Deciphering Whether Illite, a Natural Clay Mineral, Alleviates Cadmium Stress in Glycine max Plants via Modulation of Phytohormones and Endogenous Antioxidant Defense System
Abstract
1. Introduction
2. Methodology
2.1. Pot Experiment
2.2. Measurement of Seedling Characteristics and Chlorophyll Content with SPAD
2.3. Estimation of Relative Water Content (RWC)
2.4. Phytohormone Analysis (ABA and SA)
2.5. Amino Acid Evaluation
2.6. Analysis of Ions by ICP
2.7. Antioxidant Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of IL on Morphological Characteristics Under Cd Stress
3.2. Effect of Illite on RWC and Chlorophyll
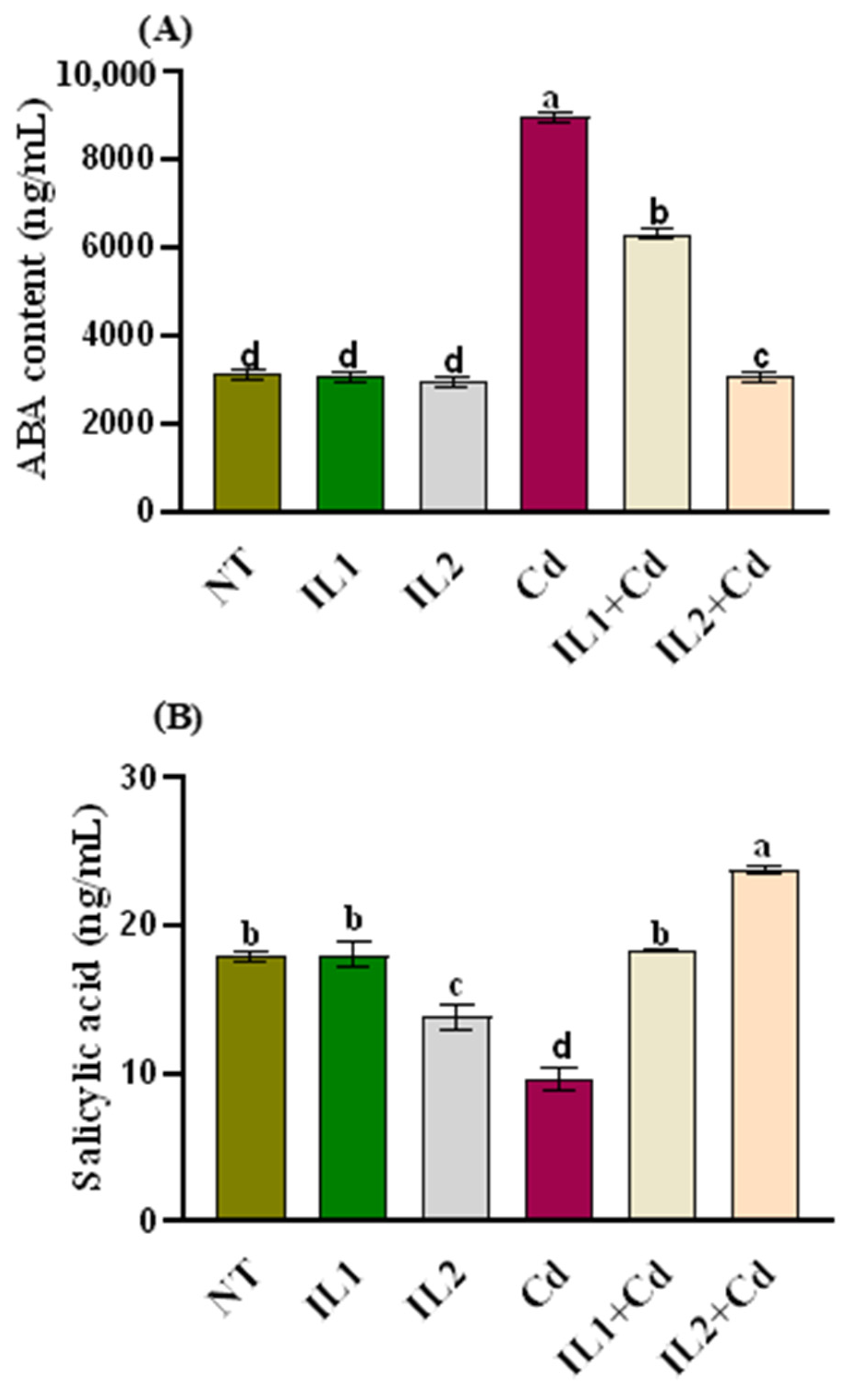
3.3. Phytohormonal Analysis
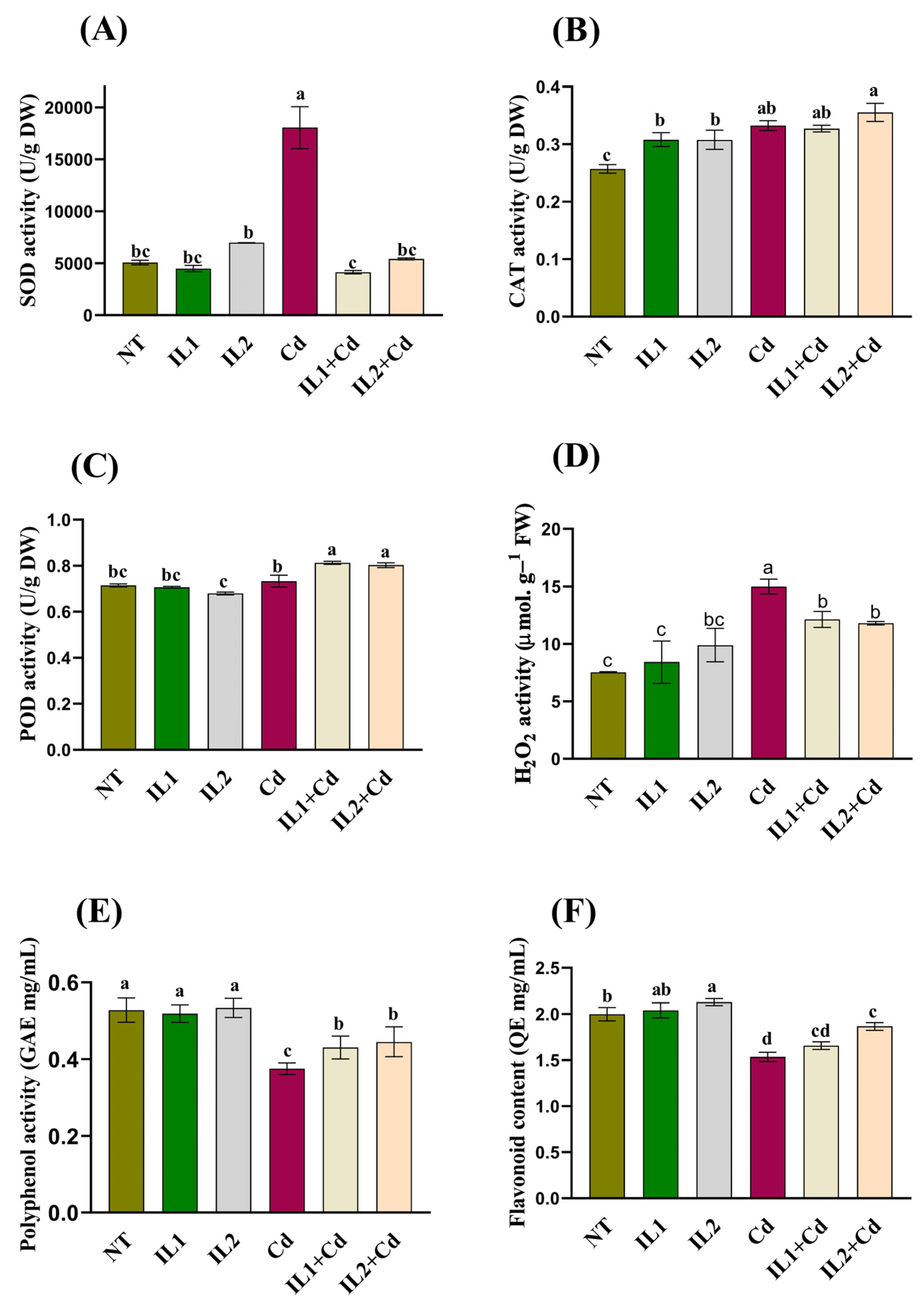
3.4. Determination of Antioxidant Analysis
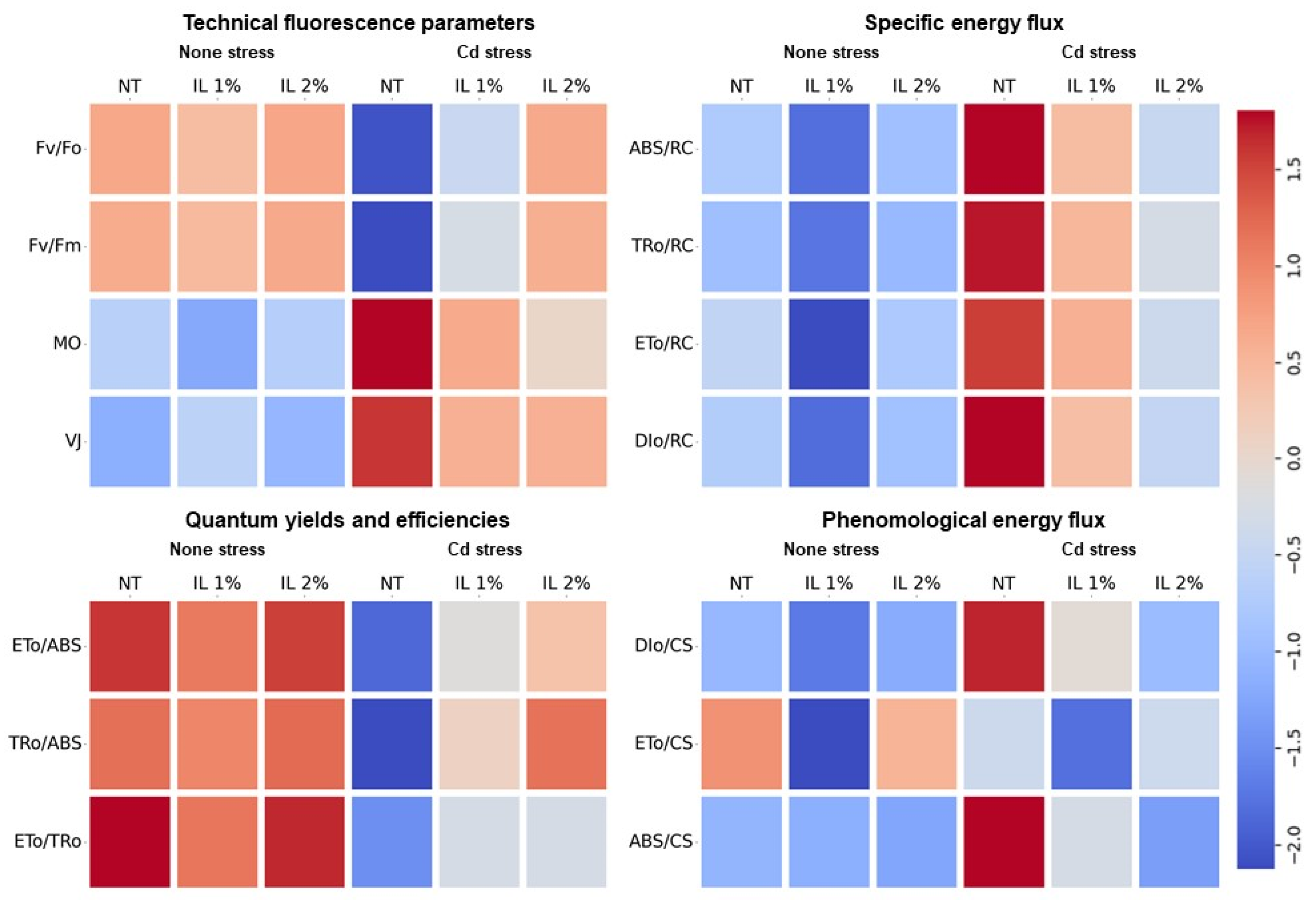
3.5. Results of the Technical Fluorescent and Energy Flux
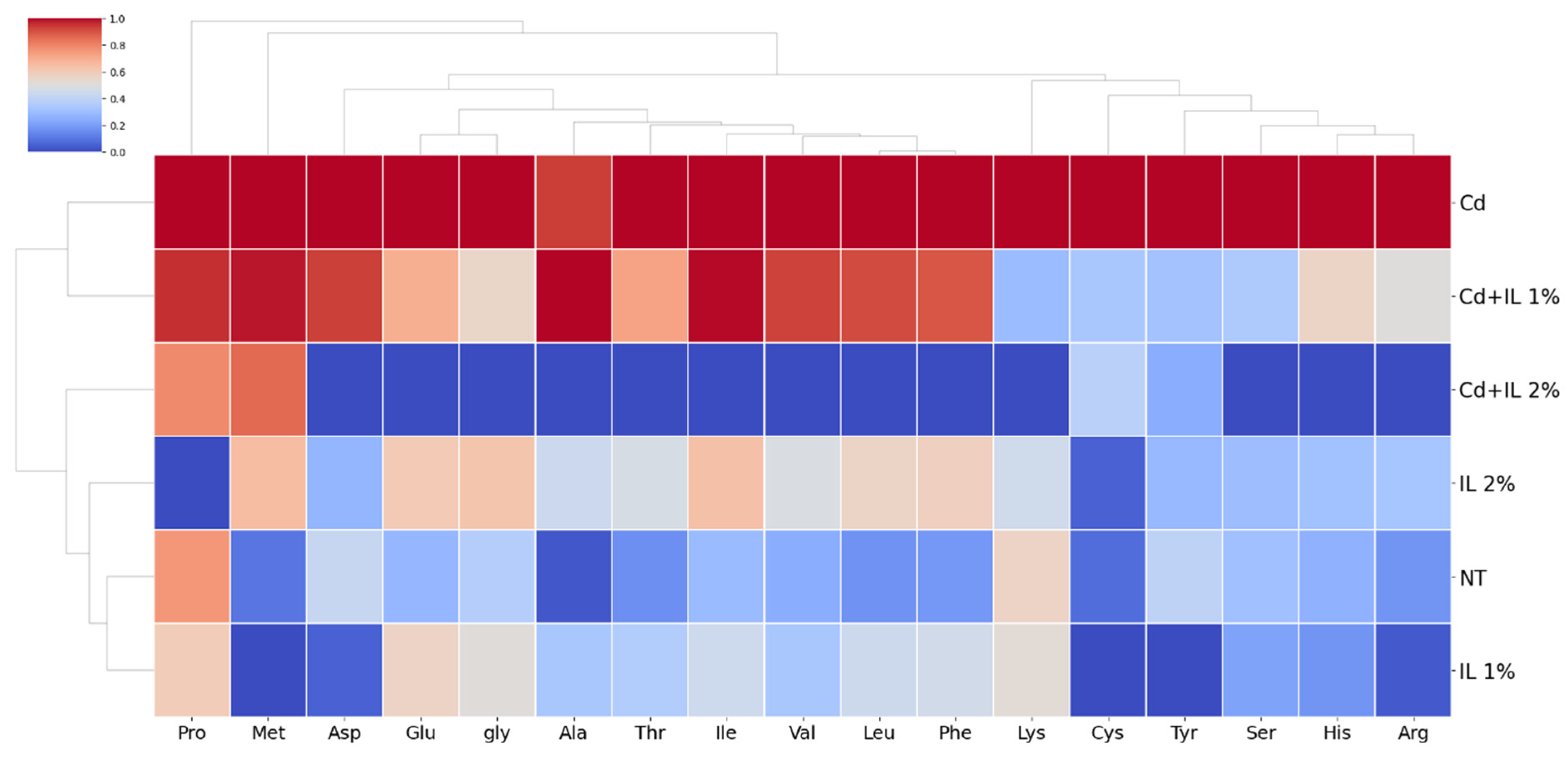
3.6. Findings of the Amino Acids
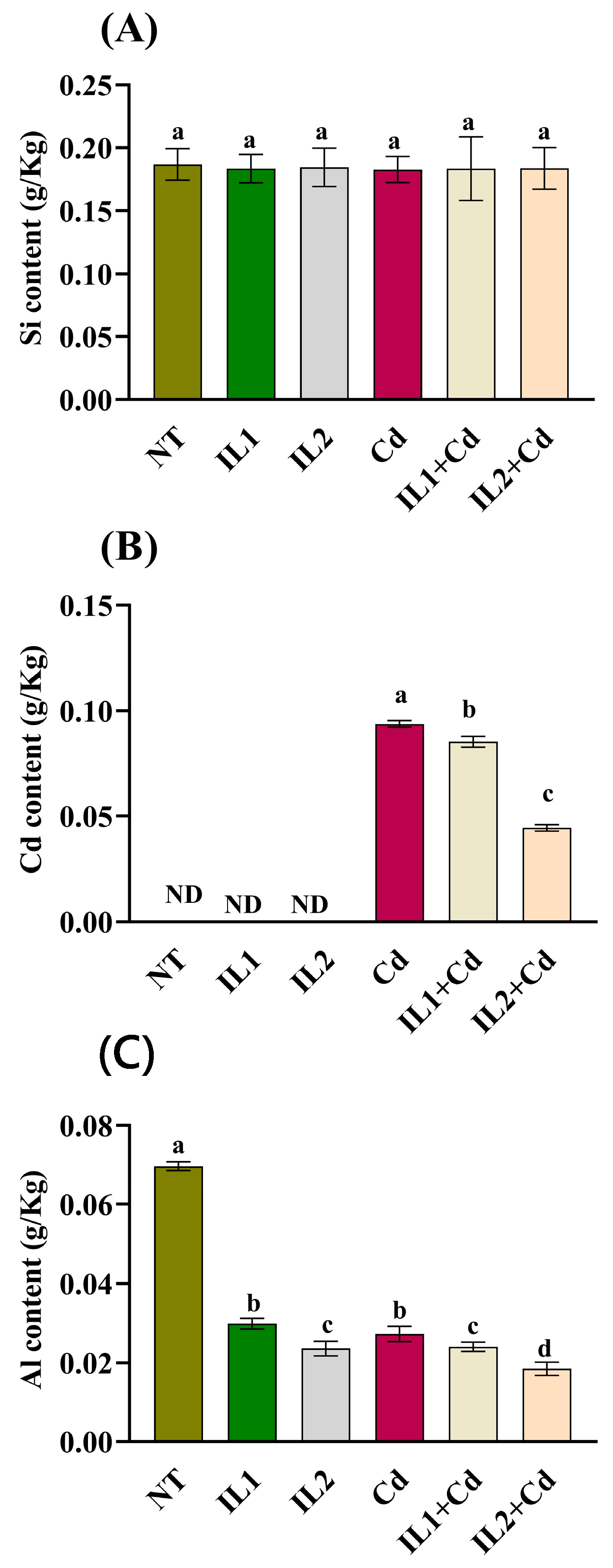
3.7. ICP Analysis
4. Discussion
5. Conclusion and Prospective Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Gill, M. Heavy metal stress in plants: A review. Int. J. Adv. Res. 2014, 2, 1043–1055. [Google Scholar]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Huang, H.; Shi, L.; Chen, R.; Yuan, J. Effect of Modified Illite on Cd Immobilization and Fertility Enhancement of Acidic Soils. Sustainability 2023, 15, 4950. [Google Scholar] [CrossRef]
- Shaffique, S.; Imran, M.; Adhikari, A.; Khan, M.A.; Rahim, W.; Alomrani, S.O.; Yun, B.W.; Kang, S.-M.; Lee, I.-J. A newly isolated Bacillus pumilus strain SH-9 modulates response to drought stress in soybean via endogenous phytohormones and gene expression (Daegu, South Korea). Plant Stress 2023, 10, 100279. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Tian, G.; Luo, Q.; Xing, R.; Zhang, J.; Li, X. Cadmium pollution exacerbated by drought: Insights from the nanoscale interaction at the clay mineral surface. Sci. Total Environ. 2024, 928, 172520. [Google Scholar] [CrossRef]
- Min, F.; Wang, X.; Li, L.; Xin, Z.; Li, X.; Zhang, T.; Sun, X.; You, H. Effects of silicate stabilizers on cadmium reduction and the quality of rice grains in acidic paddy soil. Sci. Rep. 2024, 14, 20551. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Hu, Y.; Peng, L.; Li, D.; Song, H.; Song, K.; Li, C.; Wang, Y.; He, S. Application of biological soil crusts for efficient cadmium removal from acidic mine wastewater. J. Hazard. Mater. 2024, 465, 133524. [Google Scholar] [CrossRef]
- Radziemska, M.; Blazejczyk, A.; Gusiatin, M.Z.; Cydzik-Kwiatkowska, A.; Majewski, G.; Brtnický, M. Compost-diatomite-based phytostabilization course under extreme environmental conditions in terms of high pollutant contents and low temperatures. Sci. Total Environ. 2024, 948, 174917. [Google Scholar] [CrossRef]
- Waqas, W.; Yuan, Y.; Ali, S.; Zhang, M.; Shafiq, M.; Ali, W.; Chen, Y.; Xiang, Z.; Chen, R.; Ikhwanuddin, M.; et al. Toxic effects of heavy metals on crustaceans and associated health risks in humans: A review. Environ. Chem. Lett. 2024, 22, 1391–1411. [Google Scholar] [CrossRef]
- Shaffique, S.; Farooq, M.; Kang, S.-M.; Lee, I.-J. Recent advances in biochemical reprogramming network under drought stress in soybean. J. Soil Sci. Plant Nutr. 2024, 24, 1692–1703. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; El Moneim, D.A.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Shah, A.A.; Peter, O.; Hoque, I.U.; Elansary, H.O.; Kang, S.-M.; Al Azzawi, T.N.I.; Yun, B.-W.; Lee, I.-J. The rhizobacterial Priestia megaterium strain SH-19 mitigates the hazardous effects of heat stress via an endogenous secondary metabolite elucidation network and molecular regulation signalling. BMC Plant Biol. 2024, 24, 827. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef]
- Jorjani, S.; Karakaş, F.P. Physiological and Biochemical Responses to Heavy Metals Stress in Plants. Int. J. Second. Metab. 2024, 11, 169–190. [Google Scholar] [CrossRef]
- Farid, M.; Shakoor, M.; Ehsan, S.; Ali, S.; Zubair, M.; Hanif, M.A. Morphological, physiological and biochemical responses of different plant species to Cd stress. Int. J. Chem. Biochem. Sci. 2013, 3, 53–60. [Google Scholar]
- Yasin, M.U.; Haider, Z.; Munir, R.; Zulfiqar, U.; Rehman, M.; Javaid, M.H.; Ahmad, I.; Nana, C.; Saeed, M.S.; Ali, B.; et al. The synergistic potential of biochar and nanoparticles in phytoremediation and enhancing cadmium tolerance in plants. Chemosphere 2024, 354, 141672. [Google Scholar] [CrossRef]
- Xin, J. Enhancing soil health to minimize cadmium accumulation in agro-products: The role of microorganisms, organic matter, and nutrients. Environ. Pollut. 2024, 348, 123890. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Wang, X.; Zeb, A.; Wang, Q.; Mo, F.; Shi, R.; Liu, J.; Yu, M.; Li, J.; et al. Assessing stress responses in potherb mustard (Brassica juncea var. multiceps) exposed to a synergy of microplastics and cadmium: Insights from physiology, oxidative damage, and metabolomics. Sci. Total Environ. 2024, 907, 167920. [Google Scholar] [CrossRef]
- Kübler, B.; Jaboyedoff, M. Illite crystallinity. Comptes Rendus De L’académie Des Sci.-Ser. IIA-Earth Planet. Sci. 2000, 331, 75–89. [Google Scholar] [CrossRef]
- Kim, S.-H.; Dhungana, S.K.; Kim, I.-D.; Adhikari, A.; Kim, J.-H. Effect of Illite Treatment on Quality Characteristics and Antioxidant Activity of Broccoli (Brassica oleracea L. var. italica) Sprouts. Molecules 2024, 29, 4347. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-H.; Kim, H.-J.; Kim, S.-H.; Kim, I.-D.; Adhikari, A.; Kim, J.-H. Effect of illite pretreatment on germinated Brown rice with Special Reference to amino acids, antioxidants, texture, and mineral elements. Heliyon 2024, 10, e28843. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Lee, H.-S.; Kim, G.-E.; Shin, Y.-T.; Joo, J.-Y.; Lee, J.-J.; Sung, J. Effects of illite-containing fertilizer prototype on soil chemical property and tomato growth. Korean J. Soil Sci. Fertil. 2021, 54, 338–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Fu, Q.; Hou, R.; Li, M.; Liu, D.; Shi, G.; Yang, X.; Xue, P. Drip irrigation reduces the toxicity of heavy metals to soybean: By moving heavy metals out of the root zone and improving physiological metabolism. Agric. Water Manag. 2024, 292, 108670. [Google Scholar] [CrossRef]
- Shaffique, S.; Kang, S.-M.; Hoque, I.U.; Imran, M.; Khan, M.A.; Lee, I.-J. Research progress in soybean by phytohormone modulation and metal chelation over the past decade. Agriculture 2023, 13, 1325. [Google Scholar] [CrossRef]
- Uddin, M.; Hoque, M.; Monira, S.; Bari, M. Gas exchange and chlorophyll fluorescence parameters in four maize genotypes influenced by first phase of salt stress. Progress. Agric. 2019, 30, 26–32. [Google Scholar] [CrossRef]
- Imran, M.; Khan, M.A.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.-W.; Khan, A.L.; Lee, I.-J. Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Pashaei, M.; Hassanpour, H. Phenolic, amino acids, and fatty acids profiles and the nutritional properties in the fresh and dried fruits of black rosehip (Rosa pimpinellifolia L.). Sci. Rep. 2024, 14, 19665. [Google Scholar] [CrossRef]
- Xu, L.; Ma, X.; Yang, J.; Burken, J.G.; Nam, P.; Shi, H.; Yang, H. Advancing Simultaneous Extraction and Sequential Single-Particle ICP-MS Analysis for Metallic Nanoparticle Mixtures in Plant Tissues. J. Agric. Food Chem. 2024, 72, 11251–11258. [Google Scholar] [CrossRef]
- Imran, M.; Khan, A.L.; Mun, B.-G.; Bilal, S.; Shaffique, S.; Kwon, E.-H.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Melatonin and nitric oxide: Dual players inhibiting hazardous metal toxicity in soybean plants via molecular and antioxidant signaling cascades. Chemosphere 2022, 308, 136575. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Vogelsang, L. A general concept of quantitative abiotic stress sensing. Trends Plant Sci. 2024, 29, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Plant Stress Physiology; Cabi: São Paulo, Brazil, 2017. [Google Scholar]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C.; Jouve, L.; Hausman, J.; Dommes, J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–285. [Google Scholar] [CrossRef]
- Asiminicesei, D.-M.; Fertu, D.I.; Gavrilescu, M. Impact of Heavy Metal Pollution in the Environment on the Metabolic Profile of Medicinal Plants and Their Therapeutic Potential. Plants 2024, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Fan, P.; Wu, L.; Wang, Q.; Wang, Y.; Luo, H.; Song, J.; Yang, M.; Yao, H.; Chen, S. Physiological and molecular mechanisms of medicinal plants in response to cadmium stress: Current status and future perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef]
- Dominguez-Lerena, S.; Sierra, N.H.; Manzano, I.C.; Bueno, L.O.; Rubira, J.P.; Mexal, J. Container characteristics influence Pinus pinea seedling development in the nursery and field. For. Ecol. Manag. 2006, 221, 63–71. [Google Scholar] [CrossRef]
- Choi, J.-S.; Cho, S.-W.; Kim, T.-S.; Cho, K.; Han, S.-S.; Kim, H.-K.; Woo, S.-H.; Chung, K.-Y. Proteome analysis of greenhouse-cultured lettuce with the natural soil mineral conditioner illite. Soil Biol. Biochem. 2008, 40, 1370–1378. [Google Scholar] [CrossRef]
- Ha, M.-C.; Im, D.-Y.; Park, H.-S.; Dhungana, S.K.; Kim, I.-D.; Shin, D.-H. Seed treatment with illite enhanced yield and nutritional value of soybean sprouts. Molecules 2022, 27, 1152. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.; Wang, R.; Mo, G.; Luo, S.; Luo, X.; He, L.; Gonsamo, A.; Arabian, J.; Zhang, Y.; et al. The global distribution of leaf chlorophyll content. Remote Sens. Environ. 2020, 236, 111479. [Google Scholar] [CrossRef]
- Kamble, P.N.; Giri, S.; Mane, R.; Tiwana, A. Estimation of chlorophyll content in young and adult leaves of some selected plants. Univers. J. Environ. Res. Technol. 2015, 5, 306–310. [Google Scholar]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Niu, J.; Deng, Z. Abscisic acid treatment alleviates cadmium toxicity in purple flowering stalk (Brassica campestris L. ssp. chinensis var. purpurea Hort.) seedlings. Plant Physiol. Biochem. 2017, 118, 471–478. [Google Scholar] [CrossRef]
- Hu, B.; Deng, F.; Chen, G.; Chen, X.; Gao, W.; Long, L.; Xia, J.; Chen, Z.-H. Evolution of abscisic acid signaling for stress responses to toxic metals and metalloids. Front. Plant Sci. 2020, 11, 909. [Google Scholar] [CrossRef]
- Zhu, Y.; You, Y.; Zheng, S.; Li, J.; Wang, Y.; Wu, R.; Fang, Z.; Liu, H.; Du, S. ABA-importing transporter (AIT1) synergies enhances exogenous ABA minimize heavy metals accumulations in Arabidopsis. J. Hazard. Mater. 2024, 473, 134718. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Y.; Liu, M.; Qin, L.; Wang, J.; Zu, Y. Pb Accumulation Related to Plant Hormones and Its Mechanism Based on Genes Expression and Transporter Activities in Hyperaccumulator Arabis alpina. Environ. Earth Sci. 2024. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. The role of soils in provision of genetic, medicinal and biochemical resources. Philos. Trans. R. Soc. B 2021, 376, 20200183. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, A.; Alyemeni, M.N. Salicylic Acid; Springer International Publishing: Cham, Switzerland, 2013. [Google Scholar]
- Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Szőllősi, R. Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview. In Oxidative Damage to Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 89–129. [Google Scholar]
- Berwal, M.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. Abiotic Biot. Stress Plants 2018, 7, 1–10. [Google Scholar]
- Morrison, K.D.; Underwood, J.C.; Metge, D.W.; Eberl, D.D.; Williams, L.B. Mineralogical variables that control the antibacterial effectiveness of a natural clay deposit. Environ. Geochem. Health 2014, 36, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Skorochod, I. Microbial interaction with clay minerals and its environmental and biotechnological implications. Minerals 2020, 10, 861. [Google Scholar] [CrossRef]
- Li, Q.; Tang, Y.; Dong, D.; Wang, X.; Wu, X.; Gul, S.; Li, Y.; Xie, X.; Liu, D.; Xu, W. Remediation of Pb-, Zn-, Cu-, and Cd-Contaminated Soil in a Lead–Zinc Mining Area by Co-Cropping Ilex cornuta and Epipremnum aureum with Illite Application. Agriculture 2024, 14, 867. [Google Scholar] [CrossRef]
- Kocaman, A. Combined interactions of amino acids and organic acids in heavy metal binding in plants. Plant Signal. Behav. 2023, 18, 2064072. [Google Scholar] [CrossRef] [PubMed]
- Fellah, M.; Hezil, N.; Guerfi, K.; Djellabi, R.; Montagne, A.; Iost, A.; Borodin, K.; Obrosov, A. Mechanistic pathways of cationic and anionic surfactants sorption by kaolinite in water. Environ. Sci. Pollut. Res. 2021, 28, 7307–7321. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]
- Bahabadi, F.N.; Farpoor, M.H.; Mehrizi, M.H. Removal of Cd, Cu and Zn ions from aqueous solutions using natural and Fe modified sepiolite, zeolite and palygorskite clay minerals. Water Sci. Technol. 2017, 75, 340–349. [Google Scholar] [CrossRef]


| Treatment | SL (cm) | RL (cm) | SFW (g) | RFW (g) |
|---|---|---|---|---|
| NT (Non-treated) | 13.25 ± 0.57 a | 17.90 ± 0.86 ab | 2.68 ± 0.07 a | 1.94 ± 0.06 abc |
| IL1 (Illite 1%) | 12.58 ± 0.58 a | 18.53 ± 0.64 a | 2.84 ± 0.07 a | 2.01 ± 0.11 ab |
| IL2 (Illite 2%) | 13.67 ± 0.56 a | 17.18 ± 0.76 ab | 2.88 ± 0.09 a | 2.14 ± 0.05 a |
| Cd | 10.32 ± 0.76 b | 12.77 ± 0.82 c | 1.57 ± 0.17 c | 1.57 ± 0.22 c |
| IL1 + Cd | 11.82 ± 0.28 ab | 16.55 ± 0.66 ab | 1.74 ± 0.14 bc | 1.67 ± 0.16 bc |
| IL2 + Cd | 13.42 ± 0.74 a | 15.93 ± 1.05 b | 1.93 ± 0.11 b | 1.67 ± 0.05 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-M.; Shaffique, S.; Injamum-Ul-Hoque, M.; Gam, H.-J.; Woo, J.-I.; Jeon, J.R.; Lee, D.-S.; Lee, I.-J.; Mun, B.-G. Deciphering Whether Illite, a Natural Clay Mineral, Alleviates Cadmium Stress in Glycine max Plants via Modulation of Phytohormones and Endogenous Antioxidant Defense System. Sustainability 2024, 16, 10039. https://doi.org/10.3390/su162210039
Kang S-M, Shaffique S, Injamum-Ul-Hoque M, Gam H-J, Woo J-I, Jeon JR, Lee D-S, Lee I-J, Mun B-G. Deciphering Whether Illite, a Natural Clay Mineral, Alleviates Cadmium Stress in Glycine max Plants via Modulation of Phytohormones and Endogenous Antioxidant Defense System. Sustainability. 2024; 16(22):10039. https://doi.org/10.3390/su162210039
Chicago/Turabian StyleKang, Sang-Mo, Shifa Shaffique, Md. Injamum-Ul-Hoque, Ho-Jun Gam, Ji-In Woo, Jin Ryeol Jeon, Da-Sol Lee, In-Jung Lee, and Bong-Gyu Mun. 2024. "Deciphering Whether Illite, a Natural Clay Mineral, Alleviates Cadmium Stress in Glycine max Plants via Modulation of Phytohormones and Endogenous Antioxidant Defense System" Sustainability 16, no. 22: 10039. https://doi.org/10.3390/su162210039
APA StyleKang, S.-M., Shaffique, S., Injamum-Ul-Hoque, M., Gam, H.-J., Woo, J.-I., Jeon, J. R., Lee, D.-S., Lee, I.-J., & Mun, B.-G. (2024). Deciphering Whether Illite, a Natural Clay Mineral, Alleviates Cadmium Stress in Glycine max Plants via Modulation of Phytohormones and Endogenous Antioxidant Defense System. Sustainability, 16(22), 10039. https://doi.org/10.3390/su162210039








