Abstract
Coralligenous bioconstructions are a key Mediterranean ecosystem for their associated biodiversity and role in the blue carbon cycle. They are also sensitive to environmental alterations (e.g., climate change) and other anthropic impacts related to coastal anthropization (e.g., fishing activities). Marine-coastal zone protection, conservation programs and management strategies are essential to guarantee a good ecological status of the coralligenous habitat. In this context, environmental and ecosystem accounting are useful tools to measure natural capital stocks and ecosystem service flows associated with marine ecosystems, conveying their importance in scientific and policy contexts. Indeed, the importance of marine ecosystems is often overlooked due to the difficulty of expressing their value in common units, making it challenging for decision-makers to explore trade-offs between conservation and exploitation of marine ecosystems. In this study, a biophysical and trophodynamic environmental accounting model was used to assess the biophysical value of natural capital stocks of the coralligenous habitat in three Marine Protected Areas (MPAs) of the Campania Region (Southern Italy): Punta Campanella, Santa Maria di Castellabate, and Costa degli Infreschi e della Masseta. The natural capital value per unit area associated with the coralligenous habitat ranged from 2.44 × 1012 to 4.72 × 1012 sej m−2 for Santa Maria di Castellabate and Punta Campanella, respectively. Despite the different intensive values of natural capital calculated for the MPAs, there were no significant differences both in the biomass values of the taxonomic groups and in the biomass-based Shannon diversity index. Additionally, the biophysical values were also converted into monetary units, with the aim of facilitating the understanding of the importance of natural stocks in socio-economic and political contexts. The economic equivalent of natural capital value refers to the total extent of the coralligenous habitat and ranged from about EUR 1 to 15 million for Costa degli Infreschi e della Masseta and Santa Maria di Castellabate, respectively. The results of this study could be useful for local managers and policy makers and may make them more likely to achieve biodiversity conservation and sustainable development goals in MPAs. This is the first study devoted to the assessment of natural capital value of coralligenous habitats. Future studies could complement the results of this study with biophysical and economic assessments of ecosystem service flows generated by coralligenous habitats, focusing on the role they play in human well-being.
1. Introduction
Marine ecosystems are among the most productive environments in the world. Their natural capital, defined as the stock of natural resources (both renewable and non-renewable), generates a large set of ecosystem services (e.g., food provisioning, coastal protection, carbon sequestration, and recreational activities), providing important benefits to humans [1,2,3].
In 2022, the European Commission published the Blue Economy Report where it is highlighted how the blue economy activities strongly depend on marine natural capital stocks and how, meanwhile, they generate pressures on them. Indeed, marine ecosystems experience direct and indirect anthropogenic pressures (e.g., plastic pollution, overfishing, habitat destruction, climate change and eutrophication), resulting in severe environmental problems such as, above all, the loss of biodiversity [4].
It is important to raise awareness about the role of the life-support system provided by nature for human well-being, also highlighting the need to enhance conservation and management strategies directed at sustainable exploitation of marine natural capital stocks. Therefore, in order to limit impacts caused by human activities, it is essential to assess the value of marine natural capital and the associated ecosystem services, both in biophysical and economic terms.
Among marine ecosystems in the Mediterranean Sea, coralligenous reefs represent one of the most important biodiversity hotspots [5] together with Posidonia oceanica meadows [6]. They are the main biogenic habitat constituted by calcareous structures edified by crustose coralline algae (mostly belonging to the Lithophyllum, Lithothamnium and Mesophyllum genera) occurring in the circalittoral zone from about 20 m to about 150 m deep, forming complex three-dimensional systems supporting high biodiversity levels [7,8]. The stocks develop on rocky or sandy bottoms, in waters with relatively constant values of temperature, currents and salinity. An important ecological parameter is the lighting that must be very much reduced. For the stand formed by the algae, the reduction in radiation must be in the range 2–3% and 0–0.5%, as a greater decrease would not allow the development of plant organisms essential for the formation of the coralligenous construction [9]. These ecological requirements can be found in the Mediterranean at different depths in relation to local environmental conditions. Coralligenous assemblages are characterized by high biodiversity, mostly related to the heterogeneity of the biogenic substrate [10] considering that topographic complexity is an important determinant of marine species distribution, abundance and diversity [11,12]. Moreover, their complex 3D structure hosts a very complex community of organisms dominated by filter feeders (e.g., sponges, hydrozoans, anthozoans, bryozoans, serpulids, mollusks and tunicates), while within the crevices and interstices there is a very diverse endofauna (e.g., polychaetes and crustaceans) [5,13]. Coralligenous organisms are also considered effective indicators of long-term changes in environmental conditions, both of natural and anthropic origin [14,15,16,17].
A coralligenous construction develops in particular and stable environmental conditions and any variation in these conditions can be fatal for organisms that are not adapted to change [15,18]. Indeed, this habitat and consequently these taxa are very sensitive to both global (e.g., climate change) and local (e.g., fishing activities) anthropogenic impacts [15,19,20,21,22,23].
Climate change can affect coral populations in various ways. A rise in temperature for prolonged periods below the thermocline can cause the death of stenothermal organisms either directly or by encouraging the proliferation of pathogens [24,25,26,27]. The increase in carbon dioxide in the atmosphere due to the use of fossil fuels and deforestation also causes an increase in marine waters [28].
The increase in temperature can favor the onset of phenomena such as the development of planktonic and benthic mucilage, produced by macro- (Phaeophyceae) and microalgae (Chrysophyceae) both native and introduced, which cover all the sessile organisms, causing their death. The organisms most sensitive to this phenomenon are the gorgonians [29].
Human activities can also cause the mechanical destruction of bioconstructed limestone structures. Trawling is considered the most destructive method of fishing and is causing the degradation of vast areas of coral concretions [30].
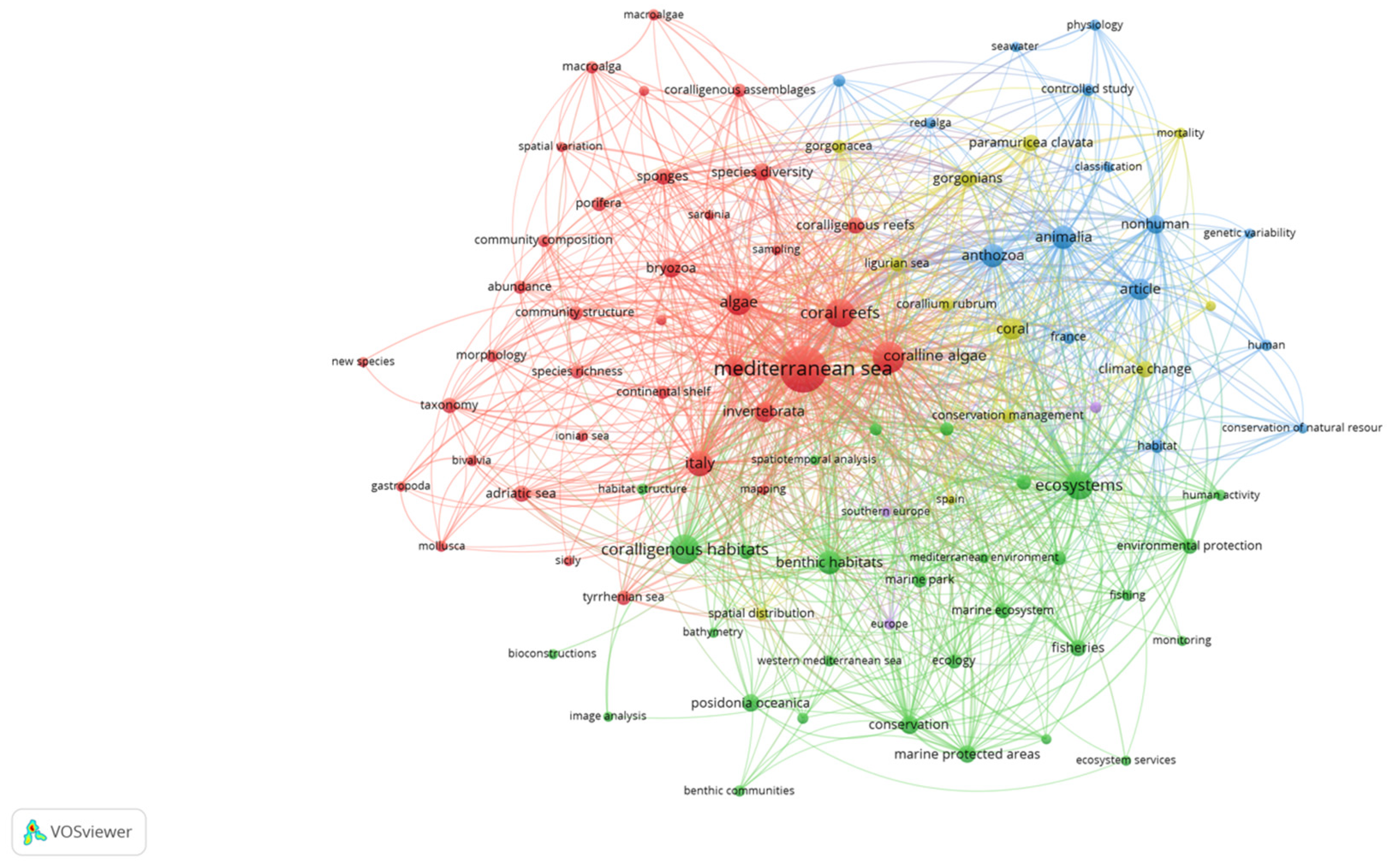
The coralligenous habitat is of great ecological and economic importance, so it has been included in the Barcelona Convention, the Habitats Directive [31] and the Marine Strategy Framework Directive [32]. However, despite its value and vulnerability, this habitat is still poorly investigated in the global scientific literature. This knowledge gap emerged through a bibliometric network analysis conducted using the VOSviewer software (version 1.6.19) by searching on the Scopus database the keyword “coralligenous”. The resulting 368 published documents from 2018 to 2024 were used to generate Figure 1.
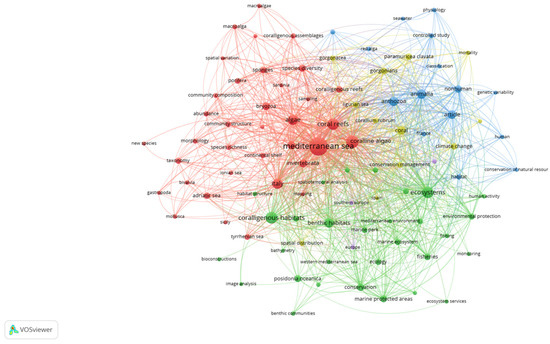
Figure 1.
Keywords related to “coralligenous” in the global scientific literature.
This figure shows the distribution and recurrence of keywords over the years, divided in clusters of different colors identifying a specific topic area (e.g., the green cluster incorporates the conservation studies). The map reveals that there has been more focus on conservation and taxonomic concepts. It is noteworthy that there is no mention of “natural capital” among keywords. This is probably due to difficulties in sampling procedures related to the elevated depths at which coralligenous reefs occur. Therefore, there is a need to fill this research gap. In fact, one of the main reasons why the importance of marine habitats is overlooked is the difficulty of finding a common unit for expressing its value. As a consequence, it is rather difficult for decision-makers to explore the trade-offs between conservation and exploitation of marine ecosystems, often leading to favoring the achievement of monetary and short-term benefits. Natural capital accounting provides biophysical and economic indicators of nature’s value that can support marine policy in implementing long-term effective strategies for the sustainable use and management of coastal and marine ecosystems.
In this study, a biophysical and trophodynamic environmental accounting model was used to assess the natural capital value of the coralligenous habitat in three Marine Protected Areas (MPAs) of the Campania Region, Southern Italy. The proposed model allows natural capital accounting based on the principle that the greater the investment of nature in capital generation, the greater its value.
2. Materials and Methods
2.1. Study Area
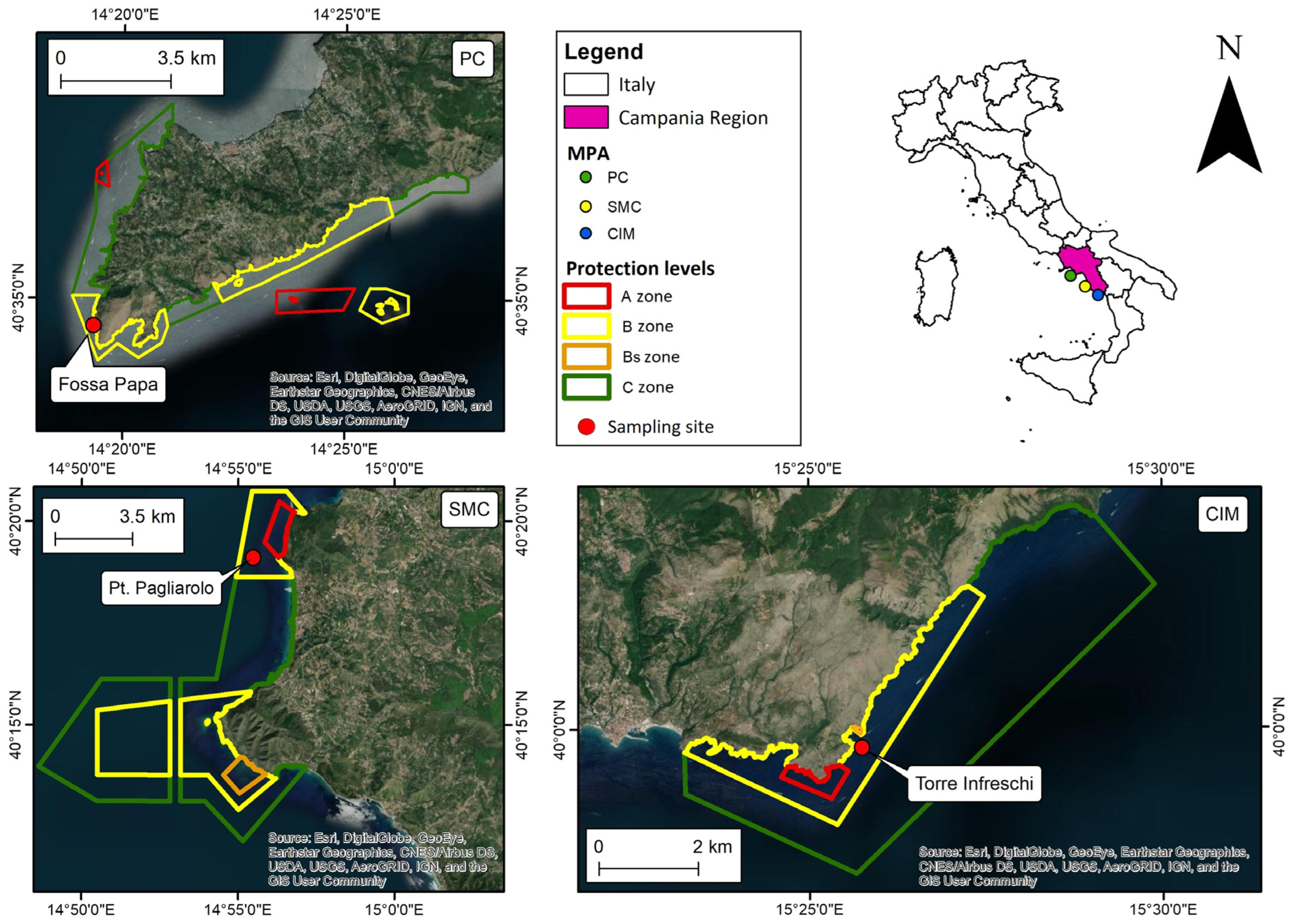
Three Italian MPAs in the Campania region (Southern Italy) were considered: “Santa Maria di Castellabate” (SMC) and “Costa degli Infreschi e della Masseta” (CIM) (40°17′ N, 14°56′ E and 40°0′ N, 15°26′ E, respectively) are located on the coast of the National Park of “Cilento, Vallo di Diano e Alburni”; while, “Punta Campanella” (PC) (40°34′8.4″ N, 14°19′29″ E) is located in the Sorrento Peninsula, in the Gulf of Naples (Figure 2). The three MPAs have extensions of 7095, 2332 and 1500 ha, with coastlines of 18, 14 and 40 km, respectively.
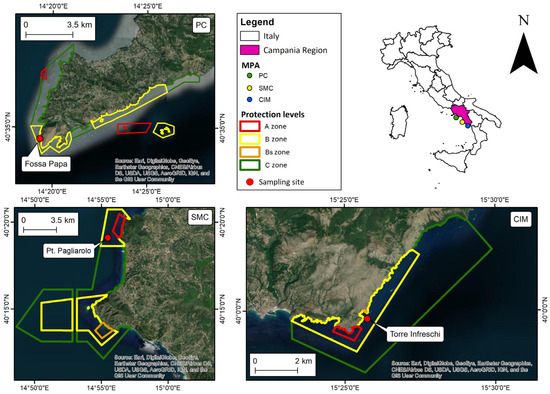
Figure 2.
Maps of the investigated MPAs. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.
Like most MPAs, SMC, CIM and PC are divided into three subareas with different levels of protection (Zone A, Zone B and Zone C). The zones A, B and C, respectively, cover about 2%, 42% and 56% of the total area in SMC, 2%, 20% and 78% in CIM and 12%, 43% and 45% in PC.
All the considered MPAs are characterized by the presence of Posidonia oceanica beds up to 30–35 m [33,34] and coralligenous bioconstructions below the bed lower limits. Both these habitats are defined as priority natural habitats by the EC Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora [35], thus requiring special conservation actions. In addition to their major ecological importance, these habitats are important touristic attractions for recreational activities, such as boating and diving.
2.2. Sampling Procedures and Data Analysis
Sampling campaigns were conducted in May and July 2022 to collect data on macrobenthic communities associated with the coralligenous habitats in the three investigated areas. The sampling protocol consisted in one sampling site for each MPA randomly selected. A total of 9 measurements (3 × 3, indicating number of replicates × number of sites, respectively) for the three MPAs were taken.
Macrobenthic fauna was collected by SCUBA using the “air lift–scraping–air lift” technique [36] within a 40 × 40 cm frame and a 1 mm-mesh sampling net. Samples were fixed in a 70% alcohol–seawater solution. All organisms were sorted and counted under a stereomicroscope. After sorting, organisms were bundled in the following main taxonomic groups: Algae, Bryozoa, Anellida, Crustacea, Echinodermata and Mollusca. To determine the biomass, organisms were first weighted after drying at 80 °C for 24 h using a drying oven. Then, gC were estimated using conversion factors [37].
Necto-benthic fish species, their abundance and their size were estimated by visual census along transects 25 m long and 5 m wide (for a total of 125 m2), following the “strip transect” method [38]. This monitoring technique provides qualitative and quantitative surveys with a limited impact on the ecosystem and is therefore particularly suited for marine reserves [39,40], as in our case study. Fish abundance was ascribed to one of the abundance categories proposed in the literature (1, 2–5, 5–10, 11–30, 31–50, 51–200, 201–500, >500 individuals), while the biomass for the different species was calculated by using the FishBase database (www.fishbase.org; accessed on 20 September 2022).
2.3. The Emergy Accounting Method
A biophysical and trophodynamic environmental accounting model based on the emergy accounting method was used to assess the natural capital value of the coralligenous habitat. This method of environmental accounting quantifies the total environmental support for a process [41]. The method aims at evaluating the environmental performance of a system, considering free environmental inputs (e.g., solar radiation, wind, rain and geothermal flow), but also human-driven material and energy flows, as well as the indirect environmental support embodied in human labor and services [41,42,43,44]. For this purpose, to consider both man-made (with a market price, considered by economics) and natural systems (without a market price, disregarded by economics), inputs are evaluated as solar emergy, defined as the total amount of available solar energy directly or indirectly required to make a given product or support a given flow, and measured as sej (solar equivalent joules). Therefore, solar radiation is used as the basic reference (unitary value of sej) to which all other energy types are scaled, because, from the ecological point of view, it represents the major driving force of the biosphere.
Emergy accounting was used for assessing the biophysical value of natural capital stocks of the coralligenous habitat in the investigated MPAs, based on the principle that the greater the investment of nature in the generation of capital, the greater its value [45].
The assessment of natural capital stocks was implemented according to the environmental accounting model described in Vassallo et al. [46] as illustrated in Figure 3. The model encompasses four main steps:
- Identification of the spatial and temporal boundaries of the habitat in the three MPAs;
- Biomass inventory and trophodynamic analysis of the main taxonomic groups identified in the habitat of the three MPAs, providing an estimate of the primary productivity used to support the benthic trophic web within the study areas;
- Biophysical accounting, providing an estimate of the biophysical value of natural capital and environmental flows (in terms of matter and energy), converting them into solar emergy units;
- Monetary conversion, expressing the biophysical value of natural capital in monetary units.
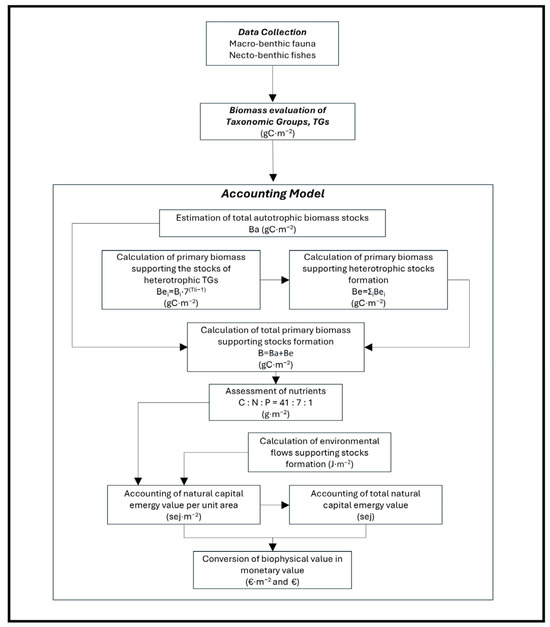
Figure 3.
Flow chart of the implemented environmental accounting model.
Figure 3.
Flow chart of the implemented environmental accounting model.
The amounts of N and P assimilated in the organic matter are calculated according to the ratio C:N:P of 41:7:1 [47]. Furthermore, natural flows (solar radiation, wind, rain, geothermal flow, currents, tides and runoff) supporting biomass production in the MPAs are calculated according to Odum [41] and accounted for the time of stock formation.
All the inputs (i.e., nutrients and natural flows) are then converted into emergy units by using specific UEVs, representing the emergy required to generate one unit of each input as a measure of the environmental support provided to the system: the higher the UEV of a product, the greater the environmental cost to produce it [48,49]. UEVs were updated to the 1.20 × 1025 sej yr−1 biosphere emergy baseline calculated by Brown et al. [43]. Once converted into emergy units, input flows were summed, according to the emergy algebra, to assess the emergy density value of natural capital in the habitat (sej m−2).
Finally, the emergy value of natural capital and environmental flows was converted into a monetary equivalent by using the Emergy to Money Ratio (EMR) indicator, calculated as the ratio between the total emergy supporting a nation and its gross domestic product (GDP) in the same year [42]. It represents the average amount of emergy needed to generate one unit of GDP in the national economy [41]. In particular, the monetary value of natural capital stocks was calculated by dividing the biophysical value with the EMR calculated for Italy of 2.63 × 1012 sej/EUR (NEAD: http://www.emergy-nead.com/home; accessed on 15 July 2024).
2.4. Statistical Analysis
Statistical analysis was implemented based on the biomass per square meter of taxonomic groups in each of the three sampling sites within the investigated MPAs. The MPAs were compared based on the mean biomass of each group by the Kruskal–Wallis non-parametric test with Dunn’s post hoc comparisons as measurements reflecting skewed distributions (Shapiro–Wilk test p > 0.05). Moreover, based on taxonomic groups the biomass-based Shannon diversity index and taxonomic equitability were calculated. For mean value comparisons, the same Kruskal–Wallis test was applied.
3. Results
The main taxonomic groups identified in the coralligenous habitat of each MPA are shown in Table 1. It includes the biomass density values of the different groups (expressed in gC per unit area).

Table 1.
Biomass density (gC m−2) and trophic levels of heterotrophic groups associated with coralligenous constructions in the investigated MPAs. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.
The biomass of the major taxonomic groups included in the habitat is the basic information provided to the trophodynamic environmental accounting model for calculating the primary productivity required to generate and maintain natural capital stocks.
The energy-flow values that support the generation of autotrophic and heterotrophic natural capital stocks are reported in Table 2 and Table 3, respectively. The input fluxes include nutrients (C, N, P) and natural fluxes (solar radiation, rainfall, wind, geothermal fluxes, tides, currents and runoff) that support the formation of both stocks at different spatio-temporal scales. The values of emergy density shown in the tables account for the total emergy per unit area, thus representing an intensive measure of the emergy support to the coralligenous habitat. Emergy supporting autotrophic stock formation ranges from 2.59 × 1011 sej m−2 for PC to 3.99 × 1011 sej m−2 for CIM (Table 2). In contrast, the emergy density that supports the formation of heterotrophic stocks is higher for PC and ranges from 2.14 × 1012 sej m−2 for SMC to 4.47 × 1012 sej m−2 for PC (Table 3).

Table 2.
Emergy flows supporting the generation of autotrophic coralligenous stocks in each investigated MPA. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.

Table 3.
Emergy flows supporting the generation of heterotrophic coralligenous stocks at each investigated MPA. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.
The values of total natural capital emergy density (by summing autotrophic and heterotrophic stocks) and the corresponding emergy-based monetary equivalents calculated for the different MPAs are reported in Table 4.

Table 4.
Total emergy density and corresponding emergy-based natural capital monetary equivalents at each investigated MPA. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.
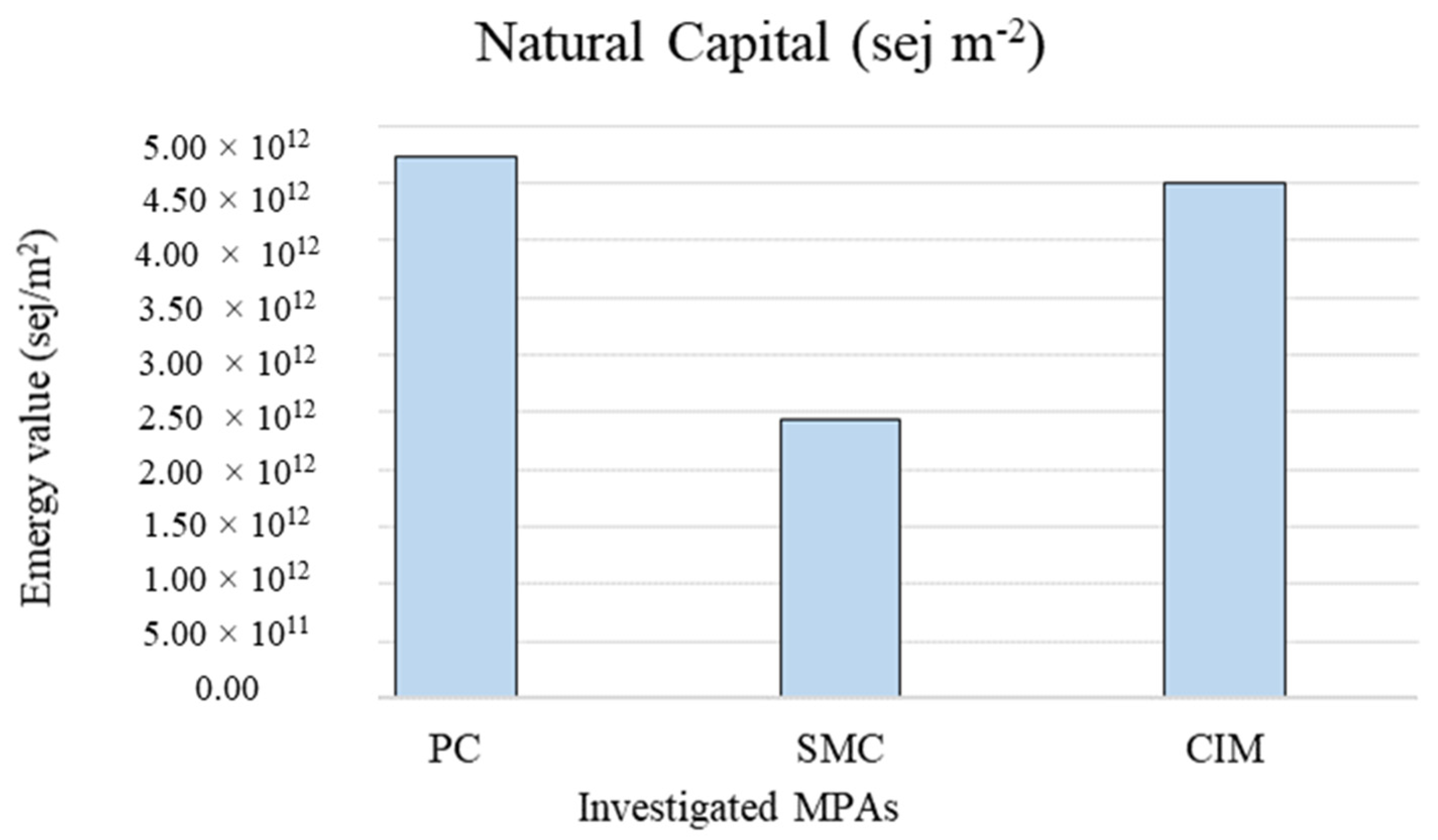
The total natural capital values per unit area are shown in Figure 4. The emergy value per unit area was higher for PC (4.72 × 1012 sej m−2).
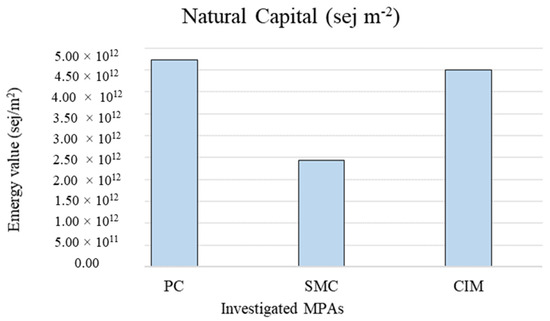
Figure 4.
Intensive natural capital values in the different investigated MPAs per unit area. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.
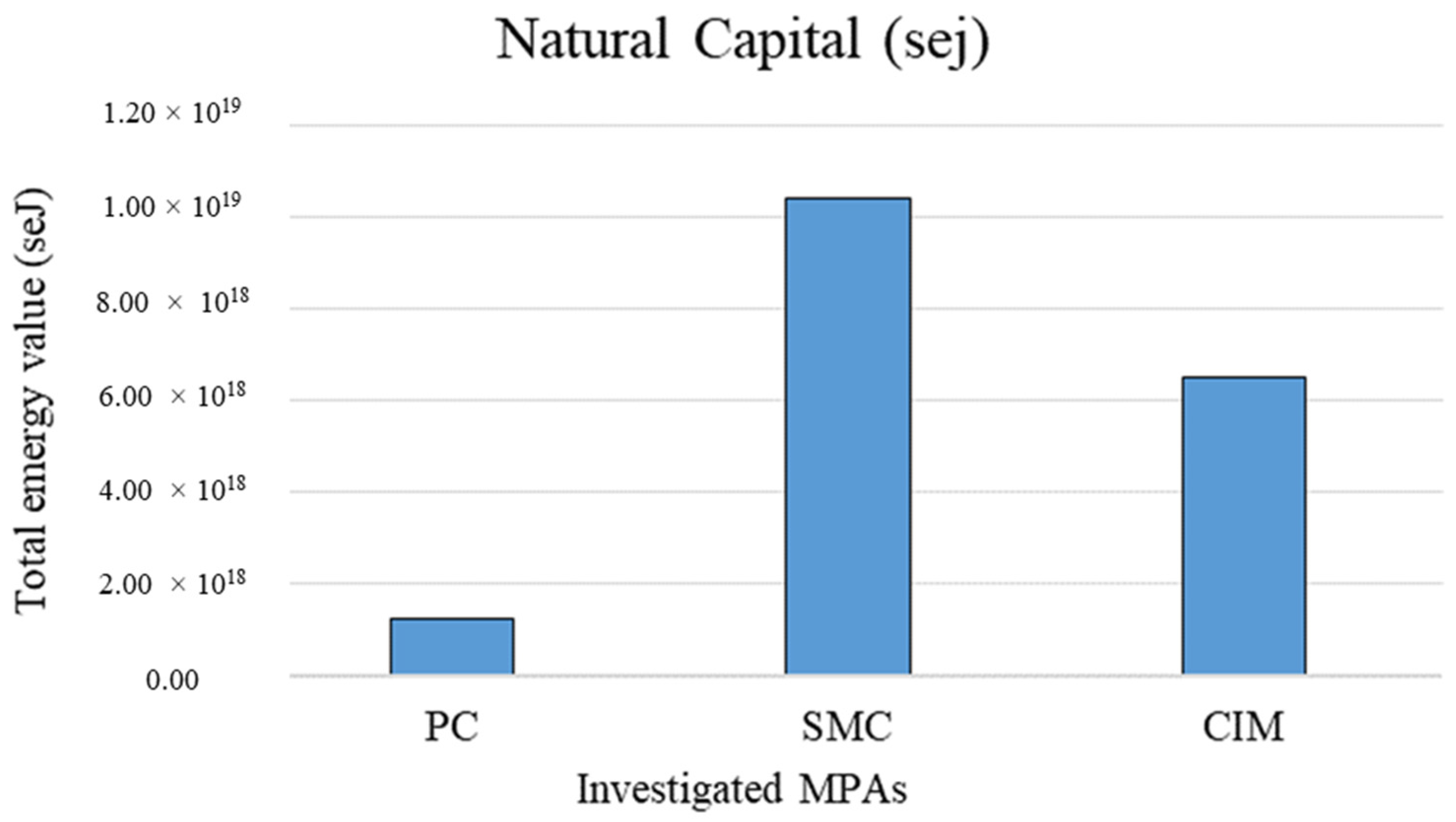
In contrast, Figure 5 shows the total natural capital value of the coralligenous habitat related to the extent of the three investigated MPAs (extensive measure of the emergy support for the habitat). SMC shows the highest value among them (1.04 × 1019).
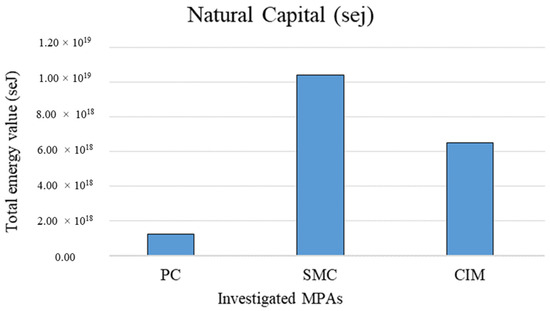
Figure 5.
Extensive natural capital values in the different investigated MPAs related to the total area of the considered biocenosis. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.
To integrate the biophysical assessment with an economic perspective, the emergy values of natural capital stocks were converted into monetary units by dividing the total value by the Emergy to Money Ratio (EMR) indicator. The economic values of natural capital in the investigated MPAs for the coralligenous habitat are EUR 5.21 m−2 for PC, EUR 4.98 m−2 for CIM and EUR 2.70 m−2 for SMC.
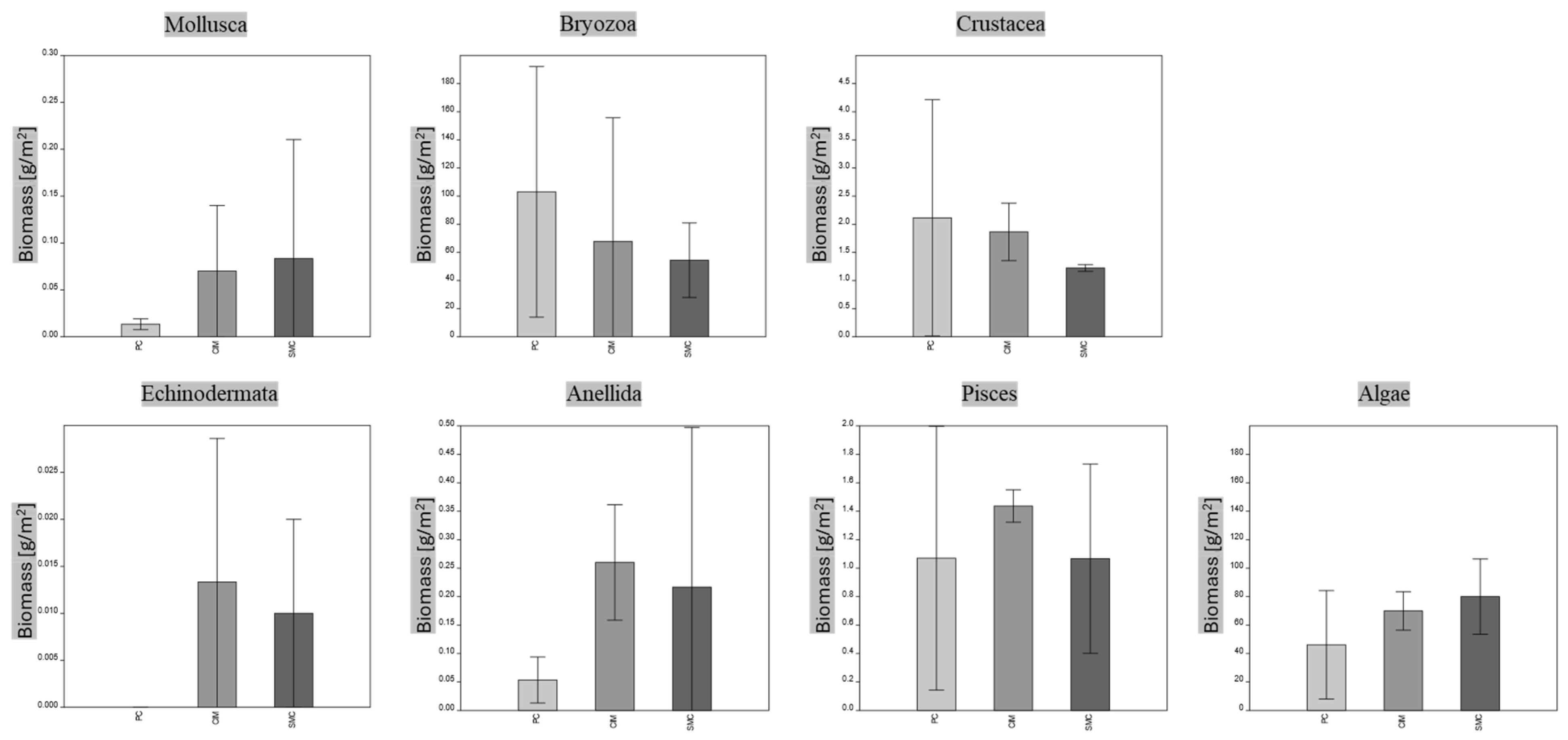
The economic equivalent of the extensive measure referring to the total extent of the coralligenous habitat in the investigated MPAs is about EUR 15 million for SMC, EUR 1 million for CIM and EUR 7 million for PC. It is worth nothing that despite the different intensive values of natural capital calculated for the MPAs, there are no significant differences in the biomass values of the taxonomic groups (Figure 6).
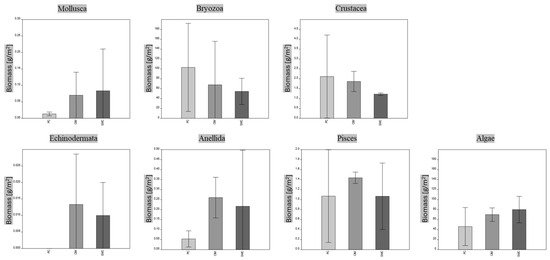
Figure 6.
MPA comparison based on mean biomass values of main taxonomic groups (Kruskal–Wallis test with Dunn’s post hoc); p > 0.05, no significant differences. Means ± SD are presented (n = 3). PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta.
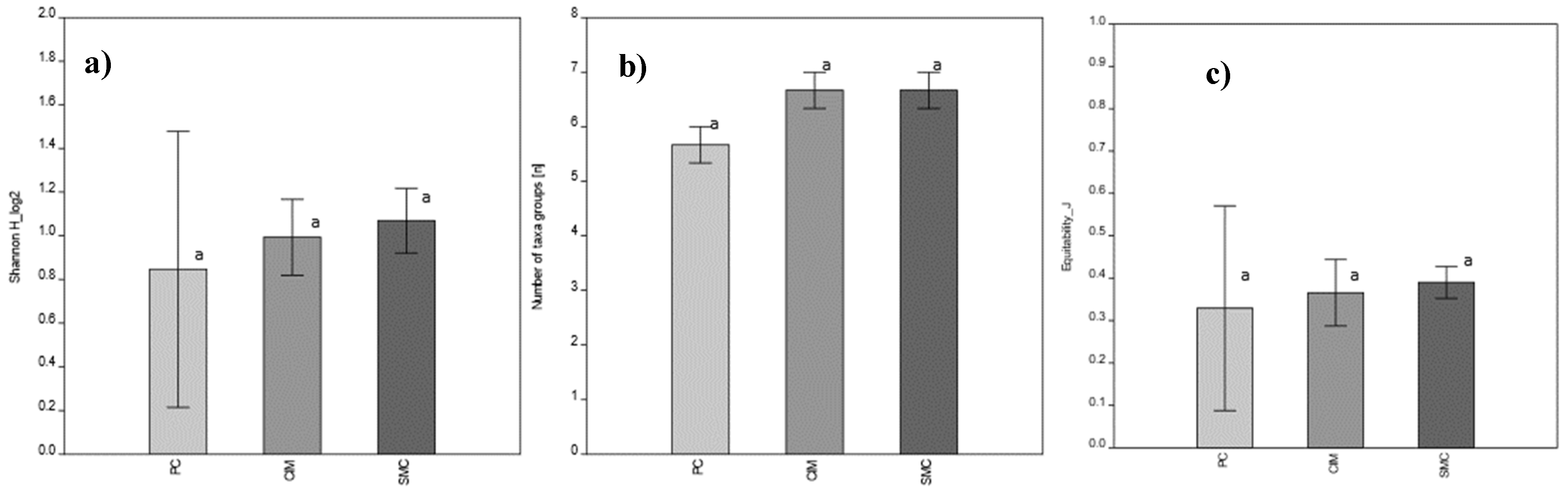
Moreover, the comparison of the biomass-based Shannon diversity index (H), the taxonomic equitability (J) and the number of taxonomic groups also did not demonstrate any significant difference among MPAs. SMC showed the highest H value while the lowest H value resulted for PC MPA (Figure 7).
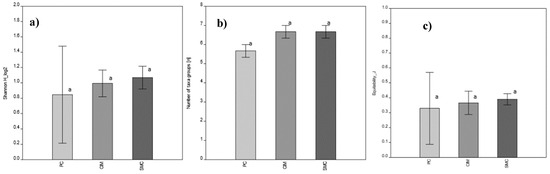
Figure 7.
MPA comparison based on mean biomass values of main taxonomic groups (Kruskal–Wallis test with Dunn’s post hoc); p > 0.05, no significant differences. Means ± SD are presented (n = 3). (a) Biomass-based Shannon Index; (b) number of taxonomic groups; (c) equitability. PC is Punta Campanella; SMC is Santa Maria di Castellabate; CIM is Costa degli Infreschi e della Masseta. No significant differences were detected, as denoted by the same letter “a”.
4. Discussion
The study of benthic habitats in MPAs is usually performed as a list of species, often neglecting the assessment of natural capital stocks.
In this study, the emergy-based accounting model based on emergy accounting allowed us to calculate the biophysical value of the natural capital stock of the coralligenous habitat in terms of the environmental costs for its generation. This kind of assessment accounts for the work performed by nature for the generation of natural capital stocks and the time needed for their formation. The assessment was based on ad hoc samplings for the coralligenous habitat performed in three MPAs of Campania Region (Southern Italy).
The results show that “algae” play a consistent role with their high biomass (ranging from 174.24 to 221.27 gC m−2) in building these heterogenic habitats. Indeed, the coralligenous habitat consists of bioconstructions, with thickness ranging from a few centimeters up to 2 m, formed by the multilayer deposition of calcium carbonate mainly produced by crustose coralline algae and the aggregation of other marine calcifiers [7,50,51,52]. Additionally, the high values of fish biomass (ranging from 88.12 to 202.6 gC m−2) highlight the key role of these complex habitats as feeding, recruitment and nursery sites for many species, often of commercial interest, also explaining why coralligenous bioconstructions are widely threatened by fishing impacts [53,54,55].
The total value of natural capital associated with the coralligenous habitat calculated with reference to the total extent of the MPAs is higher in SMC (1.51 × 1007 sej) because the coralligenous habitat covers a significantly larger area (427.28 ha) than in PC (137.28 ha) and CIM (27.42 ha). Being an extensive measure, this emergy indicator value related to the total area of the habitat allows for quantification of the total amount of stocks contained in the MPAs, but it does not allow for comparison among them. Instead, the total emergy value associated with the coralligenous habitat per unit area (sej m−2) represents an intensive indicator of the environmental support for stock formation, allowing comparison of the same habitat in different MPAs, regardless of their extent. In this case, results show a higher emergy value in PC (4.72 × 1012 sej m−2). This could be related to the particular geomorphological configuration of PC. Indeed, its submerged cliffs and the carbonate pinnacles allow the presence of pre-coralligenous formation from a few meters of water depth, while usually the rich biocenoses of coralligenous habitats occur at greater depths [51]. Moreover, the intensive value of natural capital stocks calculated in this study for the tree investigated MPAs is comparable with the values calculated by Franzese et al. [56] and Paoli et al. [57] for the coralligenous habitat in other Mediterranean MPAs.
It is worth noting that despite the different intensive values of natural capital calculated for the MPAs, statistical analyses show that there are no significant differences in the biomass values of the taxonomic groups and in the biomass-based Shannon diversity and the equitability indices. This outcome could be related to the similar conservation measures applied in the Campania Region MPAs aimed at preserving the coralligenous habitat.
Finally, the value of natural capital stock is also expressed in monetary units. This conversion does not alter the biophysical characteristics of the adopted environmental accounting model. Indeed, the results expressed in monetary equivalents still represent the environmental cost in generating natural capital stocks but, in the meantime, they provide an easier understanding of the value of the coralligenous habitat in policy and socio-economic contexts.
The integration of biophysical, ecological and economic indicators can be useful to communicate with stakeholders and support local managers and policy makers in the implementation of sustainable management and conservation strategies.
5. Conclusions
In this study, a biophysical and trophodynamic environmental accounting model was implemented for the first time to assess the natural capital value of the coralligenous habitat. The environmental accounting model was based on ad hoc sampling campaigns performed on the benthic community in the investigated area. The value of natural capital was calculated in both biophysical and economic terms. The former can support a deeper understanding of the ecological dynamics of marine habitats, while the latter is useful to better convey results to local managers, stakeholders and policy makers. Indeed, the calculated biophysical and economic indicators of nature’s value can be incorporated into marine policy to implement long-term effective plans for the sustainable management of marine ecosystems.
The environmental accounting model adopted in this study could provide a useful benchmark to calculate the natural capital value of coralligenous constructions in other MPAs of the Mediterranean Sea and for conducting potential comparative analyses. In terms of future development, the environmental accounting model presented in this study could be implemented with additional data collected in different years to assess potential variations in natural capital values over time. Finally, the assessment of natural capital stocks may be integrated with the evaluation of ecosystem service flows generated by the coralligenous habitat. For instance, its role in carbon sequestration and climate regulation is still overlooked and deserves in depth investigation. Therefore, forthcoming studies are needed based on multi-criteria perspectives that consider both the key role of the coralligenous habitat in providing ecosystem goods and services and its vulnerability to human pressures.
Author Contributions
Conceptualization, P.P.F., G.F.R. and E.B.; methodology, S.S. and L.C.; software, A.P. and U.G.; validation, F.R. and A.P.; formal analysis, E.B., U.G. and A.P.; investigation, F.R.; data curation, S.S., L.C., U.G. and A.P.; writing—original draft preparation, S.S.; writing—review and editing, F.R., U.G., P.P.F., G.F.R. and E.B.; visualization, S.S.; supervision, F.R., E.B., G.F.R. and P.P.F.; project administration, G.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We are grateful to the Campania Marine Protected Areas and the members of the Marine Ecology laboratory of the Parthenope University for their help during sampling activities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Rendina, F.; Buonocore, E.; di Montanara, A.C.; Russo, G.F. The scientific research on rhodolith beds: A review through bibliometric network analysis. Ecol. Inform. 2022, 70, 101738. [Google Scholar] [CrossRef]
- Buonocore, E.; Grande, U.; Franzese, P.P.; Russo, G.F. Trends and evolution in the concept of marine ecosystem services: An overview. Water 2021, 13, 2060. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Maritime Affairs and Fisheries, Joint Research Centre; Borriello, A., Calvo Santos, A., Ghiani, M., Eds.; The EU Blue Economy Report; Publications Office of the European Union: Brussels, Belgium, 2023. [Google Scholar]
- Ferrigno, F.; Rendina, F.; Sandulli, R.; Russo, G.F. Coralligenous assemblages: Research status and trends of a key Mediterranean biodiversity hotspot through bibliometric analysis. Ecol. Quest. 2024, 35, 1–32. [Google Scholar]
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Trav. Sci. Parc Natl. Port-Cros 2004, 20, 97–146. [Google Scholar]
- Ballesteros, E.; Gibson, R.; Atkinson, R.; Gordon, J. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar]
- Martin, S.; Gattuso, J.P. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob. Chang. Biol. 2009, 15, 2089–2100. [Google Scholar] [CrossRef]
- Garrabou, J.; Ballesteros, E. Growth of Mesophyllum alternans and Lithophyllum frondosum (Corallinales, Rhodophyta) in the northwestern Mediterranean. Eur. J. Phycol. 2000, 35, 1–10. [Google Scholar] [CrossRef]
- Cocito, S. Bioconstruction and biodiversity: Their mutual influence. Sci. Mar. 2004, 68, 137–144. [Google Scholar] [CrossRef]
- Lapointe, L.; Bourget, E. Influence of substratum heterogeneity scales and complexity on a temperate epibenthic marine community. Mar. Ecol. Prog. Ser. 1999, 189, 159–170. [Google Scholar] [CrossRef]
- Balata, D.; Piazzi, L.; Bulleri, F. Sediment deposition dampens positive effects of substratum complexity on the diversity of macroalgal assemblages. J. Exp. Mar. Biol. Ecol. 2015, 467, 45–51. [Google Scholar] [CrossRef]
- Ferrigno, F.; Appolloni, L.; Rendina, F.; Donnarumma, L.; Russo, G.F.; Sandulli, R. Red coral (Corallium rubrum) populations and coralligenous characterization within “Regno di Nettuno MPA” (Tyrrhenian Sea, Italy). Eur. Zool. J. 2020, 87, 203–213. [Google Scholar] [CrossRef]
- Deter, J.; Descamp, P.; Boissery, P.; Ballesta, L.; Holon, F. A rapid photographic method detects depth gradient in coralligenous assemblages. J. Exp. Mar. Biol. Ebarbiercology 2012, 418–419, 75–82. [Google Scholar] [CrossRef]
- Piazzi, L.; Gennaro, P.; Balata, D. Threats to macroalgal coralligenous assemblages in the Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, L.; Bianchi, C.N.; Cecchi, E.; Gatti, G.; Guala, I.; Morri, C.; Sartoretto, S.; Serena, F.; Montefalcone, M. What’s in an index? Comparing the ecological information provided by two indices to assess the status of coralligenous reefs in the NW Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1091–1100. [Google Scholar] [CrossRef]
- Sartoretto, S.; Schohn, T.; Bianchi, C.N.; Morri, C.; Garrabou, J.; Ballesteros, E.; Gatti, G. An integrated method to evaluate and monitor the conservation state of coralligenous habitats: The INDEX-COR approach. Mar. Pollut. Bull. 2017, 120, 222–231. [Google Scholar] [CrossRef]
- Gatti, G.; Bianchi, C.N.; Parravicini, V.; Rovere, A.; Peirano, A.; Montefalcone, M.; Massa, F.; Morri, C. Ecological change, sliding baselines and the importance of historical data: Lessons from combining observational and quantitative data on a temperate reef over 70 years. PLoS ONE 2015, 10, e0118581. [Google Scholar] [CrossRef]
- Gambi, M.C.; Barbieri, F.; Signorelli, S.; Saggiomo, V. Mortality events along the Campania coast (Tyrrhenian Sea) in summers 2008 and 2009 and relation to thermal conditions. Biol. Mar. Mediterr. 2010, 17, 126–127. [Google Scholar]
- Bavestrello, G.; Bo, M.; Canese, S.; Sandulli, R.; Cattaneo-Vietti, R. The red coral populations of the gulfs of Naples and Salerno: Human impact and deep mass mortalities. Ital. J. Zool. 2014, 81, 552–563. [Google Scholar] [CrossRef]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Gómez-Gras, D.; Linares, C.; Dornelas, M.; Madin, J.S.; Brambilla, V.; Ledoux, J.B.; Garrabou, J. Climate change transforms the functional identity of Mediterranean coralligenous assemblages. Ecol. Lett. 2021, 24, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneo-Vietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.; Sara, G.; Schiaparelli, S.; et al. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol. Lett. 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Garrabou, J.; Perez, T.; Sartoretto, S.; Harmelin, J.G. Mass mortality event in red coral Corallium rubrum populations in Provence region (France, NW Mediterranean). Mar. Ecol. Prog. Ser. 2001, 217, 263–272. [Google Scholar] [CrossRef]
- Bramanti, L.; Magagnini, G.; De Maio, L.; Santangelo, G. Recruitment, early survival and growth of the Mediterranean red coral Corallium rubrum (L. 1758), a 4-year study. J. Exp. Mar. Biol. Ecol. 2005, 314, 69–78. [Google Scholar] [CrossRef]
- Bramanti, L.; Movilla, J.; Guron, M.; Calvo, E.; Gori, A.; Dominguez-Carrió, C.; Grinyò, J.; Lopez-Sanz, A.; Martinez-Quintana, A.; Pelejero, C.; et al. Detrimental effects of ocean acidification on the economically important Mediterranean red coral (Corallium rubrum). Glob. Chang. Biol. 2013, 19, 1897–1908. [Google Scholar] [CrossRef]
- Lombardi, C.; Rodolfo-Metalpa, R.; Cocito, S.; Gambi, M.C.; Taylor, P.D. Structural and geochemical alterations in the Mg calcite bryozoan Myriapora truncata under elevated seawater pCO2 simulating ocean acidification. Mar. Ecol. 2011, 32, 211–221. [Google Scholar] [CrossRef]
- Giuliani, S.; Lamberti, C.V.; Sonni, C.; Pellegrini, D. Mucilage impact on gorgonians in the Tyrrhenian sea. Sci. Total Environ. 2005, 353, 340–349. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Meinesz, A.; Ballesteros, E.; Ben Maiz, N.; Boisset, F.; Cinelli, F.; Cirik, S.; Cormaci, M.; Jeudy de Grissac, A.; Laborel, J.; et al. Livre Rouge “Gérard Vuignier” des Végétaux, Peuplements et Paysages Marins Menacés de Méditerranée; MAP Technical Report Series 1990, 43. Athens: UNEP/IUCN/GIS Posidonie; IUCN: Gland, Switzerland, 1990; pp. 1–250. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Boero, F. Mediterranean bioconstructions along the Italian coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar]
- European Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for Community action in the field of marine policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, L164, 19–40. [Google Scholar]
- Buonocore, E.; Appolloni, L.; Russo, G.F.; Franzese, P.P. Assessing natural capital value in marine ecosystems through an environmental accounting model: A case study in Southern Italy. Ecol. Model. 2020, 419, 108958. [Google Scholar] [CrossRef]
- Appolloni, L.; Pagliarani, A.; Cocozza di Montanara, A.; Rendina, F.; Donnarumma, L.; Ciorciaro, D.; Ferrigno, F.; Di Stefano, F.; Sandulli, R.; Russo, G.F. Benthic Fish Communities Associated with Posidonia oceanica Beds May Reveal the Fishing Impact and Effectiveness of Marine Protected Areas: Two Case Studies in the Southern Tyrrhenian Sea. Water 2023, 15, 1967. [Google Scholar] [CrossRef]
- Catucci, E.; Buonocore, E.; Franzese, P.P.; Scardi, M. Assessing the natural capital value of Posidonia oceanica meadows in the Italian seas by integrating Habitat Suitability and Environmental Accounting Models. ICES J. Mar. Sci. 2023, 80, 739–750. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, L206, 7–50. [Google Scholar]
- Nandan, S.B.; Jayachandran, P.R.; Asha, C.V. Sampling Techniques for molluscan fauna. In Training Manual-1 St International Training Workshop On Taxonomy of Bivalve Molluscs; Cochin University of Science & Technology: Kochi, India, 2016; pp. 107–116. [Google Scholar]
- Brey, T.; Müller-Wiegmann, C.; Zittier, Z.M.; Hagen, W. Body composition in aquatic organisms—A global data bank of relationships between mass, elemental composition and energy content. J. Sea Res. 2010, 64, 334–340. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L.; Harmelin, J.G.; Chauvet, C.; Duval, C.; Galzin, R.; Lejeune, P.; Lasserre, G. Evaluation visuelle des peuplements et populations de poissons méthodes et problèmes. Rev. D’ecol. Terre Vie 1985, 40, 467–539. [Google Scholar]
- Harmelin-Vivien, M.L.; Harmelin, J.G.; Leboulleux, V. Microhabitat requirements for settlement of juvenile sparid fishes on Mediterranean rocky shores. Hydrobiologia 1995, 300, 309–320. [Google Scholar] [CrossRef]
- Odum, H.T. Self organization, transformity and information. Science 1988, 242, 1132–1139. [Google Scholar] [CrossRef]
- Odum, H.T. Environmental Accounting: Emergy and Environmental Decision Making; John Wiley and Sons: Hoboken, NJ, USA, 1996; p. 369. [Google Scholar]
- Brown, M.T.; Ulgiati, S. Emergy analysis and environmental accounting. In Encyclopedia of Energy; Elsevier: Amsterdam, The Netherlands, 2004; p. 2. [Google Scholar]
- Brown, M.T.; Campbell, D.E.; De Vilbiss, C.; Ulgiati, S. The geobiosphere emergy baseline: A synthesis. Ecol. Model. 2016, 339, 89–91. [Google Scholar] [CrossRef]
- Kamp, A.; Morandi, F.; Østergård, H. Development of concepts for human labour accounting in Emergy Assessment and other Environmental Sustainability Assessment methods. Ecol. Indic. 2016, 60, 884–892. [Google Scholar] [CrossRef]
- Grande, U.; Piernik, A.; Nienartowicz, A.; Buonocore, E.; Franzese, P.P. Measuring natural capital value and ecological complexity of lake ecosystems. Ecol. Model. 2023, 482, 110401. [Google Scholar] [CrossRef]
- Vassallo, P.; Paoli, C.; Buonocore, E.; Franzese, P.P.; Russo, G.F.; Povero, P. Assessing the value of natural capital in marine protected areas: A biophysical and trophodynamic environmental accounting model. Ecol. Model. 2017, 355, 12–17. [Google Scholar] [CrossRef]
- Redfield, A.C. The influence of organisms on the composition of seawater. Sea 1963, 2, 26–77. [Google Scholar]
- Brown, M.T.; Ulgiati, S. Emergy-based indices and ratios to evaluate sustainability: Monitoring economies and technology toward environmentally sound innovation. Ecol. Eng. 1997, 9, 51–69. [Google Scholar] [CrossRef]
- Zhang, C.; Su, B.; Beckmann, M.; Volk, M. Emergy-based evaluation of ecosystem services: Progress and perspectives. Renew. Sustain. Energy Rev. 2024, 192, 114201. [Google Scholar] [CrossRef]
- Bracchi, V.A.; Bazzicalupo, P.; Fallati, L.; Varzi, A.G.; Savini, A.; Negri, M.P.; Rosso, A.; Sanfilippo, R.; Guido, A.; Bertolino, M.; et al. The Main Builders of Mediterranean Coralligenous: 2D and 3D Quantitative Approaches for its Identification. Front. Earth Sci. 2022, 10, 910522. [Google Scholar] [CrossRef]
- Casellato, S.; Stefanon, A. Coralligenous habitat in the northern Adriatic Sea: An overview. Mar. Ecol. 2008, 29, 321–341. [Google Scholar] [CrossRef]
- Casas-Güell, E.; Teixidó, N.; Garrabou, J.; Cebrian, E. Structure and biodiversity of coralligenous assemblages over broad spatial and temporal scales. Mar. Biol. 2015, 162, 901–912. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bava, S.; Bavestrello, G.; Betti, F.; Lanteri, L.; Bo, M. Artisanal fishing impact on deep coralligenous animal forests: A Mediterranean case study of marine vulnerability. Ocean Coast. Manag. 2019, 177, 112–126. [Google Scholar] [CrossRef]
- Silva, S.; Grande, U.; Rendina, F.; Buonocore, E.; Guidato, M.; Contegiacomo, M.; Franzese, P.P. The scientific literature on coralligenous habitat and fishing impacts. Ecol. Quest. 2024, 35, 1. [Google Scholar] [CrossRef]
- Piazzi, L.; Cecchi, E.; Cinti, M.F.; Stipcich, P.; Ceccherelli, G. Impact assessment of fish cages on coralligenous reefs through the use of the STAR sampling procedure. Mediterr. Mar. Sci. 2019, 20, 627–635. [Google Scholar] [CrossRef]
- Franzese, P.P.; Buonocore, E.; Donnarumma, L.; Russo, G.F. Natural capital accounting in marine protected areas: The case of the Islands of Ventotene and S. Stefano (Central Italy). Ecol. Model. 2017, 360, 290–299. [Google Scholar] [CrossRef]
- Paoli, C.; Povero, P.; Burgos, E.; Dapueto, G.; Fanciulli, G.; Massa, F.; Scarpellini, P.; Vassallo, P. Natural capital and environmental flows assessment in marine protected areas: The case study of Liguria region (NW Mediterranean Sea). Ecol. Model. 2018, 368, 121–135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).