The Removal and Mitigation Effects of Biochar on Microplastics in Water and Soils: Application and Mechanism Analysis
Abstract
1. Introduction
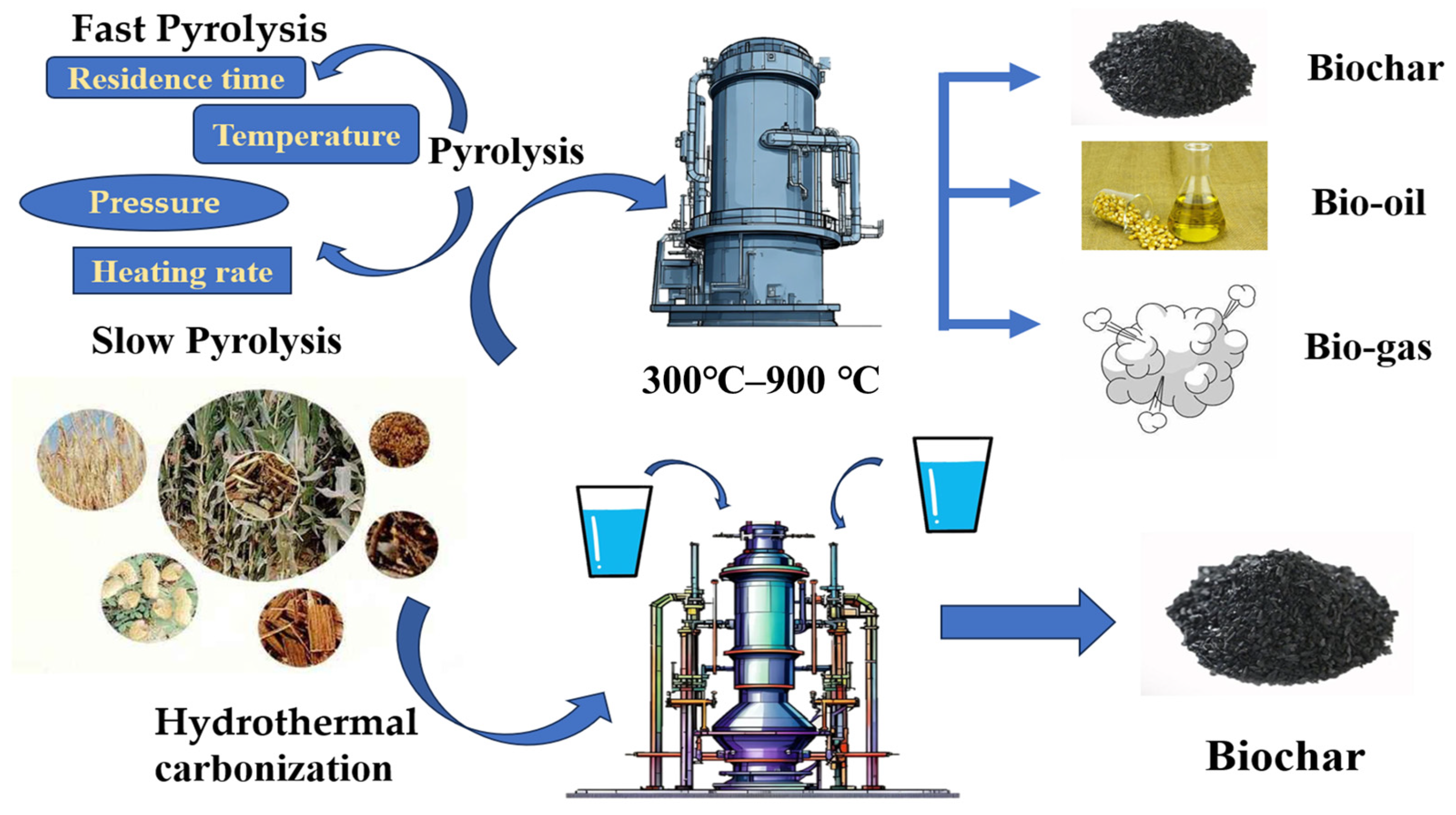
1.1. Biochar
1.1.1. Preparation, Types, and Properties of Biochar
1.1.2. Application of Biochar as a Remediation Material
| Biochar Feedstock | Pollution | Removal Efficiency | Reference |
|---|---|---|---|
| Biomass pyrolysis (waste wood, pig manure, or straw) | VOCs | 50% | [30] |
| Poplar and conifer | Phenanthrene and pentachlorophenol | Greatly reduced | [31] |
| Antibiotic | Penicillin | 44.1% | [32] |
| Oil palm frond | Tannic acid | 67.4% | [33] |
| Phenol | 62.9% | ||
| Corn straw, bamboo | PAHs in carrot root | Greatly reduced | [34] |
| Straw powders | Tetrabromobisphenol A | 97% | [35] |
| Chicken dung biochar | Cu | 45.3% | [36] |
| Casuarina biochar | Cd, Co, Cr, Cu, Ni | Varied reduction | [37] |
| Bur cucumber | Sulfamethazine | Up to 86% reduction | [38] |
| Aminocyclopyrachlor and bentazone | Wood pellet biochar | Varied reduction | [39] |
| Peanut shell | Atrazine and nisulfuron | Greatly reduced | [40] |
| Straw, willow | PAHs | 70.3% (biochar–straw), 29.3% (biochar–willow) | [41] |
| Muffle furnace | Dissolved organic matte | Increase of 5.3–17.7% | [42] |
| Sheep bone | Zn | 57% | [43] |
1.2. Occurrence, Distribution, and Breakdown of Microplastics
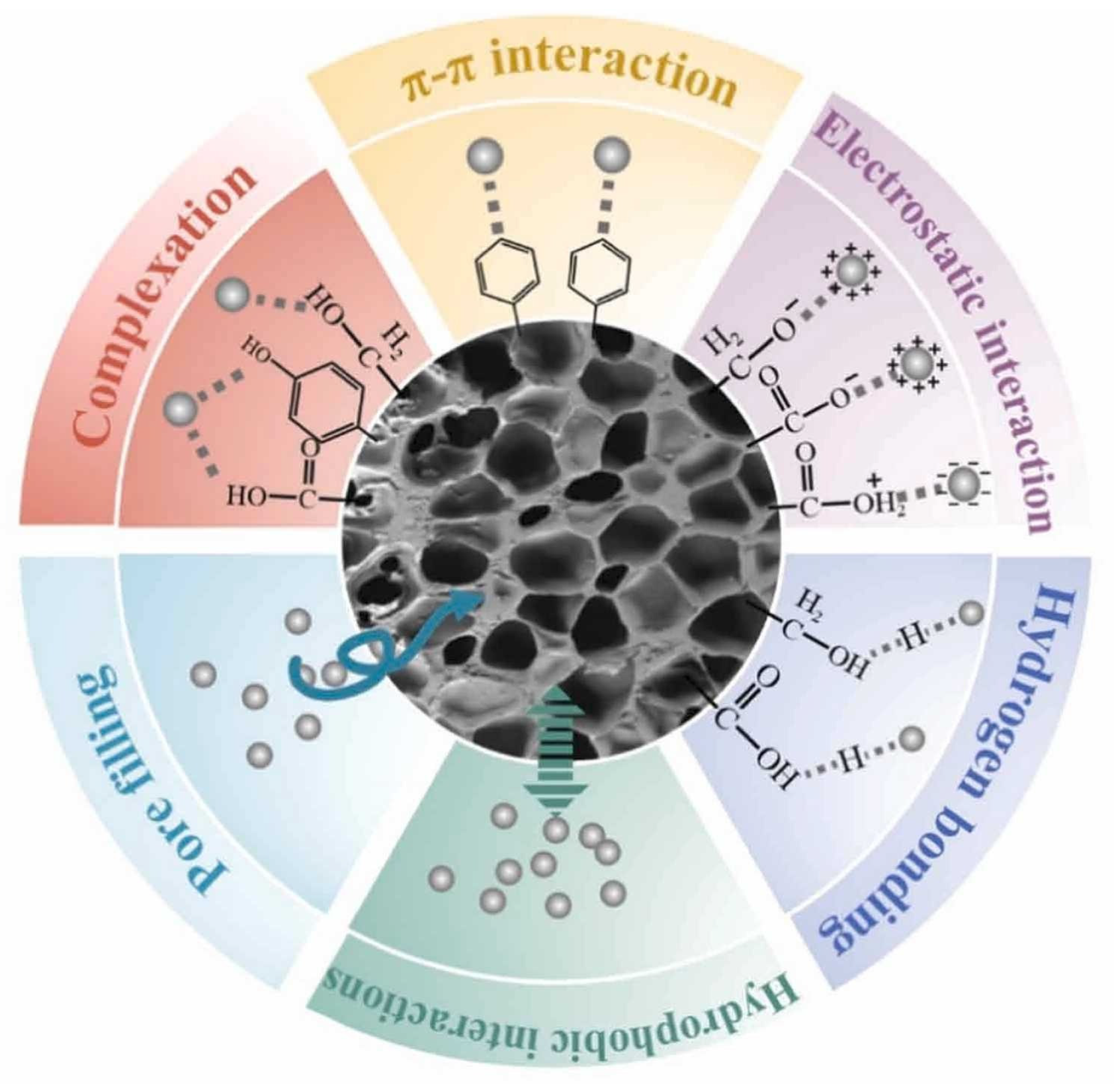
2. Biochar as an Adsorbent for MPs in Water
| Biochar Feedstock | Preparation Parameter | Microplastic | Removal Efficiency | Reference |
|---|---|---|---|---|
| Corn straw and hardwood feedstock | Pyrolysis, heated 300 °C, 400 °C, 500 °C | PSMPs | >95% (packed column) | [69] |
| Corncob biochar | Pyrolysis, heated 500 °C, 2 h, 5 °C/min | PSNPs | 90% | [91] |
| Jujube biomass | Pyrolysis, heated 700 °C, 3 h, 5 °C/min | PE pellets | >99% | [92] |
| Woodchips | Pyrolysis, 700 °C | PSMPs (1 μm) | 100% | [89] |
| Rice straw | Pyrolysis, heated 5 °C/min to 700 °C | PSNPs (300 nm) | 99.96% | [88] |
| Pinewood sawdust | Pyrolysis, heated at 550 °C for 2 h in a tube furnace with a heating rate of 5 °C/min | PSMPs (1 µm) | 94.81% | [93] |
| Sugarcane bagasse | Pyrolysis, heated at 750 °C. After the synthesis, biochar was pulverized using a ball mill at 25 hertz/min oscillating frequency for 3 min | PSNPs (<500 nm) | 99% | [94] |
| Contaminated corncobs | Pyrolysis, heated 180 °C for 6 h | PSNPs (100 nm) | 100% | [95] |
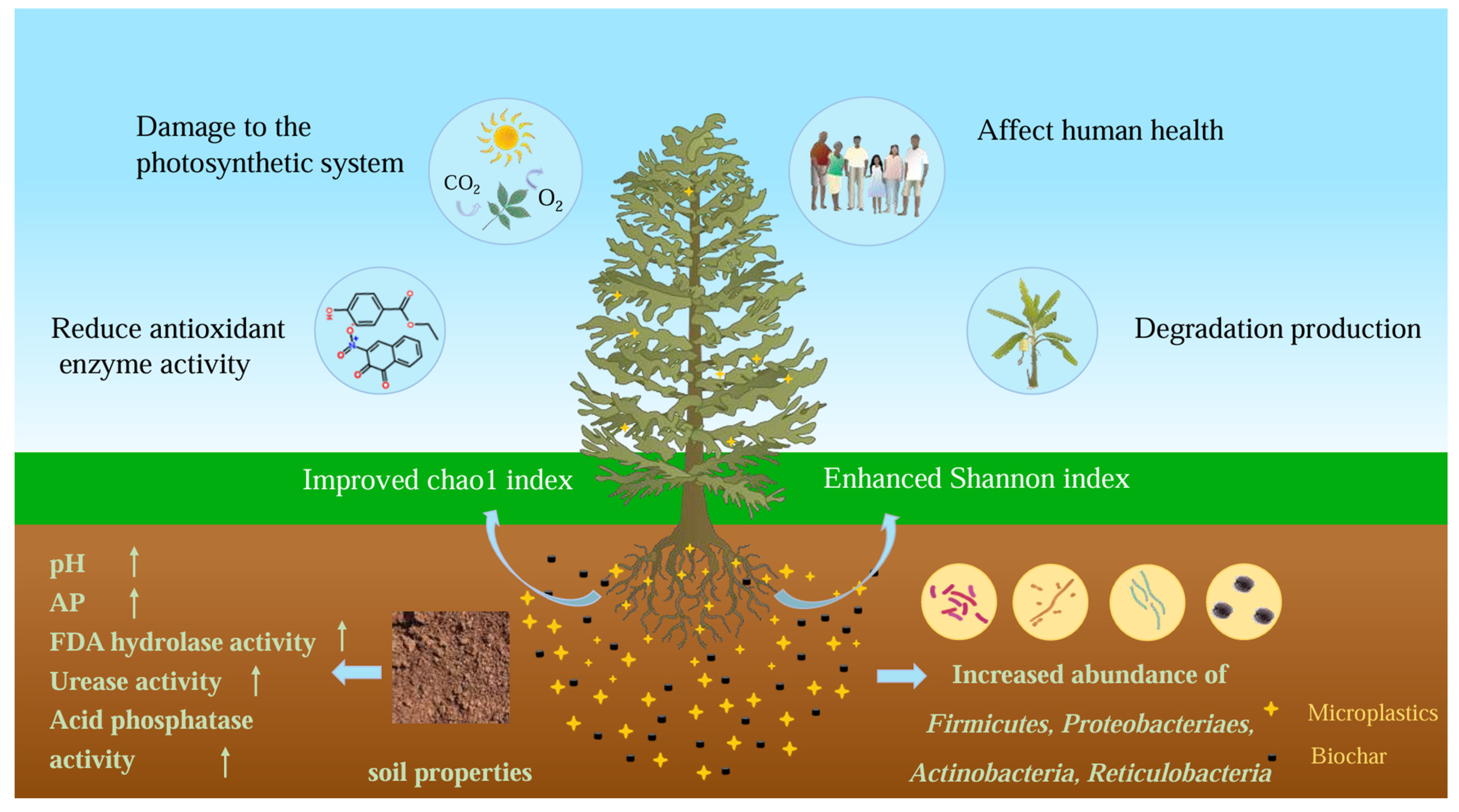
3. Combined Effects of Biochar and MPs on Soil Environment
3.1. Combined Effects of Biochar and MPs on Soil Properties
3.2. Combined Effects of Biochar and MPs on Enzyme Activities
3.2.1. Combined Effects of Biochar and MPs on Fluorescein Diacetate Hydrolase (FDAse) Activity
3.2.2. Combined Effects of Biochar and MPs on Urease Activity
3.2.3. Combined Effects of Biochar and MPs on Phosphatase Activity
3.3. Combined Effects of Biochar and MPs on Soil Organisms
3.3.1. Combined Effects of Biochar and MPs on the Diversity of Soil Microorganisms
3.3.2. Combined Effects of Biochar and MPs on Soil Microbial Community Composition
3.4. Combined Effects of Biochar and MPs on Plants
3.5. Biochar as a Remediation Material for MPs in Soils
4. Prospects and Challenges
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Zhang, T.; Piche-Choquette, S.; Wang, G.; Li, J. Microplastic Pollution in China, an Invisible Threat Exacerbated by Food Delivery Services. Bull. Environ. Contam. Toxicol. 2021, 107, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, L.; Chen, B.; Zhu, X. Low-pressure driven electrospun membrane with tuned surface charge for efficient removal of polystyrene nanoplastics from water. J. Membr. Sci. 2020, 614, 118470. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, J.; Wei, W.; Ngo, H.H.; Guo, W.; Ni, B.-J. Emerging adsorbents for micro/nanoplastics removal from contaminated water: Advances and perspectives. J. Clean. Prod. 2022, 371, 133676. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Perez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Dong, M.; He, L.; Jiang, M.; Zhu, Y.; Wang, J.; Gustave, W.; Wang, S.; Deng, Y.; Zhang, X.; Wang, Z. Biochar for the Removal of Emerging Pollutants from Aquatic Systems: A Review. Int. J. Environ. Res. Public Health 2023, 20, 1679. [Google Scholar] [CrossRef]
- Leng, L.J.; Xiong, Q.; Yang, L.H.; Li, H.; Zhou, Y.Y.; Zhang, W.J.; Jiang, S.J.; Li, H.L.; Huang, H.J. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Li, X.; Zeng, J.Y.; Zuo, S.; Lin, S.T.; Chen, G.X. Preparation, modification, and application of biochar in the printing field: A review. Materials 2023, 16, 5081. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.Y.; Cho, T.S.; Choi, J.W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Y.; Li, F.; Yang, D. Preparation of biochar by simultaneous carbonization, magnetization and activation for norfloxacin removal in water. Bioresour. Technol. 2017, 233, 159–165. [Google Scholar] [CrossRef]
- Xiao, F.; Bedane, A.H.; Zhao, J.X.; Mann, M.D.; Pignatello, J.J. Thermal air oxidation changes surface and adsorptive properties of black carbon (char/biochar). Sci. Total Environ. 2018, 618, 276–283. [Google Scholar] [CrossRef]
- Shi, R.Y.; Hong, Z.N.; Li, J.Y.; Jiang, J.; Kamran, M.A.; Xu, R.K.; Qian, W. Peanut straw biochar increases the resistance of two Ultisols derived from different parent materials to acidification: A mechanism study. J. Environ. Manag. 2018, 210, 171–179. [Google Scholar] [CrossRef]
- Wu, H.; Yip, K.; Tian, F.; Xie, Z.; Li, C. Evolution of char structure during the steam gasification of biochars produced from the pyrolysis of various mallee biomass components. Ind. Eng. Chem. Res. 2009, 48, 10431–10438. [Google Scholar] [CrossRef]
- Wiedner, K.; Rumpel, C.; Steiner, C.; Pozzi, A.; Maas, R.; Glaser, B. Chemical evaluation of chars produced by thermochemical conversion (gasification, pyrolysis and hydrothermal carbonization) of agro-industrial biomass on a commercial scale. Biomass Bioenergy 2013, 59, 264–278. [Google Scholar] [CrossRef]
- Hansen, V.; Muller Stover, D.; Munkholm, L.J.; Peltre, C.; Hauggaard Nielsen, H.; Jensen, L.S. The effect of straw and wood gasification biochar on carbon sequestration, selected soil fertility indicators and functional groups in soil: An incubation study. Geoderma 2016, 269, 99–107. [Google Scholar] [CrossRef]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Gasco, G.; Paz Ferreiro, J.; Alvarez, M.L.; Saa, A.; Mendez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef]
- Harmon, S.M.; Wyatt, D.E. Evaluation of post-Katrina flooded soils for contaminants and toxicity to the soil invertebrates Eisenia fetida and Caenorhabditis elegans. Chemosphere 2008, 70, 1857–1864. [Google Scholar] [CrossRef]
- Ameen, M.; Zamri, N.M.; May, S.T.; Azizan, M.T.; Aqsha, A.; Sabzoi, N.; Sher, F. Effect of acid catalysts on hydrothermal carbonization of Malaysian oil palm residues (leaves, fronds, and shells) for hydrochar production. Biomass Convers. Bior. 2022, 12, 103–114. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Zhou, X.X.; Moghaddam, T.B.; Chen, M.Z.; Wu, S.P.; Adhikari, S. Biochar removes volatile organic compounds generated from asphalt. Sci. Total Environ. 2020, 745, 141096. [Google Scholar] [CrossRef]
- Rao, M.A.; Simeone, G.D.R.; Scelza, R.; Conte, P. Biochar based remediation of water and soil contaminated by phenanthrene and pentachlorophenol. Chemosphere 2017, 186, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Pyrolysis of penicillin fermentation residue and sludge to produce biochar: Antibiotic resistance genes destruction and biochar application in the adsorption of penicillin in water. J. Hazard. Mater. 2021, 413, 125385. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.A.; Hassan, M.A.; Farid, M.A.A.; Yasim-Anuar, T.A.T.; Samsudin, M.H.; Yusoff, M.Z.M.; Zakaria, M.R.; Mokhtar, M.N.; Shirai, Y. Adsorption mechanism and effectiveness of phenol and tannic acid removal by biochar produced from oil palm frond using steam pyrolysis. Environ. Pollut. 2021, 269, 116197. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Song, Y.; Shi, R.; Liu, Z.; Bian, Y.; Wang, F.; Yang, X.; Gu, C.; Jiang, X. Biochar reduces the bioaccumulation of PAHs from soil to carrot (Daucus carota L.) in the rhizosphere: A mechanism study. Sci. Total Environ. 2017, 601, 1015–1023. [Google Scholar] [CrossRef]
- Ye, S.J.; Tan, X.F.; Yang, H.L.; Xiong, J.H.; Zhu, H.X.; Song, H.N.; Chen, G.N. Catalytic removal of attached tetrabromobisphenol A from microplastic surface by biochar activating oxidation and its impact on potential of disinfection by-products formation. Water Res. 2022, 225, 119191. [Google Scholar] [CrossRef]
- Meier, S.; Curaqueo, G.; Khan, N.; Bolan, N.; Cea, M.; Maria Eugenia, G.; Cornejo, P.; Ok, Y.S.; Borie, F. Chicken-manure-derived biochar reduced bioavailability of copper in a contaminated soil. J. Soils Sediments 2017, 17, 741–750. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; El-Sherbini, M.A.A.; Selim, E.-M.M. Effects of biochar on soil properties, heavy metal availability and uptake, and growth of summer squash grown in metal-contaminated soil. Sci. Hortic. 2022, 301, 111097. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Lim, J.E.; Ahmed, M.B.M.; Zhang, M.; Lee, S.S.; Ok, Y.S. Invasive plant-derived biochar inhibits sulfamethazine uptake by lettuce in soil. Chemosphere 2014, 111, 500–504. [Google Scholar] [CrossRef]
- Cabrera, A.; Cox, L.; Spokas, K.; Hermosin, M.C.; Cornejo, J.; Koskinen, W.C. Influence of biochar amendments on the sorption-desorption of aminocyclopyrachlor, bentazone and pyraclostrobin pesticides to an agricultural soil. Sci. Total Environ. 2014, 470, 438–443. [Google Scholar] [CrossRef]
- Wang, P.P.; Liu, X.G.; Yu, B.C.; Wu, X.H.; Xu, J.; Dong, F.S.; Zheng, Y.Q. Characterization of peanut-shell biochar and the mechanisms underlying its sorption for atrazine and nicosulfuron in aqueous solution. Sci. Total Environ. 2020, 702, 134767. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Godlewska, P.; Reible, D.D.; Kraska, P. Bioaccessibility of polycyclic aromatic hydrocarbons in activated carbon or biochar amended vegetated (Salix viminalis) soil. Environ. Pollut. 2017, 227, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ni, N.; Li, X.; Wang, N.; Bian, Y.; Jiang, X.; Song, Y.; Bolan, N.S.; Zhang, Q.; Tsang, D.C.W. Interactions between white and black carbon in water: A case study of concurrent aging of microplastics and biochar. Water Res. 2023, 238, 120006. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Ali, A.; Jeyasundar, P.G.S.A.; Bashir, S.; Hussain, Q.; Wahid, F.; Ali, E.F.; Abdelrahman, H.; Li, R.; Antoniadis, V.; et al. Effects of sheep bone biochar on soil quality, maize growth, and fractionation and phytoavailability of Cd and Zn in a mining-contaminated soil. Chemosphere 2021, 282, 131016. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Ya, H.B.; Xing, Y.; Zhang, T.; Lv, M.J.; Jiang, B. LDPE microplastics affect soil microbial community and form a unique plastisphere on microplastics. Appl. Soil Ecol. 2022, 180, 104623. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric transport of microplastic fibres influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Lian, J.P.; Liu, W.T.; Sun, Y.B.; Men, S.Z.; Wu, J.N.; Zeb, A.; Yang, T.Z.; Ma, L.Q.; Zhou, Q.X. Nanotoxicological effects and transcriptome mechanisms of wheat (Triticum aestivum L.) under stress of polystyrene nanoplastics. J. Hazard. Mater. 2022, 423, 127241. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotox. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Ali, M.; Xu, D.; Yang, X.; Hu, J. Microplastics and PAHs mixed contamination: An in-depth review on the sources, co-occurrence, and fate in marine ecosystems. Water Res. 2024, 257, 121622. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Thavamani, P. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, S.; Qiu, T.; Cui, Q.; Yang, Y.; Li, L.; Chen, J.; Huang, M.; Zhan, A.; Fang, L. Interaction of microplastics with heavy metals in soil: Mechanisms, influencing factors and biological effects. Sci. Total Environ. 2024, 918, 170281. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.J.; Jiang, B.; Xing, Y.; Ya, H.B.; Zhang, T.; Wang, X. Recent advances in the breakdown of microplastics: Strategies and future prospectives. Environ. Sci. Pollut. Res. 2022, 29, 65887–65903. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, J.; Baskaralingam, V.; Stalin, T.; Muthuvel, I. Mechanistic vision on polypropylene microplastics degradation by solar radiation using TiO2 nanoparticle as photocatalyst. Environ. Res. 2023, 233, 116366. [Google Scholar] [CrossRef] [PubMed]
- Alexandra, T.H.; Lucie, L.; Marion, M.; Anne Francoise, M.; Olivier, B.; Emile, P. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 2017, 227, 167–174. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Schettini, E.; Stefani, L.; Vox, G. Interaction between agrochemical contaminants and UV stabilizers for greenhouse eva plastic films. Appl. Eng. Agric. 2014, 30, 229–239. [Google Scholar]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Johansen, M.P.; Cresswell, T.; Davis, J.; Howard, D.L.; Howell, N.R.; Prentice, E. Biofilm-enhanced adsorption of strong and weak cations onto different microplastic sample types: Use of spectroscopy, microscopy and radiotracer methods. Water Res. 2019, 158, 392–400. [Google Scholar] [CrossRef]
- Sait, S.T.L.; Sorensen, L.; Kubowicz, S.; Vike-Jonas, K.; Gonzalez, S.V.; Asimakopoulos, A.G.; Booth, A.M. Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content. Environ. Pollut. 2021, 268, 115745. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.; Bacha, A.U.R.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef] [PubMed]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced visible light photodegradation of microplastic fragments with plasmonic Platinum/Zinc oxide nanorod photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.D. Enzymatic degradation of poly(ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Baker, P.J.; Poultney, C.; Liu, Z.Q.; Gross, R.; Montclare, J.K. Identification and comparison of cutinases for synthetic polyester degradation. Appl. Microbiol. Biotechnol. 2012, 93, 229–240. [Google Scholar] [CrossRef]
- Roth, C.; Wei, R.; Oeser, T.; Then, J.; Foellner, C.; Zimmermann, W.; Straeter, N. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl. Microbiol. Biotechnol. 2014, 98, 7815–7823. [Google Scholar] [CrossRef]
- Kanhai, L.D.K.; Officer, R.; Lyashevska, O.; Thompson, R.C.; O’Connor, I. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017, 115, 307–314. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Ya, H.B.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.J.; Wang, X. Recent advances on ecological effects of microplastics on soil environment. Sci. Total Environ. 2021, 798, 149338. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.B.; Li, Q.; Nunes, J.K.; Deike, L. Ocean emission of microplastic. Proc. Nat. Acad. Sci. USA Nexus 2023, 2, pgad296. [Google Scholar] [CrossRef]
- Allen, D.; Allen, S.; Abbasi, S.; Baker, A.; Bergmann, M.; Brahney, J.; Butler, T.; Duce, R.A.; Eckhardt, S.; Evangeliou, N.; et al. Microplastics and nanoplastics in the marine-atmosphere environment. Nat. Rev. Earth Environ. 2022, 3, 393–405. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y.; Lv, M.J.; Zhang, T.; Ya, H.; Jiang, B. Recent advances on the effects of microplastics on elements cycling in the environment. Sci. Total Environ. 2022, 849, 157884. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 2125. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, W.; Chen, D.; Tian, J.; Ao, J. Biochar mitigates the negative effects of microplastics on sugarcane growth by altering soil nutrients and microbial community structure and function. Plants 2024, 13, 83. [Google Scholar] [CrossRef]
- Fan, P.; Yu, H.; Xi, B.D.; Tan, W.B. A review on the occurrence and influence of biodegradable microplastics in soil ecosystems: Are biodegradable plastics substitute or threat? Environ. Int. 2022, 163, 107244. [Google Scholar] [CrossRef]
- Feng, Y.; Han, L.; Li, D.; Sun, M.; Wang, X.; Xue, L.; Poinern, G.; Feng, Y.; Xing, B. Presence of microplastics alone and co-existence with hydrochar unexpectedly mitigate ammonia volatilization from rice paddy soil and affect structure of soil microbiome. J. Hazard. Mater. 2022, 422, 126831. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.Q.; Chi, J. Impact of biochar coexistence with polar/nonpolar microplastics on phenanthrene sorption in soil. J. Hazard. Mater. 2023, 447, 130761. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.R.; Shah, T.; Asad, M.; Ali, A.; Samee, E.; Adnan, F.; Bhatti, M.F.; Marhan, S.; Kammann, C.I.; Haider, G. Biochar alleviated the toxic effects of PVC microplastic in a soil-plant system by upregulating soil enzyme activities and microbial abundance. Environ. Pollut. 2023, 332, 121810. [Google Scholar] [CrossRef]
- Liu, Q.; Khor, S.M. Emerging absorption-based techniques for removing microplastics and nanoplastics from actual water bodies. TrAC-Trends Anal. Chem. 2024, 170, 117465. [Google Scholar] [CrossRef]
- Camila Ariza-Tarazona, M.; Francisco Villarreal-Chiu, J.; Barbieri, V.; Siligardi, C.; Iveth Cedillo-Gonzalez, E. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- Ji, G.Y.; Xing, Y.C.; You, T.Y. Biochar as adsorbents for environmental microplastics and nanoplastics removal. J. Environ. Chem. Eng. 2024, 12, 113377. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.; Zhao, H.; Shi, J.; Liu, Z.; Li, C.; Song, F. Efficient removal of microplastics from aqueous solution by a novel magnetic biochar: Performance, mechanism, and reusability. Environ. Sci. Pollut. Res. 2023, 30, 26914–26928. [Google Scholar] [CrossRef]
- Hsieh, L.; He, L.; Zhang, M.; Lv, W.; Yang, K.; Tong, M. Addition of biochar as thin preamble layer into sand filtration columns could improve the microplastics removal from water. Water Res. 2022, 221, 118783. [Google Scholar] [CrossRef]
- Li, X.; Yao, S.; Wang, Z.; Jiang, X.; Song, Y.; Chang, S.X. Polyethylene microplastic and biochar interactively affect the global warming potential of soil greenhouse gas emissions. Environ. Pollut. 2022, 315, 120433. [Google Scholar] [CrossRef]
- Magid, A.S.I.A.; Islam, M.S.; Chen, Y.; Weng, L.; Li, J.; Ma, J.; Li, Y. Enhanced adsorption of polystyrene nanoplastics (PSNPs) onto oxidized corncob biochar with high pyrolysis temperature. Sci. Total Environ. 2021, 784, 147115. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lubis, N.M.A.; Usama, M.; Ahmad, J.; Al-Wabel, M.I.; Al-Swadi, H.A.; Rafique, M.I.; Al-Farraj, A.S.F. Scavenging microplastics and heavy metals from water using jujube waste-derived biochar in fixed-bed column trials. Environ. Pollut. 2023, 335, 122319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, C.; Huang, Q.-X.; Chi, Y.; Yan, J.-H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Khandelwal, N.; Tiwari, E.; Singh, N.; Darbha, G.K. Biochar-facilitated remediation of nanoplastic contaminated water: Effect of pyrolysis temperature induced surface modifications. J. Hazard. Mater. 2021, 417, 126096. [Google Scholar] [CrossRef]
- Zhu, N.; Yan, Q.; He, Y.; Wang, X.; Wei, Z.; Liang, D.; Yue, H.; Yun, Y.; Li, G.; Sang, N. Insights into the removal of polystyrene nanoplastics using the contaminated corncob-derived mesoporous biochar from mining area. J. Hazard. Mater. 2022, 433, 128756. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.; Kirkham, M.B.; et al. Multifunctional applications of biochar beyond carbon storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahushi, F.; Bolan, N.; et al. Integrating Biochar, Bacteria, and Plants for Sustainable Remediation of Soils Contaminated with Organic Pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, X.; Xue, J.M. Biofilm developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol. 2021, 55, 12780–12790. [Google Scholar] [CrossRef]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-cost biochar adsorbents for water purification including microplastics removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.u.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Ahmad, S.; Liu, X.; Tang, J.; Zhang, S. Biochar-supported nanosized zero-valent iron (nZVI/BC) composites for removal of nitro and chlorinated contaminants. Chem. Eng. J. 2022, 431, 133187. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Luo, T.; Ma, Y.; Wang, B.; Huang, Q. Remediation potential of immobilized bacterial strain with biochar as carrier in petroleum hydrocarbon and Ni co-contaminated soil. Environ. Technol. 2022, 43, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Sang, M.K.; Igalavithana, A.D.; Zhang, M.; Hou, D.; Oleszczuk, P.; Sung, J.; Ok, Y.S. Biochar alters chemical and microbial properties of microplastic-contaminated soil. Environ. Res. 2022, 209, 112807. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Withana, P.A.; Jeong, Y.; Sang, M.K.; Cho, Y.; Hwang, G.; Chang, S.X.; Ok, Y.S. Contrasting effects of food waste and its biochar on soil properties and lettuce growth in a microplastic-contaminated soil. Appl. Biol. Chem. 2024, 67, 3. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Chen, X.; Yu, S.; Cui, M.; Wang, S.; Song, F. Effects of biochar on the phytotoxicity of polyvinyl chloride microplastics. Plant Physiol. Biochem. 2023, 195, 228–237. [Google Scholar] [CrossRef]
- Yang, L.; Liang, H.; Wu, Q.; Shen, P. Biochar alleviated the toxic effects of microplastics-contaminated geocarposphere soil on peanut (Arachis hypogaea L.) pod development: Roles of pod nutrient metabolism and geocarposphere microbial modulation. J. Sci. Food Agric. 2024, 104, 2990–3001. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Palansooriya, K.N.; Sang, M.K.; Oh, D.X.; Park, J.; Hwang, S.Y.; Igalavithana, A.D.; Gu, C.; Ok, Y.S. Combined effect of biochar and soil moisture on soil chemical properties and microbial community composition in microplastic-contaminated agricultural soil. Soil Use Manag. 2022, 38, 1446–1458. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Mostafa, A.A.; Zedan, A.; Elbltagy, H.M.; Dawoud, S.F.M.; Elbanna, B.A. Potential effect of biochar on soil properties, microbial activity and vicia faba properties affected by microplastics contamination. Agronomy 2023, 13, 149. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Chai, B.; Xiao, T.; Xiao, E.; Du, S.; Yang, S.; Yin, H.; Dang, Z.; Pan, K. Enhancing microplastics removal from soils using wheat straw and cow dung-derived biochars. J. Clean. Prod. 2024, 470, 143288. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yuan, M.; Xu, R.; Bish, D.L. Mobilization of phosphate in variable-charge soils amended with biochars derived from crop straws. Soil Tillage Res. 2015, 146, 139–147. [Google Scholar] [CrossRef]
- Kamran, M.A.; Jiang, J.; Li, J.; Shi, R.; Mehmood, K.; Abdulaha Al Baquy, M.; Xu, R. Amelioration of soil acidity, Olsen-P, and phosphatase activity by manure- and peat-derived biochars in different acidic soils. Arab. J. Geosci. 2018, 11, 272. [Google Scholar] [CrossRef]
- Tesfaye, F.; Liu, X.; Zheng, J.; Cheng, K.; Bian, R.; Zhang, X.; Li, L.; Drosos, M.; Joseph, S.; Pan, G. Could biochar amendment be a tool to improve soil availability and plant uptake of phosphorus? A meta-analysis of published experiments. Environ. Sci. Pollut. Res. 2021, 28, 34108–34120. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Daughtridge, R.C.; Nakayama, Y.; Margenot, A.J. Sources of abiotic hydrolysis of chromogenic substrates in soil enzyme assays: Storage, termination, and incubation. Soil Biol. Biochem. 2021, 158, 108245. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Chen, Y.; Liu, G.; Xue, S. Responses of soil enzyme activity and soil organic carbon stability over time after cropland abandonment in different vegetation zones of the Loess Plateau of China. Catena 2021, 196, 104812. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Attina, E. Early warning indicators of changes in soil ecosystem functioning. Ecol. Indic. 2015, 48, 542–549. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Ma, H.; Wirth, S.; Bellingrath Kimura, S.D. Soil amendment with different maize biochars improves chickpea growth under different moisture levels by improving symbiotic performance with Mesorhizobium ciceri and Soil Biochemical properties to Varying Degrees. Front. Microbiol. 2019, 10, 2423. [Google Scholar] [CrossRef]
- Machado, A.A.d.S.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Feng, C.; Ma, Y.; Jin, X.; Wang, Z.; Ma, Y.; Fu, S. Soil enzyme activities increase following restoration of degraded subtropical forests. Geoderma 2019, 351, 180–187. [Google Scholar] [CrossRef]
- Ghani, M.I.; Ali, A.; Atif, M.J.; Ali, M.; Amin, B.; Anees, M.; Khurshid, H.; Cheng, Z. Changes in the soil microbiome in eggplant monoculture revealed by high-throughput illumina miseq sequencing as influenced by raw garlic stalk amendment. Int. J. Mol. Sci. 2019, 20, 2125. [Google Scholar] [CrossRef]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021, 93, 24–32. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Lopes, E.M.G.; Reis, M.M.; Frazao, L.A.; Terra, L.E.d.M.; Lopes, E.F.; dos Santos, M.M.; Fernandes, L.A. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov. 2021, 21, 101270. [Google Scholar] [CrossRef]
- Chen, Y.; Leng, Y.; Liu, X.; Wang, J. Microplastic pollution in vegetable farmlands of suburb Wuhan, central China. Environ. Pollut. 2020, 257, 113449. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- De Souza, C.L.; Procopio Lucas, L. The profile of the soil microbiota in the Cerrado is influenced by land use. Appl. Microbiol. Biotechnol. 2021, 105, 4791–4803. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; Ferreira, A.d.S.; Muller Barboza, A.D.; Wurdig Roesch, L.F. Changes in diversity, abundance, and structure of soil bacterial communities in brazilian savanna under different land use systems. Microb. Ecol. 2013, 66, 593–607. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, X.; Yan, C.; Lin, Y.; Huang, Q. Polystyrene microplastics induce microbial dysbiosis and dysfunction in surrounding seawater. Environ. Int. 2021, 156, 106724. [Google Scholar] [CrossRef]
- Zhu, D.; Li, G.; Wang, H.; Duan, G. Effects of nano or microplastic exposure combined with arsenic on soil bacterial, fungal, and protistan communities. Chemosphere 2021, 281, 130998. [Google Scholar] [CrossRef]
- Yu, H.; Qi, W.; Cao, X.; Hu, J.; Li, Y.; Peng, J.; Hu, C.; Qu, J. Microplastic residues in wetland ecosystems: Do they truly threaten the plant-microbe-soil system? Environ. Int. 2021, 156, 106708. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Qin, X.; Jia, W.; Chai, L.; Huang, M.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Y.; Song, Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.; Zhu, J.; Wang, J.; Wang, H.; Zhan, X. The joint toxicity of polyethylene microplastic and phenanthrene to wheat seedlings. Chemosphere 2021, 282, 130967. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Xu, M.; Wang, L. Cyto-Genotoxic Effect Causing Potential of Polystyrene Micro-Plastics in Terrestrial Plants. Nanomaterials 2022, 12, 2024. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tan, L.; Zhang, S.; Zuo, Y.; Han, X.; Liu, N.; Lu, W.; Liu, A. Detection of genotoxic effects of drinking water disinfection by-products using Vicia faba bioassay. Environ. Sci. Pollut. Res. 2017, 24, 1509–1517. [Google Scholar] [CrossRef]
- Taylor, S.E.; Pearce, C.I.; Sanguinet, K.A.; Hu, D.; Chrisler, W.B.; Kim, Y.; Wang, Z.; Flury, M. Polystyrene nano- and microplastic accumulation at Arabidopsis and wheat root cap cells, but no evidence for uptake into roots. Environ. Sci. Nano 2020, 7, 1942–1953. [Google Scholar] [CrossRef]
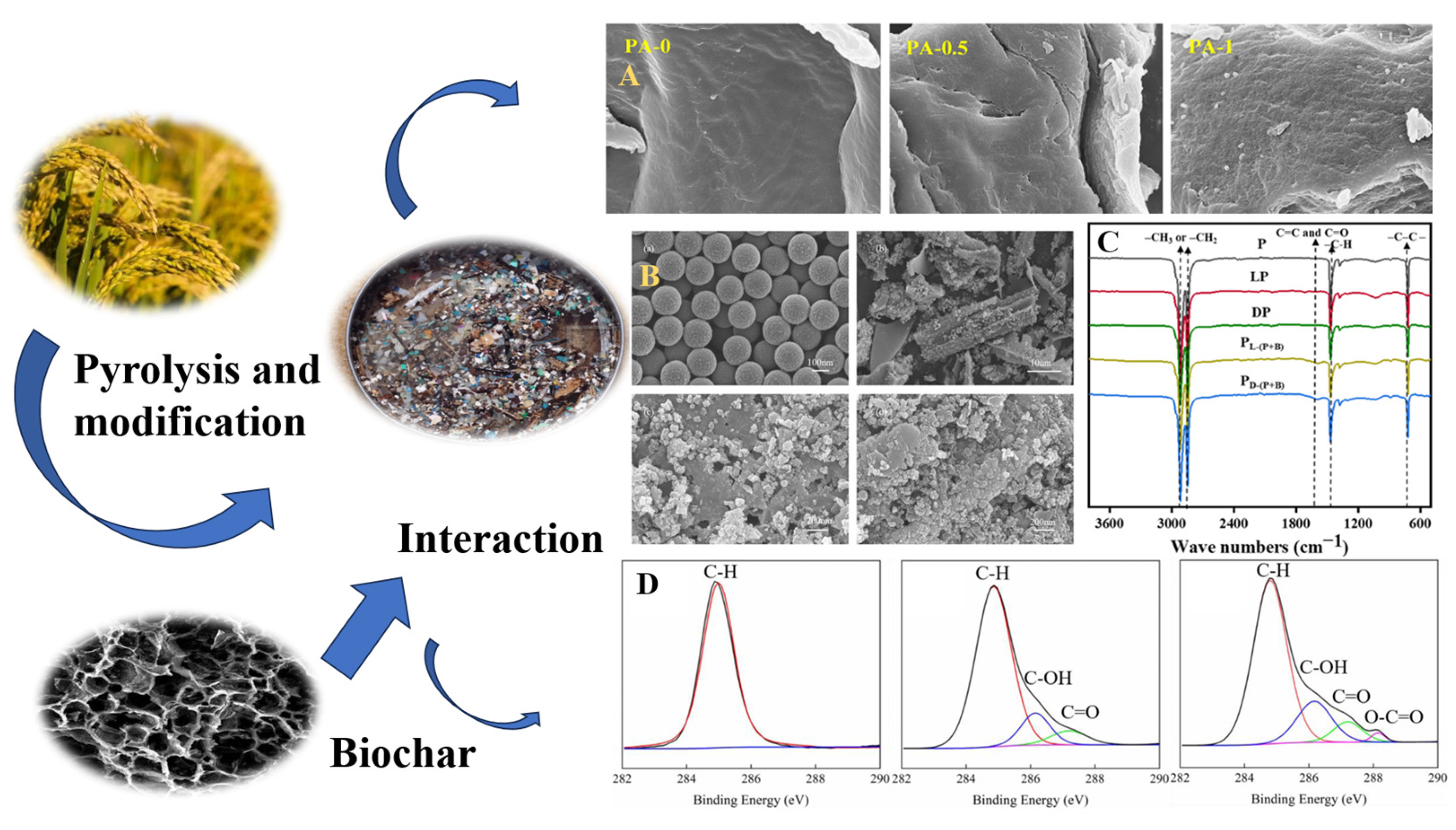
| Feedstock | Preparation Condition | Carbon Content | Application of Biochar | Reference |
|---|---|---|---|---|
| Bamboo | Pyrolysis, 500 °C, - | 83.6% | Removal of norfloxacin from water | [18] |
| Corncob | Pyrolysis, 500, 700 °C, 2 h | - | Adsorption of neutral organic compounds | [19] |
| Peanut straw | Pyrolysis, 400 °C, 3 h | - | Optimization of biochar performance | [20] |
| Wood | Gasification, 750 °C, 0.25 h | 48.4% | Optimization catalyst performance | [21] |
| Leaf | - | 59.3% | ||
| Bark | - | 50.4% | ||
| Wood chips | Gasification, 1200 °C, 0.5–0.75 h | 80.6% | Elimination of copper and cadmium | [22] |
| Wheat straw | Gasification, 750 °C | 46.8% | Lead removal adsorbent | [23] |
| Sewage sludge | 180 °C, 1.25 h | 29.8% | ||
| Peanut hull | Hydrothermal carbonization, 300 °C, 5 h | - | ||
| Switchgrass | Hydrothermal carbonization, 300 °C, 0.5 h | 70.5% | Pyrolysis of organic wastes for biochar | [24] |
| Banana peels | Hydrothermal carbonization, 230 °C, 2 h | 71.38% | Soil remediation of MP contamination | [25] |
| Pig manure | Hydrothermal carbonization, 240 °C, 2 h | 23.9% | Soil remediation of MP contamination | [26] |
| Microplastic | Breakdown Method | Catalytic Material | Duration of Breakdown | Reference |
|---|---|---|---|---|
| PE | Natural weathering | - | - | [55] |
| HDPE | Natural weathering | - | 3 years | [56] |
| PET | UV | - | 1 year | [57] |
| PS, PE | K2S2O8 to oxidize | - | 30 days | [58] |
| PS, PE, PVC | Heat treatment | - | 2000 h | [59] |
| PE, PP | Gamma irradiation | - | - | [60] |
| PET (black) | Photooxidation | - | 10 months | [61] |
| PET (white) | Photooxidation | - | 10 months | [61] |
| PS (1 mm) | Photooxidation | - | 24 h | [62] |
| LDPE | Photooxidation | - | 175 h | [63] |
| PVC | Electrocatalysis | TiO2 | 6 h | [64,65] |
| PET | Biocatalysis | T. fusca | - | [64] |
| PCL | Biocatalysis | Keratinaes | - | [66] |
| PET | Biocatalysis | Thermobifida fusca | - | [67] |
| Microplastic | Biochar Feedstock | Preparation Condition | Duration of Cultivation | Interaction Mechanism | Combined Effect | Reference |
|---|---|---|---|---|---|---|
| LDPE | Oilseed rape straw (OSR), soft wood pellet (SWP) | 550 °C, 700 °C | 100 days | Soil properties | Reduced plant-available P (23–86%) and elevated pH (0.15–0.46 units), EC (0.14–0.38 ds m−1), TN (63–120%), K (12–41%), and FDA activity (27–280%) | [104] |
| LDPE | Oilseed rape (OSR), soft wood pellet (SWP) | 550 °C, 700 °C | 100 days | Soil properties | Improved plant growth, soil microbes, and enzyme activity | [109] |
| PE | Straw biochar, manure biochar | 500 °C, 4 h | 45 days | Reducing greenhouse gas emissions | Reduced CH4 emissions by 35.8%, lowed N2O, CO2 and CH4 emissions by 24.8%, 6.2%, and 65.2% | [110] |
| PVC | Cotton stalks | 650–750 °C | 21 days | Soil properties | Shoot dry be increased matter production in PVC-MPs contaminated soil | [83] |
| PE | Wheat (Triticum aestivum) straw, cow manure | 500 °C | 45 days | Changing greenhouse gas emissions | Increased N2O emissions by 37.5% but decreased CH4 emissions by 35.8% | [90] |
| PVC | Corncob | 500 °C, 3 h | 14 days | Enzyme activities | Decreased the contents of H2O2 and MDA | [106] |
| PS | Food waste | 500 °C, 20 min | 5 weeks | Soil properties | Changed in available nitrogen (NO3-N: 325.5 mg kg−1, NH4+-N: 105.2 mg kg−1), soil electrical conductivity (EC, 2.04 ds m−1), available phosphorus (88.4 mg kg−1), and total exchangable cations (18.6 c mol (+) kg−1) | [105] |
| PS | Peanut | 450 °C, 4 h | 80 days | Soil microorganisms | Reinstate the microbial communities’ variety that was impacted by the pollution of MPs and increase the proportion of bacteria that are associated with resistance to pathogens | [107] |
| PVC | Corncob biochar | - | 20 days | Plants | significantly reduced the contents of H2O2 and MDA in lettuce sprouts but increased the content of H2O2 in roots | [111] |
| PE | Corn stalk biochar | 600 °C | 300 days | Soil microorganisms | significantly increased the abundance of Subgroup_10 for the 16S rRNA gene and treatments with MPs alone significantly increased the relative abundance of Streptomyces for the phoD gene compared to CK | [79] |
| PS | Wheat straw and cow dung | 500 °C, 600 °C, 700 °C | 24 h | Soil properties | The removal efficiencies of MPs exceeded 86% for all biochar | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xing, Y.; Guo, Y.; Zhang, D.; Tang, Y.; Chen, J.; Zhang, H.; Jiang, B. The Removal and Mitigation Effects of Biochar on Microplastics in Water and Soils: Application and Mechanism Analysis. Sustainability 2024, 16, 9749. https://doi.org/10.3390/su16229749
Li W, Xing Y, Guo Y, Zhang D, Tang Y, Chen J, Zhang H, Jiang B. The Removal and Mitigation Effects of Biochar on Microplastics in Water and Soils: Application and Mechanism Analysis. Sustainability. 2024; 16(22):9749. https://doi.org/10.3390/su16229749
Chicago/Turabian StyleLi, Wenxin, Yi Xing, Ying Guo, Duo Zhang, Yajuan Tang, Jiayu Chen, Han Zhang, and Bo Jiang. 2024. "The Removal and Mitigation Effects of Biochar on Microplastics in Water and Soils: Application and Mechanism Analysis" Sustainability 16, no. 22: 9749. https://doi.org/10.3390/su16229749
APA StyleLi, W., Xing, Y., Guo, Y., Zhang, D., Tang, Y., Chen, J., Zhang, H., & Jiang, B. (2024). The Removal and Mitigation Effects of Biochar on Microplastics in Water and Soils: Application and Mechanism Analysis. Sustainability, 16(22), 9749. https://doi.org/10.3390/su16229749