Abstract
The increased presence of cadmium in the environment can lead to its increase in the food chain, particularly due to its accumulation in the consumable parts of plants. For humans, ingesting food containing high levels of Cd is a significant exposure pathway. Being a non-essential and non-metabolized element, it is harmful to microorganisms, animals, plants, and humans, even in minimal concentrations. As a result, there is a need for the remediation of both natural and urban environments. Bioremediation is a sustainable and eco-friendly technique for cleaning up the environment and reducing contamination of living organisms. This review explores the potential of phytoremediation, a bioremediation approach that utilizes plants as agents for decontamination, as a method to restore such areas. Certain plants, particularly macrophytes, are capable of remediating Cd. In response to induced stress, plants activate various tolerance mechanisms, including antioxidant enzyme systems (as peroxidase, catalase, ascorbate peroxidase, superoxide dismutase, and glutathione peroxidase) as well as non-enzymatic pathways (like phytochelatins). However, a thorough understanding of these tolerance mechanisms is essential for optimizing this method, especially for application in aquatic environments. This study will, therefore, review the existing tolerance and detoxification mechanisms for Cd, along with bioremediation strategies. The application of this eco-friendly approach is highly correlated with the three main areas required for sustainability: economic, environmental and social.
1. Introduction
Heavy metals naturally occur in soil, aquatic environments, and living organisms, arising from rock weathering, volcanic activity [1], forest fires, biological processes, as well as marine salts from the oceans [2]. Nevertheless, human activities, including mining, industrial operations, agriculture, waste management, the disposal of biosolids (sewage sludge), pesticide use, coal combustion byproducts, and petrochemical products have led to elevated levels of these metals, drawing significant global concern [3].
Volcanic rocks or ash contain varying concentrations of heavy metals. In general, the concentrations for the metals As, Cu, Pb, and Zn are 1.2–47.8 μg g−1, 8.4–36 mg kg−1, 5.2–16 mg kg−1, and 15–73 mg kg−1, respectively. Cadmium in volcanic rocks ranges from 0.1–0.5 ppm in ocean waters of <5 and 110 ng L−1 [4,5,6]. The anthropogenic sources present the greater contribution of heavy metals levels, being responsible for releasing significant levels of these contaminants in forms that are more toxic and mobile [7,8].
This is especially true for aquatic environments, where heavy metals can remain for extended periods in bottom sediments, as well as in suspended forms, and within aquatic plants and fish [9]. When entering an aquatic environment, a great part of metals remains absorbed by suspended particles being deposited as sediments. The dynamics of heavy metals usually involve sediments acting as reservoirs for these metals and releasing them back into the water under certain chemical changes, leading to secondary pollution [10]. The heavy metals behavior in sediments is reported as long-lasting persistence [8].
Certain heavy metals, including Cu, Ni, Fe, Mo, Mn, and Zn, are micronutrients essential for animals, plants, and humans. However, when their concentrations exceed specific thresholds, they can become toxic. In contrast, cadmium (Cd), mercury (Hg), and lead (Pb) are not only non-essential but also highly toxic, even at low concentrations [11,12]. The reference values in drinking water established by the World Health Organization [13] regarding Cd, Hg and Pb are 0.003 mg L−1, 0.006 mg L−1, and 0.01 mg L−1, respectively. Cadmium is classified as a hazardous substance by the ATSDR and EPA, and it is ranked 7th in terms of priority due to its significant threat to human health, owing to its known toxicity and the high likelihood of human exposure [14]. Humans are primarily exposed to this metal through the consumption of contaminated food and water. This underscores the importance of implementing prevention and decontamination strategies to address the issue [15,16].
One approach for decontamination is bioremediation, an emerging and eco-friendly technology that employs microorganisms [17], principally plants (referred to as phytoremediation) [18,19], or a combination of these methods [20,21] to remediate metals from water and soil. Among the plant species suitable for phytoremediation, notable examples include aquatic plants such as Pistia stratiotes L., Eichhornia crassipes [21], Hydrocotyle ranunculoides [22] and Lemna minor [23,24]. These plants have been documented for their ability to remove different heavy metals from solutions. This review also explores the various mechanisms plants use to mitigate Cd toxicity.
Cadmium is classified as a heavy metal, a term used for chemical elements with a density exceeding 5 g/cm3 and an atomic mass higher than 20 [25,26]. In nature, cadmium (Cd) is commonly found in association with sulfides of zinc, lead, copper, and mercury ores [27]. It exists in two oxidation states (0 or +2), and the distribution of soluble Cd species can vary significantly [28]. When referring to aquatic environments, the solubility and movement of Cd are influenced by factors such as the levels of organic and inorganic carbon, the presence of clay and oxyhydroxides like Al, Mn and Fe, as well as the pH level. Cadmium tends to stay dissolved in water when the pH is below 6.5 and in oxygen-rich conditions. Depending on the composition of groundwater, between 55% and 90% of the Cd (total soluble) is found as divalent Cd2+ ions, with the remainder existing as various inorganic and organic complexes, such as CdNO3+, CdCl+, Cd2OH3+, CdCl20, Cd(OH)3−, CdCl3−, Cd(OH)20, Cd(SO4)22−, CdOH+, Cd(CO3)22−, CdSO40, CdCO30, CdHCO3− [28,29]. The precipitation of Cd occurs in the forms of chromates, phosphate, arsenate, and sulfides and presents stability at alkaline pH levels [30,31].
2. Cd Toxicity in Humans
The element cadmium poses a significant risk to human health due to its higher mobility and bioavailability compared to other toxic elements [30]. The primary routes of Cd exposure are through the consumption of contaminated water and food [32]. Additionally, smoking and occupational exposure are significant sources of cadmium [30]. After absorption, cadmium accumulates primarily in the liver and kidneys [15] (Figure 1). High levels of Cd dust can lead to various organ malfunctions [31,32,33], such as (a) Respiratory system issues such as pneumonia, breathlessness, and damage to the mucous membranes; (b) Reproductive system problems including testicular necrosis (which affects spermatogenesis and semen quality, particularly sperm motility and hormone production/release), breast cancer, hormonal imbalances (in testosterone and progesterone), and menstrual cycle disruptions; (c) Skeletal system conditions such as osteoporosis, reduced bone density, and Itai-Itai disease; (d) Excretory system effects like kidney stone formation, proteinuria, and damage to both glomerular and tubular kidney structures.
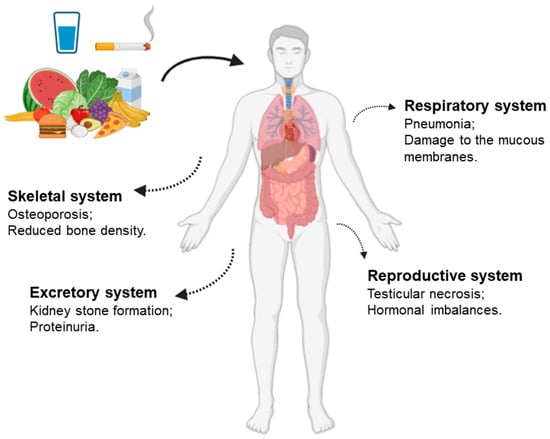
Figure 1.
Cadmium exposure effects in the human body.
Cadmium has been linked to the promotion of apoptosis, DNA methylation, damage and oxidative stress. DNA methylation is a biochemical process where a methyl group attaches to DNA, often modifying gene expression without changing the DNA sequence. It plays a role in regulating genes, development, and cellular responses to environmental factors [34]. Oxidative stress is referred to as an imbalance between free radicals (unstable molecules) and antioxidants, leading to potential cellular damage [35].
The Cd exposure also disrupts the metabolism of selenium, copper, zinc and iron. Studies have proved that cadmium reduces hepatic glycogen stores, which in turn raises blood glucose levels [15]. Once cadmium accumulates in the human body, it has an estimated average half-life of around 10 years. Therefore, finding effective and relevant solutions for removing Cd from the environment is essential [31]. The long-term effects of cadmium exposure include bone and kidney disorders, reproductive toxicity, and increased cancer risk in both animals and humans [36].
3. Effects of Cd in Plants
Exposure to elevated levels of cadmium in plants triggers biochemical, physiological, and morphological alterations, leading to a significant decrease in the roots’ ability to absorb and accumulate this element. This is largely due to an overall reduction in metabolic activity [37].
The visible symptoms of cadmium stress help determine the extent of toxicity in plants. Common signs of Cd toxicity include leaf discoloration (chlorosis), necrosis, and wilting (Figure 2), along with stunted growth (due to disruption of macro and micronutrient metabolism), decreased photosynthesis, and inhibited respiration [38]. The reduction in photosynthesis caused by cadmium toxicity is attributed to lower levels of chlorophyll and carotenoids, resulting from the inactivation of enzymes that synthesize these pigments [39]. Table 1 presents the comparison between different plant species regarding the impact of Cd on photosynthesis.
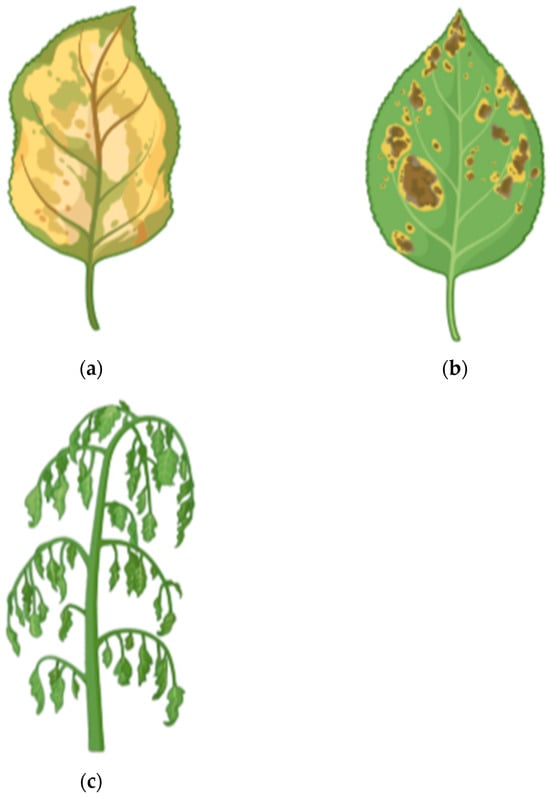
Figure 2.
The most common visible symptoms in plants of cadmium stress: (a) chlorosis, (b) necrosis, and (c) wilting.

Table 1.
Cd impact in photosynthesis in different plant species.
On the other hand, the non-visible symptoms include reduced biomass, alterations in mineral composition, and changes in subcellular structures. A decline in biomass due to Cd exposure can ultimately result in plant death. Cadmium is thought to trigger both enzyme induction and inhibition, affect stomatal function, and lead to the production of free radicals [38] as well as reactive oxygen species (ROS), such as superoxide anion (O2−), hydroxyl (OH−), and hydrogen peroxide (H2O2). At elevated levels, these compounds cause imbalances that result in oxidative stress in plants [45].
Cadmium toxicity leads to the breakdown of the plant’s plasma membrane, likely due to the leakage of electrolytes and membrane proteins, including the inhibition of H-ATPase. This disruption also affects the DNA repair system [32].
4. Mechanisms of Plant Resistance to Heavy Metals
Given that heavy metals impact the distribution, growth, and life cycle of plant species, selecting plants with mechanisms for heavy metal tolerance is essential when considering the phytoremediation process [46]. Heavy metal tolerance refers to the ability of organisms, such as plants or microbes, to survive and grow in environments with high concentrations of heavy metals that are typically toxic to other species. A plant’s tolerance to heavy metals is evident when it thrives in environments with high concentrations of these contaminants, where other plant species would typically be unable to survive due to the metals’ toxic effects. The capacity to tolerate heavy metals can be developed over time as plants adapt to environmental stress. A single plant may exhibit various strategies to cope with contamination, and these responses can differ based on the species, the specific elements causing the stress, and the unique conditions of the environment [47].
The mechanisms that plants develop to mitigate the destructive effects of heavy metals on their general tissues are categorized as either extracellular or intracellular (Figure 3).
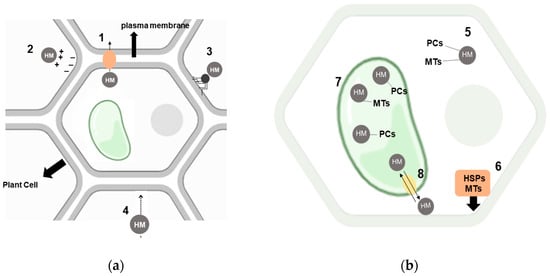
Figure 3.
Overview of cellular mechanisms that may detoxify heavy metals and enhance plant tolerance to such contamination: (a) extracellular mechanisms, including (1) active removal of heavy metals, (2) binding of heavy metals to the cell wall, (3) reduction of heavy metal mobility and availability through mycorrhizal fungi, and (4) decreased entry of heavy metals; (b) intracellular mechanisms, such as (5) high-affinity chelation of heavy metals, (6) plasma membrane repair and protection, (7) complexed metal compartmentalization, and (8) vacuole accumulation of heavy metals.
4.1. Extracellular Mechanisms
The extracellular mechanisms (Figure 3) encompass mycorrhizae functions, cell wall functions, and extracellular exudates. Additionally, tolerance might involve the plasma membrane by either decreasing heavy metal absorption or enhancing the efflux of metals that have entered the cytosol [47,48].
The German botanist Albert Bernard Frank first introduced the term “mycorrhiza” in 1885, derived from Greek, where “mico” translates to fungus and “riza” to root. Mycorrhizae refers to structures that develop from the symbiotic relationship between roots and soil fungi, present in over 80% of higher plant species [49]. An appressorium is formed by arbuscular mycorrhizal fungi and facilitates entry into the root cortex. Within the cortex, the hyphae spread through the spaces between cells and periodically penetrate the cell wall, branching extensively to form an arbuscule (Figure 4). Additionally, the hyphae growing between the cell walls may give rise to structures like vesicles, spores, or auxiliary cells [50,51].
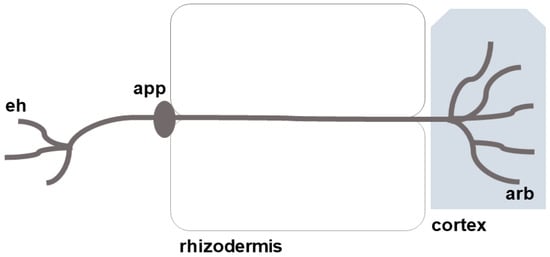
Figure 4.
Mechanisms by which mycorrhizae resist heavy metals. eh—hyphae, and arb—arbuscule.
The hyphae facilitate nutrient transport to the fungus, with the arbuscule, or branched hyphae, serving as the primary site for nutrient exchange between the mycorrhizae and the plant, thereby enhancing the plant’s nutritional status [52]. This process also applies to heavy metals, as fungal hyphae absorb heavy metals and can transfer them to the plant. As a result, mycorrhizal plants may exhibit increased heavy metal uptake and translocation to their aerial parts. In other instances, mycorrhizal fungi aid in the immobilization of soil heavy metals [53]. The immobilization process occurs through the insoluble glycoprotein or glomalin release, which can bind to metals such as Cd, Cu and Pb. Approximately 1 g of glomalin has the capacity to remove up to 0.08 mg of cadmium, 4.3 mg of copper, and 1.12 mg of lead, respectively [54]. Additionally, mycorrhizae may utilize fungal vesicles, similar to plant vacuoles, for the storage of toxic compounds, providing an extra detoxification mechanism [53]. As a result, mycorrhizae act as an effective exclusion barrier, limiting metal entry into the plant and enhancing its heavy metals’ tolerance.
Ma et al. [55] demonstrated the application of arbuscular mycorrhizal fungi (AMF) in phytoremediation as a way to address soil contamination by geogenic contaminants (GCs). The study found that AMF significantly enhances the phytoremediation process by increasing plant biomass and improving the metal uptake capacity of host plants. AMF also contributes to the stabilization of soil structure by enhancing the soil’s physicochemical and biological properties, which aid in contaminant detoxification and immobilization. However, the field application of AMF remains challenging due to the complexity of interactions between AMF and host plants. These interactions are critical for processes such as metal accumulation, detoxification, and the distribution of metals within plant systems, highlighting the need for further research to optimize AMF-assisted phytoremediation.
Wang et al. [56], studying the effects of cadmium (Cd) contamination in agricultural soils, identified that inoculating alfalfa with arbuscular mycorrhizal fungi (AMF) significantly enhances the plant’s Cd tolerance. They observed that AMF inoculation restricted Cd inflow to the shoots and protoplasm while promoting the production of organic compounds (notably increasing HM-Res4 expression by 1.2 times), thus facilitating Cd complexation and reducing its toxicity. AMF also enhanced the plant’s ROS scavenging capacity and osmotic regulation, thereby lowering oxidative stress and preserving cellular balance.
The root cell walls are continuously exposed to heavy metals in the soil solution, so the adsorption capacity of the cell wall is limited, making it one of the plant’s mechanisms for tolerating these contaminants [48]. The cell wall contains micropores with negative charges because of carboxylic groups that serve as sites for binding and exchanging cations, including many heavy metals. In response to heavy metal contamination, the plant increases the thickness of the cell wall in affected tissues. This adds more reactive sites with negative charges, helping to shield internal tissues from the toxic effects of heavy metals [47].
When considering the excluder plants, heavy metal uptake is restricted, as the metals are trapped in the roots. This containment helps prevent the spread of contamination in the environment and protects the plant’s aerial parts, particularly the photosynthetic system, from the harmful effects of these metals [57].
The root exudates play multiple roles due to their diverse composition. The most commonly mentioned components regarding metal tolerance are low-molecular-weight acids. These acids can form complexes with metal cations, reducing their extracellular activity, thereby decreasing metal availability and enhancing the plant’s resistance to these contaminants [58]
In plants growing in heavy metal-contaminated environments, the uptake of these elements can be reduced by decreasing the production of membrane transporters in root cells. This reduction limits metal absorption, helping to mitigate their toxic effects while maintaining the contamination levels in the environment. These metal transport genes encompass zinc and iron-regulated transport proteins (ZIP), natural resistance-associated macrophage proteins (Nramps), ATPases and metal tolerance or transport proteins (MTP) [59,60]. ZIP family transporters are involved in the uptake of iron (Fe) and zinc (Zn), and some members of this family are capable of transporting multiple metals with varying affinities. For example, the AtIRT1 gene has been found to mediate the transport of several divalent metal ions, including cadmium, cobalt, zinc and manganese [61]. AtIRT1 is expressed primarily in the root epidermis, with lower expression in the root cortex. So, under iron deficiency, Cd accumulation in irt1 knockout mutants is greatly reduced [33].
In the study conducted by Liu et al. [62] on Pleurotus ostreatus, thirteen genes identified as HMATs were extracted from the genome of P. ostreatus and categorized into four classes based on conserved domains: the NRAMP family, P1B-ATPase family (metal transport or tolerance proteins), ZIP family, and additional families. NRAMP is predicted to reside in the endoplasmic or vacuolar membrane, where it has been associated with facilitating the uptake of Cd2+ and Hg2+. ZIP family proteins assisted in the absorption of Cd and Hg, and in the efflux of Hg, indicated diverse roles in the transport of HM in P. ostreatus.
In the study conducted by Plaza et al. [63] on Thlaspi caerulescens and the Ganges ecotype, four ZIP gene family members (TcIRT1, TcIRT2, TcZNT1, TcZNT5) were detected in root tissues. When the plants were grown with sufficient Fe, whether in the presence or absence of Cd, TcZNT1 expression remained unaffected by Cd, and the expression levels were consistent across both ecotypes. However, TcZNT5 expression in the Ganges was lower compared to Thlaspi caerulescens. Additionally, Cd treatments did not have a noticeable impact on TcZNT5-G expression in the roots of either ecotype.
Fan et al. [64] identified 31 genes from the M. notabilis genome, including nine MnZIPs, four MnNRAMPs, eight MnHMAs, and ten MnMTPs. The MnZIPs, MnNRAMP1, 4, and MnHMA2 were found in the plasma membrane, while MnNRAMP2-3, MnMTP1, and 3–5 were located in the vacuole membrane. MnHMA3–5 and 7 were present in both the plasma membrane and Golgi apparatus, and MnMTP6–10 was localized to the Golgi apparatus and vacuolar membrane. Additionally, MnHMA1, 6, and 8 were localized to the chloroplast, and MnMTP2 was found in the mitochondria.
The higher expression of these transporters enhances the rate of heavy metal uptake, effectively impacting the absorption kinetics of these elements and promoting the plant’s potential for metal bioaccumulation [47].
4.2. Intracellular Mechanisms
The intracellular mechanisms (Figure 3) involve the repair of proteins damaged by contamination, high-affinity ligands’ ability to chelate metals, and the transport and compartmentalization of these metals within the vacuole, thereby isolating them from the cell’s metabolic processes [47].
Heat shock proteins (HSPs) represent the growth response of various organisms to temperatures exceeding their optimal range. HSPs, present in all living organisms, can be categorized by molecular size and are now recognized for their expression under various stress conditions, including exposure to heavy metals. These proteins function as molecular chaperones, assisting in normal protein folding and assembly, and also play a role in protein protection and repair during stressful conditions [48].
Chelation of heavy metals is characterized as a critical detoxification and tolerance mechanism involving high-affinity ligands such as organic acids, amino acids, and peptides like metallothioneins (MTs) and phytochelatins (PCs).
The binding of metals to these chelators helps reduce their concentration in the cytosol, lowering their reactivity, solubility, and the likelihood of toxic effects in plants [47]. Phytochelatins, in particular, are well-studied in plants, especially regarding their role in Cd and Hg tolerance, since they induce the metabolism of small peptides, produced enzymatically from glutathione and sequester metals, and aid in their detoxification [48,58].
Metal chelation triggers the process of metal compartmentalization. Once metals are bound by chelating agents, as previously described, and their toxic effects are neutralized, they are transported to subcellular structures, such as the vacuole, for accumulation, which reduces the presence of contaminants in the cytosol. The compartmentalization of heavy metals in the vacuole is widely regarded as one of the most important mechanisms for metal tolerance [47]. A well-known example involves the accumulation of Cd and phytochelatins (PCs) in the vacuole, mediated by an ABC transporter. Additionally, evidence suggests that other metals can be sequestered in the vacuole through different tonoplast transport systems. This accumulation is thought to be facilitated by a Cd/H+ antiporter and an ATP-dependent ABC transporter in the tonoplast, while the stabilization of the Cd-PC complex in the vacuole is related to the incorporation of acid-labile sulfide [48].
In the study conducted by Midhat et al. [65], 46 native plant species were collected from the three mining sites. Total metal concentrations were variable in shoots and roots and ranged respectively from (not detected to 9.45; not detected to 15.99) mg kg−1 for Cd. The Cd levels in plant tissues showed high levels, above normal, and in phototoxic concentrations. The results indicate that these plant species are tolerant to heavy metals, as they have the ability to survive in soils that are toxic to other plants and have the ability to accumulate them in their tissues. Thus, it can be said that the toxic effects of heavy metals are mitigated by resistance mechanisms, such as decreasing the absorption of heavy metals or increasing the efflux of metals, among others. Normal and phototoxic concentrations of Cd, were 0.1–3 mg kg−1 [66].
5. Evolution of Heavy Metal Tolerance Mechanisms
Oxidative stress can be triggered by heavy metals through the generation of free radicals and reactive oxygen species (ROS), which can damage plant membranes, disrupt organelle structure, impair metabolic functions, and hinder plant growth. To counter this, plants possess antioxidant systems composed of chemical compounds that neutralize free oxygen radicals, protecting healthy cells from damage.
Among these antioxidants, SOD plays a crucial role in the defense system, being the first line of protection against the toxic effects of reactive oxygen species. Its primary function is to rapidly convert superoxide radicals (O2−) into hydrogen peroxide (H2O2). The rise in SOD activity is linked to an increase in superoxide radical concentrations, likely due to the synthesis of new enzyme proteins [20].
Another key enzyme involved in plant defense is peroxidase (POD), which works synergistically with SOD and is abundant across plant species. Its great activity is closely associated with essential processes like respiration, photosynthesis, and auxin oxidation. Additionally, POD can play a direct role in lignin biosynthesis, which helps reinforce the physical barrier against heavy metals like cadmium (Cd) [67].
Another critical mechanism in the antioxidant defense against ROS involves the activity of catalase (CAT), which breaks down H2O2 into water and oxygen [68]. Catalase is present in cytosol, peroxisomes, and mitochondria [67]. Various studies have documented both increases and decreases in CAT activity in response to metal-induced stress [20]. This enzyme is highly susceptible to photoinactivation and degradation and can be rendered inactive by elevated levels of hydrogen peroxide [69].
The ascorbate peroxidase (APX) plays a crucial role in the ascorbate/glutathione pathway, where it is essential for removing H2O2, primarily generated in chloroplasts and various cellular organelles, to help preserve the cell’s redox balance. By utilizing the reducing power of ascorbic acid, APX neutralizes H2O2, which could otherwise pose a threat to the cell [70].
A commonly used bioindicator to measure the oxidative degradation of lipids is lipid peroxidation, which is triggered by an excessive rise in reactive oxygen species (ROS) [69]. This process compromises the structural integrity and function of membranes by altering their fluidity and permeability, potentially leading to K+ ion leakage, amino acid oxidation, and ultimately cell death [45]. A key byproduct of lipid peroxidation is malondialdehyde (MDA), which is typically quantified through the formation of TBARS—thiobarbituric acid reactive substances.
The phenolic compounds (PCT) play a crucial role in the morphology and physiology of plants while also offering protection against oxidative stress. These compounds, which include secondary metabolites such as phenols and flavonoids, have the ability to degrade heavy metals. Antioxidant activity is commonly evaluated using the free radical DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, a widely utilized test. An increase in DPPH scavenging activity may be associated with a rise in certain secondary metabolites, which enhance antioxidant defenses [71]. Table 2 presents different plant species’ responses to antioxidant activity after cadmium stress.

Table 2.
Different plant species respond to antioxidant defense activity after cadmium stress.
6. Bioremediation Strategies
The growing concern over environmental pollution caused by human activities stems primarily from its harmful effects on the environment, which in turn pose risks to human health [75]. Advances in remediation techniques have been observed, particularly in terms of their cost-effectiveness, ecological approach, and ability to restore contaminated environments [76].
Unlike conventional remediation methods such as adsorption, chemical oxidation, pyrolysis, soil washing, flocculation, landfill, and incineration, biological remediation offers a more efficient approach to detoxifying and degrading pollutants to restore contaminated environments. This method is regarded as sustainable, economical, and natural, utilizing organisms to clean polluted sediments, soil, and water, making it a favorable alternative to traditional techniques [77].
Bioremediation techniques will involve the use of biological systems, primarily microorganisms such as algae, bacteria, green plants, and their enzymes, or fungi, to control environmental pollution by degrading, mineralizing, detoxifying, removing, or transforming contaminants. These pollutants are converted into CO2, H2O, microbial biomass, and metabolites, which are less toxic than the original substances and can meet the concentration limits set by the regulatory authorities [78].
The effectiveness of pollutant removal is largely influenced by the type of contaminant, e.g., benzene, pharmaceutical compounds, plastics, pesticides, halogenated hydrocarbons from petroleum, nuclear waste, sewage, xylene, polyaromatic hydrocarbons, and heavy metals, and their concentration. Additionally, successful bioremediation depends not only on the presence of suitable microorganisms but also on environmental conditions that support microbial growth and activity. This process often requires adjusting environmental factors such as soil type, oxygen levels or other electron acceptors, temperature, nutrients, and a suitable pH to enhance microbial growth and accelerate degradation [76,79,80].
Microorganisms can be found in nearly all environmental conditions, as they are highly adaptable and capable of thriving in diverse habitats such as anaerobic conditions, hot springs, oxygen-rich environments, deserts, oceans, glaciers, saline lakes, and even in the presence of waste streams or hazardous compounds. Their primary needs for adaptation are an energy source and a carbon source [78]. According to Harekrushna and Kumar [76], these microorganisms can be classified into different groups, each specialized in removing a specific type of contaminant, as outlined below:
Aerobic microorganisms are capable of surviving in oxygen-rich environments. Examples include bacteria such as Pseudomonas, Alcaligenes, Sphingomonas, Rhodococcus, and Mycobacterium. These organisms are commonly involved in the degradation of hydrocarbons and pesticides, including both polyaromatic compounds and alkanes.
Anaerobic microorganisms can survive in environments without oxygen. Although they are less commonly used than aerobic bacteria, there is increasing interest in their application for bioremediation, particularly for the dechlorination of solvents like trichloroethylene (TCE) and chloroform and for removing polychlorinated biphenyls (PCBs) in river sediments.
Ligninolytic fungi, such as the white rot fungus Phanerochaete chrysosporium, are known for their capacity to break down a wide variety of toxic and persistent environmental pollutants.
Methylotrophs are aerobic bacteria that utilize methane as both an energy source and a carbon source. The enzyme methane monooxygenase, which initiates the aerobic degradation process, operates on various substrates and is used to break down chlorinated aliphatic compounds like 1,2-dichloroethane and trichloroethylene. For effective degradation, the bacteria must come into contact with the contaminant, which can be challenging due to the uneven distribution of both microbes and pollutants in the soil or substrate. Some bacteria are motile and exhibit chemotaxis, allowing them to detect and move towards the contaminant, while others, such as fungi, grow in a filamentous pattern towards it. The mobilization of contaminants can be enhanced by using surfactants like sodium dodecyl sulfate.
Bioremediation technology, in regard to the removal and transport of pollutants from contaminated areas, can be divided into two main types: in situ and ex situ (Table 3). In situ bioremediation is often preferred due to its lower costs and minimal site disruption, as it treats contaminants directly at the location without requiring excavation or transportation. However, the effectiveness of in situ methods is restricted by the soil depth that can be treated. The other type is ex situ bioremediation, which involves excavating or removing the contaminated soil to a designated treatment location. The use of ex situ techniques must take into account factors such as contaminant type, pollution depth, geographical location, treatment cost, pollution severity, and the site’s geology [79,81].

Table 3.
Comparison between pros and cons of in situ and ex situ bioremediation.
Table 4 provides a summary of the various bioremediation methods.

Table 4.
Overview of bioremediation techniques and their underlying principle.
Bioremediation presents several challenges that must be addressed for effective implementation. One significant issue is the complexity of contaminants found at polluted sites, where chemical mixtures can hinder complete degradation through standard bioremediation techniques. Additionally, site heterogeneity poses a challenge, as environmental conditions—such as pH, temperature, moisture, and nutrient levels—can vary widely, complicating the development of adaptable bioremediation strategies. Another concern is the slow degradation rates associated with bioremediation, as microorganisms may need time to acclimate and develop effective populations for degradation. Furthermore, while bioremediation is effective for many organic pollutants, its applicability is limited when it comes to heavy metals and radioactive materials, which may require integration with alternative treatment methods [82]. Understanding these challenges is crucial for optimizing bioremediation strategies and ensuring their successful implementation in addressing environmental contamination.
7. Phytoremediation
The term “phytoremediation” combines the Greek word “phyto” (that means plant) and the Latin word “remedium” (that means to remove or correct a harmful element) [83]. Phytoremediation is an innovative bioremediation approach that employs plants and their roots to clean contaminated water and soil. This technique is based on the natural processes of plants, their interactions with associated microorganisms, and conditions such as organic matter that degrade, remove, or immobilize contaminants, thereby reducing their toxicity to the environment [84,85]. Phytoremediation follows various contrivances, such as phytoextraction, rhizofiltration, rhizodegradation, phytostabilization, phytodegradation, and phytovolatilization. Phytoremediation follows several procedures such as phytoextraction, which is the absorption of contaminants from soil or water by plant roots, which are stored in their above-ground parts, such as stems and leaves [86]; rhizofiltration is a method specifically designed for water purification, in which roots are used to absorb, retain, and sediment contaminants, mainly metals, within the roots, ensuring the limited movement of these contaminants in different environments. In this process, aquatic or terrestrial plants can be used. Aquatic species (hyacinth, azolla, duckweed, cattail, and poplar) are often used for the remediation of marsh waters.
Environmental factors, such as pH in the rhizosphere, root exudates and root turnover, play a vital role in the sedimentation of metal contaminants on the root surface. In this process, the plant and the microbial community have a symbiotic association, generally using Pseudomonas aeruginosa, Mycobacterium spp. and Rhodococcus spp. as microorganisms. Therefore, to improve the efficiency of phytoremediation in this region of the rhizosphere, other bioremediation techniques discussed in the Bioremediation Strategies section can be applied. Regular harvesting of plants is essential to maintaining the effectiveness of rhizofiltration [87]; phytodegradation is more appropriate for moderately hydrophobic organic compounds. Contaminants are decomposed externally to the plant through the effect of enzymes [dehalogenase, peroxidase, nitroreductase, nitrilase and phosphatase] produced by the plant and/or through metabolic processes within the plant [87]. However, the phytodegradation process can occur associated with the rhizodegradation mechanism, which consists of the breakdown of organic contaminants in the soil through microbial activity that secretes specific enzymes that degrade or transform organic pollutants. The disadvantage of rhizodegradation is the depth limitation, generally 20 to 25 cm, which makes the process time-consuming. Rhizodegradation is influenced by the type of soil and the species of plants selected [87]; phytostabilization is a process that takes advantage of the ability of plant roots to alter the environmental conditions of the soil, such as pH and soil moisture content.
Several root exudates cause the precipitation of metals, thus reducing bioavailability [86]; phytovolatilization is the transformation of pollutants into different volatile compounds in the atmosphere through transpiration with the assistance of stomata. This mechanism can be used for organic contaminants and some inorganic contaminants that also occur in volatile forms, such as mercury and arsenic. The use of this mechanism is limited and considered the most controversial because it is understood that it does not remove the contaminant completely but only transfers it to another segment of the environment. Plants such as Nicotiana tabacum, Crinum americanum, Triticum aestivum, Arabidopsis thaliana, Bacopa monnieri and Trifolium repens are commonly used for phytovolatilization [86,87].
Additionally, it offers the potential for utilizing contaminated biomass in profitable applications like energy production of energy [88] and biofiltering [89]. Plants can also serve as indicators of metal pollution, as they tend to accumulate metals in their above-ground parts, which often reflect the environmental metal concentrations [90]. A list of some plants that show capacity for cadmium accumulation is given in Table 5.
Phytoremediation is a method used to clean sediments, groundwater, soils, and surface waters that are contaminated with a broad range of pollutants, including heavy metals [52,91,92,93], organic substances (such as pesticides, chlorinated solvents, and petroleum-derived hydrocarbons, inorganic materials (like nitrates, cyanides and sulfates), as well as radionuclides, explosives, and landfill leachates, which can be present up to 20 m deep [94].
Certain plants have the ability to detoxify their surrounding environment while maintaining their utility and contributing to soil fertility by supplying organic matter in the form of humus and root exudates, which support greater biodiversity in the soil. Additionally, they can be employed for plume hydraulic control (contaminated groundwater), as well as for managing infiltration through vegetation cover, surface runoff containment, and stabilizing soil to prevent erosion and the subsequent leaching of contaminants [83,95,96].
Plants used for phytoremediation should exhibit certain key traits, including (a) significant above-ground biomass production; (b) the capacity to transfer heavy metals from roots to shoots; (c) good adaptability to local environmental and climatic conditions; (d) ease of cultivation and also harvesting; (e) rapid growth rate; (f) resistance to pests and pathogens; (g) an extensive, well-branched root system and dense foliage; (h) resistance to herbivores to prevent contamination of the food chain; and (i) the ability to tolerate toxic metals and accumulate them at substantial levels [83,97,98].
As noted by Susarla et al. [94], several factors influence the uptake and distribution of pollutants in living plants, including (1) plant-specific traits, such as the type of root system and the presence of certain enzymes; (2) environmental conditions such as soil moisture, organic matter content, pH and temperature; (3) properties of the contaminant (physical and chemical) such as water solubility, molecular mass, vapor pressure, and the octanol-water partition coefficient (Kow).
Regarding the plant material after use for removing contaminants, the integration of circular economy principles in phytoremediation could greatly enhance both sustainability and efficiency in handling contaminated biomass. For example, biomass from plants can be transformed into biochar, which serves as an effective adsorbent for removing pollutants from water and soil. This approach has been successfully implemented in some cases, demonstrating the potential to turn waste into a valuable resource [96,99]. A study conducted by Wang et al. [100] assessed the efficacy of biochar derived from three different plants post-phytoremediation to remove heavy metals, all processed under identical pyrolysis conditions. The findings indicated that biochar from Conyza canadensis showed the highest adsorption capacity for lead (Pb(II)) and cadmium (Cd(II)), with peak adsorption values reaching 139.16 mg/g and 8.82 mg/g, respectively. This suggests that the selection of plant species significantly influences biochar’s performance in heavy metal adsorption, with Conyza canadensis offering notable potential in remediating Pb and Cd in contaminated environments.


Table 5.
Plants tested for accumulation of cadmium metal.
Table 5.
Plants tested for accumulation of cadmium metal.
| Examples of Plants | Growing Conditions | Cd Concentrations (mg.kg−1) | Efficiency | Effect | References |
|---|---|---|---|---|---|
| Sedum plumbizincicola | Phytoextraction was conducted for two growing seasons in an 8-ha polluted field with Cd. | 170 in shoots first crop 172 in shoots second crop | soil Cd decline based on plant uptake 40% soil Cd decline after phytoextraction 10% | Decline in biomass from the first to the second crop is 53.3%. | [101] |
| Sedum plumbizincicola | Phytoextraction was conducted for three growing seasons in a 140-ha polluted field with Cd and ad the hydroxyapatite. | 13.8 in shoots first crop 14 in shoots second crop 14 in shoots third crop | soil Cd decline first 8.49% soil Cd decline second 8.88% soil Cd decline third 13.79% | Greater immobilization of Cd in the soil due to the addition of hydroxyapatite which raised the pH from 4.24 to 5.17. | [102] |
| Setaria lutescens | 1.5 in shoots first crop 1.8 in shoots second crop 1.8 in shoots third crop | soil Cd decline first 2.91% soil Cd decline second 0.76% soil Cd decline third 4.18% | |||
| Elsholtzia splendens | 2.3 in shoots first crop 2.3 in shoots second crop 2.3 in shoots third crop | soil Cd decline first 5.33% soil Cd decline second 5.58% soil Cd decline third 10.34% | |||
| Pennisetum sp. | 1.9 in shoots first crop 2 in shoots second crop 2 in shoots third crop | soil Cd decline first 7.76% soil Cd decline second 10.65% soil Cd decline third 13.79% | |||
| Lolium perenne | Polluted field with Cd 3.06 mg.kg−1and mycorrhizae or not. | 2 in shoot-only plants 1 in shoots plant + mycorrhizae 0.5 in root-only plants 1.5 in root plants + mycorrhizae | - | Increased plant tolerance to Cd higher concentration of CD in the root zone. | [103] |
| Sedum alfredii | Hydroponic system and 25 μM Cd for 4 weeks. | 3500 in shoot-only plants 4000 in shoots plant + Pseudomonas fluorescens 800 in root-only plants 1500 in root plants + Pseudomonas fluorescens | - | ↑ Chlorophyll Biomass Absorption nutrition Absorption Cd in shoot | [104] |
| Brassica juncea | Plastic pots with 8 dm−3 of soil and levels of Cd 0–30 mg.kg−1. | 14.8 in grains 75.8 in shoots | - | ↓ biomass | [105] |
| Brassica campestris | Plastic pots with 8 dm−3 of soil and levels of Cd 0–30 mg.kg−1. | 16.5 in grains 95.8 in shoots | - | ↓ biomass | |
| Brassica napus | Plastic pots with 5 kg soil and levels of Cd 0–80 mg.kg−1. | 15.3 in grains 85.7 in shoots | - | ↓ biomass | |
| Crambe abyssinica | Plastic pots with 8 dm−3 of soil and levels of Cd 0–30 mg.kg−1. | 3598 in leaf 95 in stems 124.75 in roots | - | ↓ Chlorophyll Biomass Absorsion nutrition | [106] |
| Arachis hypogaea L. | Plastic pots with 20 kg soil and 0.438 mg.kg−1 of Cd and levels of Cl 10–136%. | 3.2 in shoots 4.39 in roots 4.5 in leaves | - | ↑ Accumulation Cd | [107] |
| Tagetes erecta | Plastic pots with 2.8 kg soil and mean 36.9 mg.kg−1 of Cd and either pig or cattle manure, or organic fertilizer. | 12.2 in shoots 9.1 in roots 1.25 in flowers | - | little biomass reduction increase in phenol production | [108] |
| Tagetes erecta | 125 m2 polluted field with 38.2 mg.kg−1 of Cd and either pig or cattle manure, or organic fertilizer. | 9.3 in shoots 7.9 in roots 0.48 in flowers | soil Cd decline based on plant uptake 78.6% | little biomass reduction increase in phenol production | [108] |
| Canna indica | Hydroponic system and levels of Cd 0–400 mg and 18 days. | Whole plant 1088.61 mg.g−1 | 89.5% to 96.8% calculated from the residual substrate concentration | ↓ Chlorophyll biomass | [109] |
8. Evaluation of Phytoremediation Efficiency
Plant phytoremediation potential is generally measured by the concentration of pollutants accumulated in various parts (e.g., roots, stems, leaves, fruits, seeds, flowers, oil derivatives, and flour) of the plant and the percentage of pollutant removal from water or soil [110]. However, different efficiency indices have been used in the past two decades in the field of metal phytoremediation to study plant–soil interaction, transport mechanisms, and accumulation patterns in plants, which are the topics most studied [111,112].
BCF—bioconcentration factor (The ratio of the concentration of metal in the roots to that in soil) Equation (1).
TF—translocation factor (The ratio of metal concentration in the shoots to metal concentration in the roots) Equation (2).
where the C shoot, C root, and C soil are the concentrations of heavy metal in the shoot, root and soil, respectively.
The BCF and TF for Cd from three zinc mining areas in China were evaluated, and soil and plants were collected for analysis (Table 6). Erigeron sumatrensis, which thrives in several mining areas, is extremely vigorous and has a large biomass, which has the potential for phytoremediation. Its invasive success is mainly due to its prolific production of tiny, lightweight seeds, over 20,000 per plant, allowing easy dispersal by wind [112]. Amaranthus spp. are characterized by fast growth rate, tolerance to heavy metals, and high soil utilization for remediation of heavy metal-contaminated soils [113].

Table 6.
Parameters for evaluating the efficiency of phytoremediation.
The BCF and TF for Cd from three zinc mining areas in China were evaluated, and soil and plants (E. sumatrensis, Amaranthus spp.) were collected for analysis (Table 6). E. sumatrensis, which thrives in several mining areas, is extremely vigorous and has a large biomass, which has the potential for phytoremediation. Its invasive success is mainly due to its prolific production of tiny, lightweight seeds, over 20,000 per plant, allowing easy dispersal by wind [112]. Amaranthus spp. are characterized by fast growth rate, tolerance to heavy metals, and high soil utilization for remediation of heavy metal-contaminated soils [113]. The BCF of Cd for E. sumatrensis was highest in the Liuyang and Yueyang areas, and TF was highest in the Huayuan and Yueyang areas. Since Cd contamination was lower in Liuyang and Yueyang and at the same time, BCF was higher in these two areas than in the other areas, this suggests that E. sumatrensis may be more effective in enriching Cd in areas with low pollution. Cd has high toxicity that can induce oxidative stress in plants and impair the function of Cd transporters and translocators [114]. The same applies to TF, but in Liuyang, the translocation factor may be affected by the presence of other heavy metals, such as Pb, which is the area with the highest contamination of this metal, as well as soil pH and soil types [115]. For the plant Amaranthus spp., BCF and TF were higher in the Huayuan and Yueyang areas, to which the same considerations attributed to the plant E. sumatrensis can be applied. However, other factors must be considered, such as the presence of microorganisms that can positively interfere with BCF and TF of Cd [113] and soil organic matter content, since in soil with levels below 1%, certain species demonstrated hyperaccumulation behavior [116], as well as other factors reported in the previous section. To be considered a Cd hyperaccumulator, a plant must accumulate more than 100 mg kg−1 dry weight of Cd in the shoots (stems or leaves), 1000 mg/kg for Pb, 3000 mg/kg for Zn, 300 mg/kg for Cr or Co [111] or present BCF and TF values > 1 [116].
9. Conclusions and Future Prospects
The rise in environmental contamination from heavy metals, like cadmium, largely due to human activities, has led to a decline in the quality of aquatic ecosystems and soils worldwide. Cadmium (Cd) has been classified by the EPA and ATSDR as a non-essential, non-beneficial, and potentially toxic metal with a high likelihood of contaminating the food chain and posing risks to human health. Consequently, employing aquatic or terrestrial plants for the phytoremediation of such environments is considered a promising, eco-friendly, and cost-effective green technology. Nevertheless, not every plant has the ability to absorb or adsorb cadmium (Cd) from the environment, making it crucial to study the mechanisms involved in Cd accumulation and detoxification in plants to determine their optimal use. One example of this mechanism is the plant’s association with mycorrhizal fungi, which aids in the uptake or immobilization of Cd, helping to reduce its toxic effects.
To enhance the efficiency of the use of mycorrhizal fungi in cadmium remediation, future research could explore genetic modification and biotechnology to optimize these fungi’s resilience and efficacy in contaminated environments. By genetically engineering AMF strains, studies could potentially increase metal tolerance and binding capabilities, thus improving cadmium uptake and immobilization in soil. For example, introducing or overexpressing genes that boost metal transport and sequestration, or those regulating oxidative stress tolerance, could make AMF more robust in adverse conditions.
Additionally, advances in biotechnology could support the development of AMFs that are more adaptable to diverse environmental conditions, such as variable pH or nutrient availability, which often impact bioremediation success in the field. Enhanced strains could also help stabilize cadmium in soils, reducing its bioavailability and environmental toxicity. Genetic modification could even enable AMF to interact more effectively with specific plants known for phytoremediation, creating symbiotic pairs that optimize both metal uptake and detoxification pathways. Exploring these aspects may overcome some bioremediation limitations.
Cadmium toxicity can lead to excessive production of reactive oxygen species (ROS). To cope with the oxidative stress caused by Cd, plants possess a highly efficient defense system that includes both oxidants and antioxidant enzymes. The involvement of several antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), has been documented in different plant species.
The molecular mechanisms involved in cadmium (Cd) transport remain poorly understood. These mechanisms include zinc and iron-regulated transport proteins (ZIP), metal transport or tolerance proteins (MTP), and natural macrophage resistance proteins (Nramps). Consequently, future research may uncover how Cd is distributed within various plant parts (such as leaves, stems, seeds, and fruits). Additionally, it is essentially the development of effective methods for managing Cd-contaminated biomass, including the production of adsorbents.
To address the gaps in understanding of cadmium transport mechanisms, future research could focus on specific molecular pathways and gene families involved in Cd uptake, sequestration, and detoxification in plants and their associated mycorrhizal fungi. For instance, investigating the roles of NRAMP and ZIP could help elucidate how Cd is absorbed and translocated within plants and fungi.
Additionally, identifying and characterizing regulatory networks involving metal-binding proteins, chelators, and enzymes responsible for oxidative stress response (e.g., glutathione-S-transferase) would clarify the biochemical and physiological adaptations these organisms employ to tolerate Cd. Exploring gene expression patterns under Cd stress through transcriptomics and proteomics could reveal the upregulation or suppression of key genes involved in Cd homeostasis, further guiding genetic or biotechnological interventions.
By focusing on these molecular pathways, it could be possible to uncover potential targets for enhancing Cd resistance and accumulation efficiency in bioremediation systems, ultimately making phytoremediation a more viable and efficient approach in Cd-contaminated environments.
Environmental contamination by heavy metals is a significant concern, primarily because of their toxicity at low concentrations and their persistence in ecosystems. Cadmium, in particular, is not only non-essential but also highly toxic, even at minimal levels. This underscores the urgent need to develop effective strategies for its removal.
A promising option for removing contaminants is phytoremediation, where plants act as the primary agents for decontamination. This review highlights the key tolerance mechanisms plants use to cope with elevated cadmium (Cd) levels. Extracellular mechanisms include active efflux, heavy metal binding to the cell wall, reduced heavy metal uptake, and decreased mobility and availability of heavy metals through mycorrhizal fungi. Additionally, recent studies have explored intracellular mechanisms such as the chelation of heavy metals by ligands with high affinity, repair and protection of the plasma membrane, metal compartmentalization, and the accumulation of heavy metals within vacuoles.
An additional element supporting plants in detoxifying heavy metals is the presence of mycorrhizal fungi, which act as regulators by absorbing heavy metals through their hyphae and transporting them to the plant. In some cases, plants associated with mycorrhizal fungi show enhanced heavy metal uptake and increased translocation to the aerial parts, while in others, the fungi contribute to immobilizing heavy metals within the soil.
Additionally, metal transport genes such as ATPases, natural resistance-associated macrophage proteins (Nramps), metal transport or tolerance proteins (MTP), and zinc and iron-regulated transport proteins (ZIP) can directly affect the heavy metal levels in plants and their detoxification processes.
Plants can develop various tolerance mechanisms through the activation of antioxidant enzyme systems (such as CAT, POD, and SOD) and non-enzymatic mechanisms (like PCs). Understanding these tolerance mechanisms is crucial for effective heavy metal removal from the environment. This knowledge is vital for optimizing cadmium (Cd) remediation strategies and assessing their potential for in situ applications, particularly in aquatic environments.
The use of different bioremediation techniques contributes to sustainability since they reduce environmental impacts compared to traditional remediation techniques. The possibility of using living organisms to remove, degrade or immobilize is in accordance with sustainable development as long as we avoid the usage of synthetic chemicals that can generate even more waste and pollutants. This phenomenon leads to a less aggressive and eco-friendly environmental remediation.
Another aspect that highlights the technique as an excellent alternative for the sustainable recovery of degraded environments is the rational use of natural resources since the application of the technique prioritizes the use of local organisms that are already adapted to the environment and present pre-existing biological processes, promoting a more sustainable life cycle.
Furthermore, the application of bioremediation allows risk reduction by reducing human exposure to highly toxic compounds. This environmental recovery helps and contributes to the maintenance of biodiversity, which is one of the vital components of sustainable development. Another aspect to be mentioned is the reduction in costs and energy required to recover contaminated areas using bioremediation techniques, given that priority is given to the use of biological processes, which are natural and do not normally require large infrastructures to be performed.
The promotion of a circular economy is clear with the application of bioremediation techniques, where the contaminants, such as heavy metals, can be removed by plants, and this biomass can be used for the production of adsorbents through the pyrolysis process. The new adsorbents produced can be used for the removal of other contaminants, improving the product life cycle.
Considering the three main pillars of sustainable development (environmental, economic and social factors), the use of bioremediation techniques contributes significantly to all factors. Regarding the environmental factor, the application of this type of technique allows the restoration of degraded ecosystems while minimizing environmental damage. Regarding the economic factor, the use of bioremediation contributes by reducing operational costs and increasing the efficiency of contaminant removal, generating direct economic benefits for several sectors. Finally, the social aspect is widely met since it allows the protection of human health and the maintenance of the population’s quality of life by reducing exposure to highly toxic compounds.
Undoubtedly, there is still much to be discovered and learned from the study of plants, especially regarding the molecular mechanisms and genetic engineering of hyperaccumulator plants that would improve the remediation of environmental contaminants, including emerging contaminants such as pharmaceuticals. Future prospects for field applications of phytoremediation with genetically modified plants lack legal approval. Therefore, public approval must be obtained for the preferential use of crops versus non-crop hyperaccumulators.
Author Contributions
Conceptualization, J.P.F. and R.A.; methodology, J.P.F. and R.F.d.S.; investigation, J.P.F., F.S.C., B.C.O., S.P., C.F.D. and R.A.; resources, R.A. and M.S.Q.; writing—original draft preparation, J.P.F.; writing—review and editing, J.P.F., C.F.D., M.A.d.S. and R.A.; supervision, R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance code 001, by the CNPq (National Council for Scientific and Technological Development), and FAPERGS (Research Support Foundation of the State of Rio Grande do Sul).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shahzad, A.; Zahra, A.; Li, H.Y.; Qin, M.; Wu, H.; Wen, M.Q.; Ali, M.; Iqbal, Y.; Xie, S.H.; Sattar, S.; et al. Modern Perspectives of Heavy Metals Alleviation from Oil Contaminated Soil: A Review. Ecotoxicol. Environ. Saf. 2024, 282, 116698. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud Univ.-Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Novoselov, A.A.; Hodson, M.E.; Tapia-Gatica, J.; Dovletyarova, E.A.; Yáñez, C.; Neaman, A. The Effect of Rock Lithology on the Background Concentrations of Trace Elements in Alluvial Soils: Implications for Environmental Regulation. Appl. Geochem. 2022, 146, 105440. [Google Scholar] [CrossRef]
- Torres, P.; Llopis, A.L.; Melo, C.S.; Rodrigues, A. Environmental Impact of Cadmium in a Volcanic Archipelago: Research Challenges Related to a Natural Pollution Source. J. Mar. Sci. Eng. 2023, 11, 100. [Google Scholar] [CrossRef]
- Bia, G.; García, M.G.; Cosentino, N.J.; Borgnino, L. Dispersion of Arsenic Species from Highly Explosive Historical Volcanic Eruptions in Patagonia. Sci. Total Environ. 2022, 853, 158389. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S.; et al. Environmental Persistence, Bioaccumulation, and Ecotoxicology of Heavy Metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Kolarova, N.; Napiórkowski, P. Trace Elements in Aquatic Environment. Origin, Distribution, Assessment and Toxicity Effect for the Aquatic Biota. Ecohydrol. Hydrobiol. 2021, 21, 655–668. [Google Scholar] [CrossRef]
- Algül, F.; Beyhan, M. Concentrations and Sources of Heavy Metals in Shallow Sediments in Lake Bafa, Turkey. Sci. Rep. 2020, 10, 11782. [Google Scholar] [CrossRef]
- Orhue, E.R.; Frank, U.O. Fate of Some Heavy Metals in Soils: A Review. J. Appl. Nat. Sci. 2011, 3, 131–138. [Google Scholar] [CrossRef]
- Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- WHO Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 29 October 2024).
- Substance Priority List|ATSDR. Available online: https://www.atsdr.cdc.gov/programs/substance-priority-list.html (accessed on 3 September 2024).
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Nazir, S.; Irshad, R.; Tahir, K.; ur Rehman, K.; Islam, R.U.; Wahab, Z. Toxicity of Heavy Metals in Plants and Animals and Their Uptake by Magnetic Iron Oxide Nanoparticles. J. Mol. Liq. 2021, 321, 114455. [Google Scholar] [CrossRef]
- Andreazza, R.; Okeke, B.C.; Pieniz, S.; Brandelli, A.; Lambais, M.R.; Camargo, F.A.O. Bioreduction of Cu(II) by Cell-Free Copper Reductase from a Copper Resistant Pseudomonas Sp. NA. Biol. Trace Elem. Res. 2011, 143, 1182–1192. [Google Scholar] [CrossRef]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Camargo, F.A.O.; Quadro, M.S.; Andreazza, R. Potential of Solanum Viarum Dunal in Use for Phytoremediation of Heavy Metals to Mining Areas, Southern Brazil. Environ. Sci. Pollut. Res. Int. 2019, 26, 24132–24142. [Google Scholar] [CrossRef]
- Xin, J.; Ma, S.; Li, Y.; Zhao, C.; Tian, R. Pontederia Cordata, an Ornamental Aquatic Macrophyte with Great Potential in Phytoremediation of Heavy-Metal-Contaminated Wetlands. Ecotoxicol. Environ. Saf. 2020, 203, 111024. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of Pseudomonas aeruginosa as PGPR on Oxidative Stress Tolerance in Wheat under Zn Stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef]
- Rajkumar, M.; Freitas, H. Influence of Metal Resistant-Plant Growth-Promoting Bacteria on the Growth of Ricinus Communis in Soil Contaminated with Heavy Metals. Chemosphere 2008, 71, 834–842. [Google Scholar] [CrossRef]
- Demarco, C.F.; Bonemann, D.H.; Ribeiro, A.S.; Cadaval, T.R.S.; Gelesky, M.A.; Godinho, M.; Quadro, M.S.; Pieniz, S.; Andreazza, R. Resistance Mechanisms of Hydrocotyle ranunculoides to Cr(VI): A Biolfilter Plant. J. Clean. Prod. 2023, 405, 136721. [Google Scholar] [CrossRef]
- Jayasri, M.A.; Suthindhiran, K. Effect of Zinc and Lead on the Physiological and Biochemical Properties of Aquatic Plant Lemna Minor: Its Potential Role in Phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef]
- Bianconi, D.; Pietrini, F.; Massacci, A.; Iannelli, M.A. Uptake of Cadmium by Lemna Minor, a (Hyper?-) Accumulator Plant Involved in Phytoremediation Applications. E3S Web Conf. 2013, 1, 13002. [Google Scholar] [CrossRef]
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.S.; Hou, D.; Tack, F.M.G. The Term “Heavy Metal(s)”: History, Current Debate, and Future Use. Sci. Total Environ. 2021, 789, 147951. [Google Scholar] [CrossRef]
- Riaz, U.; Aslam, A.; uz Zaman, Q.; Javeid, S.; Gul, R.; Iqbal, S.; Javid, S.; Murtaza, G.; Jamil, M. Cadmium Contamination, Bioavailability, Uptake Mechanism and Remediation Strategies in Soil-Plant-Environment System: A Critical Review. Curr. Anal. Chem. 2021, 17, 49–60. [Google Scholar] [CrossRef]
- Zhang, H.; Reynolds, M. Cadmium Exposure in Living Organisms: A Short Review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, Bioaccumulation, Health Risks and Remediation of Potentially Toxic Metal(Loid)s (As, Cd, Cr, Pb and Hg): An Epitomised Review. Environ. Monit. Assess. 2020, 192, 108. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J. Role of Phytoremediation in Reducing Cadmium Toxicity in Soil and Water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- Luo, J.-S.; Zhang, Z. Mechanisms of Cadmium Phytoremediation and Detoxification in Plants. Crop J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Pajares, M.J.; Palanca-Ballester, C.; Urtasun, R.; Alemany-Cosme, E.; Lahoz, A.; Sandoval, J. Methods for Analysis of Specific DNA Methylation Status. Methods 2021, 187, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology—Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.R.; Corrêa, E.S.; Moraes, B.S.; Rossato, M.V.; Vestena, S. Toxicidade de cádmio em plantas. Rev. Eletrônica Gestão Educ. Tecnol. Ambient. 2015, 19, 1574–1588. [Google Scholar] [CrossRef]
- Shaari, N.E.M.; Tajudin, M.T.F.M.; Khandaker, M.M.; Majrashi, A.; Alenazi, M.M.; Abdullahi, U.A.; Mohd, K.S. Cadmium Toxicity Symptoms and Uptake Mechanism in Plants: A Review. Braz. J. Biol. 2024, 84, e252143. [Google Scholar] [CrossRef]
- Souza, V.L.; Silva, D.d.C.; Santana, K.B.; Mielke, M.S.; Almeida, A.-A.F.d.; Mangabeira, P.A.O.; Rocha, E.A. Efeitos do cádmio na anatomia e na fotossíntese de duas macrófitas aquáticas. Acta Bot. Bras. 2009, 23, 343–354. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, J.; Li, L.; Yin, Y.; Xue, B.; Li, J.; Sun, F. Exploration of the Effects of Cadmium Stress on Photosynthesis in Oenanthe Javanica (Blume) DC. Toxics 2024, 12, 307. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, G.S.; El-Esawi, M.A. Effect of Cadmium-Tolerant Rhizobacteria on Growth Attributes and Chlorophyll Contents of Bitter Gourd under Cadmium Toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef]
- Bari, M.A.; Akther, M.S.; Reza, M.A.; Kabir, A.H. Cadmium Tolerance Is Associated with the Root-Driven Coordination of Cadmium Sequestration, Iron Regulation, and ROS Scavenging in Rice. Plant Physiol. Biochem. 2019, 136, 22–33. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Kováčik, J.; Pochwatka, M.; Hanaka, A.; Strzemski, M.; Sowa, I.; Wójciak-Kosior, M. Allantoin Attenuates Cadmium-Induced Toxicity in Cucumber Plants. Ecotoxicol. Environ. Saf. 2019, 170, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Kohli, S.K.; Ohri, P.; Bhardwaj, R.; Ahmad, P. Agroecotoxicological Aspect of Cd in Soil–Plant System: Uptake, Translocation and Amelioration Strategies|Environmental Science and Pollution Research. Available online: https://link.springer.com/article/10.1007/s11356-021-18232-5 (accessed on 31 October 2024).
- Li, Y.; Zhang, S.; Jiang, W.; Liu, D. Cadmium Accumulation, Activities of Antioxidant Enzymes, and Malondialdehyde (MDA) Content in Pistia stratiotes L. Environ. Sci. Pollut. Res. Int. 2013, 20, 1117–1123. [Google Scholar] [CrossRef]
- Andreazza, R.; Camargo, F.A.d.O.; Antoniolli, Z.I.; Quadro, M.S.; Barcelos, A.A. Biorremediação de áreas contaminadas com cobre. Rev. Ciências Agrárias 2013, 36, 127–136. [Google Scholar] [CrossRef]
- Rodrigues, A.C.D.; Santos, A.M.d.; Santos, F.S.d.; Pereira, A.C.C.; Sobrinho, N.M.B.A. Mecanismos de Respostas das Plantas à Poluição por Metais Pesados: Possibilidade de Uso de Macrófitas para Remediação de Ambientes Aquáticos Contaminados. Rev. Virtual Química 2016, 8, 262–276. [Google Scholar]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.d.; Silva, R.A.d.; Cardoso, G.D.; Barreto, A.F. Estudos sobre fungos micorrízicos. Rev. Bras. Eng. Agríc. Ambient. 2006, 10, 612–618. [Google Scholar] [CrossRef]
- Gomes, S.I.F.; Merckx, V.S.F.T.; Kehl, J.; Gebauer, G. Mycoheterotrophic Plants Living on Arbuscular Mycorrhizal Fungi Are Generally Enriched in 13C, 15N and 2H Isotopes. J. Ecol. 2020, 108, 1250–1261. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition Metal Transport in Plants and Associated Endosymbionts: Arbuscular Mycorrhizal Fungi and Rhizobia. Front. Plant Sci. 2016, 7, 1088. [Google Scholar] [CrossRef]
- Chauhan, S.; Mahawar, S.; Jain, D.; Udpadhay, S.K.; Mohanty, S.R.; Singh, A.; Maharjan, E. Boosting Sustainable Agriculture by Arbuscular Mycorrhiza under Stress Condition: Mechanism and Future Prospective. BioMed Res. Int. 2022, 2022, 5275449. [Google Scholar] [CrossRef]
- Göhre, V.; Paszkowski, U. Contribution of the Arbuscular Mycorrhizal Symbiosis to Heavy Metal Phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef]
- González-Chávez, M.C.; Carrillo-González, R.; Wright, S.F.; Nichols, K.A. The Role of Glomalin, a Protein Produced by Arbuscular Mycorrhizal Fungi, in Sequestering Potentially Toxic Elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ankit; Tiwari, J.; Bauddh, K. Frontiers|Plant-Mycorrhizal Fungi Interactions in Phytoremediation of Geogenic Contaminated Soils. Available online: https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2022.843415/full (accessed on 31 October 2024).
- Wang, H.-R.; Zhao, X.-Y.; Zhang, J.-M.; Lu, C.; Feng, F.-J. Arbuscular Mycorrhizal Fungus Regulates Cadmium Accumulation, Migration, Transport, and Tolerance in Medicago Sativa. J. Hazard. Mater. 2022, 435, 129077. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Silva, G.H. Utilização do Salgueiro (Salix humboldtiana Willd) como espécie fitorremediadora em rejeitos da indústria de Zinco. Sci. For. 2011, 39, 117–123. [Google Scholar]
- Souza, E.P.d.; Silva, I.d.F.d.; Ferreira, L.E. Mecanismos de tolerância a estresses por metais pesados em plantas. Curr. Agric. Sci. Technol. 2011, 17, 167–173. [Google Scholar]
- Yang, Z.; Yang, F.; Liu, J.-L.; Wu, H.-T.; Yang, H.; Shi, Y.; Liu, J.; Zhang, Y.-F.; Luo, Y.-R.; Chen, K.-M. Heavy Metal Transporters: Functional Mechanisms, Regulation, and Application in Phytoremediation. Sci. Total Environ. 2022, 809, 151099. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, T.; Saleem, M.; He, G. Metalloprotein-Specific or Critical Amino Acid Residues: Perspectives on Plant-Precise Detoxification and Recognition Mechanisms under Cadmium Stress. Int. J. Mol. Sci. 2022, 23, 1734. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 Iron Transporter Is Involved in Cd Accumulation in Rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, X.; Du, R.; Shao, Y.; Wen, Q.; Shen, X.; Wang, F.; Qi, Y.; Shen, J.; Hu, Y. Enrichment Characteristics of Cd and Hg and Regulation of Heavy Metal Transporter Signaling in Pleurotus ostreatus. Sci. Total Environ. 2024, 955, 176909. [Google Scholar] [CrossRef]
- Plaza, S.; Tearall, K.L.; Zhao, F.-J.; Buchner, P.; McGrath, S.P.; Hawkesford, M.J. Expression and Functional Analysis of Metal Transporter Genes in Two Contrasting Ecotypes of the Hyperaccumulator Thlaspi Caerulescens. J. Exp. Bot. 2007, 58, 1717–1728. [Google Scholar] [CrossRef]
- Fan, W.; Liu, C.; Cao, B.; Qin, M.; Long, D.; Xiang, Z.; Zhao, A. Genome-Wide Identification and Characterization of Four Gene Families Putatively Involved in Cadmium Uptake, Translocation and Sequestration in Mulberry. Front. Plant Sci. 2018, 9, 879. [Google Scholar] [CrossRef]
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of Heavy Metals in Metallophytes from Three Mining Sites (Southern Centre Morocco) and Evaluation of Their Phytoremediation Potential. Ecotoxicol. Environ. Saf. 2019, 169, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. Metal Hyperaccumulation in Plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, L.; Salahuddin; Khan, A.; Zhou, Y.; He, M.; Alrefaei, A.F.; Khan, M.; Ali, S. Physiological and Ultrastructural Changes in Dendranthema Morifolium Cultivars Exposed to Different Cadmium Stress Conditions. Agriculture 2023, 13, 317. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Zia Ur Rehman, M.; Rinklebe, J.; Tsang, D.C.W.; Bashir, A.; Maqbool, A.; Tack, F.M.G.; Ok, Y.S. Cadmium Phytoremediation Potential of Brassica Crop Species: A Review. Sci. Total Environ. 2018, 631–632, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Dubey, R.S. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Demarco, C.F.; Quadro, M.S.; Selau Carlos, F.; Pieniz, S.; Morselli, L.B.G.A.; Andreazza, R. Bioremediation of Aquatic Environments Contaminated with Heavy Metals: A Review of Mechanisms, Solutions and Perspectives. Sustainability 2023, 15, 1411. [Google Scholar] [CrossRef]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Li, C.; Zhao, P. Cadmium Stress Increases Antioxidant Enzyme Activities and Decreases Endogenous Hormone Concentrations More in Cd-Tolerant than Cd-Sensitive Wheat Varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Sürücü, A.; Ashraf, M. Alleviating Effect of Nitric Oxide on Oxidative Stress and Antioxidant Defence System in Pepper (Capsicum Annuum L.) Plants Exposed to Cadmium and Lead Toxicity Applied Separately or in Combination. Sci. Hortic. 2019, 255, 52–60. [Google Scholar] [CrossRef]
- Sepehri, A.; Gharehbaghli, N. Selenium Alleviate Cadmium Toxicity by Improving Nutrient Uptake, Antioxidative and Photosynthetic Responses of Garlic. Russ. J. Plant Physiol. 2019, 66, 152–159. [Google Scholar] [CrossRef]
- Vasconcellos, M.C.; Pagliuso, D.; Sotomaior, V.S. Fitorremediação: Uma proposta de descontaminação do solo. Estud. Biol. 2012, 34, 261–267. [Google Scholar] [CrossRef]
- Sutar, H.; Kumar, D. A Review on: Bioremediation. Int. J. Res. Chem. Environ. 2012, 2, 13–21. [Google Scholar]
- Kumar, V.; SHahi, S.; Singh, S. Bioremediation: An Eco-Sustainable Approach for Restoration of Contaminated Sites. In Microbial Bioprospecting for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2018; pp. 115–136. ISBN 9789811300530. [Google Scholar]
- Tyagi, B.; Kumar, N. Chapter 1—Bioremediation: Principles and Applications in Environmental Management. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–28. ISBN 978-0-12-820524-2. [Google Scholar]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation Techniques–Classification Based on Site of Application: Principles, Advantages, Limitations and Prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, S.; Ali, M.; Song, X.; Tang, Z.; Zhang, Z.; Zhang, M.; Luo, Y. Thermally Enhanced Bioremediation: A Review of the Fundamentals and Applications in Soil and Groundwater Remediation. J. Hazard. Mater. 2022, 433, 128749. [Google Scholar] [CrossRef] [PubMed]
- Kavamura, V.N.; Esposito, E. Biotechnological Strategies Applied to the Decontamination of Soils Polluted with Heavy Metals. Biotechnol. Adv. 2010, 28, 61–69. [Google Scholar] [CrossRef]
- Kuppan, N.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A Comprehensive Review of Sustainable Bioremediation Techniques: Eco Friendly Solutions for Waste and Pollution Management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Zhakypbek, Y.; Kossalbayev, B.D.; Belkozhayev, A.M.; Murat, T.; Tursbekov, S.; Abdalimov, E.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation. Plants 2024, 13, 1534. [Google Scholar] [CrossRef]
- Ranjan, S.; Sow, S. Phytoremediation: An Eco-Friendly Approach towards Clean and Green Future. Pharma Innov. 2021, 10, 839–850. [Google Scholar] [CrossRef]
- Oubohssaine, M.; Dahmani, I. Phytoremediation: Harnessing Plant Power and Innovative Technologies for Effective Soil Remediation. Plant Stress 2024, 14, 100578. [Google Scholar] [CrossRef]
- Sabreena; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Todde, G.; Carboni, G.; Marras, S.; Caria, M.; Sirca, C. Industrial Hemp (Cannabis sativa L.) for Phytoremediation: Energy and Environmental Life Cycle Assessment of Using Contaminated Biomass as an Energy Resource. Sustain. Energy Technol. Assess. 2022, 52, 102081. [Google Scholar] [CrossRef]
- Demarco, C.F.; Afonso, T.F.; Schoeler, G.P.; dos Santos Barboza, V.; dos Santos Rocha, L.; Pieniz, S.; Giongo, J.L.; de Almeida Vaucher, R.; Igansi, A.V.; Cadaval Jr, T.R.S. New Low-Cost Biofilters for SARS-CoV-2 Using Hymenachne Grumosa as a Precursor. J. Clean. Prod. 2022, 331, 130000. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Role of Hyperaccumulators in Phytoextraction of Metals From Contaminated Mining Sites: A Review. Crit. Rev. Environ. Sci. Technol. 2011, 41, 168–214. [Google Scholar] [CrossRef]
- Kumar, J.I.N.; Soni, H.; Kumar, R.N.; Bhatt, I. Macrophytes in Phytoremediation of Heavy Metal Contaminated Water and Sediments in Pariyej Community Reserve, Gujarat, India. Turk. J. Fish. Aquat. Sci. 2008, 8, 193–200. [Google Scholar]
- Guimaraes, F.P.; Aguiar, R.; Oliveira, J.A.; Silva, J.a.A.; Karam, D. Potential of Macrophyte for Removing Arsenic from Aqueous Solution. Planta Daninha 2012, 30, 683–696. [Google Scholar] [CrossRef]
- Dahmani-Muller, H.; van Oort, F.; Gélie, B.; Balabane, M. Strategies of Heavy Metal Uptake by Three Plant Species Growing near a Metal Smelter. Environ. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Susarla, S.; Medina, V.; Mccutcheon, S. Phytoremediation: An Ecological Solution to Organic Chemical Contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Document Display|NEPIS|US EPA. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/30003T7G.txt?ZyActionD=ZyDocument&Client=EPA&Index=1995%20Thru%201999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C95THRU99%5CTXT%5C00000016%5C30003T7G.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r65g4/r65g4/x150y150g16/i360&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=2 (accessed on 6 September 2024).
- Khan, A.H.A.; Kiyani, A.; Santiago-Herrera, M.; Ibáñez, J.; Yousaf, S.; Iqbal, M.; Martel-Martín, S.; Barros, R. Sustainability of Phytoremediation: Post-Harvest Stratagems and Economic Opportunities for the Produced Metals Contaminated Biomass. J. Environ. Manag. 2023, 326, 116700. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernandez-Allica, J.; Becerril, J.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent Findings on the Phytoremediation of Soils Contaminated with Environmentally Toxic Heavy Metals and Metalloids Such as Zinc, Cadmium, Lead, and Arsenic. Rev. Environ. Sci. Bio/Technol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, B.; Manchanda, V.K. Phytoremediation: Role of Terrestrial Plants and Aquatic Macrophytes in the Remediation of Radionuclides and Heavy Metal Contaminated Soil and Water. Environ. Sci. Pollut. Res. Int. 2015, 22, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Khan, A.H.A.; Hussain, I.; Farooqi, A.; Muhammad, Y.S.; Iqbal, M.; Arslan, M.; Yousaf, S. Soil Conditioners Improve Rhizodegradation of Aged Petroleum Hydrocarbons and Enhance the Growth of Lolium Multiflorum. Environ. Sci. Pollut. Res. 2022, 29, 9097–9109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, B.; Ma, Y.; Zhang, X.; Lyu, W.; Chen, M. Stabilization of Heavy Metals in Biochar Derived from Plants in Antimony Mining Area and Its Environmental Implications. Environ. Pollut. 2022, 300, 118902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, T.; Wang, W.; Zhao, J.; Li, Z.; Ge, Y.; Wang, Z.; Wu, L.; Christie, P. Phytoextraction of Highly Cadmium-Polluted Agricultural Soil by Sedum plumbizincicola: An Eight-Hectare Field Study. Sci. Total Environ. 2023, 905, 167216. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xing, X.; Liang, J.; Peng, J.; Zhou, J. In Situ Phytoremediation of Copper and Cadmium in a Co-Contaminated Soil and Its Biological and Physical Effects. RSC Adv. 2019, 9, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga, J.F.; López, J.E.; Díaz-García, L.; Montoya-Ruiz, C. Changes in Lolium Perenne L. Rhizosphere Microbiome during Phytoremediation of Cd- and Hg-Contaminated Soils. Environ. Sci. Pollut. Res. 2023, 30, 49498–49511. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Liu, Q.; Sikder, M.M.; Vestergård, M.; Zhou, K.; Wang, Q.; Yang, X.; Feng, Y. Pseudomonas fluorescens Promote Photosynthesis, Carbon Fixation and Cadmium Phytoremediation of Hyperaccumulator Sedum alfredii. Sci. Total Environ. 2020, 726, 138554. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Kaur, J.; Shukla, A.K.; Singh, J.; Singh, P. Cadmium Phytoremediation Potential of Brassica Genotypes Grown in Cd Spiked Loamy Sand Soils: Accumulation and Tolerance. Chemosphere 2022, 302, 134842. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Schwantes, D.; Braga de Sousa, R.F.; Benetoli da Silva, T.R.; Guimarães, V.F.; Campagnolo, M.A.; Soares de Vasconcelos, E.; Zimmermann, J. Phytoremediation Capacity, Growth and Physiological Responses of Crambe abyssinica Hochst on Soil Contaminated with Cd and Pb. J. Environ. Manag. 2020, 262, 110342. [Google Scholar] [CrossRef]
- Mo, Y.; Zou, D.; Xiong, J.; Kang, J.; Yang, Y.; Wu, Q.; Zeng, X.; Xiao, Z. Enhanced Cd Accumulation and Yield in Peanut (Arachis hypogaea L.) via Combined Chlorine-Containing Fertiliser Application. Ind. Crops Prod. 2024, 222, 119698. [Google Scholar] [CrossRef]
- Meeinkuirt, W.; Phusantisampan, T.; Kubola, J.; Chumroenphat, T.; Pichtel, J. Phytomanagement of Cadmium Using Tagetes Erecta in Greenhouse and Field Conditions. J. Hazard. Mater. Adv. 2024, 16, 100481. [Google Scholar] [CrossRef]
- Ozurumba, Z.N.; Tanee, F.B.G.; Agbagwa, I.O. Phytoextraction Potential of Canna indica (L.) for Cd Removal from a Hydroponic System. Chem. Ecol. 2024, 40, 466–485. [Google Scholar] [CrossRef]
- Schnabel, B.; Wright, S.; Miller, R.; Bryant, L.D.; Kjeldsen, T.R.; Maconachie, R.; Gbanie, S.P.; Bangura, K.S.; Kamara, A.J. Urban Surface Water Quality and the Potential of Phytoremediation to Improve Water Quality in Peri-Urban and Urban Areas in Sub-Saharan Africa—A Review. Water Supply 2022, 22, 8372–8404. [Google Scholar] [CrossRef]
- Hosseinniaee, S.; Jafari, M.; Tavili, A.; Zare, S.; Cappai, G.; De Giudici, G. Perspectives for Phytoremediation Capability of Native Plants Growing on Angouran Pb–Zn Mining Complex in Northwest of Iran. J. Environ. Manag. 2022, 315, 115184. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Zhou, Q.; Li, J.; Liu, M.; Deng, Y.; Huang, Y.; Zhou, Y.; Yang, H.; Xiao, Y. Investigation of Cd and Pb Enrichment Capacities of Erigeron sumatrensis across Three Polluted Regions: Insights into Soil Parameters and Microbial Communities. Environ. Res. 2024, 262, 119868. [Google Scholar] [CrossRef]
- Zhou, Y.; Lan, W.; Yang, F.; Zhou, Q.; Liu, M.; Li, J.; Yang, H.; Xiao, Y. Invasive Amaranthus Spp. for Heavy Metal Phytoremediation: Investigations of Cadmium and Lead Accumulation and Soil Microbial Community in Three Zinc Mining Areas. Ecotoxicol. Environ. Saf. 2024, 285, 117040. [Google Scholar] [CrossRef]
- Zakaria, Z.; Zulkafflee, N.S.; Mohd Redzuan, N.A.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Tóth, G.; Abdull Razis, A.F. Understanding Potential Heavy Metal Contamination, Absorption, Translocation and Accumulation in Rice and Human Health Risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, F.; Zafar, A.; Khan, Z.I.; Ahmad, K.; Ch, S.A.; Batool, A.I.; Nadeem, M. Assessment of Potential Toxicological Risk for Public Health of Heavy Metal Iron in Diverse Wheat Varieties Irrigated with Various Types of Waste Water in South Asian Country. Agric. Water Manag. 2023, 276, 108044. [Google Scholar] [CrossRef]
- Soubasakou, G.; Cavoura, O.; Damikouka, I. Phytoremediation of Cadmium-Contaminated Soils: A Review of New Cadmium Hyperaccumulators and Factors Affecting Their Efficiency. Bull. Environ. Contam. Toxicol. 2022, 109, 783–787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).