Analysis of the Morphological Characteristics of PM2.5 and Its Microbiological Composition in a Fattening Pig House
Abstract
1. Introduction
2. Materials and Methods
2.1. Condition of Pig Housing and Feeding Management
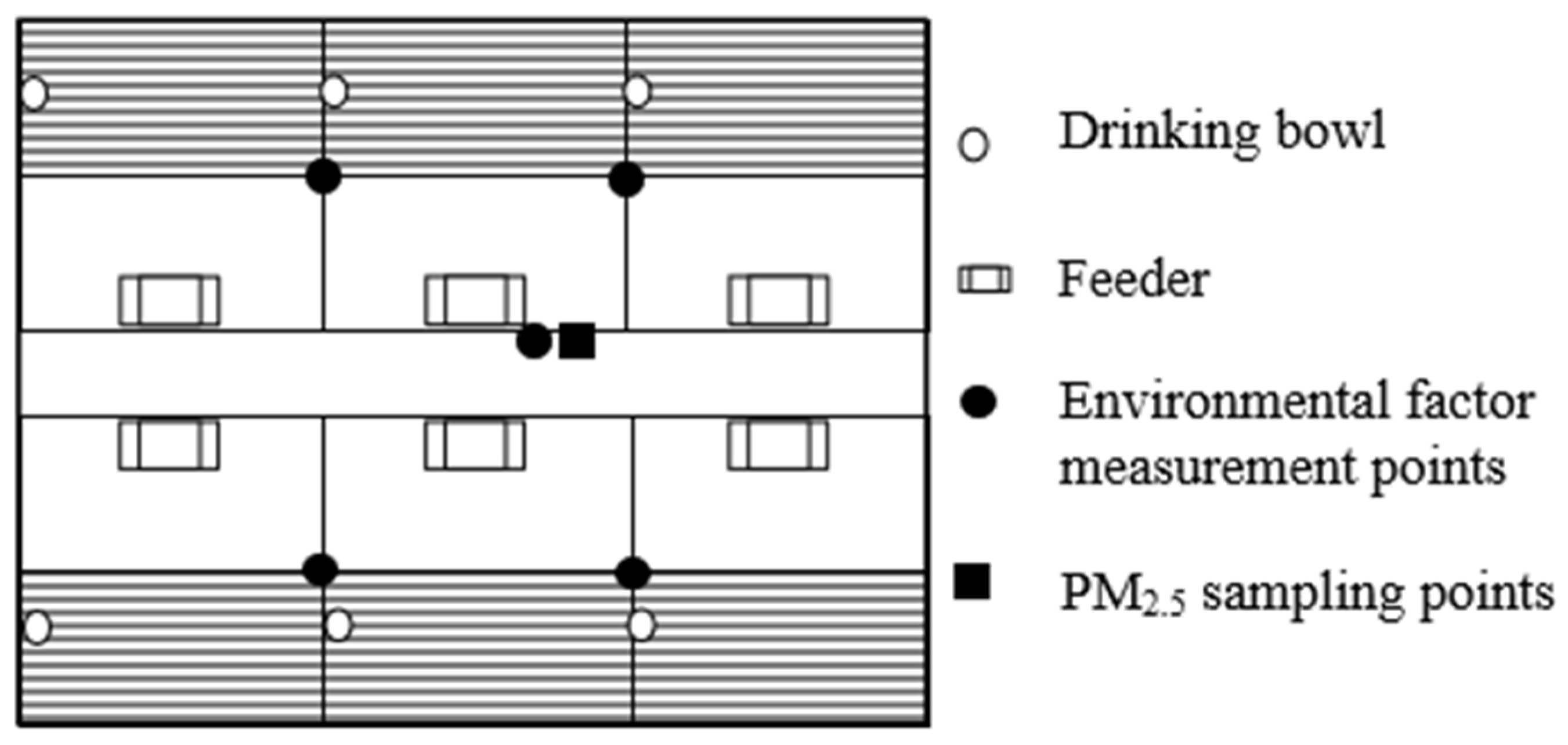
2.2. Measurement of Microclimate Variables and PM2.5 Collection in the Pig House
2.3. Micro-Morphological Analysis of PM2.5 Samples
2.4. DNA Extraction and Bacterial/Fungal Sequencing
2.5. Data Analysis
3. Results
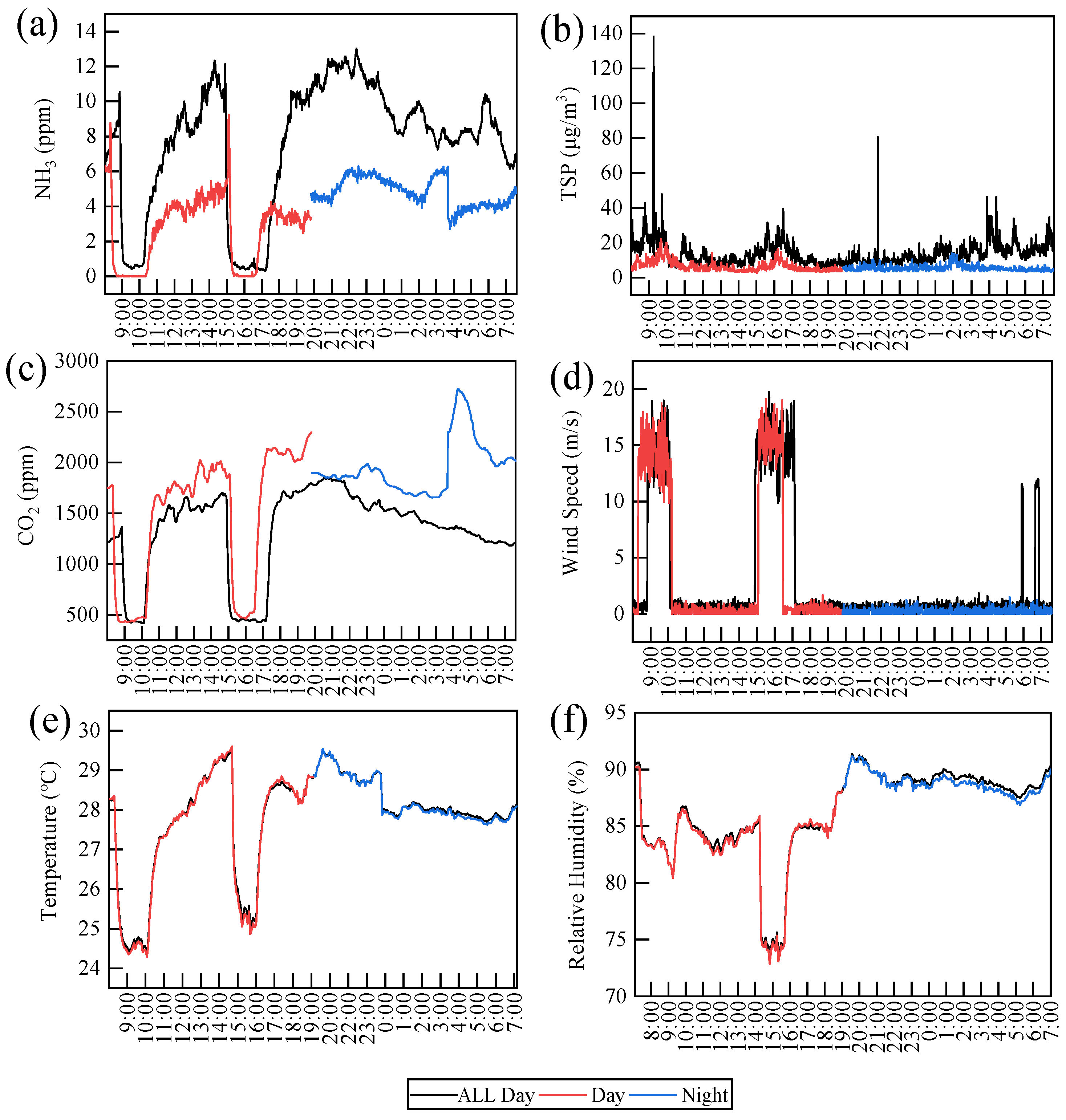
3.1. Microclimate Variables in the Pig House
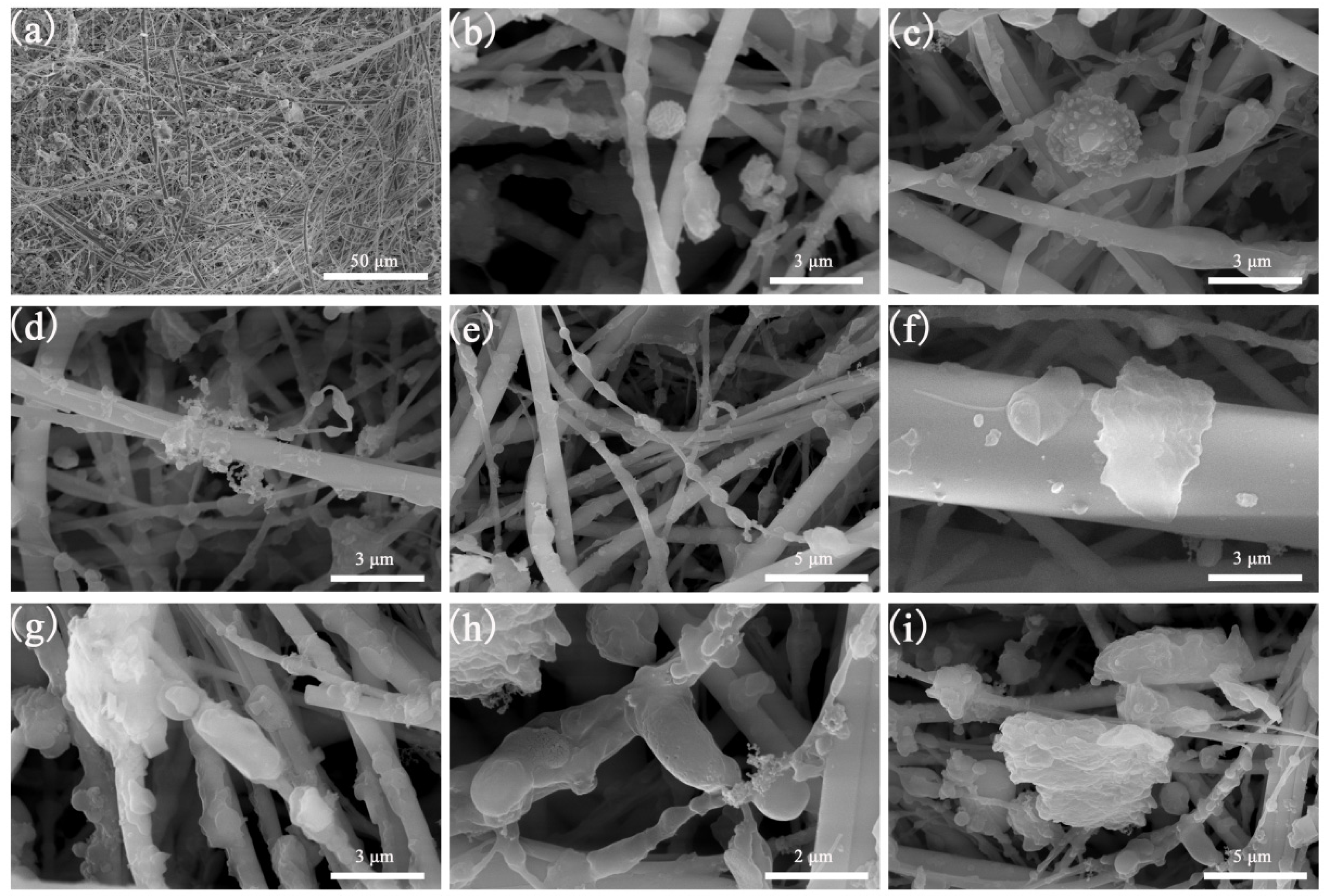
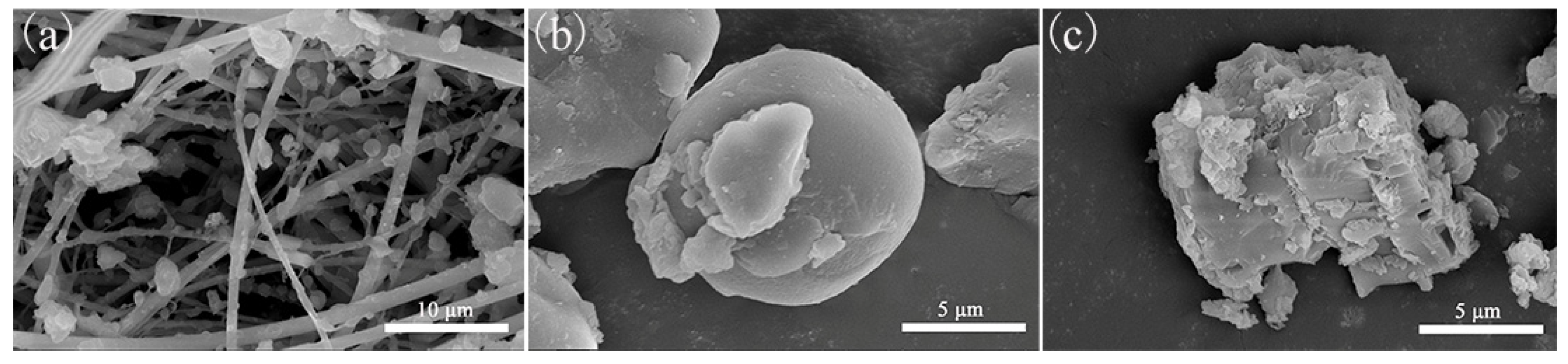
3.2. Analysis of Microscopic Morphology of PM2.5
3.3. Analysis of Microbial Diversity in PM2.5
3.4. Analysis of Microbiological Composition in PM2.5
3.5. Correlation Analysis Between PM2.5 Microorganisms and Environmental Factors in the Pig House
3.6. Correlation Analysis of PM2.5 Microorganisms with Feed and Faeces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knetter, S.M.; Tuggle, C.K.; Wannemuehler, M.J.; Ramer-Tait, A.E. Organic barn dust extract exposure impairs porcine macrophage function in vitro: Implications for respiratory health. Vet. Immunol. Immunopathol. 2014, 157, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Borlée, F.; Yzermans, C.J.; Aalders, B.; Rooijackers, J.; Krop, E.; Maassen, C.B.; Smit, L.A. Air pollution from livestock farms is associated with airway obstruction in neighboring residents. Am. J. Respir. Crit. Care Med. 2017, 196, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Jahne, M.A.; Rogers, S.W.; Holsen, T.M.; Grimberg, S.J.; Ramler, I.P. Emission and dispersion of bioaerosols from dairy manure application sites: Human health risk assessment. Environ. Sci. Technol. 2015, 49, 9842–9849. [Google Scholar] [CrossRef] [PubMed]
- Weichenthal, S.A.; Godri Pollitt, K.; Villeneuve, P.J. PM2.5, oxidant defence and cardiorespiratory health: A review. Environ. Health. 2013, 12, 1612. [Google Scholar] [CrossRef]
- Audi, C.; Baïz, N.; Maesano, C.N.; Ramousse, O.; Reboulleau, D.; Magnan, A.; Caillaud, D.; Annesi-Maesano, I. Serum cytokine levels related to exposure to volatile organic compounds and PM2.5 in dwellings and workplaces in French farmers—A mechanism to explain nonsmoking COPD. Int. J. Chron. Obstruct Pulmon Dis. 2017, 12, 1363. [Google Scholar] [CrossRef]
- Hedelin, A.S.; Sundblad, B.M.; Sahlander, K.; Wilkinson, K.; Seisenbaeva, G.; Kessler, V.; Larsson, K.; Palmberg, L. Comparing human respiratory adverse effects after acute exposure to particulate matter in conventional and particle-reduced swine building environments. Occup. Environ. Med. 2016, 73, 648–655. [Google Scholar] [CrossRef]
- Viegas, S.; Mateus, V.; Almeida-Silva, M.; Carolino, E.; Viegas, C. Occupational exposure to particulate matter and respiratory symptoms in Portuguese swine barn workers. J. Toxicol. Environ. Health A 2013, 76, 1007–1014. [Google Scholar] [CrossRef]
- Pateraki, S.; Asimakopoulos, D.N.; Flocas, H.A.; Maggos, T.; Vasilakos, C. The role of meteorology on different sized aerosol fractions (PM₁₀, PM2.5, PM2.5–10). Sci. Total Environ. 2012, 419, 124–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Aarnink, A.J.A.; De, M.C.M.; Groot, P.W.G. Airborne Microorganisms From Livestock Production Systems and Their Relation to Dust. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1071–1128. [Google Scholar] [CrossRef]
- Rooij, M.M.T.; Smit, L.A.M.; Erbrink, H.J.; Hagenaars, T.J.; Hoek, G.; Ogink, N.W.M.; Winkel, A.; Heederik, D.J.J.; Wouters, I.M. Endotoxin and particulate matter emitted by livestock farms and respiratory health effects in neighboring residents. Environ. Int. 2019, 132, 105009. [Google Scholar] [CrossRef]
- Peng, S.; Luo, M.; Long, D.; Liu, Z.; Tan, Q.; Huang, P.; Shen, J.; Pu, S. Full-length 16S rRNA gene sequencing and machine learning reveal the bacterial composition of inhalable particles from two different breeding stages in a piggery. Ecotoxicol. Environ. Saf. 2023, 253, 114712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mo, J.; Li, Y.; Sundell, J.; Wargocki, P.; Zhang, J.; Little, J.C.; Corsi, R.; Deng, Q.; Leung, M.H.K.; et al. Can commonly-used fan-driven air cleaning technologies improve indoor air quality? A literature review. Atmos. Environ. 2011, 45, 4329–4343. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, D.; Li, B.; Sun, S.; Yabo, S.D.; Geng, J.; Ma, L.; Qi, H. Exploring the disparity of inhalable bacterial communities and antibiotic resistance genes between hazy days and non-hazy days in a cold megacity in Northeast China. J. Hazard. Mater. 2020, 398, 122984. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.Y.; Li, X.; Yang, X.; Shinkai, T.; Zhang, Y.; Wang, X.; Mackie, R.I. Monitoring airborne biotic contaminants in the indoor environment of pig and poultry confinement buildings. Environ. Microbiol. 2012, 14, 1420–1431. [Google Scholar] [CrossRef]
- White, J.K.; Nielsen, J.L.; Madsen, A.M. Microbial species and biodiversity in settling dust within and between pig farms. Environ. Res. 2019, 171, 558–567. [Google Scholar] [CrossRef]
- Griffin, D.W.; Gonzalez-Martin, C.; Hoose, C.; Smith, D.J. Global-scale atmospheric dispersion of microorganisms. In Microbiology of Aerosols, 1st ed.; Anne-Marie, D., Pierre, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 155–194. [Google Scholar]
- Reche, I.; D’Orta, G.; Mladenov, N.; Winget, D.M.; Suttle, C.A. Deposition rates of viruses and bacteria above the atmospheric boundary layer. ISME J. 2018, 12, 1154–1162. [Google Scholar] [CrossRef]
- Dee, S.; Otake, S.; Oliveira, S.; Deen, J. Evidence of long distance airborne transport of porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Vet. Res. 2009, 40, 39. [Google Scholar] [CrossRef]
- Xue, F.; Yang, Y.; Zou, S.; Zhang, Y.; Yue, D.; Zhao, Y.; Lai, S. Characterization of airborne bacteria and fungi at a land-sea transition site in Southern China. Sci. Total Environ. 2022, 849, 157786. [Google Scholar] [CrossRef]
- Bai, W.; Li, Y.; Xie, W.; Ma, T.; Hou, J.; Zeng, X. Vertical variations in the concentration and community structure of airborne microbes in PM2.5. Sci. Total Environ. 2021, 760, 143396. [Google Scholar] [CrossRef]
- Xu, C.; Chen, H.; Liu, Z.; Sui, G.; Li, D.; Kan, H.; Chen, J. The decay of airborne bacteria and fungi in a constant temperature and humidity test chamber. Environ. Int. 2021, 157, 106816. [Google Scholar] [CrossRef]
- Cambra-López, M.; Hermosilla, T.; Aarnink, A.J.A.; Ogink, N.W.M. A methodology to select particle morpho-chemical characteristics to use in source apportionment of particulate matter from livestock houses. Comput. Electron. Agric. 2012, 81, 14–23. [Google Scholar] [CrossRef]
- Pu, S.; Peng, S.; Zhu, J.; Liu, Z.; Long, D.; Lim, T. Characteristics of PM2.5 and Its Correlation with Feed, Manure and NH3 in a Pig-Fattening House. Toxics 2022, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Donham, K.J. Hazardous agents in agricultural dusts and methods of evaluation. Am. J. Ind. Med. 1986, 10, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, P.G.; Yadav, S. Characterization of PM2.5 by X-ray diffraction and scanning electron microscopy–energy dispersive spectrometer: Its relation with different pollution sources. Int. J. Environ. Sci. Technol. 2014, 11, 217–232. [Google Scholar] [CrossRef]
- Aloyan, A.E.; Arutyunyan, V.O.; Lushnikov, A.A.; Zagaynov, V.A. Transport of coagulating aerosol in the atmosphere. J. Aerosol Sci. 1997, 28, 67–85. [Google Scholar] [CrossRef]
- Xie, Z.; Li, Y.; Lu, R.; Li, W.; Fan, C.; Liu, P.; Wang, W. Characteristics of total airborne microbes at various air quality levels. J. Aerosol Sci. 2018, 116, 57–65. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Zhou, M.; Zhang, H.; Li, Z.; Zhang, H.; Hu, J.; Yao, X.; Lou, L.; Xi, C.; et al. Airborne microorganisms exacerbate the formation of atmospheric ammonium and sulfate. Environ. Pollut. 2020, 263, 114293. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, X.; Wang, T.; Wang, B.; Li, C.; Zeng, G. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. [Google Scholar] [CrossRef]
- Almaguer, M.; Aira, M.J.; Rodríguez-Rajo, F.J.; Rojas, T.I. Temporal dynamics of airborne fungi in Havana (Cuba) during dry and rainy seasons: Influence of meteorological parameters. Int. J. Biometeorol. 2014, 58, 1459–1470. [Google Scholar] [CrossRef]
- Smets, W.; Moretti, S.; Denys, S.; Lebeer, S. Airborne bacteria in the atmosphere: Presence, purpose, and potential. Atmos. Environ. 2016, 139, 214–221. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, T.; Su, J.; Zhao, L.L.; Wang, H.; Fang, X.M.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and Composition of Airborne Fungal Community Associated with Particulate Matters in Beijing during Haze and Non-haze Days. Front. Microbiol. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Park, J.; Jeong, H.; Kim, M. Evaluation of the microbiome composition in particulate matter inside and outside of pig houses. J. Anim. Sci. Technol. 2021, 63, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Xie, S.; Zeng, M.; Xu, X.; Li, Y.; Liu, X.; Wang, X. Impacts of pile temperature on antibiotic resistance, metal resistance and microbial community during swine manure composting. Sci. Total Environ. 2020, 744, 140920. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Du, R.; Ren, W.; Lu, Z.; Fu, P. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 2018, 610, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.D.; Polson, S.W.; Don, R.; Jacques, R.; Jack, G.; Robin, M.; Wommack, K.E. Impacts of poultry house environment on poultry litter bacterial community composition. PLoS ONE 2011, 6, e24785. [Google Scholar] [CrossRef]
- Freedman, J.C.; Shrestha, A.; McClane, B.A. Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxics 2016, 8, 73. [Google Scholar] [CrossRef]
- Sukumaran, V.; Senanayake, S. Bacterial skin and soft tissue infections. Aust. Prescr. 2016, 39, 159–163. [Google Scholar] [CrossRef]
- Vacher, G.; Niculita-Hirzel, H.; Roger, T. Immune responses to airborne fungi and non-invasive airway discases. Semin. Immunopathol. 2014, 37, 83–96. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
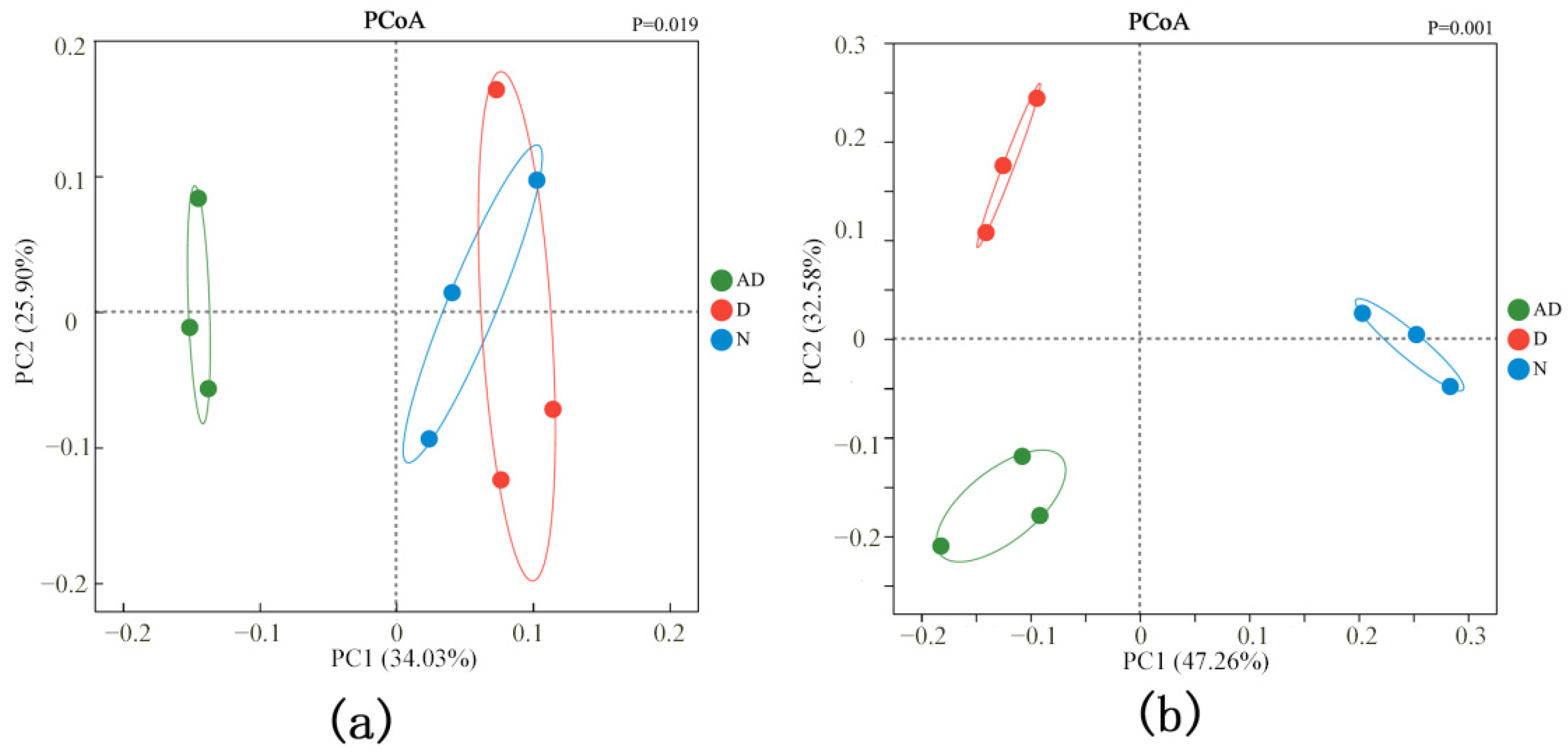
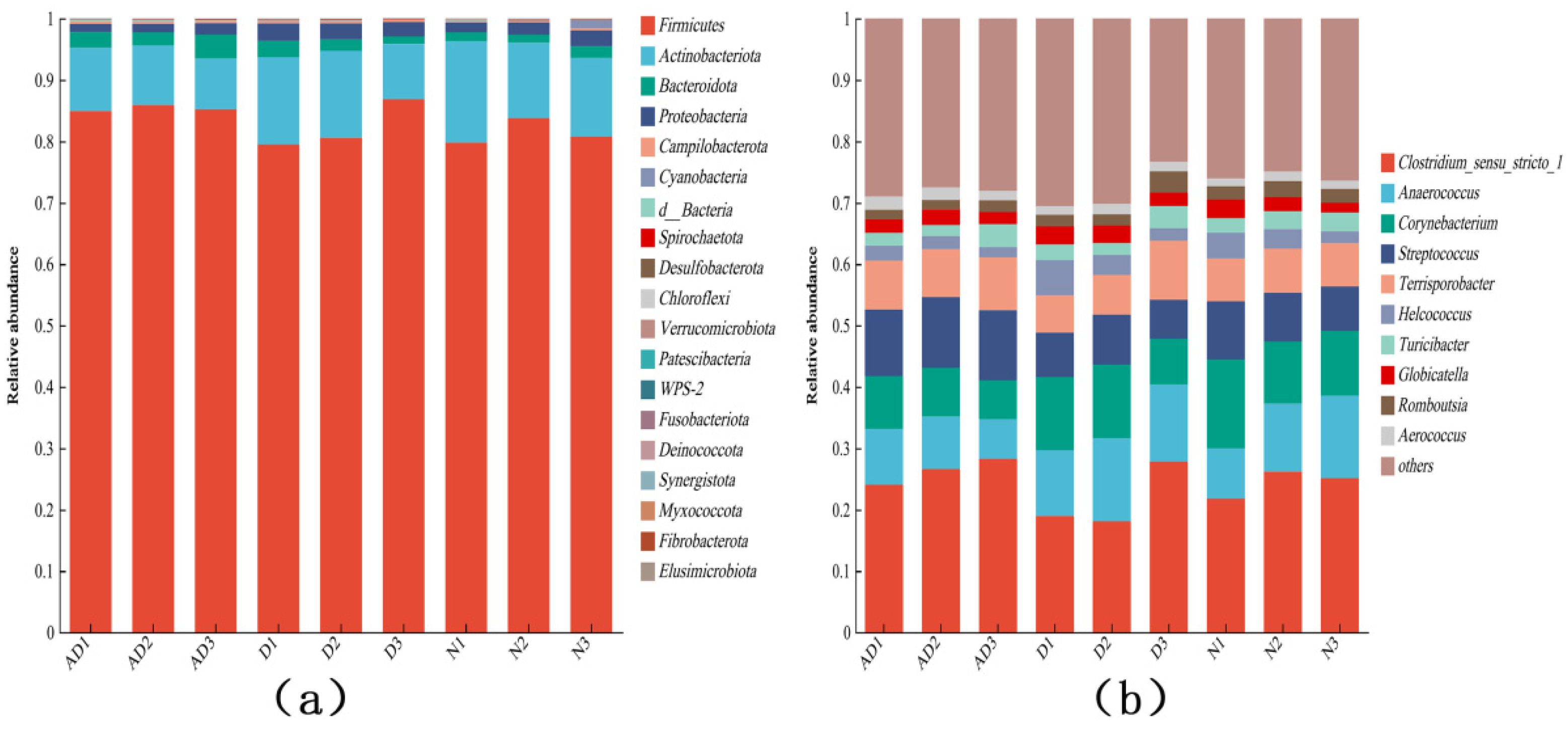

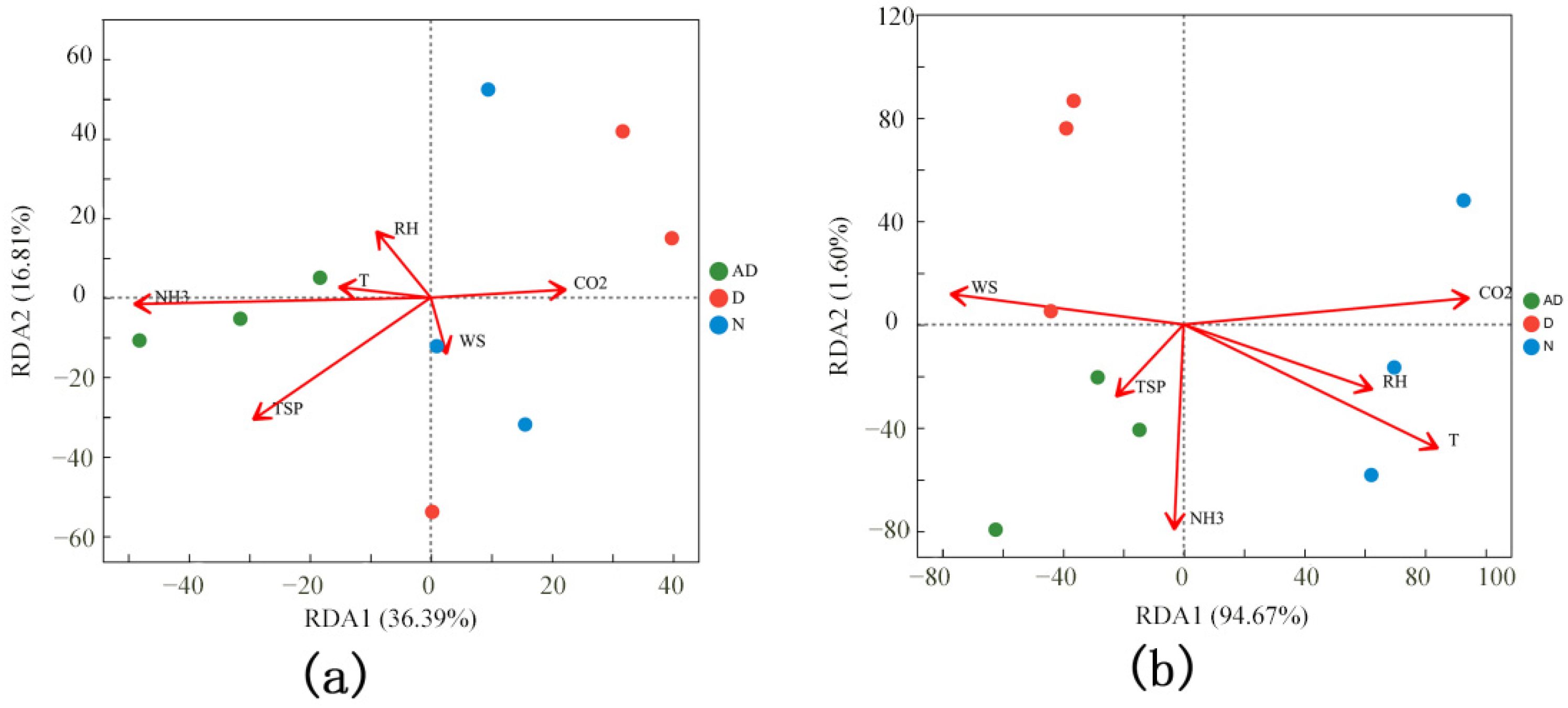
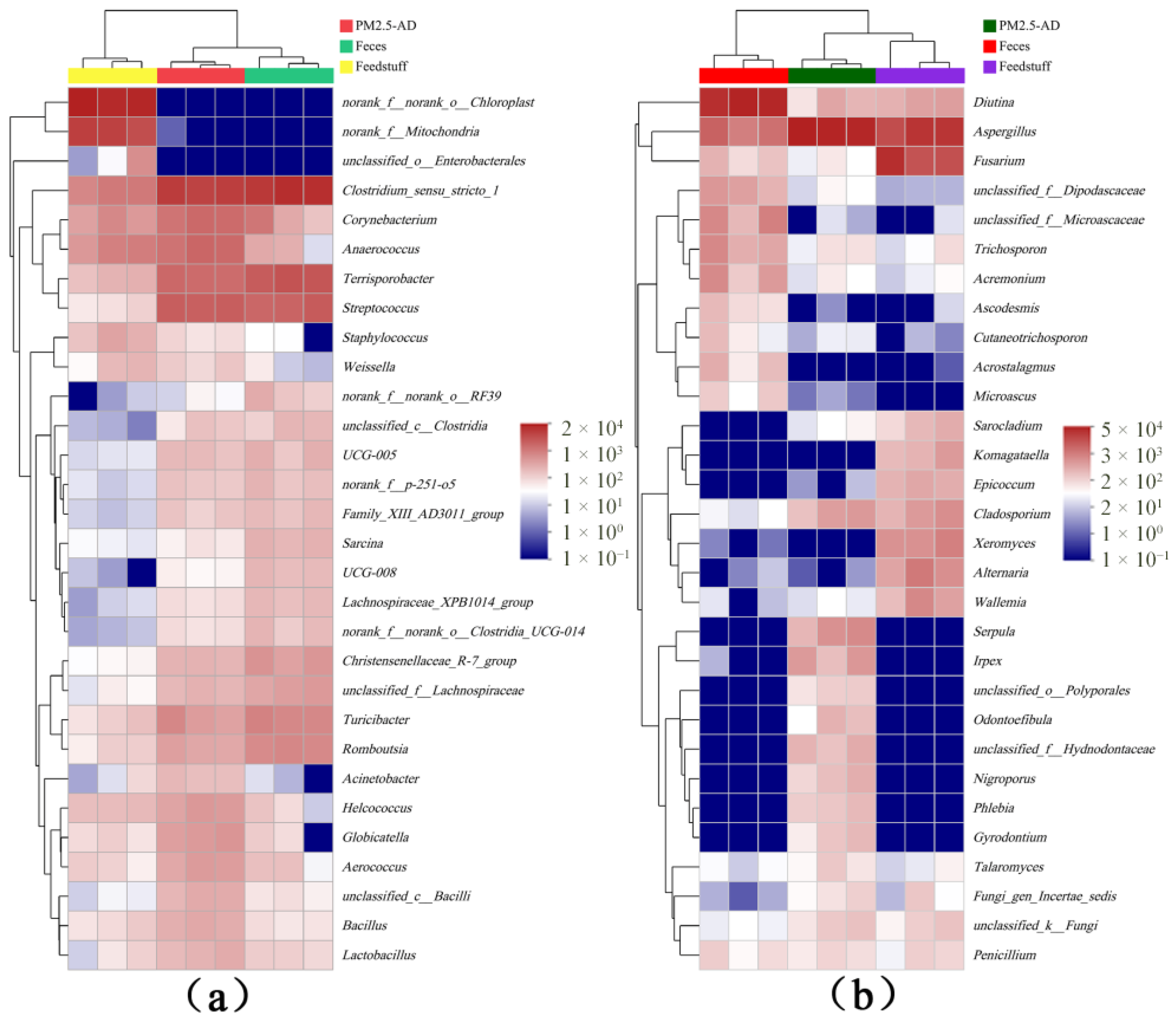
| Sample Name | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| AD | 329.5 ± 72.45 B | 327.23 ± 72.89 B | 2.71 ± 0.44 B | 0.18 ± 0.07 a |
| D | 325.8 ± 57.33 B | 325.10 ± 57.09 B | 2.82 ± 0.07 B | 0.13 ± 0.02 ab |
| N | 606.30 ± 5.94 A | 601.40 ± 6.50 A | 4.18 ± 0.12 A | 0.05 ± 0.01 b |
| Sample Name | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| AD | 918.90 ± 54.42 Aa | 899.30 ± 53.15 Aa | 4.45 ± 0.09 a | 0.05 ± 0.01 a |
| D | 701.30 ± 82.88 Bb | 690.30 ± 81.07 Bb | 4.39 ± 0.28 a | 0.04 ± 0.01 a |
| N | 725.90 ± 19.93 b | 711.20 ± 16.07 b | 4.31 ± 0.07 a | 0.05 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Jian, Y.; Zhu, J.; Tian, K.; Tan, Q.; Zhao, R. Analysis of the Morphological Characteristics of PM2.5 and Its Microbiological Composition in a Fattening Pig House. Sustainability 2024, 16, 10249. https://doi.org/10.3390/su162310249
Tang M, Jian Y, Zhu J, Tian K, Tan Q, Zhao R. Analysis of the Morphological Characteristics of PM2.5 and Its Microbiological Composition in a Fattening Pig House. Sustainability. 2024; 16(23):10249. https://doi.org/10.3390/su162310249
Chicago/Turabian StyleTang, Mingfeng, Yue Jian, Jiaming Zhu, Kun Tian, Qiong Tan, and Run Zhao. 2024. "Analysis of the Morphological Characteristics of PM2.5 and Its Microbiological Composition in a Fattening Pig House" Sustainability 16, no. 23: 10249. https://doi.org/10.3390/su162310249
APA StyleTang, M., Jian, Y., Zhu, J., Tian, K., Tan, Q., & Zhao, R. (2024). Analysis of the Morphological Characteristics of PM2.5 and Its Microbiological Composition in a Fattening Pig House. Sustainability, 16(23), 10249. https://doi.org/10.3390/su162310249







