Composting as a Sustainable Solution for the Management of Plant Biomass Contaminated with Hg and As from Puddles Generated by Small-Scale Gold Mining in the Municipality of Unión Panamericana, Colombian Pacific
Abstract
1. Introduction
2. Materials and Methods
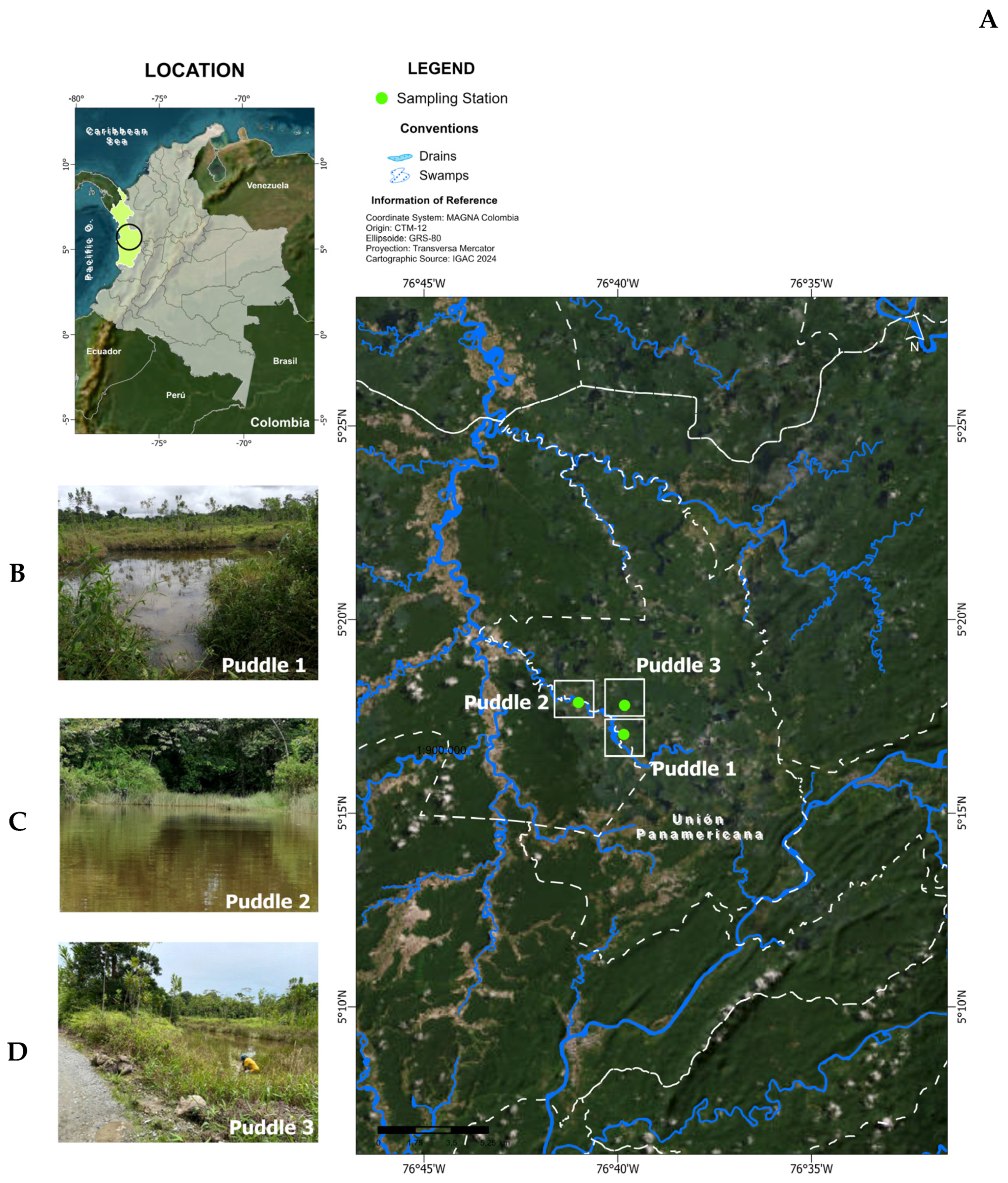
2.1. Study Area
2.2. Sample Collection
2.3. Sample Analysis
2.4. Analysis of Bioavailability of Hg During the Composting Process
2.5. Quality Assurance
2.6. Analysis of Physicochemical Properties of Composting
2.7. Experimental Design
2.8. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Compost Properties in Different Treatments
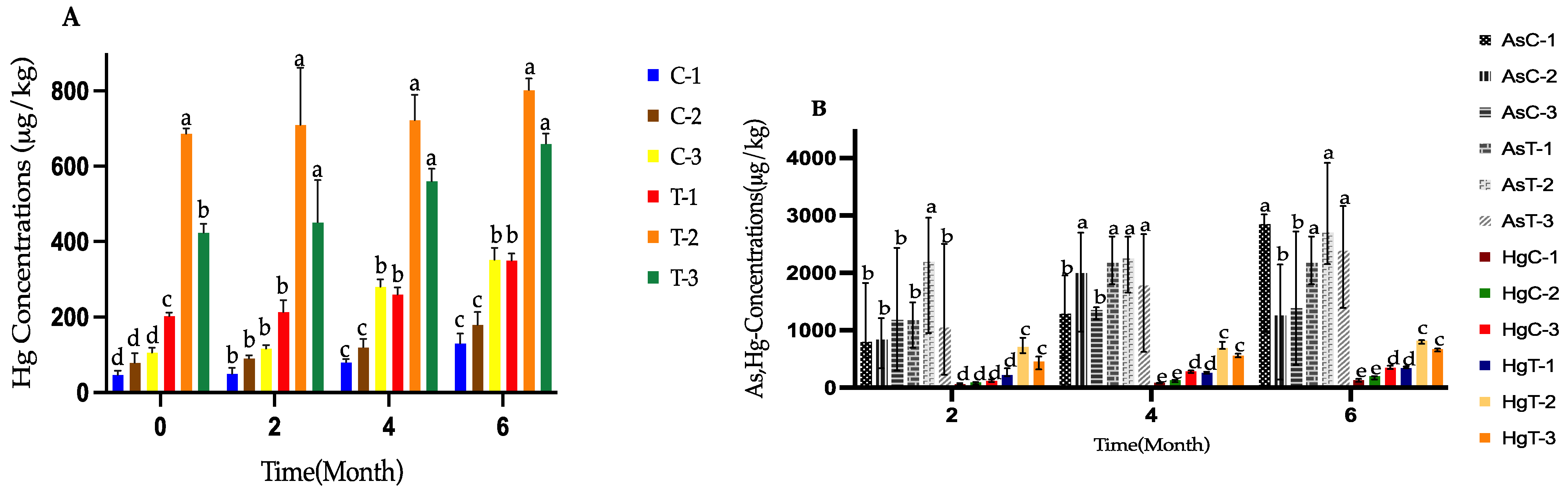
3.2. Concentration of Hg and As in the Treatments During the Composting Process
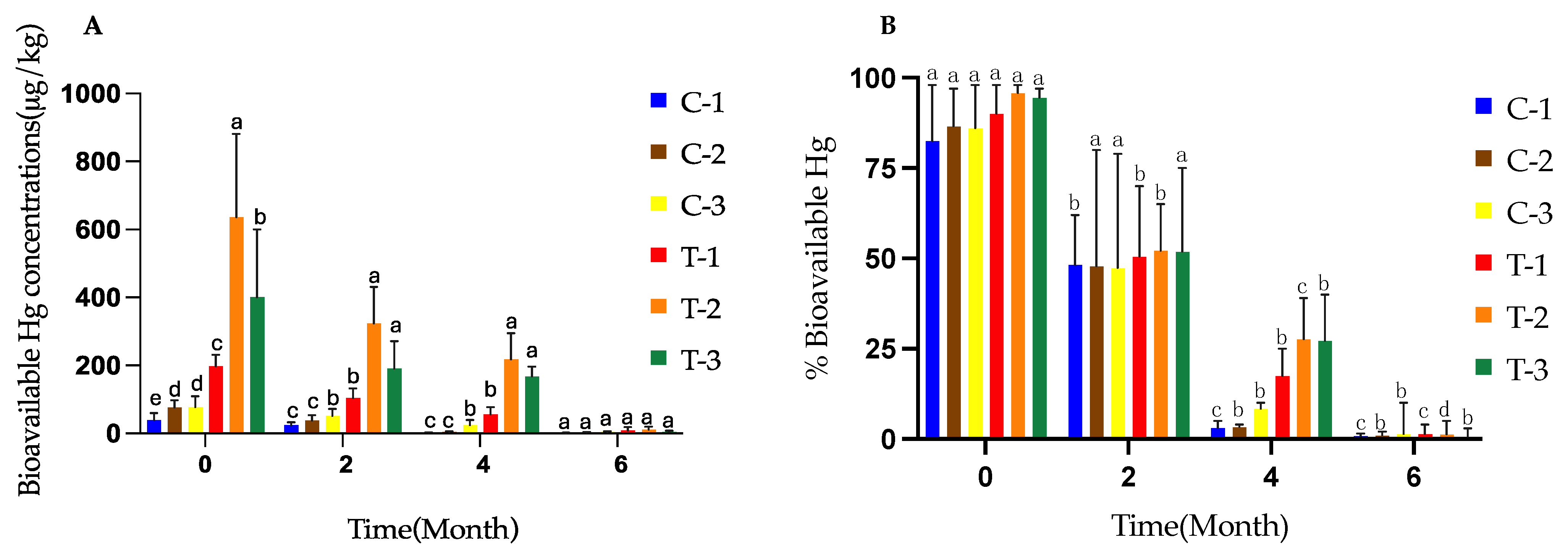
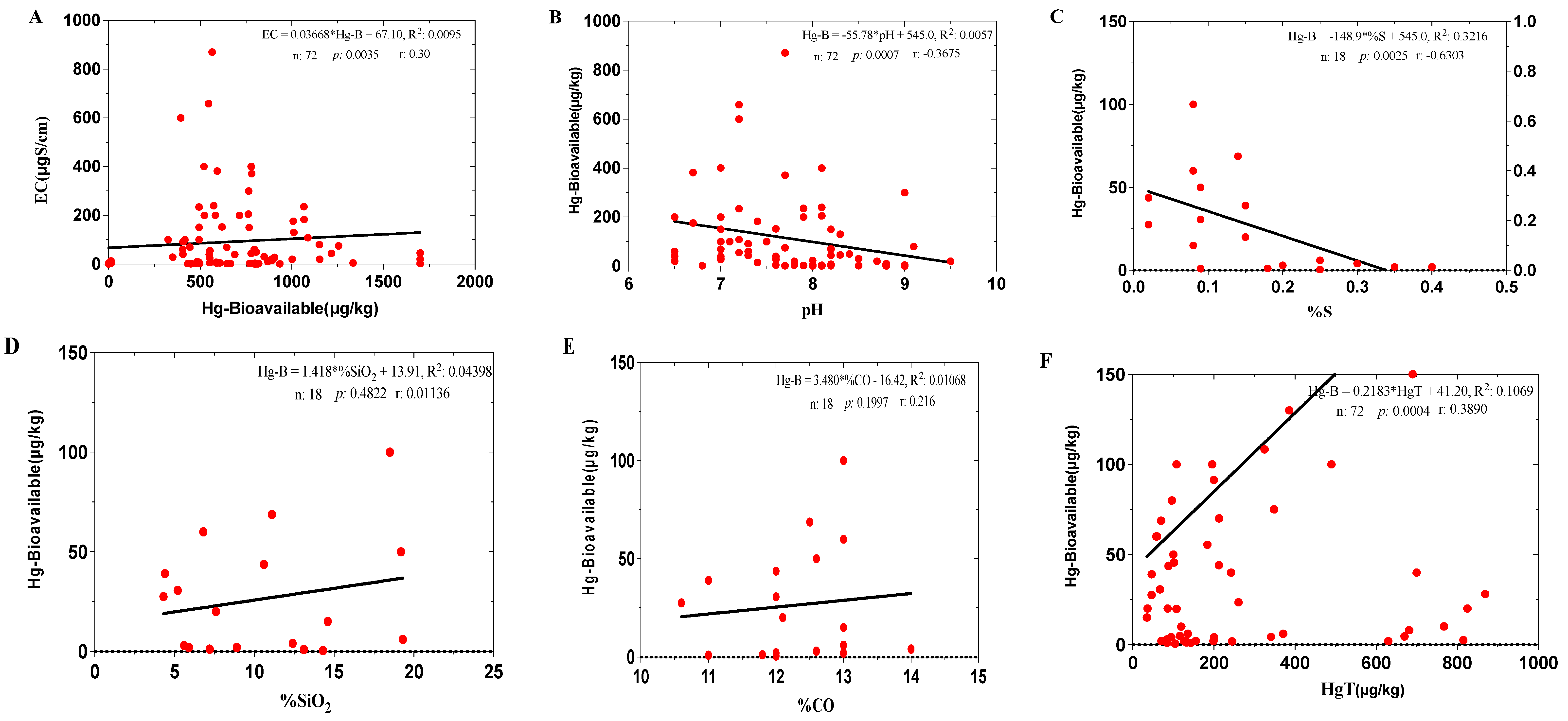
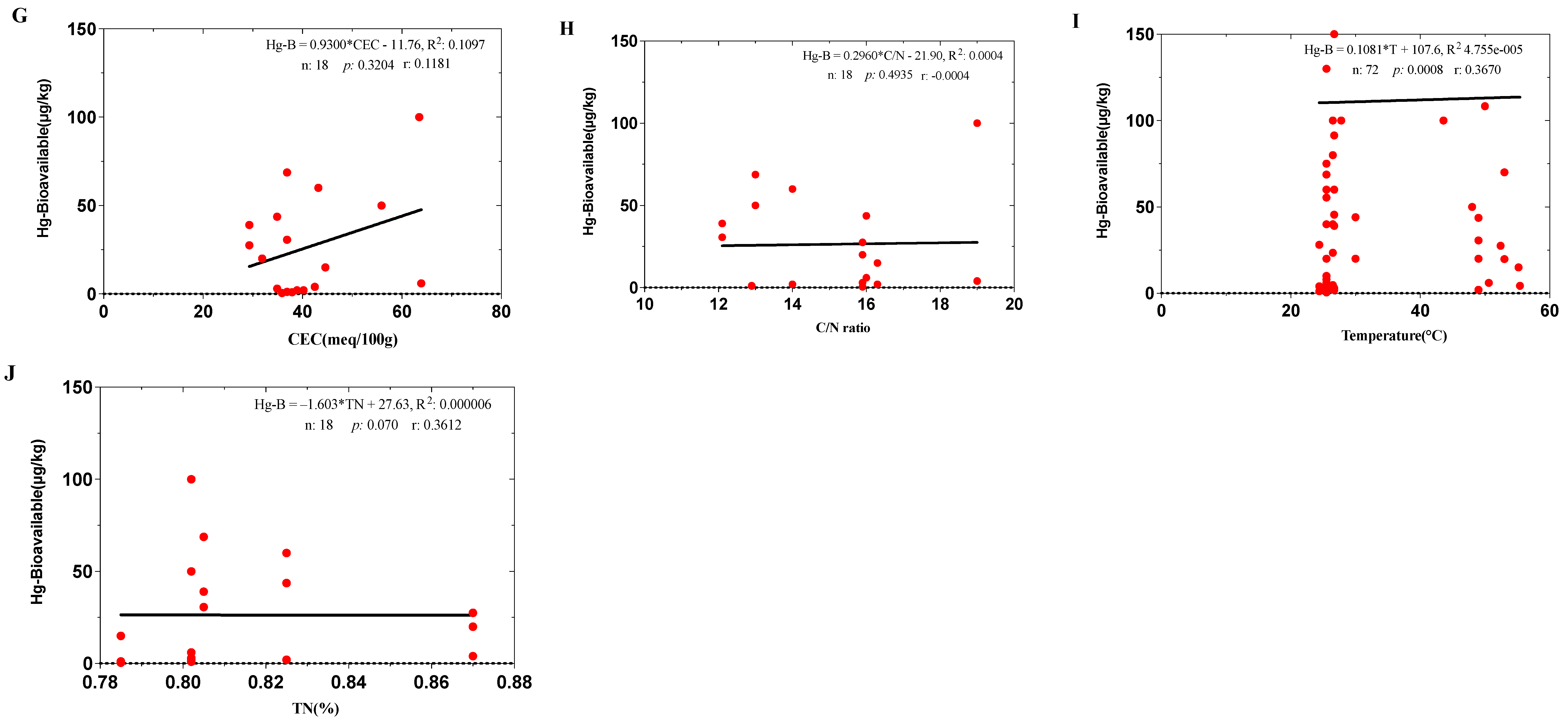
3.3. Bioavailability of Hg in the Experimental Treatments During the Composting Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Amit, R.J.; Mishra, N.; Singh, D.K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Li, Y.; Yan, M.; Zeng, G.; Zhang, J.; Zhang, J.; Tan, X. Microbiological study on bioremediation of 2,2‚Ä≤,4,4‚Ä≤-tet bromodiphenyl ether (BDE-47) con- taminated soil by agricultural waste composting. Appl. Microbiol. Biotechnol. 2017, 100, 9709–9718. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Zeng, C.; Li, Y.; Zhu, L.; Wu, J.; Chen, J.; Wei, Z. Assessment contributions of physicochemical properties and bacterial community to mitigate the bioavailability of heavy metals during composting based on structural equation models. Bioresour. Technol. 2019, 289, 121657. [Google Scholar] [CrossRef]
- Wei, Y.; Li, R.; Lu, N.; Zhang, B. Stabilization of Soil Co-Contaminated with Mercury and Arsenic by Different Types of Biochar. Sustainability 2022, 14, 13637. [Google Scholar] [CrossRef]
- Gilli, R.; Karlen, C.; Weber, M.; Rüegg, J.; Barmettler, K.; Biester, H.; Boivin, P.; Kretzschmar, R. Speciation and Mobility of Mercury in Soils Contaminated by Legacy Emissions from a Chemical Factory in the Rhône Valley in Canton of Valais, Switzerland. Soil Syst. 2018, 2, 44. [Google Scholar] [CrossRef]
- Gao, M.; Su, Y.; Gao, J.; Zhong, X.; Li, H.; Wang, H.; Lü, C.; He, J. Arsenic speciation transformation in soils with high geological background: New insights from the governing role of Fe. Chemosphere 2022, 302, 134860. [Google Scholar] [CrossRef]
- UN Environment. Global Mercury Assessment 2018; UN Environment Programme, Chemicals and Health Branch: Geneva, Switzerland, 2019; 62p. [Google Scholar]
- Xiang, Y.; Zhu, A.; Guo, Y.; Liu, G.; Chen, B.; He, B.; Liang, Y.; Yin, Y.; Cai, Y.; Jiang, G. Decreased bioavailability of both inorganic mercury and methylmercury in anaerobic sediments by sorption on iron sulfide nanoparticles. J. Hazard. Mater. 2022, 424, 127399. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef]
- Wang, F.; Yao, W.; Zhang, W.; Miao, L.; Wang, Y.; Zhang, H.; Ding, Y.; Zhu, W. Humic acid characterization and heavy metal behaviour during vermicomposting of pig manure amended with 13C-labelled rice straw. Waste Manag. Res. 2022, 40, 736–744. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, Y.; Wu, Y.; Chen, Y.; Zeng, G.; Zhang, J.; Li, H. Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour Technol. 2017, 243, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, Z.; Wang, C.; Wei, J.; Wei, Y.; Chen, M. Understanding of mercury and methylmercury transformation in sludge composting by metagenomic analysis. Water Res. 2022, 226, 119204. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Li, Y.; Liu, Y.; Zhang, D.; Wu, Y.; Li, H.; Xu, F.; Li, M. The Effect of Composting on the Multiple Heavy Metals in the River Sediment Investigated by Multivariate Analysis. Water Air Soil Pollut. 2021, 232, 20. [Google Scholar] [CrossRef]
- Manea, E.E.; Bumbac, C.; Dinu, L.R.; Bumbac, M.; Nicolescu, C.M. Composting as a Sustainable Solution for Organic Solid Waste Management: Current Practices and Potential Improvements. Sustainability 2024, 16, 6329. [Google Scholar] [CrossRef]
- Guo, H.-N.; Wang, L.-X.; Liu, H.-T. Potential mechanisms involving the immobilization of Cd, As and Cr during swine manure composting. Sci. Rep. 2020, 10, 16632. [Google Scholar] [CrossRef]
- Martínez-Madrid, D.E.; Marrugo-Negrete, J. Effects of adding amendments on the immobilization of heavy metals in mining soils of southern Bolívar, Colombia. Cienc. Tecnol. Agropecu. 2021, 22, e2272. [Google Scholar]
- Rivera, J.; Reyes, J.; Cuervo, J.; Martínez-Cordón, M.; Zamudio, A. Effect of biochar amendments on the growth and development of ‘Vera’ crisp lettuce in four soils contaminated with cadmium. Chil. J. Agric. Res. 2022, 82, 244–255. [Google Scholar] [CrossRef]
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, J.; Díez, S.; Morales-Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M.P. Distribution of chemical forms of mercury in sediments from abandoned ponds created during former gold mining operations in Colombia. Chemosphere 2020, 258, 127319. [Google Scholar] [CrossRef]
- PDUP. Plan de Desarrollo Municipio de Unión Panamericana—Comprometidos con el Cambio para Servir 2020—2023—Alcaldía Municipal de Unión Panamericana Chocó. 2020. Available online: https://www.unionpanamericana-choco.gov.co/normatividad/plan-de-desarrollo-municipio-de-union-panamericana-comprometidos (accessed on 14 August 2024).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- PMGRD. Plan de Gestión del Riesgo de Desastres del Municipio de Unión Panamericana-Chocó. 2020–2023—Alcaldía Municipal de Unión Panamericana Chocó. Available online: https://www.unionpanamericana-choco.gov.co/normatividad/plan-de-gestion-del-riesgo-de-desastres-del-municipio (accessed on 14 August 2024).
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, J.; Díez, S.; Morales-Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M.P. Mercury distribution in different environmental matrices in aquatic systems of abandoned gold mines, western Colombia: Focus on human health. J. Hazard. Mater. 2020, 404, 124080. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, R.; Szteke, B.; Reczajska, W. Mercury determination in food of plant origin by cold vapour atomic absorption spectrometry (CVAAS). Roczniki Panstwowego Zakladu Higieny 1996, 47, 223–230. [Google Scholar] [PubMed]
- Szkoda, J.; Zmudzki, J.; Grzebalska, A. Determination of arsenic in biological material by hydride generation atomic absorption spectrometry method. Bull. Vet. Inst. Pulawy 2006, 50, 269–272. [Google Scholar]
- Vázquez, F.; Pérez, B.; Río, S. Assessment of metal bioavailability in the vineyard soil-grapevine system using different extraction methods. Food Chem. 2016, 208, 199–208. [Google Scholar] [CrossRef]
- Kjeldahl, J. “Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern” (New method for the determination of nitrogen in organic substances). Z. Anal. Chem. 1883, 22, 366–383. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Wenk, H.R.; Bulakh, A. Minerals. Their Constitution and Origin; University Press Cambridge: Cambridge, UK, 2004. [Google Scholar]
- Jain, M.S.; Kalamdhad, A.S. Efficacy of batch mode rotary drum composter for management of aquatic weed (Hydrilla verticillata (L.f.) Royle). J. Environ. Manag. 2018, 221, 20–27. [Google Scholar] [CrossRef]
- Maturi, K.C.; Banerjee, A.; Kalamdhad, A.S. Assessing mobility and chemical speciation of heavy metals during rotary drum composting of Ageratum conyzoides. Environ. Technol. Innov. 2021, 24, 101871. [Google Scholar] [CrossRef]
- Clima Colombia. 2024. Available online: https://www.clima.com/colombia/choco/las-animas (accessed on 12 August 2024).
- Plan de Desarrollo Municipio Unión Panamericana, 2024–2027. Available online: https://www.unionpanamericana-choco.gov.co/noticias/contruccion-del-plan-desarrollo-20242027 (accessed on 12 August 2024).
- Hussain, S.; Yang, J.; Hussain, J.; Sattar, A.; Ullah, S.; Hussain, I.; Rahman, S.; Zandi, P.; Xia, X.; Zhang, L. Mercury fractionation, bioavailability, and the major factors predicting its transfer and accumulation in soil–wheat systems. Sci. Total Environ. 2022, 847, 157432. [Google Scholar] [CrossRef]
- Li, X.; Shi, X.-S.; Lu, M.-Y.; Zhao, Y.-Z.; Li, X.; Peng, H.; Guo, R.-B. Succession of the bacterial community and functional characteristics during continuous thermophilic composting of dairy manure amended with recycled ceramsite. Bioresour. Technol. 2019, 294, 122044. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Guo, Q.J.; Yang, J.X.; Ma, J.; Chen, G.; Chen, T.B.; Zhu, G.X.; Wang, J.; Zhang, G.X.; Wang, X.; et al. Comparison of chelates for enhancing Ricinus communis L. phytoremediation of Cd and Pb contaminated soil. Ecotoxicol. Environ. Saf. 2016, 133, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Gao, X.; Tu, X.; Mao, Y.; Jiang, L.; Lu, Z.; Du, Y. Heavy metal remediation in sludge compost: Recent progress. J. Renew. Mater. 2022, 10, 469–486. [Google Scholar] [CrossRef]
- Sardroudi, N.P.; Sorolla, S.; Casas, C.; Bacardit, A. A Study of the Composting Capacity of Different Kinds of Leathers, Leatherette and Alternative Materials. Sustainability 2024, 16, 2324. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, W.; Men, M.; Bello, A.; Xu, X.; Xu, B.; Deng, L.; Jiang, X.; Sheng, S.; Wu, X.; et al. Microbial Community Successionand Response to Environmental Variables During Cow Manure and Corn Straw Composting. Front. Microbiol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, G.; Chen, Y.; Yu, M.; Yu, Z.; Li, H.; Yu, Y.; Huang, H. Effects of physico- chemical parameters on the bacterial and fungal communities during agricultural waste composting. Bioresour. Technol. 2011, 102, 2950–2956. [Google Scholar] [CrossRef]
- Al-Bataina, B.B.; Young, T.M.; Ranieri, E. Effects of compost age on the release of nutrients. Int. Soil Water Conserv. Res. 2016, 4, 230–236. [Google Scholar] [CrossRef]
- Tibu, C.; Annang, T.Y.; Solomon, N.; Yirenya-Tawiah, D. Effect of the composting process on physicochemical properties and concentration of heavy metals in market waste with additive materials in the Ga West Municipality, Ghana. Int. J. Recycl. Org. Waste Agric. 2019, 8, 393–403. [Google Scholar] [CrossRef]
- Qian, X.; Shen, G.; Wang, Z.; Guo, C.; Liu, Y.; Lei, Z.; Zhang, Z. Co- composting of livestock manure with rice straw: Characteriza- tion and establishment of maturity evaluation system. Waste Manag. 2014, 34, 530–535. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil. Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Khater, E.S.G. Some Physical and Chemical Properties of Compost. Int. J. Waste Resour. 2015, 5, 72–79. [Google Scholar] [CrossRef]
- Sidi, N.; Aris, A.Z.; Talib, S.N.; Johan, S.; Tengku, M. Yusoff, T.S.; Ismail, M.Z. Influential factors on the cation exchange capacity in sediment of merambong shoal, Johor. Procedia Environ. Sci. 2015, 30, 186–189. [Google Scholar] [CrossRef]
- Paradelo, R.; Barral, M.T. Evaluation of the potential capacity as biosorbents of two Msw composts with different Cu, Pb and Zn concentrations. Bioresour. Technol. 2012, 104, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Nartey, E.G. Faecal Sludge Reuse in Urban and Peri-Urban Crop Production. Master’s Thesis, University of Ghana, Accra, Ghana, 2013. [Google Scholar]
- Rashad, F.M.; Saleh, W.D.; Moselhy, M.A. Bioconversion of rice straw and certain agro-industrial wastes to amendments for organic farming systems: 1. Composting, quality, stability and maturity indices. Bioresour. Technol. 2010, 101, 5952–5960. [Google Scholar] [CrossRef] [PubMed]
- Diacono, M.; Montemurro, F. Effectiveness of organic wastes as fertilizers and amendments in salt-affected soils. Agriculture 2015, 5, 221–230. [Google Scholar] [CrossRef]
- Janowska, B.; Szymański, K.; Sidełko, R.; Siebielska, I.; Walendzik, B. Assessment of mobility and bioavailability of mercury compounds in sewage sludge and composts. Environ. Res. 2017, 156, 394–403. [Google Scholar] [CrossRef]
- Xu, S.; Li, L.; Zhan, J.; Guo, X. Variation and factors on heavy metal speciation during co-composting of rural sewage sludge and typical rural organic solid waste. J. Environ. Manag. 2022, 306, 114418. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Zhang, C.; Luo, Y.; Zhang, M.; Zhang, Y.; Wang, D. Variation characteristics of mercury during municipal sludge composting and its land use. Environ. Chem. 2021, 40, 2226–2233. [Google Scholar]
- United state Environmental Protection Agency. Method 7473 (SW-846). Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absortion Spectrophotometry. Methods (1998). Available online: https://www.epa.gov/sites/production/files/2015-07/documents/epa-7473.pdf (accessed on 14 August 2024).
- Salazar-Camacho, C.; Salas-Moreno, M.; Marrugo-Madrid, S.; Paternina-Uribe, R.; Marrugo-Negrete, J.; Díez, S. A human health risk assessment of methylmercury, arsenic and metals in a tropical river basin impacted by gold mining in the Colombian Pacific región. Environ. Res. 2022, 212, 113120. [Google Scholar] [CrossRef]
- Caicedo-Rivas, G.; Salas-Moreno, M.; Marrugo-Negrete, J. Health Risk Assessment for Human Exposure to Heavy Metals via Food Consumption in Inhabitants of Middle Basin of the Atrato River in the Colombian Pacific. Int. J. Environ. Res. Public Health 2023, 20, 435. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Bencko, V.; Foong, F.Y.L. The history of arsenical pesticides and health risks related to the use of Agent Blue. Ann. Agric. Environ. Med. 2016, 24, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.; Wang, X.; Wu, L.; Wang, F.; Chen, Y.; Yang, H.; Zhang, Z.; Zhao, X. Arsenic accumulation and speciation in rice grown in arsanilic acid-elevated paddy soil. Ecotoxicol. Environ. Saf. 2017, 137, 172–178. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Gong, J.; Xue, X.; Yang, H. Preparation of zinc chloride and sulfur modified cornstalk biochar and its stabilizatiom effect on mercury contaminated soil. Chin. J. Environ. Eng. 2021, 15, 1403–1408. [Google Scholar]
- Pannu, R.; Siciliano, S.D.; Driscoll, N.J.O. Quantifying the effects of soil temperature, moisture and sterilization on elemental mercury formation in boreal soils. Environ. Pollut. 2014, 193, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Papale, M.; Romano, I.; Finore, I.; Lo Giudice, A.; Piccolo, A.; Cangemi, S.; Di Meo, V.; Nicolaus, B.; Poli, A. Prokaryotic Diversity of the Composting Thermophilic Phase: The Case of Ground Coffee Compost. Microorganisms 2021, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.Q.; Reinfelder, J.R.; Hines, M.E.; Barkay, T. Syntrophic pathways for microbial mercury methylation. ISME J. 2018, 12, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xi, B.; Tan, W. Effects of sulfur-rich biochar amendment on microbial methylation of mercury in rhizosphere paddy soil and methylmercury accumulation in rice. Environ. Pollut. 2021, 286, 117290. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Li, G.; Wang, S.; Duan, L.; Mulder, J.; Cornelissen, G.; Cheng, Z.; Yang, S.; Hou, D. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 2018, 621, 819–826. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Shen, Z.; Tsang, D.C.W.; Rinklebe, J.; Hou, D. Sulfur-modified biochar as a soil amendment to stabilize mercury pollution: An accelerated simulation of long-term aging effects. Environ. Pollut. 2020, 264, 114687. [Google Scholar] [CrossRef]
- Hu, H.; Gao, Y.; Tan, W.; Xi, B. Effects of dissolved organic matter on mercury speciation in rice rhizosphere amended with sulfur rich biochar. Soil Environ. Health 2023, 1, 100022. [Google Scholar] [CrossRef]

| Property | Treatments | |||||
|---|---|---|---|---|---|---|
| C-1 | C-2 | C-3 | T-1 | T-2 | T-3 | |
| Hg Total (μg/kg) | 130.9 ± 20.9 d | 180.7 ± 26.8 d | 353.1 ± 25.2 c | 350.9 ± 14.2 c | 802.5 ± 25.1 a | 660.5 ± 22.1 b |
| Hg Bioavailable (μg/kg) | 0.9 ± 0.6 | 1.1 ± 0.8 | 1.4 ± 0.9 | 1.4 ± 0.8 | 1.3 ± 0.5 | 0.7 ± 0.5 |
| As Total (μg/kg) | 2844.5 ± 792.7 a | 748.5 ± 841.5 e | 1381.0 ± 950.0 d | 2174.6 ± 310.6 c | 2699.9 ± 621.0 a | 2384.4 ± 706.5 b |
| pH | 8.5 ± 0.5 a | 8.3 ± 0.4 a | 7.9 ± 0.2 b | 8.7 ± 0.9 a | 8.5 ± 0.5 a | 7.6 ± 0.7 b |
| EC (μS/cm) | 853.6 ± 105.6 b | 562.2 ± 49.8 bc | 491.4 ± 28.2 c | 1275.2 ± 114.5 a | 743.2 ± 49.7b c | 543.2 ± 18.1 bc |
| S (%) | 0.12 ± 0.07 | 0.19 ± 0.19 | 0.23 ± 0.11 | 0.14 ± 0.09 | 0.14 ± 0.09 | 0.15 ± 0.14 |
| SiO2 (%) | 5.85 ± 0.83 d | 4.86 ± 0.9 d | 7.93 ± 0.88 d | 14 ± 0.79 b | 19 ± 0.43 a | 11.4 ± 0.93 c |
| CEC (meq/100g) | 38.3 ± 4.3 b | 32.5 ± 5.5 b | 36.2 ± 4.2 b | 39.5 ± 4.6 b | 61.1 ± 4.5 a | 38.1 ± 3.9 b |
| TN (%) | 0.870 ± 0.02 | 0.805 ± 0.02 | 0.802 ± 0.1 | 0.785 ± 0.03 | 0.802 ± 0.04 | 0.826 ± 0.01 |
| C/N ratio | 14 ± 0.5 | 14 ± 0.7 | 15 ± 0.8 | 15 ± 0.9 | 16 ± 0.6 | 16 ± 1.0 |
| CO (%) | 12.5 ± 0.5 ab | 11.2 ± 0.7 b | 12.3 ± 0.6 ab | 12 ± 1.0 b | 12.5 ± 0.5 ab | 13.1 ± 0.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rengifo-Mosquera, G.; Salas-Moreno, M.; Gutierréz-Palacios, H.; Palacios-Torres, Y.; Romaña-Palacios, A.; Marrugo-Negrete, J. Composting as a Sustainable Solution for the Management of Plant Biomass Contaminated with Hg and As from Puddles Generated by Small-Scale Gold Mining in the Municipality of Unión Panamericana, Colombian Pacific. Sustainability 2024, 16, 9940. https://doi.org/10.3390/su16229940
Rengifo-Mosquera G, Salas-Moreno M, Gutierréz-Palacios H, Palacios-Torres Y, Romaña-Palacios A, Marrugo-Negrete J. Composting as a Sustainable Solution for the Management of Plant Biomass Contaminated with Hg and As from Puddles Generated by Small-Scale Gold Mining in the Municipality of Unión Panamericana, Colombian Pacific. Sustainability. 2024; 16(22):9940. https://doi.org/10.3390/su16229940
Chicago/Turabian StyleRengifo-Mosquera, Gysela, Manuel Salas-Moreno, Harry Gutierréz-Palacios, Yuber Palacios-Torres, Allien Romaña-Palacios, and José Marrugo-Negrete. 2024. "Composting as a Sustainable Solution for the Management of Plant Biomass Contaminated with Hg and As from Puddles Generated by Small-Scale Gold Mining in the Municipality of Unión Panamericana, Colombian Pacific" Sustainability 16, no. 22: 9940. https://doi.org/10.3390/su16229940
APA StyleRengifo-Mosquera, G., Salas-Moreno, M., Gutierréz-Palacios, H., Palacios-Torres, Y., Romaña-Palacios, A., & Marrugo-Negrete, J. (2024). Composting as a Sustainable Solution for the Management of Plant Biomass Contaminated with Hg and As from Puddles Generated by Small-Scale Gold Mining in the Municipality of Unión Panamericana, Colombian Pacific. Sustainability, 16(22), 9940. https://doi.org/10.3390/su16229940








