESs provided by the King’s Lagoon wetland.
3. Results
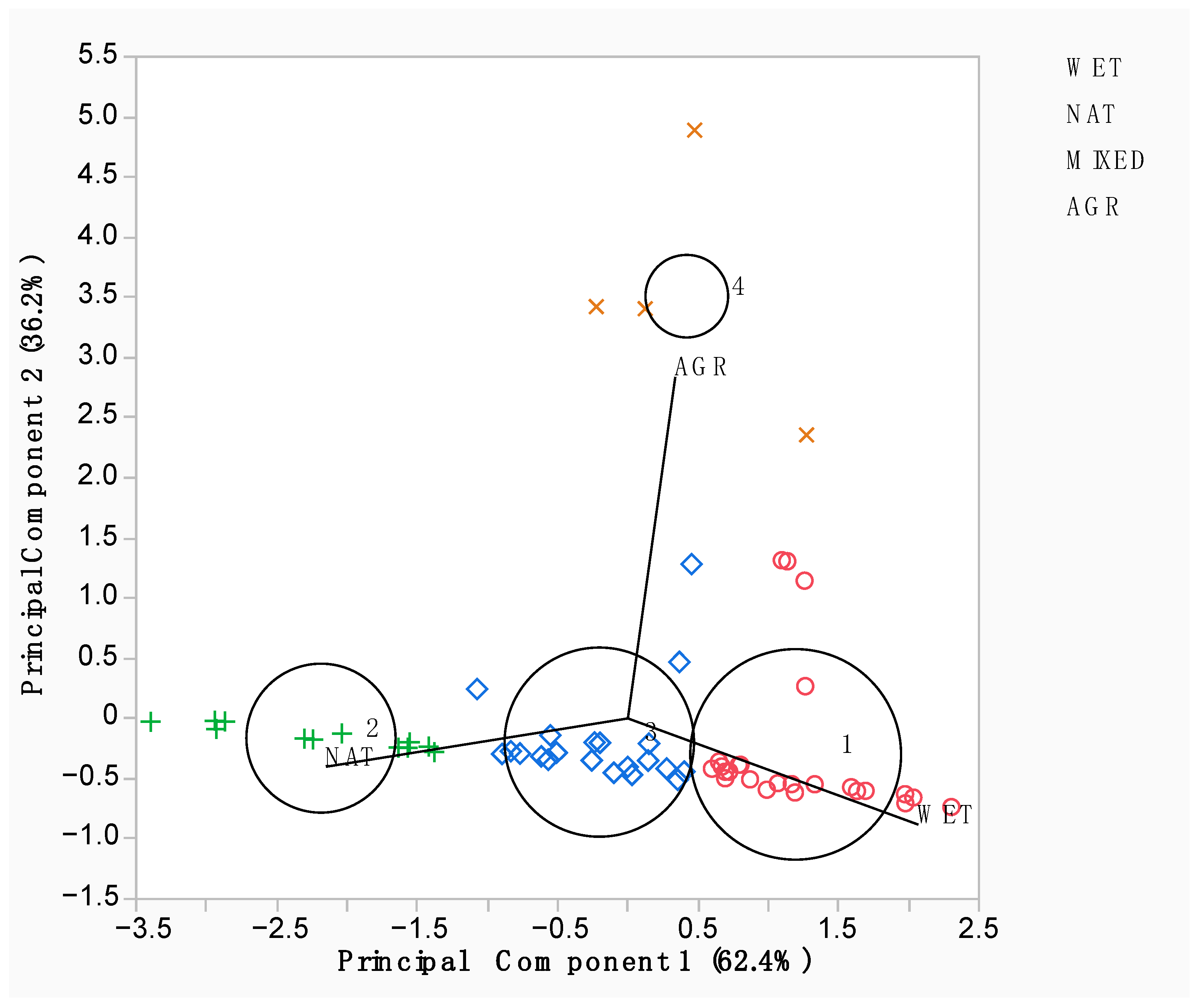
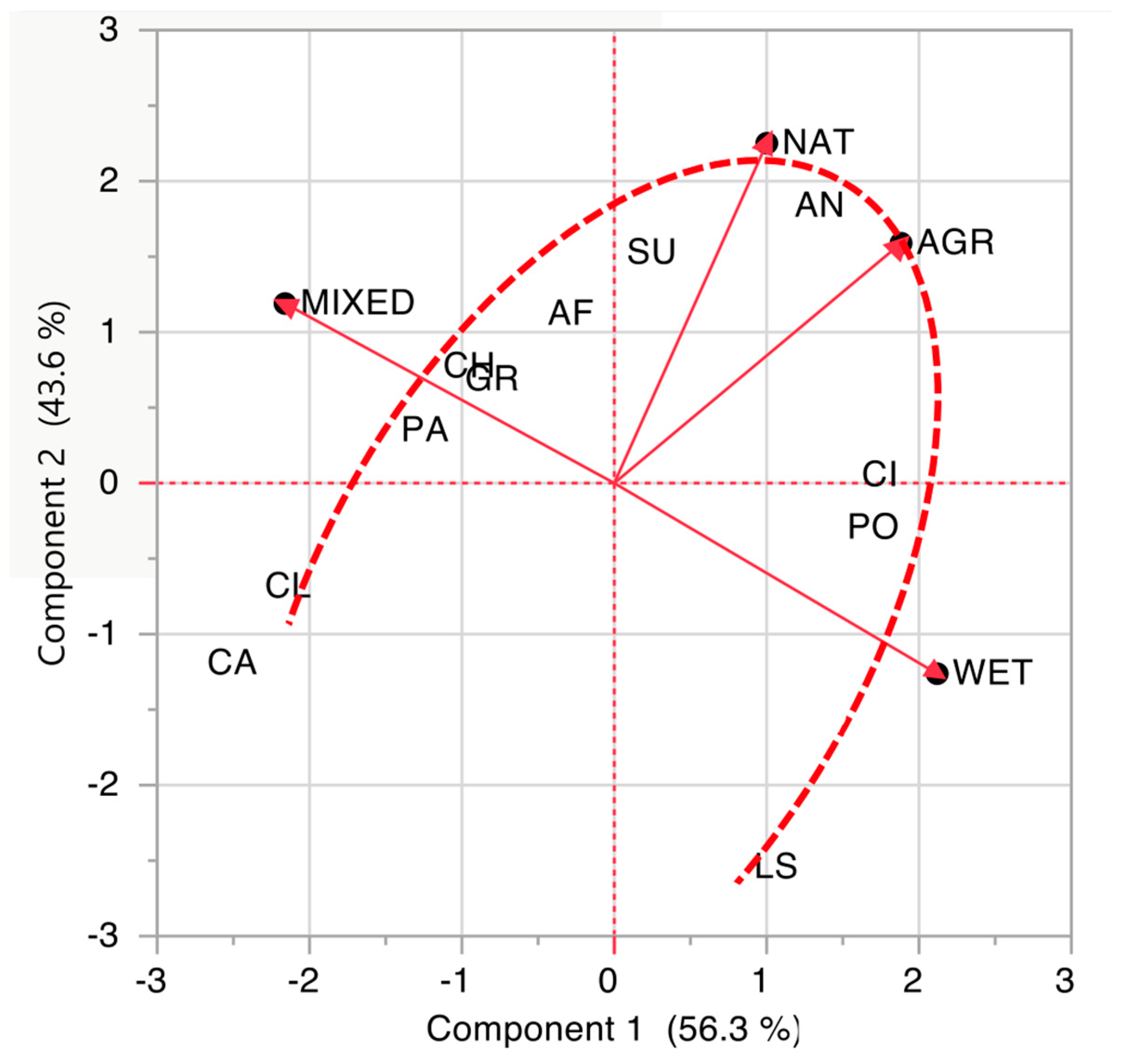
3.1. Wetland Bird Community as a Whole
3.2. Visitor Identification, Preferences and Orientations
4. Discussion
4.1. Suitability of the Bird Counting Method Used in the Survey
4.2. Bird Community and Biotopes: The Importance of Ecotones and Flagship Species Selection
4.3. Interpretation of Visitor Survey Results
4.4. Conservation and Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anthony, A.; Atwood, J.; August, P.; Byron, C.; Cobb, S.; Foster, C.; Fry, C.; Gold, A.; Hagos, K.; Heffner, L.; et al. Coastal Lagoons and Climate Change: Ecological and Social Ramifications in U.S. Atlantic and Gulf Coast Ecosystems. Ecol. Soc. 2009, 14, art8. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment; Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005.
- Pastur, G.M.; Peri, P.L.; Lencinas, M.V.; García-Llorente, M.; Martín-López, B. Spatial Patterns of Cultural Ecosystem Services Provision in Southern Patagonia. Landsc. Ecol. 2016, 31, 383–399. [Google Scholar] [CrossRef]
- Mao, Q.; Hu, C.; Guo, Q.; Li, Y.; Liu, M. How Does Vegetation Landscape Structure of Urban Green Spaces Affect Cultural Ecosystem Services at Multiscale: Based on PLS-SEM Model. Forests 2023, 14, 1401. [Google Scholar] [CrossRef]
- Kennish, M.; Paerl, H. Coastal Lagoons: Critical Habitats of Environmental Change. In Coastal Lagoons; Kennish, M., Paerl, H., Eds.; Marine Science; CRC Press: Boca Raton, FL, USA, 2010; Volume 20103358, pp. 1–15. ISBN 978-1-4200-8830-4. [Google Scholar]
- Sánchez, B.; Rodríguez, D. Avifauna Associated with the Aquatic and Coastal Ecosystems of Cayo Coco, Cuba. J. Caribb. Ornithol. 2000, 13, 68–75. [Google Scholar]
- Kularatne, R.K.A.; Harris, J.M.; Vinobaba, P.; Thanusanth, S.; Kishoran, S.; Kankanamge, C.E. Use of Habitats by Aquatic and Terrestrial Avifauna in Tropical Coastal Lagoons. Reg. Stud. Mar. Sci. 2021, 47, 101926. [Google Scholar] [CrossRef]
- Whelan, C.J.; Wenny, D.G.; Marquis, R.J. Ecosystem Services Provided by Birds. Ann. N. Y. Acad. Sci. 2008, 1134, 25–60. [Google Scholar] [CrossRef]
- Santos, F.C.V.; Nascimento, M.S.; Braga, S.S.; Guzi, A. O Potencial Do Birdwatching Na Área de Proteção Ambiental Do Delta Do Parnaíba (Piauí, Brasil). Rev. Bras. Ecoturismo 2019, 12, 854–865. [Google Scholar] [CrossRef]
- Gregory, R.D.; Strien, A.V. Wild Bird Indicators: Using Composite Population Trends of Birds as Measures of Environmental Health. Ornithol. Sci. 2010, 9, 3–22. [Google Scholar] [CrossRef]
- Sundar, K.S.G.; Kittur, S. Can Wetlands Maintained for Human Use Also Help Conserve Biodiversity? Landscape-Scale Patterns of Bird Use of Wetlands in an Agricultural Landscape in North India. Biol. Conserv. 2013, 168, 49–56. [Google Scholar] [CrossRef]
- Graves, R.A.; Pearson, S.M.; Turner, M.G. Effects of Bird Community Dynamics on the Seasonal Distribution of Cultural Ecosystem Services. Ambio 2019, 48, 280–292. [Google Scholar] [CrossRef]
- Rodrigues-Filho, J.L.; Macêdo, R.L.; Sarmento, H.; Pimenta, V.R.A.; Alonso, C.; Teixeira, C.R.; Pagliosa, P.R.; Netto, S.A.; Santos, N.C.L.; Daura-Jorge, F.G.; et al. From Ecological Functions to Ecosystem Services: Linking Coastal Lagoons Biodiversity with Human Well-Being. Hydrobiologia 2023, 850, 2611–2653. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Demissew, S.; Carabias, J.; Joly, C.; Lonsdale, M.; Ash, N.; Larigauderie, A.; Adhikari, J.R.; Arico, S.; Báldi, A.; et al. The IPBES Conceptual Framework—Connecting Nature and People. Curr. Opin. Environ. Sustain. 2015, 14, 1–16. [Google Scholar] [CrossRef]
- Ebner, M.; Schirpke, U.; Tappeiner, U. Combining Multiple Socio-Cultural Approaches—Deeper Insights into Cultural Ecosystem Services of Mountain Lakes? Landsc. Urban Plan. 2022, 228, 104549. [Google Scholar] [CrossRef]
- Sherrouse, B.C.; Semmens, D.J.; Ancona, Z.H.; Brunner, N.M. Analyzing Land-Use Change Scenarios for Trade-Offs among Cultural Ecosystem Services in the Southern Rocky Mountains. Ecosyst. Serv. 2017, 26, 431–444. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P. Valuation of the Ecosystem Services: A Psycho-Cultural Perspective. Ecol. Econ. 2008, 64, 808–819. [Google Scholar] [CrossRef]
- Martín-López, B.; Gómez-Baggethun, E.; García-Llorente, M.; Montes, C. Trade-Offs across Value-Domains in Ecosystem Services Assessment. Ecol. Indic. 2014, 37, 220–228. [Google Scholar] [CrossRef]
- Perman, R. (Ed.) Natural Resource and Environmental Economics, 3rd ed.; Pearson Addison Wesley: Harlow, UK; Munich, Germany, 2003. [Google Scholar]
- Nesbitt, L.; Adamczewski, J. Decline and Recovery of the Bathurst Caribou Herd: Workshops Held in Yellowknife, NWT; Government of NWT: Yellowknife, NWT, Canada, 2009. [Google Scholar]
- Brambilla, M.; Ilahiane, L.; Assandri, G.; Ronchi, S.; Bogliani, G. Combining Habitat Requirements of Endemic Bird Species and Other Ecosystem Services May Synergistically Enhance Conservation Efforts. Sci. Total Environ. 2017, 586, 206–214. [Google Scholar] [CrossRef]
- Zhou, L.; Guan, D.; Huang, X.; Yuan, X.; Zhang, M. Evaluation of the Cultural Ecosystem Services of Wetland Park. Ecol. Indic. 2020, 114, 106286. [Google Scholar] [CrossRef]
- Hale, R.L.; Cook, E.M.; Beltrán, B.J. Cultural Ecosystem Services Provided by Rivers across Diverse Social-Ecological Landscapes: A Social Media Analysis. Ecol. Indic. 2019, 107, 105580. [Google Scholar] [CrossRef]
- Sinclair, M.; Mayer, M.; Woltering, M.; Ghermandi, A. Using Social Media to Estimate Visitor Provenance and Patterns of Recreation in Germany’s National Parks. J. Environ. Manag. 2020, 263, 110418. [Google Scholar] [CrossRef]
- Sinclair, M.; Ghermandi, A.; Signorello, G.; Giuffrida, L.; De Salvo, M. Valuing Recreation in Italy’s Protected Areas Using Spatial Big Data. Ecol. Econ. 2022, 200, 107526. [Google Scholar] [CrossRef]
- Brown, G.; Fagerholm, N. Empirical PPGIS/PGIS Mapping of Ecosystem Services: A Review and Evaluation. Ecosyst. Serv. 2015, 13, 119–133. [Google Scholar] [CrossRef]
- Ondiek, R.A.; Kitaka, N.; Oduor, S.O. Assessment of Provisioning and Cultural Ecosystem Services in Natural Wetlands and Rice Fields in Kano Floodplain, Kenya. Ecosyst. Serv. 2016, 21, 166–173. [Google Scholar] [CrossRef]
- Johansson, M.; Pedersen, E.; Weisner, S. Assessing Cultural Ecosystem Services as Individuals’ Place-Based Appraisals. Urban For. Urban Green. 2019, 39, 79–88. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Tudor, M.; Doroftei, M.; Covaliov, S.; Năstase, A.; Onără, D.-F.; Mierlă, M.; Marinov, M.; Doroșencu, A.-C.; Lupu, G.; et al. Changes in Ecosystem Services from Wetland Loss and Restoration: An Ecosystem Assessment of the Danube Delta (1960–2010). Ecosyst. Serv. 2019, 39, 100965. [Google Scholar] [CrossRef]
- Satz, D.; Gould, R.K.; Chan, K.M.A.; Guerry, A.; Norton, B.; Satterfield, T.; Halpern, B.S.; Levine, J.; Woodside, U.; Hannahs, N.; et al. The Challenges of Incorporating Cultural Ecosystem Services into Environmental Assessment. AMBIO 2013, 42, 675–684. [Google Scholar] [CrossRef]
- Lavorel, S.; Bayer, A.; Bondeau, A.; Lautenbach, S.; Ruiz-Frau, A.; Schulp, N.; Seppelt, R.; Verburg, P.; Teeffelen, A.V.; Vannier, C.; et al. Pathways to Bridge the Biophysical Realism Gap in Ecosystem Services Mapping Approaches. Ecol. Indic. 2017, 74, 241–260. [Google Scholar] [CrossRef]
- Kremen, C. Managing Ecosystem Services: What Do We Need to Know about Their Ecology? Ecol. Lett. 2005, 8, 468–479. [Google Scholar] [CrossRef]
- Cumming, G.S.; Maciejewski, K. Reconciling Community Ecology and Ecosystem Services: Cultural Services and Benefits from Birds in South African National Parks. Ecosyst. Serv. 2017, 28, 219–227. [Google Scholar] [CrossRef]
- Booth, J.E.; Gaston, K.J.; Evans, K.L.; Armsworth, P.R. The Value of Species Rarity in Biodiversity Recreation: A Birdwatching Example. Biol. Conserv. 2011, 144, 2728–2732. [Google Scholar] [CrossRef]
- Villamagna, A.M.; Angermeier, P.L.; Niazi, N. Evaluating Opportunities to Enhance Ecosystem Services in Public Use Areas. Ecosyst. Serv. 2014, 7, 167–176. [Google Scholar] [CrossRef]
- Paracchini, M.L.; Zulian, G.; Kopperoinen, L.; Maes, J.; Schägner, J.P.; Termansen, M.; Zandersen, M.; Perez-Soba, M.; Scholefield, P.A.; Bidoglio, G. Mapping Cultural Ecosystem Services: A Framework to Assess the Potential for Outdoor Recreation across the EU. Ecol. Indic. 2014, 45, 371–385. [Google Scholar] [CrossRef]
- Assandri, G.; Bogliani, G.; Pedrini, P.; Brambilla, M. Beautiful Agricultural Landscapes Promote Cultural Ecosystem Services and Biodiversity Conservation. Agric. Ecosyst. Environ. 2018, 256, 200–210. [Google Scholar] [CrossRef]
- Cammerino, A.R.B.; Ingaramo, M.; Monteleone, M. Complementary Approaches to Planning a Restored Coastal Wetland and Assessing the Role of Agriculture and Biodiversity: An Applied Case Study in Southern Italy. Water 2023, 16, 153. [Google Scholar] [CrossRef]
- Müller, S.M.; Peisker, J.; Bieling, C.; Linnemann, K.; Reidl, K.; Schmieder, K. The Importance of Cultural Ecosystem Services and Biodiversity for Landscape Visitors in the Biosphere Reserve Swabian Alb (Germany). Sustainability 2019, 11, 2650. [Google Scholar] [CrossRef]
- Gosal, A.S.; Newton, A.C.; Gillingham, P.K. Comparison of Methods for a Landscape-Scale Assessment of the Cultural Ecosystem Services Associated with Different Habitats. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2018, 14, 91–104. [Google Scholar] [CrossRef]
- Whitehead, A.L.; Kujala, H.; Ives, C.D.; Gordon, A.; Lentini, P.E.; Wintle, B.A.; Nicholson, E.; Raymond, C.M. Integrating Biological and Social Values When Prioritizing Places for Biodiversity Conservation. Conserv. Biol. 2014, 28, 992–1003. [Google Scholar] [CrossRef]
- Plieninger, T.; Bieling, C.; Fagerholm, N.; Byg, A.; Hartel, T.; Hurley, P.; López-Santiago, C.A.; Nagabhatla, N.; Oteros-Rozas, E.; Raymond, C.M.; et al. The Role of Cultural Ecosystem Services in Landscape Management and Planning. Curr. Opin. Environ. Sustain. 2015, 14, 28–33. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wu, Y.; Zhang, W.; Yang, X.; Lv, H.; Xia, S.; Zhao, S.; Tian, J.; Cui, P.; et al. Selection of Flagship Species and Their Use as Umbrellas in Bird Conservation: A Case Study in Lishui, Zhejiang Province, China. Animals 2023, 13, 1825. [Google Scholar] [CrossRef]
- Polgar, G.; Jaafar, Z. Flagship Species. In Endangered Forested Wetlands of Sundaland; Springer International Publishing: Cham, Switzerland, 2018; pp. 57–88. [Google Scholar]
- LIFE+09 NAT/IT/000150 Layman’s Report. Available online: https://pugliacon.regione.puglia.it/web/sit-puglia-paesaggio/-/rapporto-finale-progetto-life-interventi-di-conservazione-degli-habitat-delle-zone-umide-costiere-nel-sic-zone-umide-della-capitanata- (accessed on 21 September 2024).
- Cammerino, A.R.B.; Piacquadio, L.; Ingaramo, M.; Gioiosa, M.; Monteleone, M. Wild Edible Plant Species in the ‘King’s Lagoon’ Coastal Wetland: Survey, Collection, Mapping and Ecological Characterization. Horticulturae 2024, 10, 632. [Google Scholar] [CrossRef]
- Dylewski, Ł.; Tobolka, M. Unused Railway Lines as a Contributor to Bird Abundance, Species Richness and Diversity in Intensively Managed Farmland. Agric. Ecosyst. Environ. 2022, 326, 107820. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Whittaker, R.H. Dominance and Diversity in Land Plant Communities: Numerical Relations of Species Express the Importance of Competition in Community Function and Evolution. Science 1965, 147, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Peet, R.K. The Measurement of Species Diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A Tribute to Claude Shannon (1916–2001) and a Plea for More Rigorous Use of Species Richness, Species Diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale. LEGGE 6 Dicembre 1991, n. 394 Legge Quadro Sulle Aree Protette; GU Serie Generale n.292 Del 13-12-1991—Supplemento Ordinario n. 83; Gazzetta Ufficiale: Rome, Italy, 1991. [Google Scholar]
- Pascual, U.; Balvanera, P.; Anderson, C.B.; Chaplin-Kramer, R.; Christie, M.; González-Jiménez, D.; Martin, A.; Raymond, C.M.; Termansen, M.; Vatn, A.; et al. Diverse Values of Nature for Sustainability. Nature 2023, 620, 813–823. [Google Scholar] [CrossRef]
- Van Oudenhoven, A.P.E.; Petz, K.; Alkemade, R.; Hein, L.; De Groot, R.S. Framework for Systematic Indicator Selection to Assess Effects of Land Management on Ecosystem Services. Ecol. Indic. 2012, 21, 110–122. [Google Scholar] [CrossRef]
- Shoyama, K.; Yamagata, Y. Local Perception of Ecosystem Service Bundles in the Kushiro Watershed, Northern Japan—Application of a Public Participation GIS Tool. Ecosyst. Serv. 2016, 22, 139–149. [Google Scholar] [CrossRef]
- Hausner, V.H.; Brown, G.; Lægreid, E. Effects of Land Tenure and Protected Areas on Ecosystem Services and Land Use Preferences in Norway. Land Use Policy 2015, 49, 446–461. [Google Scholar] [CrossRef]
- Karimi, A.; Raymond, C.M. Assessing the Diversity and Evenness of Ecosystem Services as Perceived by Residents Using Participatory Mapping. Appl. Geogr. 2022, 138, 102624. [Google Scholar] [CrossRef]
- Haines-Young, R.; Potschin-Young, M.; Czúcz, B. Report on the Use of CICES to Identify and Characterise the Biophysical, Social and Monetary Dimensions of ES Assessments; Deliverable D4.2, EU Horizon 2020 ESMERALDA Project, Grant Agreement No. 642007; European Commision: Brussel, Belgium, 2018. [Google Scholar]
- Kobori, H.; Dickinson, J.L.; Washitani, I.; Sakurai, R.; Amano, T.; Komatsu, N.; Kitamura, W.; Takagawa, S.; Koyama, K.; Ogawara, T.; et al. Citizen Science: A New Approach to Advance Ecology, Education, and Conservation. Ecol. Res. 2016, 31, 1–19. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Newton, I.; Green, R.E. Bird Ecology and Conservation: A Handbook of Techniques; Techniques in Ecology and Conservation Series; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Fletcher, R.J.; Dhundale, J.A.; Dean, T.F. Estimating non-breeding season bird abundance in prairies: A comparison of two survey techniques. J. Field Ornithol. 2000, 71, 321–329. [Google Scholar] [CrossRef]
- Diefenbach, D.R.; Brauning, D.W.; Mattice, J.A. Variability in grassland bird counts related to observer differences and species detection rates. Auk 2003, 120, 1168. [Google Scholar] [CrossRef]
- Silveira, L.; Jácomo, A.T.A.; Diniz-Filho, J.A.F. Camera Trap, Line Transect Census and Track Surveys: A Comparative Evaluation. Biol. Conserv. 2003, 114, 351–355. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S. Bird Census Techniques, 2nd ed.; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Tinarelli, R.; Marchesi, F. Inserto: Zone Umide—Sono Molte Le Aziende Che Tutelano La Natura. Biol. Ambient. 1996, 5, 11–12. [Google Scholar]
- Kark, S. Effects of Ecotones on Biodiversity. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–10. ISBN 978-0-12-226865-6. [Google Scholar]
- Schlacher, T.A.; Gilby, B.L.; Olds, A.D.; Henderson, C.J.; Connolly, R.M.; Peterson, C.H.; Voss, C.M.; Maslo, B.; Weston, M.A.; Bishop, M.J.; et al. Key Ecological Function Peaks at the Land–Ocean Transition Zone When Vertebrate Scavengers Concentrate on Ocean Beaches. Ecosystems 2020, 23, 906–916. [Google Scholar] [CrossRef]
- Holland, M.M.; Risser, P.G.; Naiman, R.J. (Eds.) Ecotones; Springer: Boston, MA, USA, 1991; ISBN 978-1-4615-9688-2. [Google Scholar]
- Platteeuw, M.; Foppen, R.P.B.; Van Eerden, M.R. The Need for Future Wetland Bird Studies: Scales of Habitat Use as Input for Ecological Restoration and Spatial Water Management. Ardea 2010, 98, 403–416. [Google Scholar] [CrossRef]
- Mulamoottil, G.; Warner, B.G.; McBean, E.A. Wetlands: Environmental Gradients, Boundaries, and Buffers; Lewis Publishers: Boca Raton, FL, USA, 1996; ISBN 978-0-203-73388-2. [Google Scholar]
- Kushlan, J.A.; Hancock, J.A. Herons: Bird Families of the World; Oxford University Press: Oxford, UK, 2005; ISBN 978-0-19-854981-9. [Google Scholar]
- O’Donnell, C.F.J.; Fjeldså, J. Grebes: Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 1997; ISBN 978-2-8317-0421-0. [Google Scholar]
- Fjeldså, J. The Grebes: Podicipedidae (Bird Families of the World); Oxford University Press: Oxford, UK, 2004; ISBN 978-0-19-850064-3. [Google Scholar]
- Schreiber, E.A.; Burger, J. (Eds.) Biology of Marine Birds; CRC marine biology series; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-8493-9882-7. [Google Scholar]
- Simberloff, D. Flagships, Umbrellas, and Keystones: Is Single-Species Management Passé in the Landscape Era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Ogden, J.C.; Baldwin, J.D.; Bass, O.L.; Browder, J.A.; Cook, M.I.; Frederick, P.C.; Frezza, P.E.; Galvez, R.A.; Hodgson, A.B.; Meyer, K.D.; et al. Waterbirds as Indicators of Ecosystem Health in the Coastal Marine Habitats of Southern Florida: 1. Selection and Justification for a Suite of Indicator Species. Ecol. Indic. 2014, 44, 148–163. [Google Scholar] [CrossRef]
- Jepson, P.; Barua, M. A Theory of Flagship Species Action. Conserv. Soc. 2015, 13, 95. [Google Scholar] [CrossRef]
- Xie, Y.; Zou, J.; Chen, Y.; Li, F.; Jiang, Q. Are Wading Birds the Ideal Focal Species for Broader Bird Conservation? A Cost-Effective Approach to Ecological Network Planning. Ecol. Indic. 2024, 160, 111785. [Google Scholar] [CrossRef]
- Luoni, F.; Morganti, M. Report Workshop LIFE14 IPE/IT/000018—26 May 2017—Le Aree Umide per Gli Uccelli in Lombardia: Di Cosa Hanno Bisogno? Raccolta di Suggerimenti e Buone Pratiche; University of Pavia: Pavia, Italy, 2017. [Google Scholar]
- Morganti, M.; Manica, M.; Bogliani, G.; Gustin, M.; Luoni, F.; Trotti, P.; Perin, V.; Brambilla, M. Multi-Species Habitat Models Highlight the Key Importance of Flooded Reedbeds for Inland Wetland Birds: Implications for Management and Conservation. Avian Res. 2019, 10, 15. [Google Scholar] [CrossRef]
- Trasforini, S.; Clerici, S.; Bendotti, R.; Romanò, A.; Barenghi, B.; Ioculano, D.; Bucchini, A.; Porrini, S. Interventi Idraulici Ittiocompatibili: Linee Guida. Quaderni della Ricerca. Regione Lombardia, Italy, 2011. Available online: http://www.graia.eu/wp-content/uploads/2017/07/Interventi-idraulici-ittiocompatibili.pdf (accessed on 21 November 2024).
- White, C.L.; Frederick, P.C.; Main, M.B.; Rodgers, J.A., Jr. Nesting Island Creation for Wading Birds. EDIS 2019, 2005. [Google Scholar] [CrossRef]
- Draulans, D. The Effect of Prey Density on Foraging Behaviour and Success of Adult and First-Year Grey Herons (Ardea Cinerea). J. Anim. Ecol. 1987, 56, 479. [Google Scholar] [CrossRef]

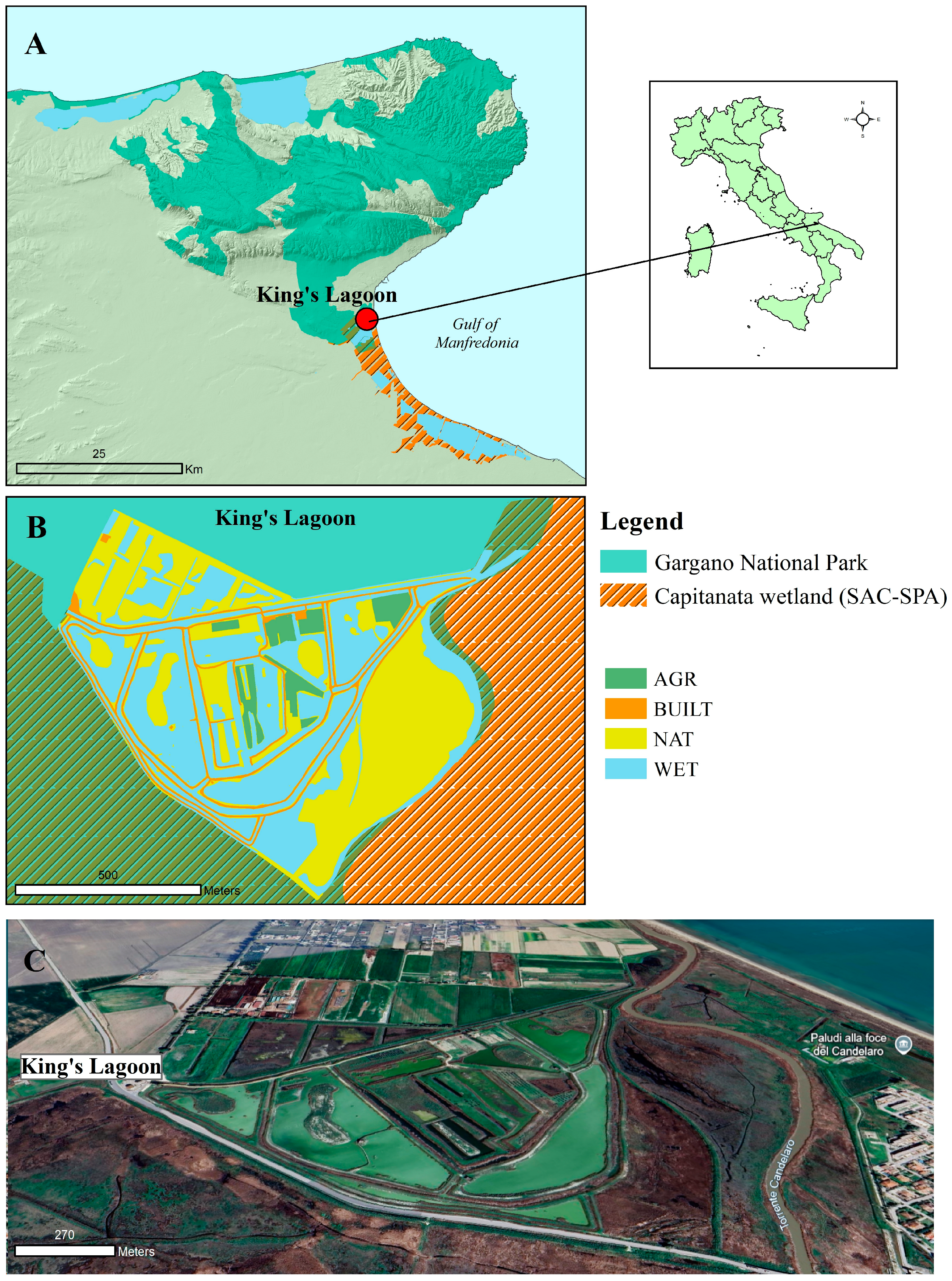



| First-Order Land Cover Classes | Second-Order Land Cover Classes |
|---|---|
| WET Wetlands and aquatic/riparian ecosystems | reeds and reedbeds wetlands and lagoons channels non-permanent channels temporary ponds |
| NAT Semi-natural vegetation areas (meadows) | sparse semi-natural vegetation herbaceous vegetation and scattered trees |
| BUILT Built-up areas | roads and paths rural buildings rural building annexes roof terraces and rural sheds |
| AGR Agricultural areas | arable agricultural land tree-lined and wooded agricultural areas complex cropping and parcel systems, including small orchards and olive groves abandoned olive groves |
| Jan. 2023 | Feb. 2023 | Mar. 2023 | Apr. 2023 | May 2023 | June 2023 | July 2023 | Aug. 2023 | Sept. 2023 | Oct. 2023 | Nov. 2023 | Dec. 2023 | Total Species Abundance | Occurrence (No. of Cells) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ardea alba | 2–3 | 2–3 | = | = | 1 | = | = | = | = | 2–3 | 1 | = | 13 | 7 |
| Ardea cinerea | >50 | 8–20 | 8–20 | 8–20 | 1 | 8–20 | >50 | 21–50 | 8–20 | 8–20 | 8–20 | 221 | 20 | |
| Ardea purpurea | = | = | 1 | 2–3 | 1 | 2–3 | 4–7 | 1 | = | = | 13 | 10 | ||
| Ardeola ralloides | = | = | = | 2–3 | 4–7 | 2–3 | 2–3 | 8–20 | 2–3 | = | = | = | 33 | 16 |
| Botaurus stellaris | 1 | = | = | = | = | = | = | = | = | = | = | = | 1 | 1 |
| Bubulcus ibis | 8–20 | 8–20 | = | = | 8–20 | 8–20 | >50 | 21–50 | = | = | 8–20 | 8–20 | 167 | 20 |
| Egretta garzetta | = | = | 2–3 | 2–3 | 8–20 | 8–20 | 8–20 | 8–20 | 21–50 | 8–20 | 8–20 | 8–20 | 122 | 30 |
| Ixobrychus minutus | = | = | = | = | = | 1 | = | = | = | = | = | = | 1 | 1 |
| Biotope Categories | No. of Cells | WET Land Cover | NAT Land Cover | AGR Land Cover | BUILT Land Cover | |||
|---|---|---|---|---|---|---|---|---|
| WET | 26 | 0.673 | ±0.095 | 0.221 | ±0.065 | 0.023 | ±0.056 | 0.083 ± 0.056 |
| NAT | 13 | 0.196 | ±0.099 | 0.776 | ±0.101 | = | = | 0.028 ± 0.101 |
| MIXED | 21 | 0.459 | ±0.077 | 0.433 | ±0.083 | 0.015 | ±0.040 | 0.094 ± 0.040 |
| AGR | 4 | 0.330 | ±0.123 | 0.228 | ±0.085 | 0.370 | ±0.079 | 0.072 ± 0.085 |
| Bird Taxonomic Order | Code Order | N Species | WET Biotope | NAT Biotope | MIXED Biotope | AGR Biotope | Biotope Assigned |
|---|---|---|---|---|---|---|---|
| Caprimulgiformes and Apodiformes | CA | 3 | 0.017 | 0.007 | 0.973 | 0.002 | MIXED |
| Columbiformes | CL | 3 | 0.026 | 0.012 | 0.958 | 0.004 | MIXED |
| Passeriformes | PA | 34 | 0.066 | 0.020 | 0.907 | 0.008 | MIXED |
| Charadriformes | CH | 12 | 0.071 | 0.023 | 0.896 | 0.009 | MIXED |
| Gruiformes | GR | 3 | 0.118 | 0.024 | 0.849 | 0.009 | MIXED |
| Accipitriformes and Falconiformes | AF | 9 | 0.155 | 0.026 | 0.807 | 0.011 | MIXED |
| Suliformes | SU | 2 | 0.204 | 0.029 | 0.753 | 0.014 | MIXED |
| Anseriformes | AN | 8 | 0.391 | 0.034 | 0.559 | 0.016 | MIXED |
| Ciconiiformes | CI | 11 | 0.714 | 0.024 | 0.248 | 0.014 | WET |
| Podicipediformes | PO | 3 | 0.759 | 0.023 | 0.206 | 0.013 | WET |
| Charadriformes (Laridae and Sternidae) | LS | 5 | 0.944 | 0.009 | 0.041 | 0.007 | WET |
| Variable | Attribute | N = 239 |
|---|---|---|
| Age | under 18 years old | 3.8% |
| from 19 to 35 years old | 25.5% | |
| from 36 to 60 years old | 40.6% | |
| over 60 years old | 30.1% | |
| Gender | Female | 54.4% |
| Male | 41.8% | |
| No answer | 3.8% | |
| Education level | Primary school | 0.8% |
| Middle school/Junior high school | 5.9% | |
| High school | 32.2% | |
| Bachelor’s degree | 11.7% | |
| Master’s degree or higher education | 48.5% | |
| Primary occupation | Student | 16.3% |
| Employee | 15.9% | |
| Retired | 13.4% | |
| Freelancer | 12.6% | |
| Teacher/Professor | 12.6% | |
| Other | 29.3% | |
| Country of origin | Italy | 92.9% |
| Other | 7.1% | |
| Distance travelled | 0–20 km | 29.3% |
| >20–40 km | 38.9% | |
| >40–100 km | 22.2% | |
| >100 km | 9.2% | |
| No answer | 0.4% | |
| First time visiting | Yes | 79.9% |
| No | 20.1% | |
| Awareness of the Nature 2000 Network | Yes | 47.7% |
| No | 52.3% |
| ES | Description | Preferences (%) | |
|---|---|---|---|
| Biodiversity | Biodiversity | 19.1 | 19.1 |
| Regulating | Flood control | 4.5 | 20.4 |
| Natural water treatment and improved water quality | 6.7 | ||
| Climate change mitigation | 9.1 | ||
| Provisioning | Agriculture | 2.8 | 15.2 |
| Fishing | 1.6 | ||
| Harvesting wild edible plants | 4.6 | ||
| Water availability | 6.2 | ||
| Cultural | Pristine nature and landscape | 15.8 | 45.3 |
| Nature-based tourism | 14.8 | ||
| Human health and well-being | 7.6 | ||
| Opportunities to socialize | 3.0 | ||
| Ancient tradition and cultural heritage | 4.2 | ||
| Total | 100.0 | 100.0 | |
| ES | Description | WET | AGR | NAT | MIXED |
|---|---|---|---|---|---|
| Biodiversity | Biodiversity | 23.4 | 3.1 | 4.7 | 68.8 |
| Regulating | Flood control | 42.9 | 5.7 | 2.9 | 48.6 |
| Natural water treatment and improved water quality | 48.8 | 9.3 | 2.3 | 39.5 | |
| Climate change mitigation | 39.7 | 4.8 | 14.3 | 41.3 | |
| Provisioning | Agriculture | 0.0 | 65.2 | 17.4 | 17.4 |
| Fishing | 90.9 | 0.0 | 0.0 | 9.1 | |
| Harvesting wild edible plants | 3.6 | 17.9 | 53.6 | 25.0 | |
| Water availability | 57.5 | 10.6 | 8.5 | 23.4 | |
| Cultural | Pristine nature and landscape | 31.4 | 7.6 | 8.6 | 52.4 |
| Nature-based tourism | 34.3 | 2.0 | 4.0 | 59.6 | |
| Human health and well-being | 7.8 | 17.7 | 7.8 | 66.7 | |
| Opportunities to socialize | 4.4 | 8.7 | 8.7 | 78.3 | |
| Ancient tradition and cultural heritage | 14.8 | 11.1 | 3.7 | 70.4 | |
| Total | 30.0 | 9.1 | 8.8 | 52.1 |
| Fields of Action | Objectives | Conservation Actions |
|---|---|---|
| Habitat | Create uneven-aged environmental mosaics | Managing vegetation for the maintenance of ecological corridors and ecotone |
| Feeding resources | Guarantee adequate water levels for feeding behavior | Creating or maintaining shallow water zones (30–50 cm) |
| Creating and/or maintaining the gradual transition between aquatic and terrestrial areas | ||
| Guarantee adequate aquatic and marsh vegetation | Increasing the distribution of floating hydrophytes (e.g., Lemna minor) | |
| Managing reedbeds to prevent the rapid accumulation of sediment (they should be maintained over 30 to 70 percent of the submerged area) | ||
| Guarantee healthy populations of fish, amphibians and aquatic invertebrates | Managing water quality by reducing runoff and preventing nutrient over-enrichment through sustainable agricultural practices (e.g., regenerative agriculture) | |
| Increase fish density [84] | Laying of dead trees, submerged stumps, and wood to encourage fish reproduction and fry growth [82] | |
| Breeding and nesting resources | Provide adequate conditions for nesting | Planting trees |
| Positioning wooden artificial platforms [83] | ||
| Human disturbance | Increase reproductive success | Creating buffer zones of dense vegetation around nesting sites |
| Limiting and/or regulating visitors’ access | ||
| Predation | Increase reproductive success | Creating buffer zones of dense vegetation around nesting sites |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingaramo, M.; Cammerino, A.R.B.; Rizzi, V.; Gioiosa, M.; Monteleone, M. Birds as Cultural Ambassadors: Bridging Ecosystem Services and Biodiversity Conservation in Wetland Planning. Sustainability 2024, 16, 10286. https://doi.org/10.3390/su162310286
Ingaramo M, Cammerino ARB, Rizzi V, Gioiosa M, Monteleone M. Birds as Cultural Ambassadors: Bridging Ecosystem Services and Biodiversity Conservation in Wetland Planning. Sustainability. 2024; 16(23):10286. https://doi.org/10.3390/su162310286
Chicago/Turabian StyleIngaramo, Michela, Anna Rita Bernadette Cammerino, Vincenzo Rizzi, Maurizio Gioiosa, and Massimo Monteleone. 2024. "Birds as Cultural Ambassadors: Bridging Ecosystem Services and Biodiversity Conservation in Wetland Planning" Sustainability 16, no. 23: 10286. https://doi.org/10.3390/su162310286
APA StyleIngaramo, M., Cammerino, A. R. B., Rizzi, V., Gioiosa, M., & Monteleone, M. (2024). Birds as Cultural Ambassadors: Bridging Ecosystem Services and Biodiversity Conservation in Wetland Planning. Sustainability, 16(23), 10286. https://doi.org/10.3390/su162310286












