Development and Characterization of PVA Membranes Modified with In(BTC) Metal–Organic Framework for Sustainable Pervaporation Separation of Isopropanol/Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.2.1. Dense Membrane
2.2.2. Supported Membranes
2.3. Pervaporation Experiment
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Specific Surface Area Measurement
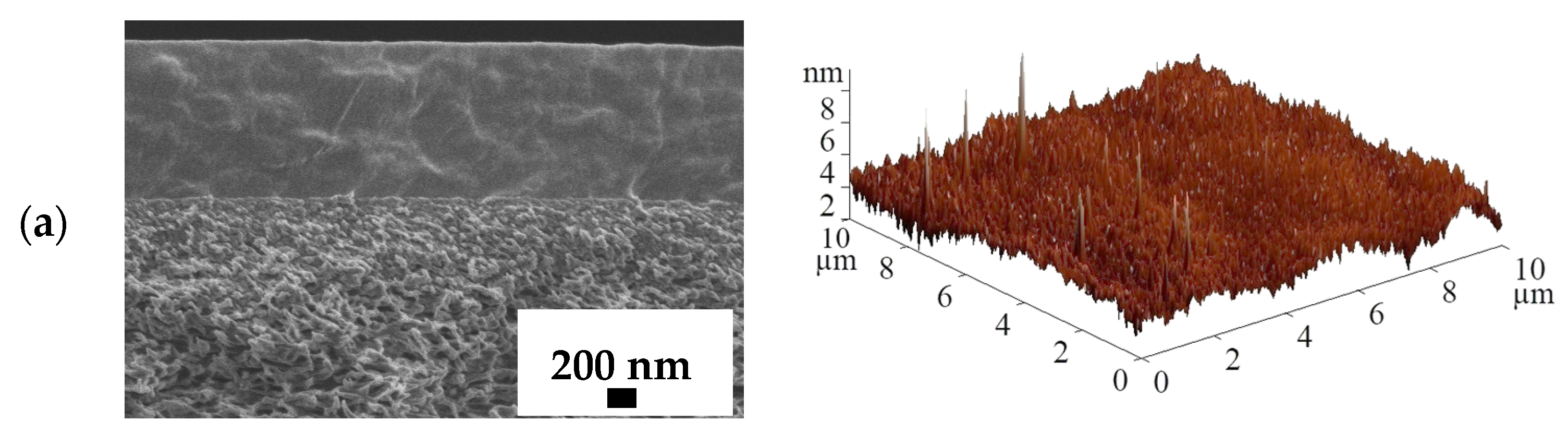
2.6. Scanning Electron Microscopy (SEM)
2.7. Atomic Force Microscopy (AFM)
2.8. Contact Angle Measurements
2.9. Swelling Degree
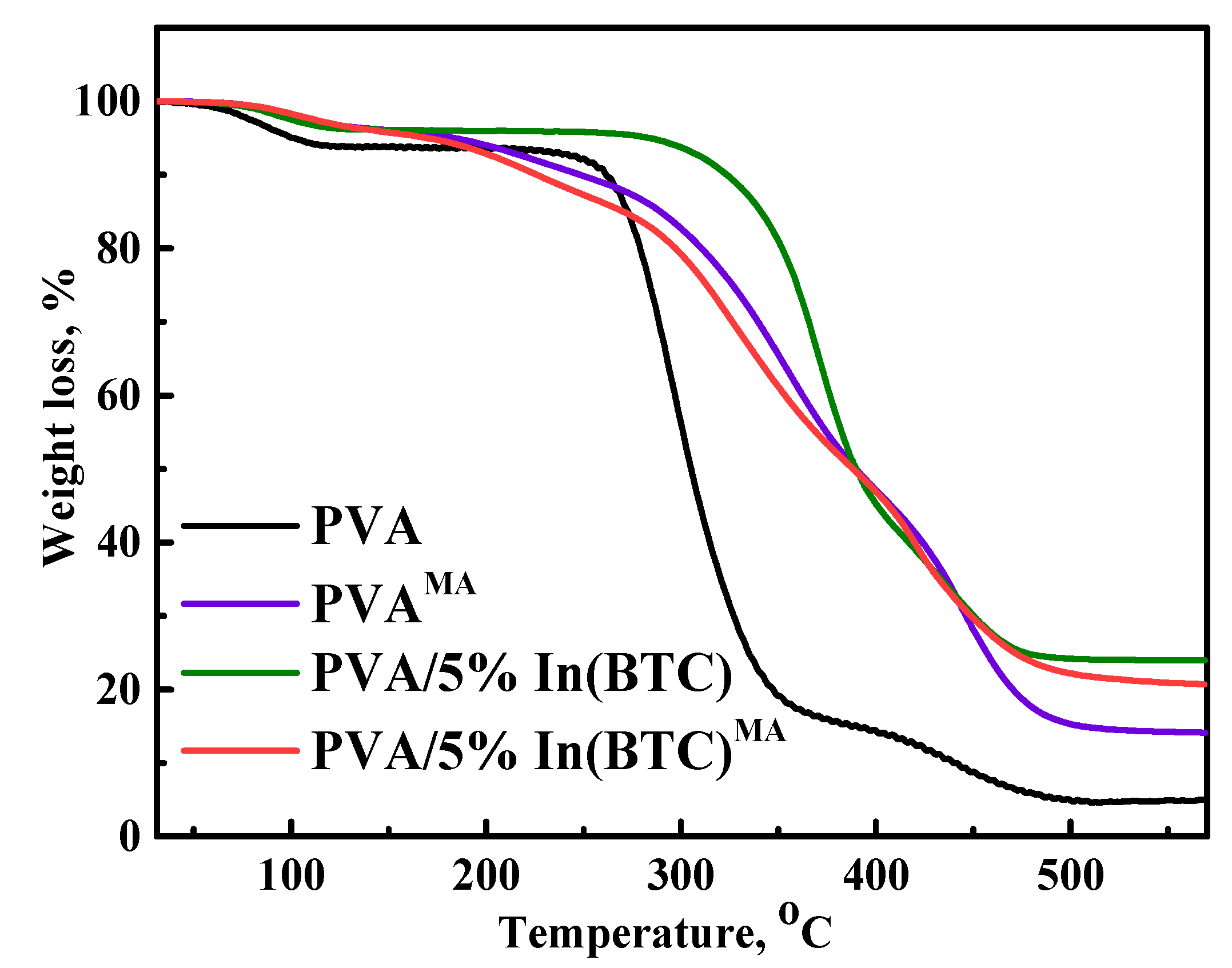
2.10. Thermogravimetric Analysis (TGA)
2.11. Computational Methods
3. Results and Discussion
3.1. Dense PVA and PVA/In(BTC) Membrane Investigation
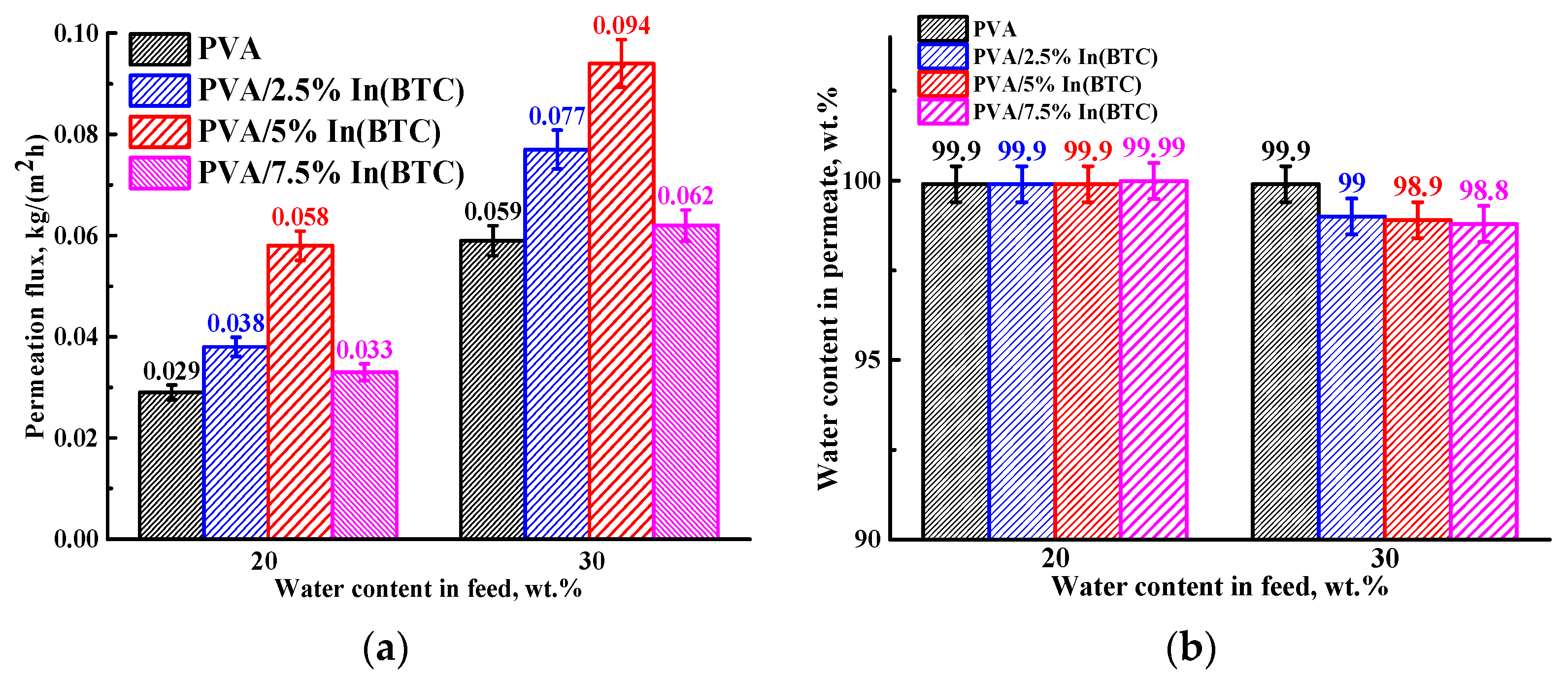
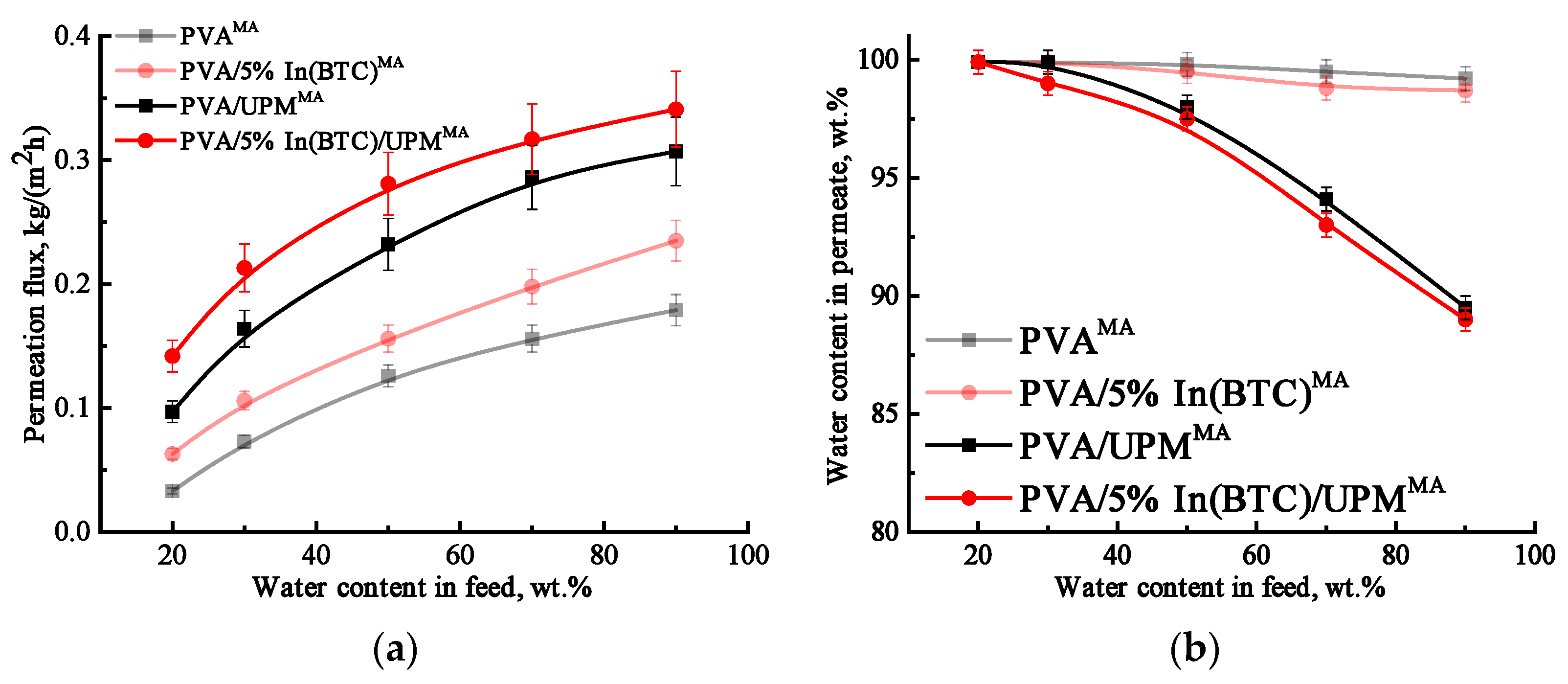
3.1.1. Transport Characteristics in Pervaporation Dehydration of IPA
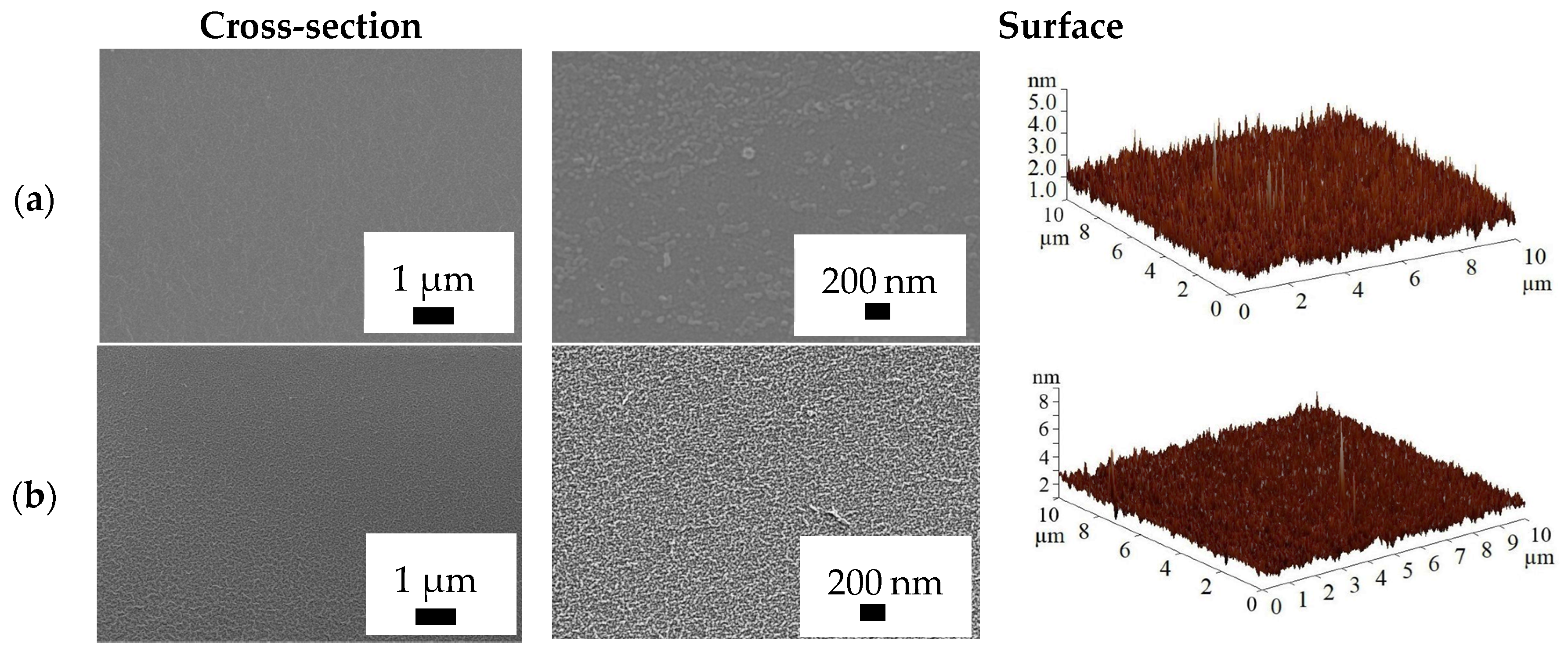
3.1.2. Structure and Physicochemical Properties
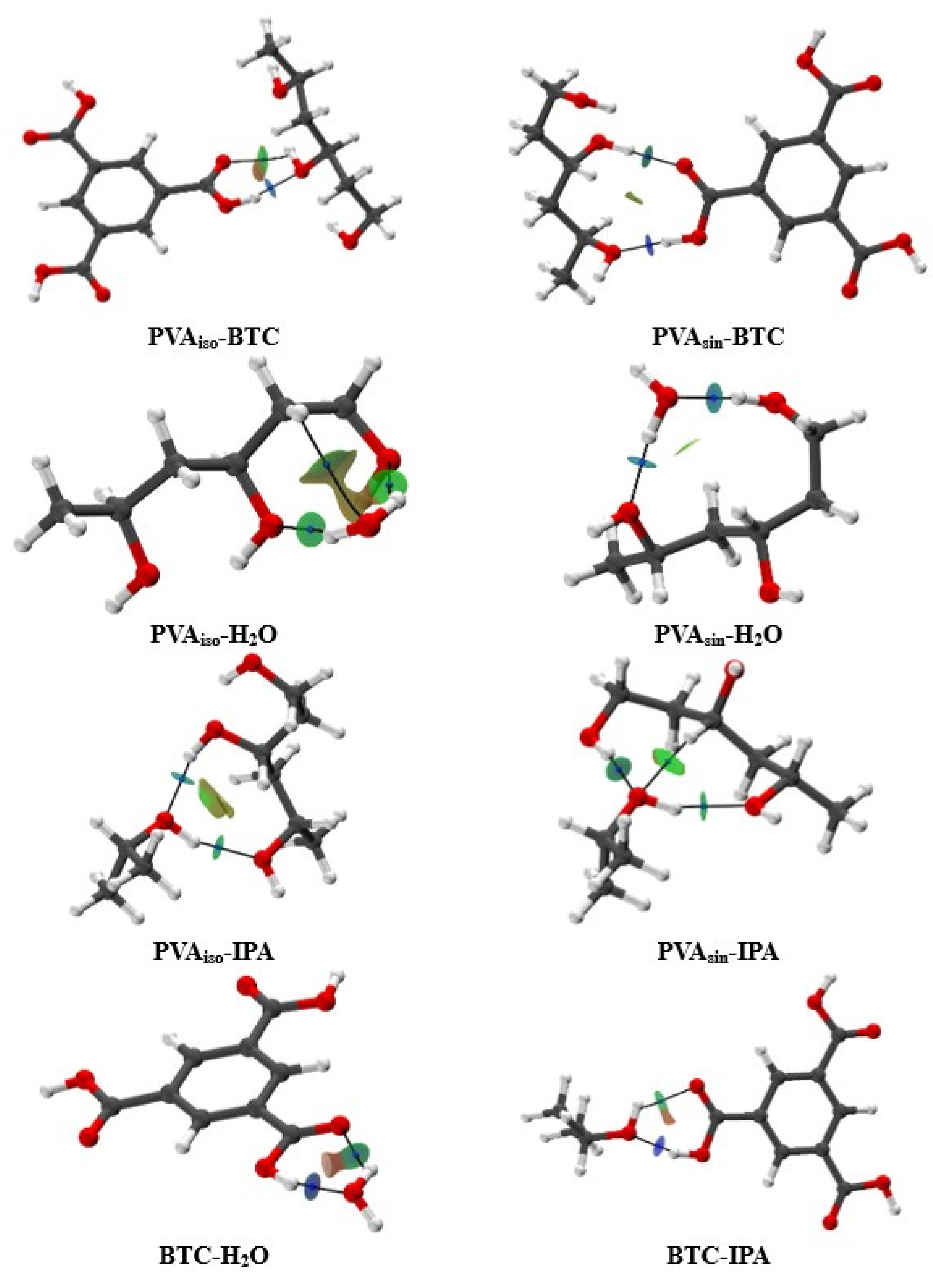
3.2. Computational Investigation
3.2.1. Formation of Hypothetical Associates
3.2.2. Non-Covalent Interaction Investigation
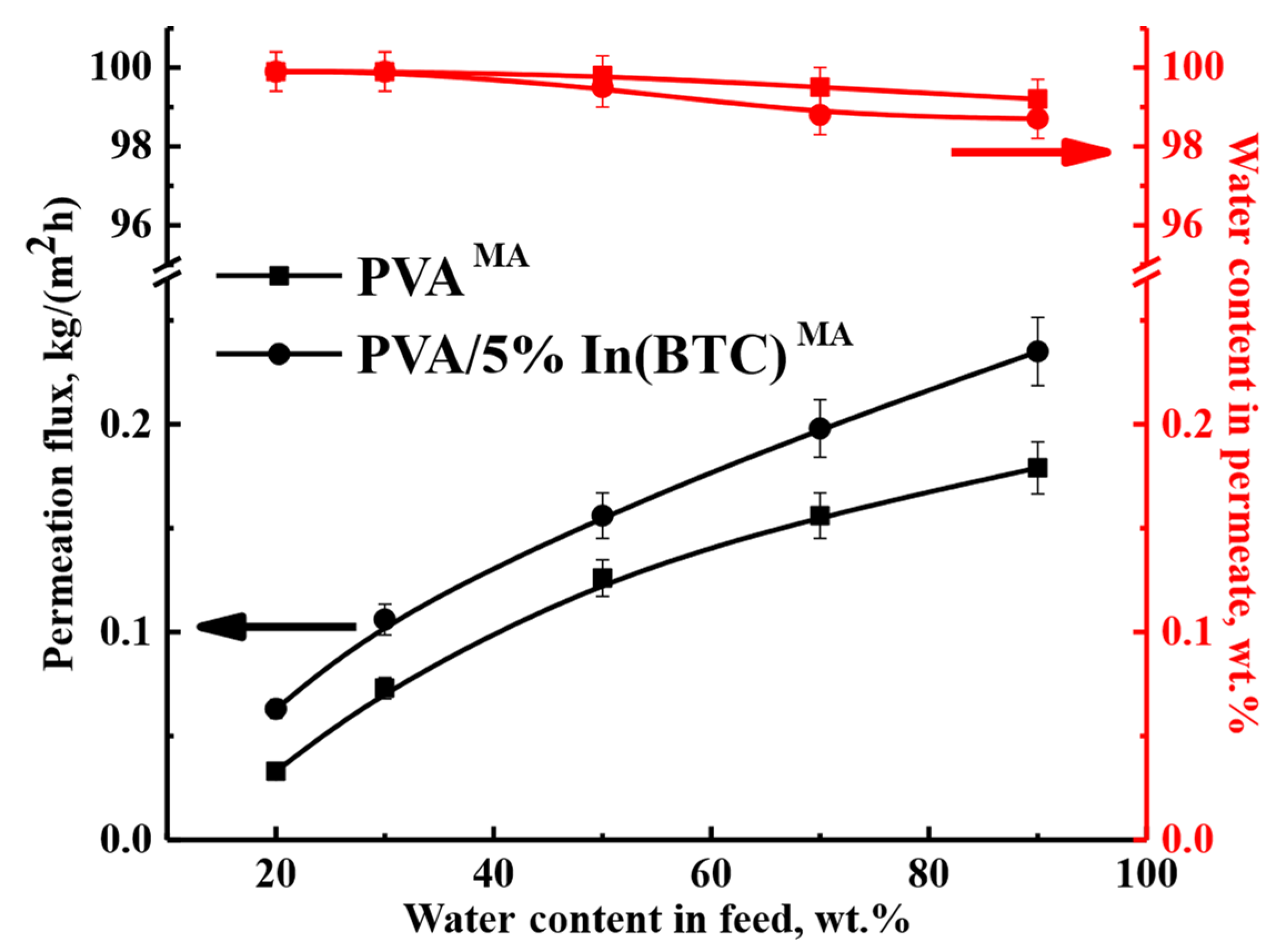
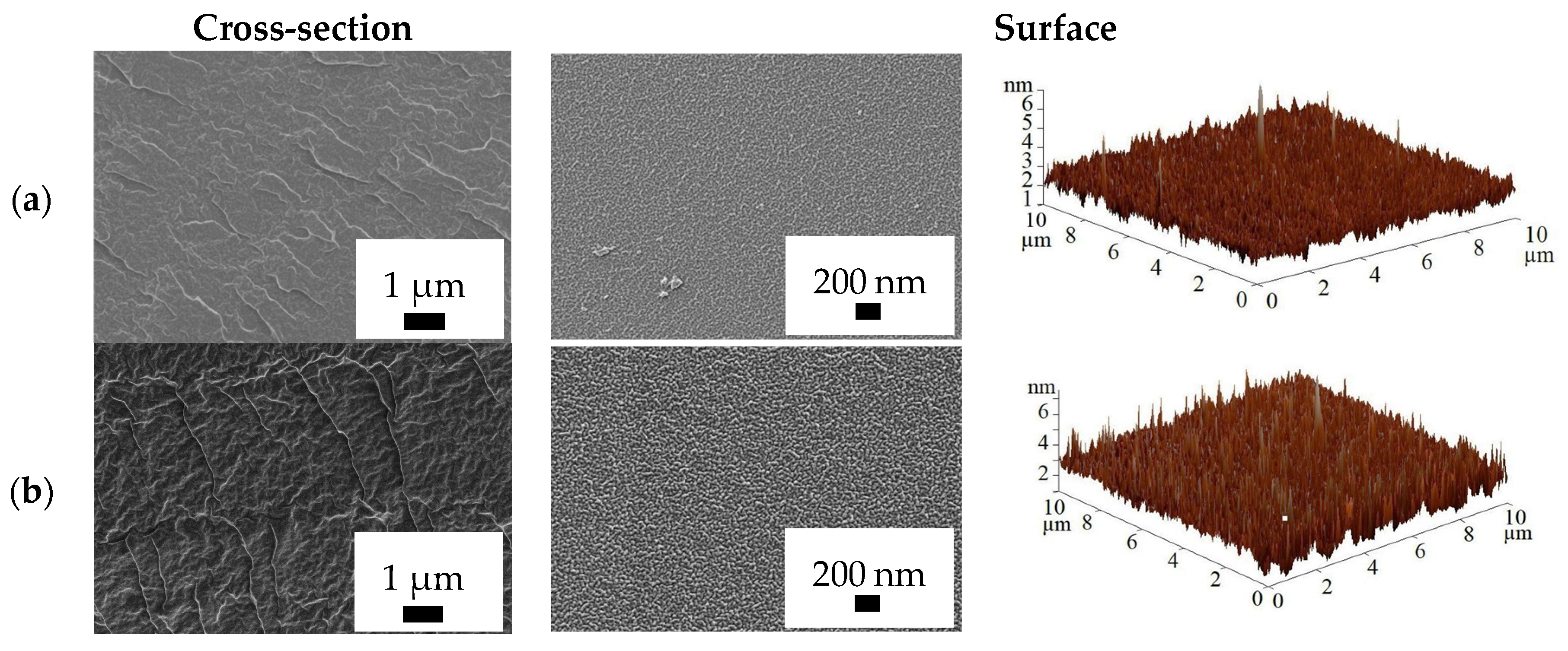
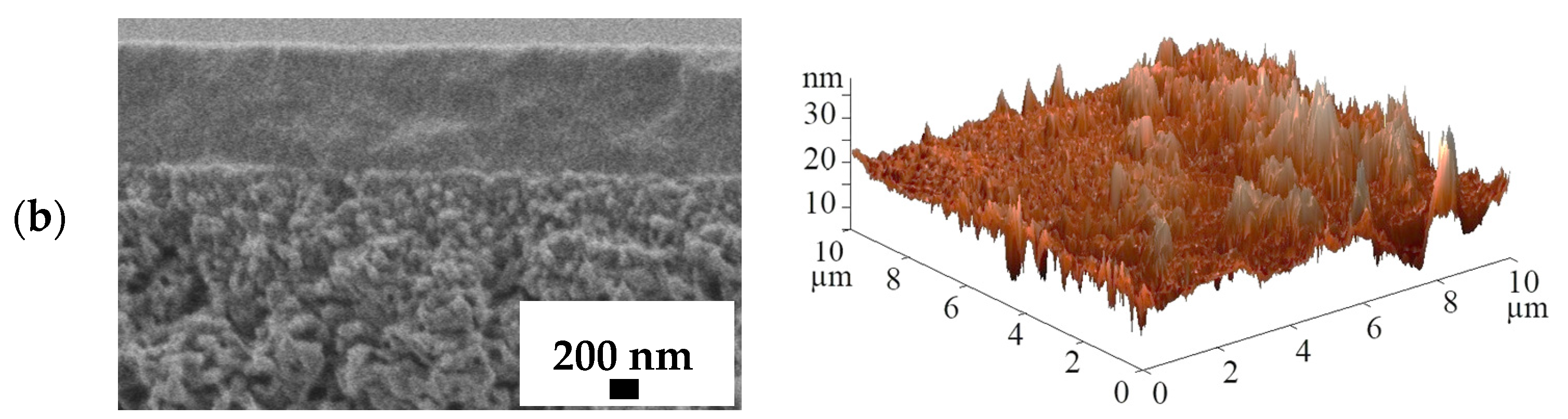
3.3. Supported PVA and PVA/In(BTC) Membrane Investigation
3.4. Comparison of the Performance with PVA-Based Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Issaoui, M.; Jellali, S.; Zorpas, A.A.; Dutournie, P. Membrane technology for sustainable water resources management: Challenges and future projections. Sustain. Chem. Pharm. 2022, 25, 100590. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Petukhov, A.N.; Gumerova, O.R.; Vorotyntsev, A.V.; Nyuchev, A.V.; Vorotyntsev, I.V. Solubility of H2S and CO2 in imidazolium-based ionic liquids with bis(2-ethylhexyl) sulfosuccinate anion. J. Chem. Thermodyn. 2019, 130, 173–182. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Pleshivtseva, T.S.; Ignatenko, V.Y.; Antonov, S.V.; Volkov, A.V. Fabrication of composite nanofiltration membranes from cellulose solutions in an [Emim]OAc–DMSO mixture. Pet. Chem. 2017, 57, 477–482. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Hliavitskaya, T.A.; Ulbricht, M. One-Step Preparation of Antifouling Polysulfone Ultrafiltration Membranes via Modification by a Cationic Polyelectrolyte Based on Polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Ermakov, S.; Roizard, D.; Penkova, A. Enhanced Pervaporation Properties of PVA-Based Membranes Modified with Polyelectrolytes. Application to IPA Dehydration. Polymers 2019, 12, 14. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Missyul, A.B.; Kuzminova, A.I.; Markelov, D.A.; Ermakov, S.S.; Roizard, D. Development and investigation of mixed-matrix PVA-fullerenol membranes for acetic acid dehydration by pervaporation. Sep. Purif. Technol. 2017, 187, 285–293. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Luis, P. Chapter Four—Pervaporation. In Progress in Filtration and Separation; Tarleton, S., Ed.; Oxford University Press: Oxford, UK, 2015; pp. 101–154. ISBN 012384746X. [Google Scholar]
- Dai, Y.; Li, S.; Meng, D.; Yang, J.; Cui, P.; Wang, Y.; Zhu, Z.; Gao, J.; Ma, Y. Economic and Environmental Evaluation for Purification of Diisopropyl Ether and Isopropyl Alcohol via Combining Distillation and Pervaporation Membrane. ACS Sustain. Chem. Eng. 2019, 7, 20170–20179. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Yang, J.; Tsuru, T. Progress in pervaporation membranes for dehydration of acetic acid. Sep. Purif. Technol. 2021, 262, 118338. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, Y.; Yang, T.; Chung, T.-S. ZIF-90/P84 mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 453, 155–167. [Google Scholar] [CrossRef]
- Oun, A.A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Recent advances in polyvinyl alcohol-based composite films and their applications in food packaging. Food Packag. Shelf Life 2022, 34, 100991. [Google Scholar] [CrossRef]
- Liu, L.; Kentish, S.E. Pervaporation performance of crosslinked PVA membranes in the vicinity of the glass transition temperature. J. Membr. Sci. 2018, 553, 63–69. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Missyul, A.; Ermakov, S.; Roizard, D. Development and characterization of new pervaporation PVA membranes for the dehydration using bulk and surface modifications. Polymers 2018, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Guan, H.; Qiao, X.; Kulprathipanja, S. Pervaporation study of aqueous ethanol solution through zeolite-incorporated multilayer poly (vinyl alcohol) membranes: Effect of zeolites. J. Membr. Sci. 2006, 276, 260–271. [Google Scholar] [CrossRef]
- Falath, W. Novel stand-alone PVA mixed matrix membranes conjugated with graphene oxide for highly improved reverse osmosis performance. Arab. J. Chem. 2021, 14, 103109. [Google Scholar] [CrossRef]
- Sheng, F.; Afsar, N.U.; Zhu, Y.; Ge, L.; Xu, T. PVA-Based Mixed Matrix Membranes Comprising ZSM-5 for Cations Separation. Membranes 2020, 10, 114. [Google Scholar] [CrossRef]
- Agafonov, M.A.; Alexandrov, E.V.; Artyukhova, N.A.; Bekmukhamedov, G.E.; Blatov, V.A.; Butova, V.V.; Gayfulin, Y.M.; Garibyan, A.A.; Gafurov, Z.N.; Gorbunova, Y.G.; et al. Metal-organic frameworks in Russia: From the synthesis and structure to functional properties and materials. J. Struct. Chem. 2022, 63, 671–843. [Google Scholar] [CrossRef]
- Halder, S.; Ghosh, P.; Rizzoli, C.; Banerjee, P.; Roy, P. Nitroaromatic explosives detection by a luminescent Cd(II) based metal organic framework. Polyhedron 2017, 123, 217–225. [Google Scholar] [CrossRef]
- Butova, V.V.; Bulanova, E.A.; Polyakov, V.A.; Guda, A.A.; Aboraia, A.M.; Shapovalov, V.V.; Zahran, H.Y.; Yahia, I.S.; Soldatov, A.V. The effect of cobalt content in Zn/Co-ZIF-8 on iodine capping properties. Inorganica Chim. Acta 2019, 492, 18–22. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, G.; Zhang, J.; Gray, S.; Xie, Z. Dual-layer membranes with a thin film hydrophilic MOF/PVA nanocomposite for enhanced antiwetting property in membrane distillation. Desalination 2021, 518, 115268. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.; Geng, Y.; Lu, X.; Jia, Z. Adjustable pervaporation performance of Zr-MOF/poly(vinyl alcohol) mixed matrix membranes. J. Chem. Technol. Biotechnol. 2019, 94, 973–981. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Regmi, C.; Friess, K. Interfacial Design of Mixed Matrix Membranes via Grafting PVA on UiO-66-NH2 to Enhance the Gas Separation Performance. Membranes 2021, 11, 419. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, H.; Chen, X.; Jiang, Z.; Lu, J.; Xin, F. Incorporating MXene@MOF-303 Composites into Poly(vinyl alcohol) (PVA) to Fabricate Pervaporation Membranes for Desalination. ACS Appl. Polym. Mater. 2024, 6, 8277–8290. [Google Scholar] [CrossRef]
- Sharma, U.; Shalini, S.; Basu, S.; Saravanan, P.; Jang, M. Active layer modification of commercial nanofiltration membrane using CuBTC/PVA matrix for improved surface and separation characteristics. J. Appl. Polym. Sci. 2021, 138, app50508. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Ying, Y.; Zhang, W. Improve MOF-801 dispersibility in PVA membranes by a pre-crosslinking strategy for enhanced pervaporation performance. J. Membr. Sci. 2023, 687, 122043. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Myznikov, D.; Selyutin, A.; Su, R.; Penkova, A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes 2022, 12, 908. [Google Scholar] [CrossRef]
- Hou, S.-Z.; Zhang, X.-D.; Yuan, W.-W.; Li, Y.-X.; Gu, Z.-Y. Indium-Based Metal–Organic Framework for High-Performance Electroreduction of CO2 to Formate. Inorg. Chem. 2020, 59, 11298–11304. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wang, G.; Wang, B.; Yan, B.; Liu, L.; Xu, L.; Ma, T.; Yao, S.; Fu, Y.; Zhang, L.; et al. A new indium-based MOF as the highly stable luminescent ultra-sensitive antibiotic detector. Chin. J. Struct. Chem. 2023, 42, 100062. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wu, T.; Feng, P.; Bu, X. Multiroute Synthesis of Porous Anionic Frameworks and Size-Tunable Extraframework Organic Cation-Controlled Gas Sorption Properties. J. Am. Chem. Soc. 2009, 131, 16027–16029. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-T.; Bu, J.T.; Li, Y.; Wu, T.; Zuo, F.; Feng, P.; Bu, X. Pore Space Partition and Charge Separation in Cage-within-Cage Indium−Organic Frameworks with High CO2 Uptake. J. Am. Chem. Soc. 2010, 132, 17062–17064. [Google Scholar] [CrossRef] [PubMed]
- Penkova, A.V.; Acquah, S.F.A.; Dmitrenko, M.E.; Chen, B.; Semenov, K.N.; Kroto, H.W. Transport properties of cross-linked fullerenol–PVA membranes. Carbon N. Y. 2014, 76, 446–450. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Frisch, M.J. Gaussian 16, Revision A.03; Gaussian, Inc., Wallingford CT, 2016. Available online: https://gaussian.com/citation_a03/ (accessed on 17 October 2024).
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shen, L.; Li, C.; Wang, Y. Properties and pervaporation performance of poly (vinyl alcohol) membranes crosslinked with various dianhydrides. J. Appl. Polym. Sci. 2018, 135, 46159. [Google Scholar] [CrossRef]
- Grigoropoulos, A.; Whitehead, G.F.S.; Perret, N.; Katsoulidis, A.P.; Chadwick, F.M.; Davies, R.P.; Haynes, A.; Brammer, L.; Weller, A.S.; Xiao, J.; et al. Encapsulation of an organometallic cationic catalyst by direct exchange into an anionic MOF. Chem. Sci. 2016, 7, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Omkaram, I.; Sreekanth Chakradhar, R.P.; Lakshmana Rao, J. EPR, optical, infrared and Raman studies of VO2+ ions in polyvinylalcohol films. Phys. B Condens. Matter 2007, 388, 318–325. [Google Scholar] [CrossRef]
- Franca, T.; Goncalves, D.; Cena, C. ATR-FTIR spectroscopy combined with machine learning for classification of PVA/PVP blends in low concentration. Vib. Spectrosc. 2022, 120, 103378. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nate, M.M.; Kurup, M.B.; Bambole, V.A.; Sabharwal, S. Effect of γ-radiation on the structure and morphology of polyvinyl alcohol films. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 2005, 237, 585–592. [Google Scholar] [CrossRef]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Zemzem, M.; Vinches, L.; Hallé, S. Morphological investigation of maleic anhydride-grafted nitrile/nanoclay nanocomposites. Mater. Res. Express 2022, 9, 085302. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Atta, R.; Zolotarev, A.; Kuzminova, A.; Ermakov, S.; Penkova, A. Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol. Sustainability 2022, 14, 3561. [Google Scholar] [CrossRef]
- Chen, R.; Chai, M.; Hou, J. Metal-organic framework-based mixed matrix membranes for gas separation: Recent advances and opportunities. Carbon Capture Sci. Technol. 2023, 8, 100130. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, S.; Li, M.; Wang, Y.; Mei, D. Metal–Organic Framework/Polyvinyl Alcohol Composite Films for Multiple Applications Prepared by Different Methods. Membranes 2023, 13, 755. [Google Scholar] [CrossRef] [PubMed]
- Gohil, J.M.; Bhattacharya, A.; Ray, P. Studies on the Crosslinking of Poly (Vinyl Alcohol). J. Polym. Res. 2006, 13, 161–169. [Google Scholar] [CrossRef]
- Goodyer, C.E.; Bunge, A.L. Mass transfer through membranes with surface roughness. J. Membr. Sci. 2012, 409–410, 127–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Lucier, B.E.G.; McKenzie, S.M.; Arhangelskis, M.; Morris, A.J.; Friščić, T.; Reid, J.W.; Terskikh, V.V.; Chen, M.; Huang, Y. Welcoming Gallium- and Indium-Fumarate MOFs to the Family: Synthesis, Comprehensive Characterization, Observation of Porous Hydrophobicity, and CO2 Dynamics. ACS Appl. Mater. Interfaces 2018, 10, 28582–28596. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K.; Kuila, S.B.; Samanta, H.S.; Singha, N.R. Systematic choice of crosslinker and filler for pervaporation membrane: A case study with dehydration of isopropyl alcohol–water mixtures by polyvinyl alcohol membranes. Sep. Purif. Technol. 2011, 81, 159–173. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, P.; Luo, Y. Dehydration of caprolactam–water mixtures through cross-linked PVA composite pervaporation membranes. J. Membr. Sci. 2007, 306, 93–102. [Google Scholar] [CrossRef]
- Xia, L.L.; Li, C.L.; Wang, Y. In-situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Membr. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Microporous and Mesoporous Materials Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Lin, R.; Villacorta Hernandez, B.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Mikhailovskaya, O.; Dubovenko, R.; Kuzminova, A.; Myznikov, D.; Mazur, A.; Semenov, K.; Rusalev, Y.; Soldatov, A.; Ermakov, S.; et al. Pervaporation Membranes Based on Polyelectrolyte Complex of Sodium Alginate/Polyethyleneimine Modified with Graphene Oxide for Ethanol Dehydration. Polymers 2024, 16, 1206. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Morshed, M.; Larionov, M.I.; Alem, H.; Zolotarev, A.A.; Ermakov, S.S.; Roizard, D. Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation. Appl. Surf. Sci. 2018, 450, 527–537. [Google Scholar] [CrossRef]
- Chong, W.; Mohd Nawawi, M.; Sadikin, A. Pervaporation of isopropanol-water mixture using poly(vinyl) alcohol-zsm-5 membranes. J. Teknol. 2008, 49, 159–166. [Google Scholar]
- Sairam, M.; Naidu, B.V.K.; Nataraj, S.K.; Sreedhar, B.; Aminabhavi, T.M. Poly(vinyl alcohol)-iron oxide nanocomposite membranes for pervaporation dehydration of isopropanol, 1,4-dioxane and tetrahydrofuran. J. Membr. Sci. 2006, 283, 65–73. [Google Scholar] [CrossRef]
- Sabouni, R.; Kazemian, H.; Rohani, S. Microwave Synthesis of the CPM-5 Metal Organic Framework. Chem. Eng. Technol. 2012, 35, 1085–1092. [Google Scholar] [CrossRef]
- Muzart, J. N,N-Dimethylformamide: Much more than a solvent. Tetrahedron 2009, 65, 8313–8323. [Google Scholar] [CrossRef]



| Membrane Designation | Additive Amount Relative to PVA, wt% | UPM Support | |
|---|---|---|---|
| In(BTC) | MA | ||
| dense uncross-linked | |||
| PVA | - | - | - |
| PVA/2.5%In(BTC) | 2.5 | ||
| PVA/5%In(BTC) | 5 | ||
| PVA/7.5%In(BTC) | 7.5 | ||
| dense cross-linked | |||
| PVAMA | - | 35 | - |
| PVA/5%In(BTC)MA | 5 | ||
| supported cross-linked | |||
| PVA/UPMMA | - | 35 | + |
| PVA/5%In(BTC)/UPMMA | 5 | ||
| Membranes | Ra, nm | Rq, nm | Contact Angle of Water, ° |
|---|---|---|---|
| PVA | 0.23 | 0.31 | 67 ± 3 |
| PVA/2.5%In(BTC) | 0.26 | 0.34 | 65 ± 3 |
| PVA/5%In(BTC) | 0.28 | 0.35 | 62 ± 5 |
| PVA/7.5%In(BTC) | 0.27 | 0.34 | 59 ± 5 |
| PVAMA | 0.28 | 0.35 | 66 ± 2 |
| PVA/5%In(BTC)MA | 0.33 | 0.46 | 60 ± 5 |
| Membrane | Swelling Degree, % | ||
|---|---|---|---|
| Water/IPA Mixture | Water | ||
| 20/80 wt.% | 30/70 wt.% | ||
| PVA | 67 | 106 | - |
| PVA/5%In(BTC) | 71 | 113 | - |
| PVAMA | 49 | 81 | 109 |
| PVA/5%In(BTC)MA | 50 | 82 | 123 |
| ∆Gmin, kJ/mol | |||
|---|---|---|---|
| B3LYP/6-311++G** | BTC | H2O | IPA |
| PVAiso | 16.6 | 17.9 | 21.7 |
| PVAsin | −6.7 | 7.6 | 9.4 |
| BTC | ~ | −1.0 | −0.8 |
| H2O | ~ | 10.4 | |
| B3LYP/6-311++G** | ||||||
|---|---|---|---|---|---|---|
| Associate | Interaction | WBI | FBO | d, Å | R, % | |
| PVAiso | BTC | O///H(COOH) | 0.131 | 0.091 | 1.79163 | 65.9% |
| H(OH)///O(CO) | 0.029 | 0.020 | 2.36285 | 86.9% | ||
| H2O | O///H | 0.059 | 0.051 | 2.1167 | 77.8% | |
| O*///H | 0.040 | 0.036 | 2.33704 | 85.9% | ||
| H(CH2)///O | 0.014 | 0.018 | 2.86991 | 105.5% | ||
| IPA-2 | O(OH)///H(OH) | 0.072 | 0.062 | 1.97676 | 72.7% | |
| H*(O*H*)///O | 0.094 | 0.074 | 1.90327 | 70.0% | ||
| IPA-4 | H(CH3)///O | 0.016 | 0.029 | 2.85031 | 104.8% | |
| H(CH2)///O | 0.019 | 0.030 | 2.59598 | 95.4% | ||
| PVAsin | BTC | O*///H(COOH) | 0.166 | 0.113 | 1.69133 | 62.2% |
| H(OH)///O(CO) | 0.085 | 0.065 | 1.91268 | 70.3% | ||
| H2O | O*///H | 0.113 | 0.084 | 1.82976 | 67.3% | |
| H(OH)///O | 0.113 | 0.090 | 1.86973 | 68.7% | ||
| IPA-1 | H(CHOH)///O | 0.029 | 0.038 | 2.31903 | 85.3% | |
| H(OH)///O | 0.080 | 0.065 | 1.97507 | 72.6% | ||
| O*(OH)///H(OH) | 0.066 | 0.057 | 2.02537 | 74.5% | ||
| IPA-3 | H(CH2)///O | 0.016 | 0.025 | 2.73736 | 100.6% | |
| H*(C*H*2)///H(CH3) | 0.001 | 0.001 | 3.53497 | 147.3% | ||
| BTC | H2O | O(CO)///H | 0.075 | 0.056 | 2.03656 | 74.9% |
| H(OH)///O | 0.143 | 0.110 | 1.76897 | 65.0% | ||
| IPA | O(CO)///H | 0.057 | 0.045 | 2.14353 | 78.8% | |
| H(OH)///O | 0.164 | 0.112 | 1.71299 | 63.0% | ||
| Membranes | Ra, nm | Rq, nm |
|---|---|---|
| PVA/UPMMA | 0.44 | 0.57 |
| PVA/5%In(BTC)/UPMMA | 2.29 | 3.19 |
| Membranes | Thickness, μm | Water Content in Feed, wt.% | Temperature, °C | Permeation Flux, g/(m2h) | Water Content in Permeate, wt.% | References |
|---|---|---|---|---|---|---|
| PVA/5%In(BTC)/UPMMA | 0.6 | 20 | 22 | 142 | 99.9 | This study |
| PERVAPTM 1201 | - | 20 | 22 | 34 | 99.9 | [67] |
| PVA/3% Pluronic F127/PA*MA | 1.5 | 20 | 22 | 296 | 98.2 | [52] |
| PVA/20%chitosan/UPMMA | 1 | 20 | 22 | 233 | 93.9 | [15] |
| PVA/5%fullerenol/20%chitosan/UPMMA | 1 | 20 | 22 | 241 | 96.8 | |
| PVA/ZSM-5/PS ** | - | 20 | 27.5 | 200 | 66.7 | [68] |
| PVA/3% Fe(II)/Fe(III)/polyester fabrics | 10 | 20 | 30 | 98 | 92.3 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyakov, V.; Dmitrenko, M.; Kalmakhelidze, M.; Kuzminova, A.; Dubovenko, R.; Mukhanova, E.; Soldatov, A.; Penkova, A. Development and Characterization of PVA Membranes Modified with In(BTC) Metal–Organic Framework for Sustainable Pervaporation Separation of Isopropanol/Water. Sustainability 2024, 16, 10257. https://doi.org/10.3390/su162310257
Polyakov V, Dmitrenko M, Kalmakhelidze M, Kuzminova A, Dubovenko R, Mukhanova E, Soldatov A, Penkova A. Development and Characterization of PVA Membranes Modified with In(BTC) Metal–Organic Framework for Sustainable Pervaporation Separation of Isopropanol/Water. Sustainability. 2024; 16(23):10257. https://doi.org/10.3390/su162310257
Chicago/Turabian StylePolyakov, Vladimir, Mariia Dmitrenko, Meri Kalmakhelidze, Anna Kuzminova, Roman Dubovenko, Elizaveta Mukhanova, Alexander Soldatov, and Anastasia Penkova. 2024. "Development and Characterization of PVA Membranes Modified with In(BTC) Metal–Organic Framework for Sustainable Pervaporation Separation of Isopropanol/Water" Sustainability 16, no. 23: 10257. https://doi.org/10.3390/su162310257
APA StylePolyakov, V., Dmitrenko, M., Kalmakhelidze, M., Kuzminova, A., Dubovenko, R., Mukhanova, E., Soldatov, A., & Penkova, A. (2024). Development and Characterization of PVA Membranes Modified with In(BTC) Metal–Organic Framework for Sustainable Pervaporation Separation of Isopropanol/Water. Sustainability, 16(23), 10257. https://doi.org/10.3390/su162310257








