Enhanced Production of Microalgal Metabolites Through Aeration Coupled with Stirring
Abstract
1. Introduction
| Microalga | Mixing Method | Culture Volume (L) | Culture Time (d) | Dry Mass (g/L) | Carbohydrates (%) | Lipids (%) | Proteins (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Mychonastes homosphaera | Aeration | 3.8 | 21 | 5.3 ± 0.4 | 25.8 ± 0.7 | 42.5 ± 1.2 | [27] | |
| Nannochloropsis sp. | Aeration + stirring | 3.27 | 5 | 3.2 | [28] | |||
| Arthrospira platensis | 3.9 | |||||||
| Tetradesmus obliquus CPCC05 | Aeration | 1.5 | 4 | 2.8 | [29] | |||
| Chlorella sorokiniana | Aeration | 0.02 | 10 | 7.5 | [30] | |||
| Chlorella vulgaris | 25.9 | |||||||
| Chlorella vulgaris | Aeration | 8.6 | 6 | 7.21 | [31] | |||
| Chlorella sp. | Aeration | 2000 | 6 | 1.83 | [32] | |||
| Scenedesmus dimorphus | 2.23 | |||||||
| Dunaliella salina | Aeration + stirring | 3 | 9 | 2.423 | 32 | 16 | 25 | [10] |
| Nannochloropsis oculata | 3.06 | 11 | 16 | 34 | ||||
| Ecdysichlamys minuta | Stirring | 1.535 | 31 | 21 | 16 | |||
| Neochloris conjuncta | 0.644 | 22 | 13 | 34 | ||||
| Chlorella sorokiniana SDEC-18 | Aeration | 2.5 | 20 | 2.08 | 25.1 | [33] | ||
| Chlorella sorokiniana SDEC-18 | Aeration + stirring | 3 | 14 | 3.62 | 41.4 | 21.7 | 28.4 | This study |
2. Materials and Methods
2.1. Microalgal Strain
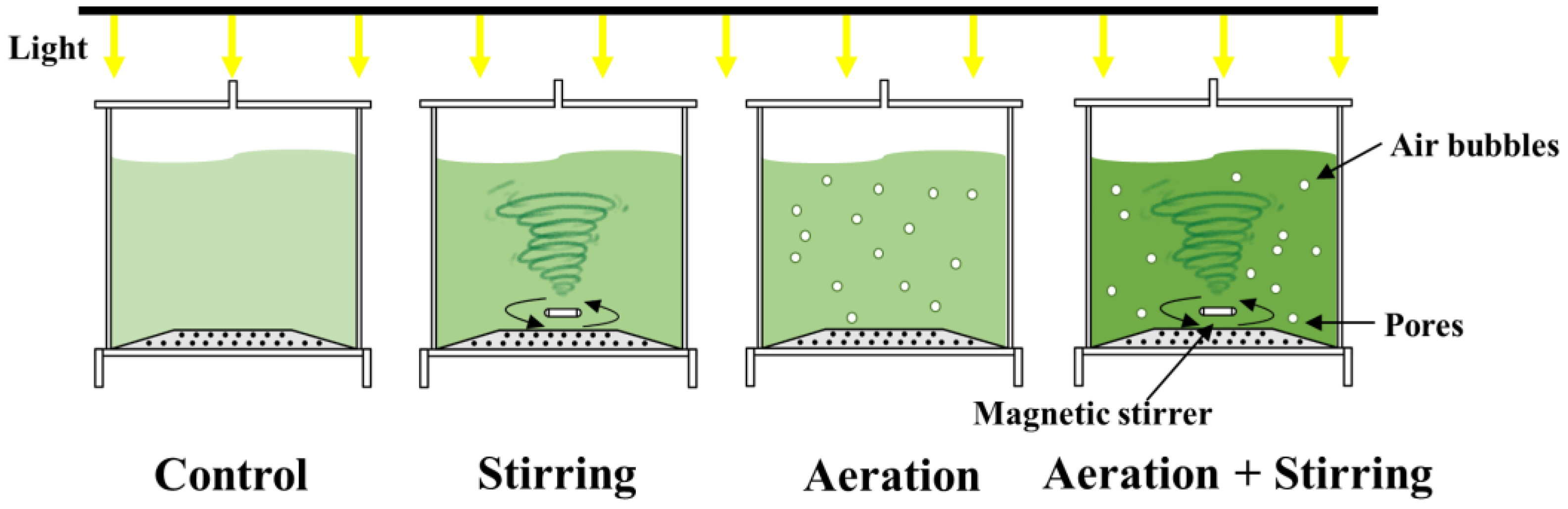
2.2. Experimental Design
2.3. Microalga Biomass and Biochemical Composition Determination
2.3.1. Dry Mass and Cell Density
2.3.2. Cellular Pigments
2.3.3. Lipid and Fatty Acids
2.3.4. Carbohydrates
2.3.5. Protein
2.4. Data Analysis
3. Results and Discussion
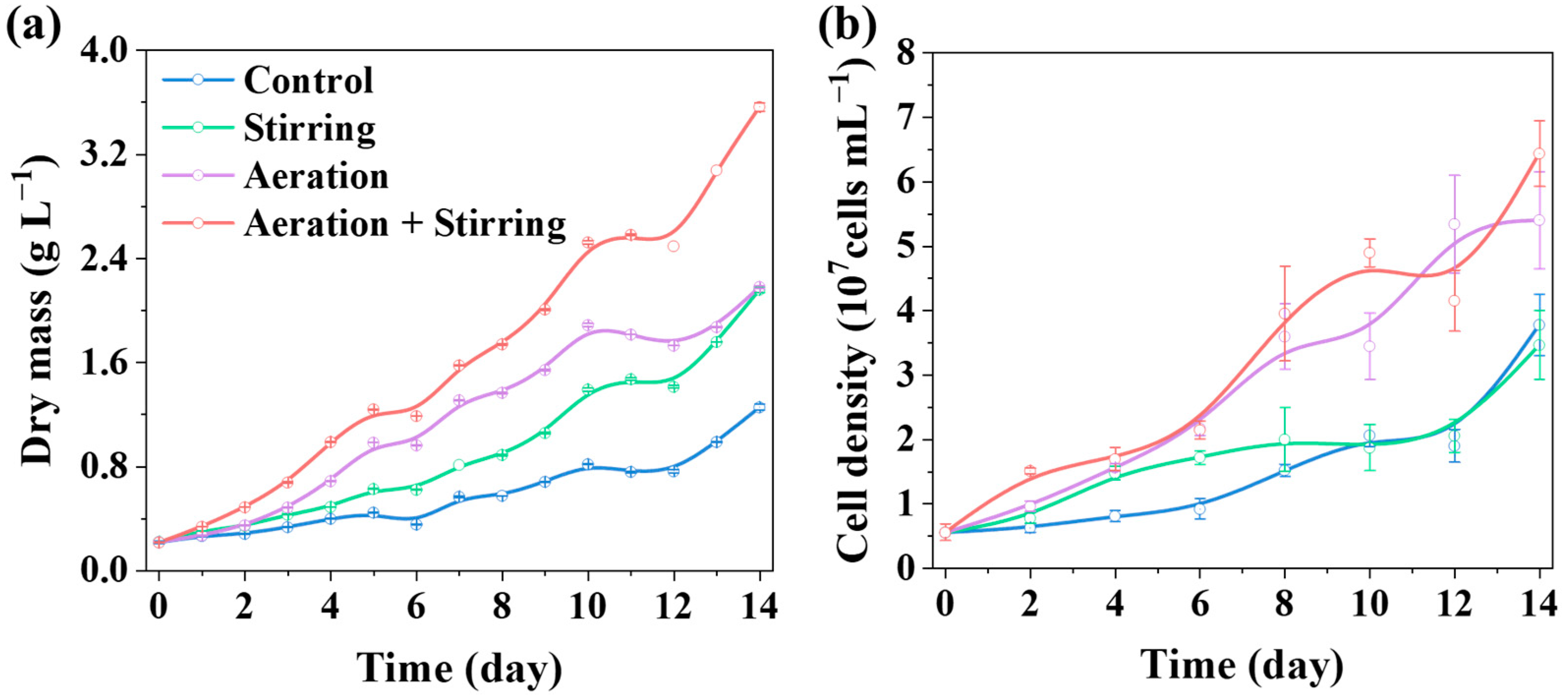
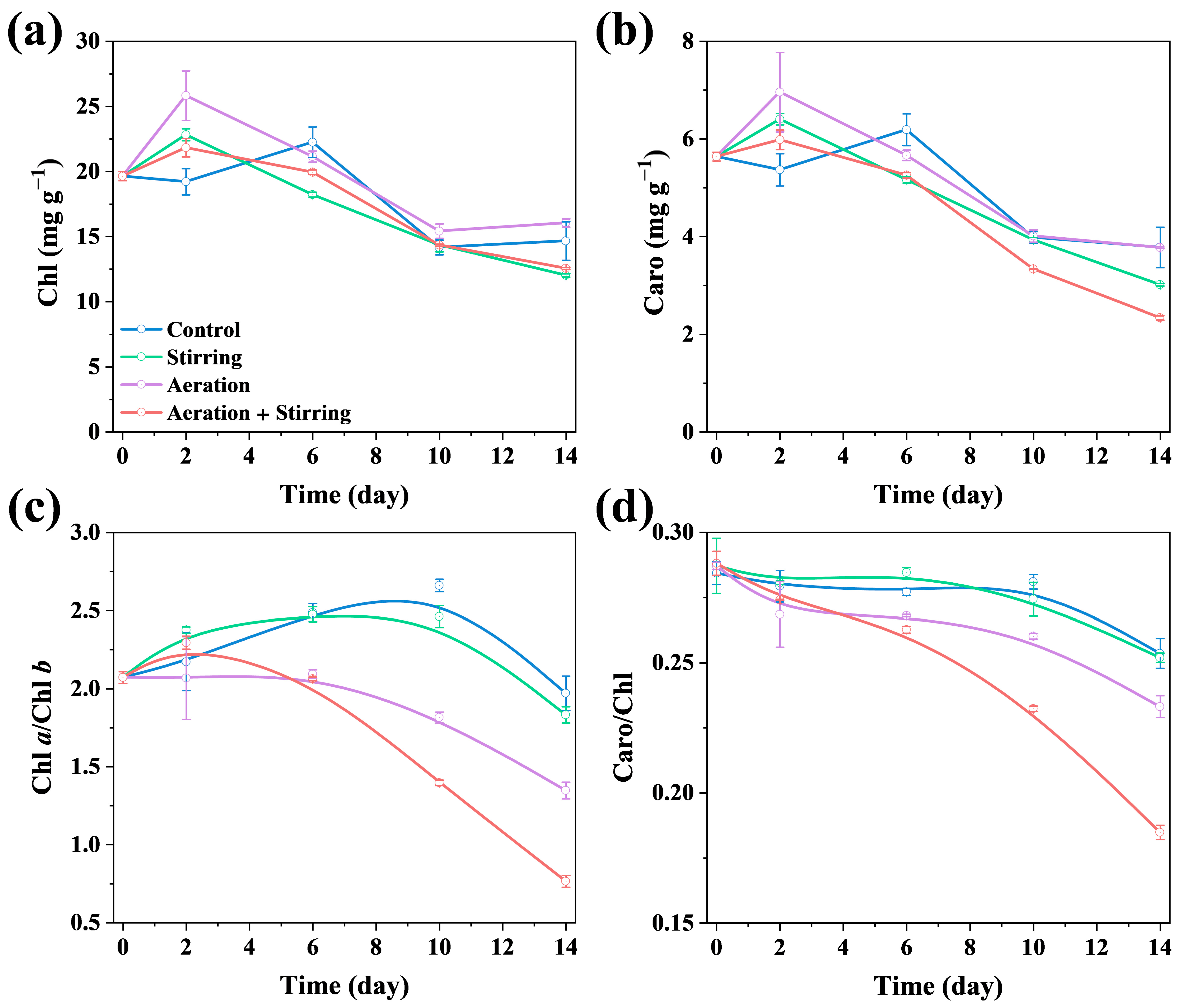
3.1. Effects of Mixing Method on Microalgal Growth and Biochemical Composition
3.1.1. Microalgal Biomass and Photosynthetic Pigments
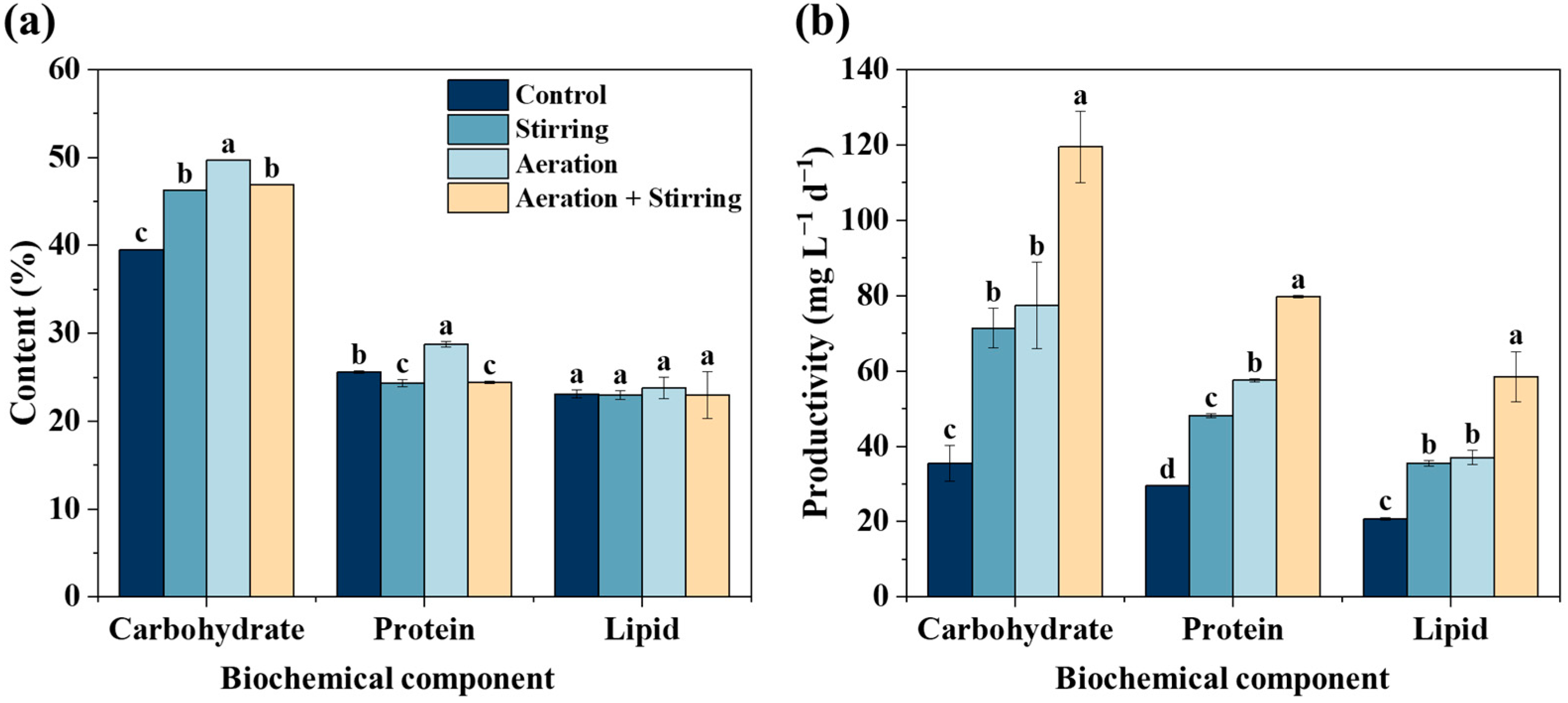
3.1.2. Carbohydrate Production
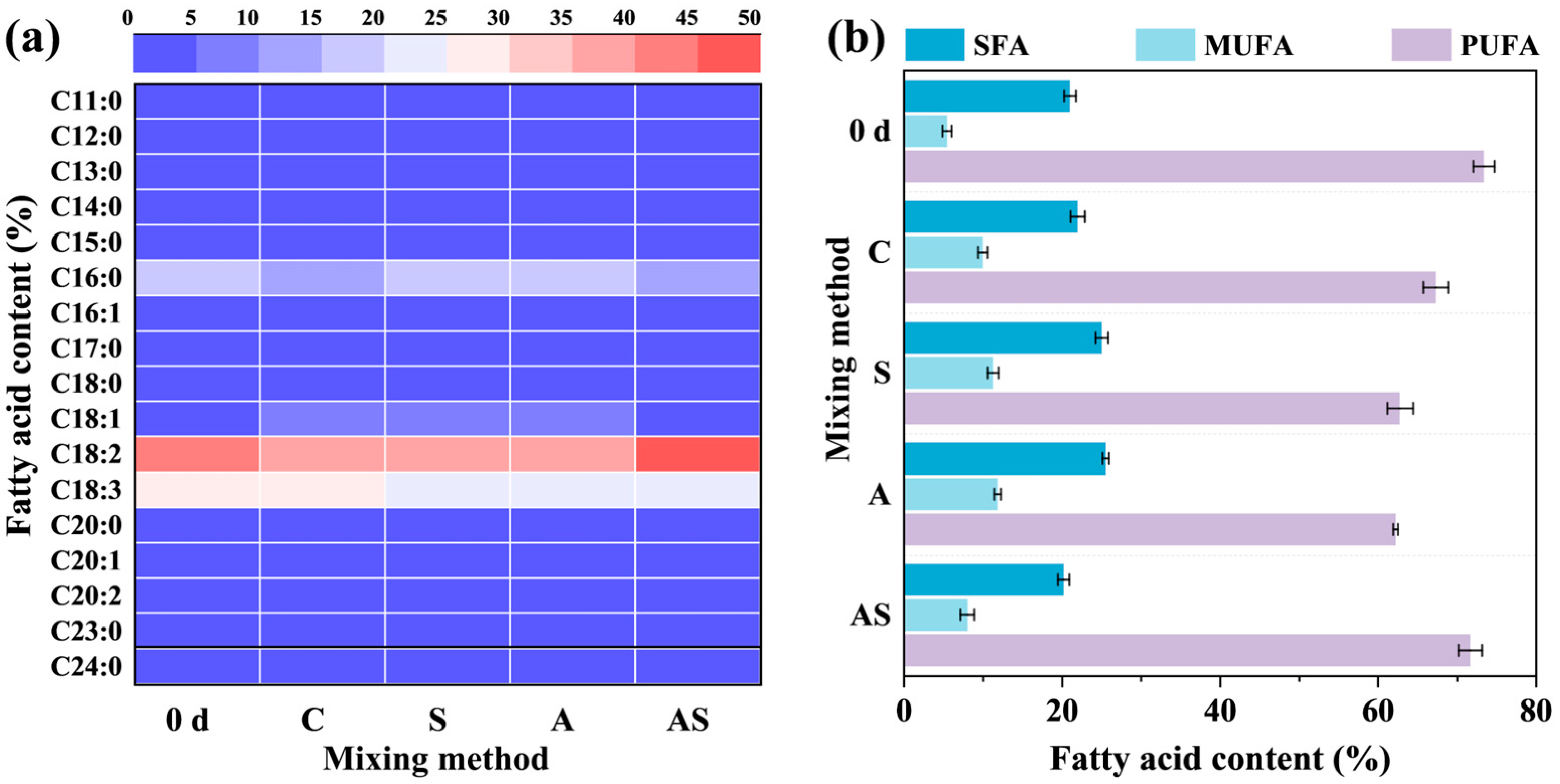
3.1.3. Protein, Lipid, and Fatty Acid Production
3.2. Effects of Stirring Speeds on Microalgal Growth and Biochemical Composition for Aeration Coupled with Stirring
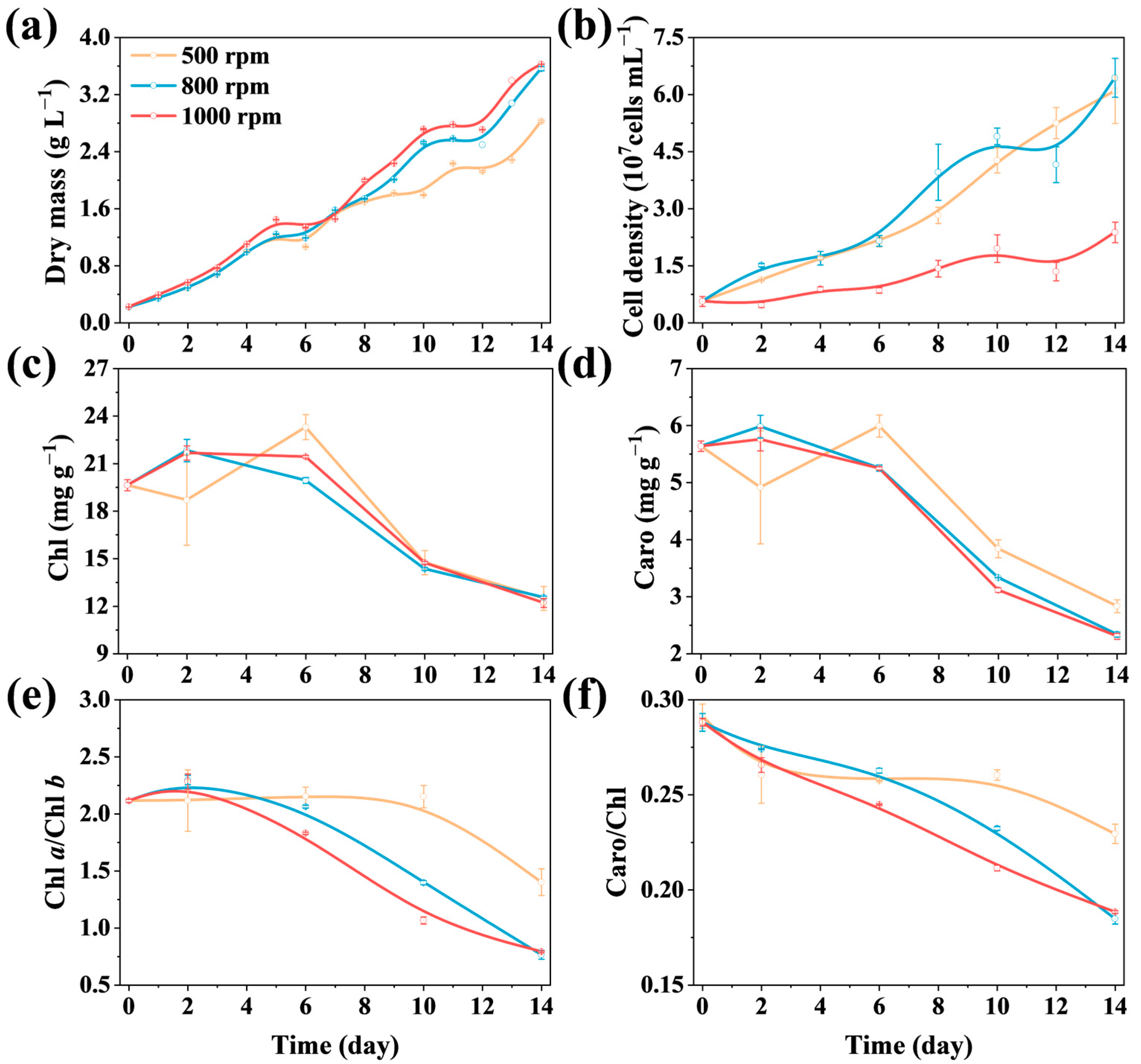
3.2.1. Microalgal Biomass and Photosynthetic Pigments
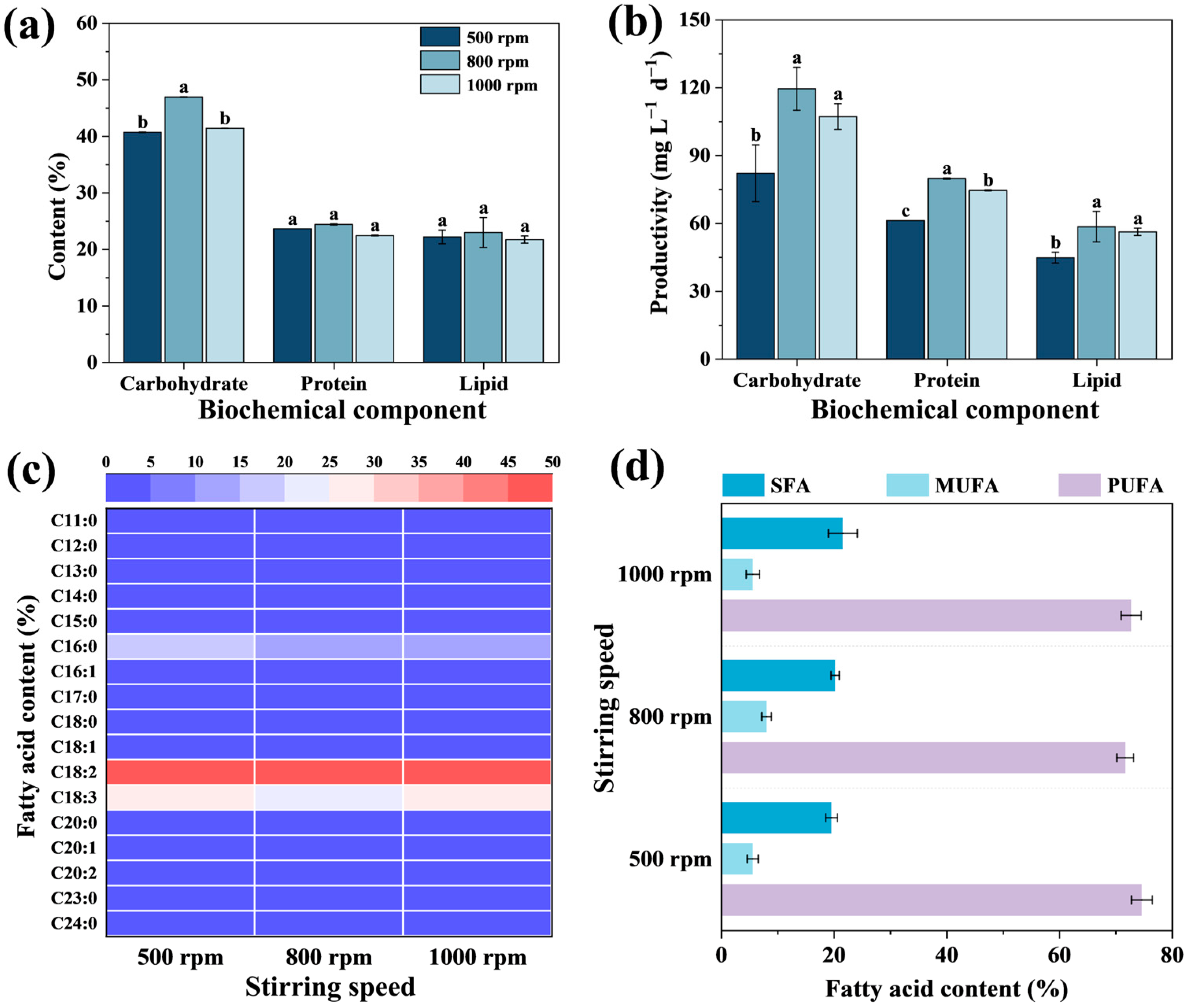
3.2.2. Biochemical Composition and Productivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Tounsi, L.; Hentati, F.; Ben Hlima, H.; Barkallah, M.; Smaoui, S.; Fendri, I.; Michaud, P.; Abdelkafi, S. Microalgae as feedstock for bioactive polysaccharides. Int. J. Biol. Macromol. 2022, 221, 1238–1250. [Google Scholar] [CrossRef]
- Ibrahim, T.N.B.T.; Feisal, N.A.S.; Kamaludin, N.H.; Cheah, W.Y.; How, V.; Bhatnagar, A.; Ma, Z.; Show, P.L. Biological active metabolites from microalgae for healthcare and pharmaceutical industries: A comprehensive review. Bioresour. Technol. 2023, 372, 128661. [Google Scholar] [CrossRef]
- Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Sustainable aquaculture and animal feed from microalgae—Nutritive value and techno-functional components. Renew. Sustain. Energy Rev. 2021, 150, 111549. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- He, Y.; Lian, J.; Wang, L.; Tan, L.; Khan, F.; Li, Y.; Wang, H.; Rebours, C.; Han, D.; Hu, Q. Recovery of nutrients from aquaculture wastewater: Effects of light quality on the growth, biochemical composition, and nutrient removal of Chlorella sorokiniana. Algal Res. 2023, 69, 102965. [Google Scholar] [CrossRef]
- Xie, Z.; Meng, X.; Yu, S.; Jiang, L.; Pei, H. Continuous extraction and application potential of value-added products from a promising microalga Coelastrella sp. SDEC-28 for green microalgae-based industry. J. Clean. Prod. 2023, 428, 139364. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef]
- de Carvalho Silvello, M.A.; Gasparotto, G.A.; Ferreira, G.F.; Santos, L.O.; Fregolente, L.V.; Goldbeck, R. Nutrient optimization strategy to increase the carbohydrate content of Chlorella vulgaris and evaluation of hydrolysis and fermentation performance. BioEnergy Res. 2023, 16, 2058–2067. [Google Scholar] [CrossRef]
- Ajala, S.O.; Alexander, M.L. Evaluating the effects of agitation by shaking, stirring and air sparging on growth and accumulation of biochemical compounds in microalgae cells. Biofuels 2020, 13, 371–381. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Contreras, A.; García, F.; Molina, E.; Merchuk, J.C. Interaction between CO2-mass transfer, light availability, and hydrodynamic stress in the growth of Phaeodactylum tricornutum in a concentric tube airlift photobioreactor. Biotechnol. Bioeng. 1998, 60, 317–325. [Google Scholar] [CrossRef]
- Hodaifa, G.; Martínez, M.E.; Órpez, R.; Sánchez, S. Influence of hydrodynamic stress in the growth of Scenedesmus obliquus using a culture medium based on olive-mill wastewater. Chem. Eng. Process. Process Intensif. 2010, 49, 1161–1168. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, S.; Guo, X.; Wang, R.; Li, M.; Fan, C.; Wu, H. Effects of disturbance modes and carbon sources on the physiological traits and nutrient removal performance of microalgae (S. obliquus) for treating low C/N ratio wastewater. Chemosphere 2024, 347, 140672. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Sarıyıldız, S.; Cholakov, R.; Nalbantsoy, A.; Baler, B.; Aslan, E.; Düzel, A.; Sargın, S.; Göksungur, Y.; Kışla, D. A novel Lactiplantibacillus plantarum strain: Probiotic properties and optimization of the growth conditions by response surface methodology. World J. Microbiol. Biotechnol. 2024, 40, 66. [Google Scholar] [CrossRef]
- Karmainski, T.; Dielentheis-Frenken, M.R.E.; Lipa, M.K.; Phan, A.N.T.; Blank, L.M.; Tiso, T. High-quality physiology of Alcanivorax borkumensis SK2 producing glycolipids enables efficient stirred-tank bioreactor cultivation. Front. Bioeng. Biotechnol. 2023, 11, 1325019. [Google Scholar] [CrossRef]
- Uyar, B.; Ali, M.D.; Uyar, G.E.O. Design parameters comparison of bubble column, airlift and stirred tank photobioreactors for microalgae production. Bioprocess Biosyst. Eng. 2024, 47, 195–209. [Google Scholar] [CrossRef]
- Yaqoubnejad, P.; Rad, H.A.; Taghavijeloudar, M. Development a novel hexagonal airlift flat plate photobioreactor for the improvement of microalgae growth that simultaneously enhance CO2 bio-fixation and wastewater treatment. J. Environ. Manag. 2021, 298, 113482. [Google Scholar] [CrossRef]
- Rezvani, F.; Rostami, K. Photobioreactors for utility-scale applications: Effect of gas–liquid mass transfer coefficient and other critical parameters. Environ. Sci. Pollut. Res. 2023, 30, 76263–76282. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, G.; Chen, J.; Wang, H.; Yuan, L.; Han, D.; Hu, Q.; Jin, H. Heterotrophically ultrahigh-cell-density cultivation of a high protein-yielding unicellular alga Chlorella with a novel nitrogen-supply strategy. Front. Bioeng. Biotechnol. 2021, 9, 774854. [Google Scholar] [CrossRef]
- Silva, H.J.; Cortifas, T.; Ertola, R.J. Effect of hydrodynamic stress on dunaliella growth. J. Chem. Technol. Biotechnol. 1987, 40, 41–49. [Google Scholar] [CrossRef]
- Sobczuk, T.M.; Camacho, F.G.; Grima, E.M.; Chisti, Y. Effects of agitation on the microalgae Phaeodactylum tricornutum and Porphyridium cruentum. Bioprocess Biosyst. Eng. 2006, 28, 243–250. [Google Scholar] [CrossRef]
- García Camacho, F.; Contreras Gómez, A.; Mazzuca Sobczuk, T.; Molina Grima, E. Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem. 2000, 35, 1045–1050. [Google Scholar] [CrossRef]
- Yu, Z.; Song, M.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. The effects of combined agricultural phytohormones on the growth, carbon partitioning and cell morphology of two screened algae. Bioresour. Technol. 2017, 239, 87–96. [Google Scholar] [CrossRef]
- Xie, Z.; Pei, H.; Zhang, L.; Yang, Z.; Nie, C.; Hou, Q.; Yu, Z. Accelerating lipid production in freshwater alga Chlorella sorokiniana SDEC-18 by seawater and ultrasound during the stationary phase. Renew. Energy 2020, 161, 448–456. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Z.; Jiang, L.; Hou, Q.; Xie, Z.; Ma, M.; Yu, S.; Pei, H. Monosodium glutamate wastewater assisted seawater to increase lipid productivity in single-celled algae. Renew. Energy 2021, 179, 1793–1802. [Google Scholar] [CrossRef]
- Tagliaferro, G.V.; Filho, H.J.I.; Chandel, A.K.; da Silva, S.S.; Silva, M.B.; Santos, J.C. Effect of nitrogen concentration on the production and composition of Chlorella minutissima biomass in a batch bubble-tank photobioreactor. Biomass Convers. Biorefin. 2023, 14, 23545–23555. [Google Scholar] [CrossRef]
- Verma, R.; Kumar, R.; Mehan, L.; Srivastava, A. Modified conventional bioreactor for microalgae cultivation. J. Biosci. Bioeng. 2018, 125, 224–230. [Google Scholar] [CrossRef]
- Deprá, M.C.; Mérida, L.G.R.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. A new hybrid photobioreactor design for microalgae culture. Chem. Eng. Res. Des. 2019, 144, 1–10. [Google Scholar] [CrossRef]
- Janoska, A.; Barten, R.; de Nooy, S.; van Rijssel, P.; Wijffels, R.H.; Janssen, M. Improved liquid foam-bed photobioreactor design for microalgae cultivation. Algal Res. 2018, 33, 55–70. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, Z.; Gu, Y.; Shan, Y.; Tang, T. Investigate the cross-flow flat-plate photobioreactor for high-density culture of microalgae. Asia-Pac. J. Chem. Eng. 2018, 13, 2247. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Q.; Xue, S.; Sun, Z.; Wu, X.; Wang, Z.; Lu, Y.; Cong, W. A novel low-cost thin-film flat plate photobioreactor for microalgae cultivation. Biotechnol. Bioprocess Eng. 2016, 21, 103–109. [Google Scholar] [CrossRef]
- Yang, Z.; Pei, H.; Han, F.; Wang, Y.; Hou, Q.; Chen, Y. Effects of air bubble size on algal growth rate and lipid accumulation using fine-pore diffuser photobioreactors. Algal Res. 2018, 32, 293–299. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, H.; Chen, S.; Jiang, L.; Hou, Q.; Yang, Z.; Yu, Z. Salinity-induced cellular cross-talk in carbon partitioning reveals starch-to-lipid biosynthesis switching in low-starch freshwater algae. Bioresour. Technol. 2018, 250, 449–456. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- González López, C.V.; Cerón García, M.d.C.; Acién Fernández, F.G.; Segovia Bustos, C.; Chisti, Y.; Fernández Sevilla, J.M. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef]
- Ma, M.; Yu, Z.; Jiang, L.; Hou, Q.; Xie, Z.; Liu, M.; Yu, S.; Pei, H. Alga-based dairy wastewater treatment scheme: Candidates screening, process advancement, and economic analysis. J. Clean. Prod. 2023, 390, 136105. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakamoto, K.; Yamanaka, N.; Wang, L.; Yoshikawa, K. Light acclimation of needle pigment composition in Sabina vulgaris seedlings under nurse plant canopy. J. Arid Environ. 2006, 67, 403–415. [Google Scholar] [CrossRef]
- Arora, N.; Patel, A.; Pruthi, P.A.; Pruthi, V. Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour. Technol. 2016, 213, 79–87. [Google Scholar] [CrossRef]
- Nakajima, Y.; Ueda, R. Improvement of photosynthesis in dense microalgal suspension by reduction of light harvesting pigments. J. Appl. Phycol. 1997, 9, 503–510. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.I. Chlorophyll and carotenoid composition in leaves of Euonymus kiautschovicus acclimated to different degrees of light stress in the field. Aust. J. Plant Physiol. 1996, 5, 649–659. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Yang, J.; Zhou, Y.; Wang, X.; Dou, S.; Li, L.; Liu, G.; Yang, M. Effect of sulfur limitation strategies on glucose-based carbohydrate production from Chlorella Sorokiniana. Renew. Energy 2022, 200, 449–456. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Pei, H.; Pan, J.; Jiang, L.; Hou, Q.; Han, F. Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew. Energy 2018, 115, 276–287. [Google Scholar] [CrossRef]
- Demirel, Z.; Imamoglu, E.; Dalay, M.C. Growth kinetics of nanofrustulum shiloi under different mixing conditions in flat-plate photobioreactor. Braz. Arch. Biol. Technol. 2020, 63, e20190201. [Google Scholar] [CrossRef]
- Anjos, M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A.; Dragone, G. Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 2013, 139, 149–154. [Google Scholar] [CrossRef]
- Feng, C.; Johns, M.R. Effect of C/N ratio and aeration on the fatty-acid composition of heterotrophic Chlorella sorokiniana. J. Appl. Phycol. 1991, 3, 203–209. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Mimouni, V.; Ulmann, L.; Tremblin, G. Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. J. Exp. Mar. Biol. Ecol. 2009, 369, 136–143. [Google Scholar] [CrossRef]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology; John Wiley & Sons: Oxford, UK, 2013. [Google Scholar]
- Pancha, I.; Chokshi, K.; Maurya, R.; Trivedi, K.; Patidar, S.K.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 189, 341–348. [Google Scholar] [CrossRef]
- Ugya, A.Y.; Imam, T.S.; Li, A.; Ma, J.; Hua, X. Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production: A mini review. Chem. Ecol. 2019, 36, 174–193. [Google Scholar] [CrossRef]
- Hazeem, L.J.; Yesilay, G.; Bououdina, M.; Perna, S.; Cetin, D.; Suludere, Z.; Barras, A.; Boukherroub, R. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Mar. Pollut. Bull. 2020, 156, 111278. [Google Scholar] [CrossRef]
- Katsaros, C.; Karyophyllis, D.; Galatis, B. Cytoskeleton and morphogenesis in brown algae. Ann Bot 2006, 97, 679–693. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- Benavides, A.M.S.; Torzillo, G.; Kopecky, J.; Masojídek, J. Productivity and biochemical composition of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in tubular photobioreactors and open ponds. Biomass Bioenergy 2013, 54, 115–122. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef]
- Williamson, E.; Ross, I.L.; Wall, B.T.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2024, 29, 370–382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; He, Y.; Zhao, T.; Zhao, Y.; Yu, Z.; Pei, H. Enhanced Production of Microalgal Metabolites Through Aeration Coupled with Stirring. Sustainability 2024, 16, 9001. https://doi.org/10.3390/su16209001
Wu Y, He Y, Zhao T, Zhao Y, Yu Z, Pei H. Enhanced Production of Microalgal Metabolites Through Aeration Coupled with Stirring. Sustainability. 2024; 16(20):9001. https://doi.org/10.3390/su16209001
Chicago/Turabian StyleWu, Yangyingdong, Yuqing He, Tuo Zhao, Yang Zhao, Ze Yu, and Haiyan Pei. 2024. "Enhanced Production of Microalgal Metabolites Through Aeration Coupled with Stirring" Sustainability 16, no. 20: 9001. https://doi.org/10.3390/su16209001
APA StyleWu, Y., He, Y., Zhao, T., Zhao, Y., Yu, Z., & Pei, H. (2024). Enhanced Production of Microalgal Metabolites Through Aeration Coupled with Stirring. Sustainability, 16(20), 9001. https://doi.org/10.3390/su16209001






