Agroecological Nutrient Management Strategy for Attaining Sustainable Rice Self-Sufficiency in Indonesia
Abstract
1. Introduction
2. Paddy Rice Soils and Their Nutrient Status
2.1. Paddy Rice Soils
2.2. Essential Nutrients
2.3. Nutrient Status of Indonesian Paddy Fields
3. Agroecological Nutrient Management
3.1. Nitrogen, Phosphorous and Potassium Fertilization
3.2. Micronutrient Fertilization
3.3. Enhanced Use of Organic Fertilizers
3.3.1. Organic Matter
3.3.2. Biofertilizers
3.3.3. N-Fixing Biofertilizer
3.3.4. Phosphate-Solubilizing Microbes
3.3.5. K-Solubilizing Microbes
3.3.6. Bioorganic Fertilizers
3.4. Other Locally Exploitable Nutrient Sources
3.5. Improved Water Management Systems
3.6. Farming Systems and Crop Rotation
4. Improvement in Agroecological Functions
4.1. Mitigation and Adaptation to Climate Change
4.1.1. Mitigation
4.1.2. Adaptation
4.1.3. Mitigation and Adaptation Synergy
4.2. Nutrient Recycling
4.3. Water Conservation
4.4. Biodiversity Conservation
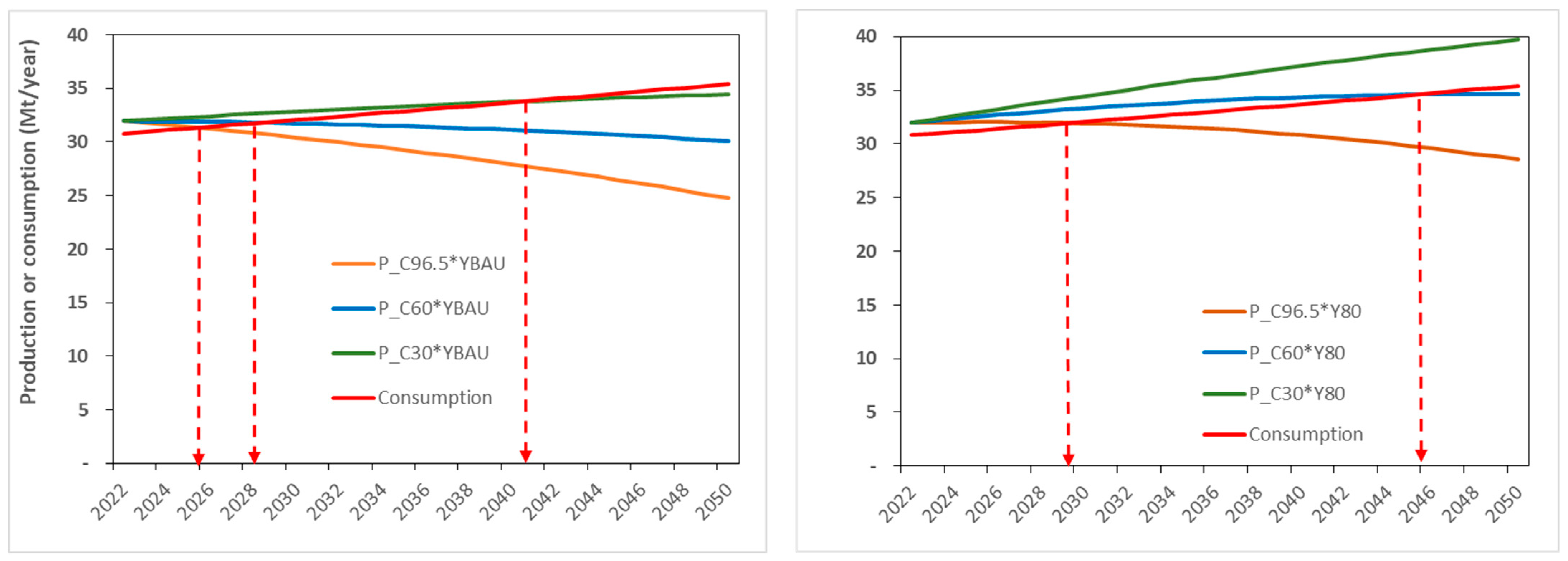
5. Intensification and Control of Paddy Field Conversion
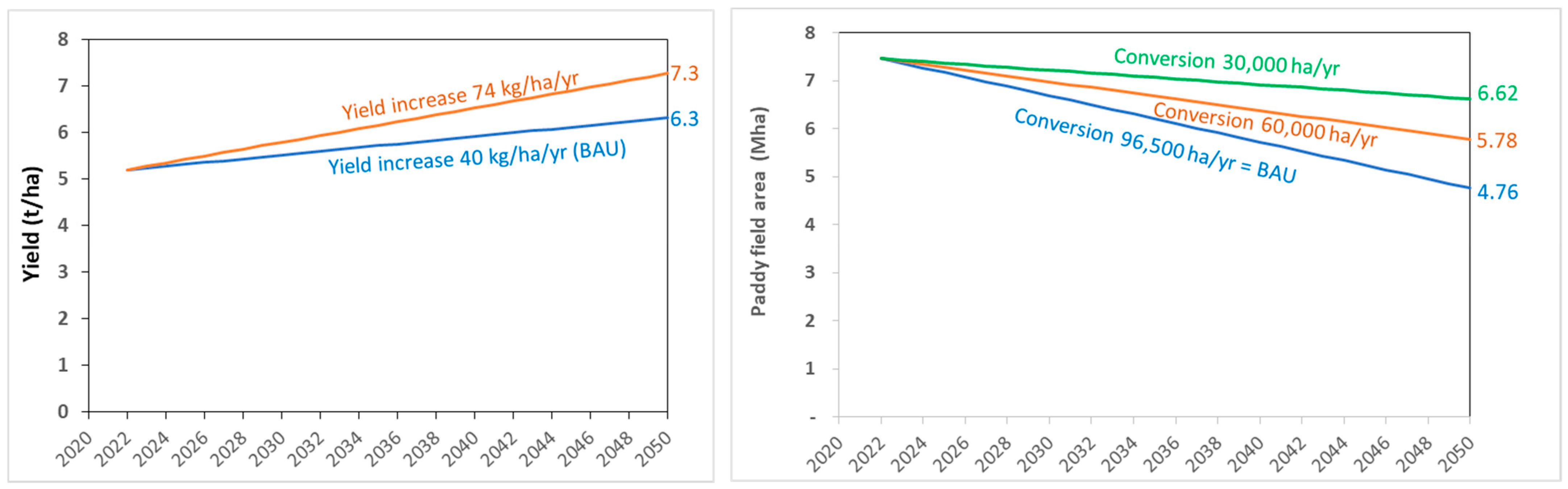
- Rice yield increase scenario:
- Y_BAU = The yield, starting at 5.2 t ha−1 in 2022, increases 40 kg ha−1 yr−1 in accordance with the historical (business as usual, BAU) trend (see the Central Bureau of Statistics for more information, https://bps.go.id, accessed on 10 January 2023).
- Y_80% = The yield, starting at 5.2 t ha−1 in 2022, increases 74 kg ha−1 yr−1 to attain 80% of the yield potential of 9.1 t ha−1 (7.3 t ha−1) in 2050 (Yuan et al., 2022) [14].
- Paddy field conversion scenario:
- C96.5 = The business-as-usual (BAU) level of paddy field conversion of 96,500 ha year−1.
- C60 = The medium level of paddy field conversion of 60,000 ha year−1.
- C30 = The optimistic low level of paddy field conversion of 30,000 ha year−1.
6. Challenges in Implementing Agroecological Management
- Financial incentives in the form of subsidies and/or financial support for farmers transitioning to organic fertilizers.
- Training and extension services to inform farmers about the benefits of organic fertilizers, proper application methods, and their impact on soil health and crop yield.
- Training on techniques to make compost and the use of local nutrient sources.
- Research and development funding aimed at improving the effectiveness and efficient production of organic fertilizers, including on the simpler storage and longer viability of biofertilizers.
- Regulatory support that favors the use of organic fertilizers. This includes setting the standards for organic fertilizers and certification programs to ensure quality.
- Investing in infrastructure such as composting and biochar facilities and organic waste management systems to facilitate the production and distribution of organic fertilizers.
- The establishment of pilot programs at selected farmers’ fields to demonstrate the effectiveness of organic fertilizers is essential. These pilots should aim to demonstrate the beneficial effects of organic fertilizers, in addition to showing the ideal combination of synthetic and organic fertilizers.
| Agroecological Indicator | Improved Management System | Challenges in Implementation | Strategies to Overcome the Challenges |
|---|---|---|---|
| Lower methane (CH4) emissions | Intermittent irrigation, including implementation of the System of Rice Intensification | Water may be excessive when draining is needed or deficient when flooding is needed | Improved irrigation and drainage systems |
| Use of low CH4 emission varieties | Low-emission varieties may not be the high-yielding or high-quality varieties; hence, they are not preferable | Long-term genetic program to produce low-emission, high-yielding and high-quality rice varieties | |
| Lower nitrous oxide (N2O) emissions | Improve efficiency of nitrogen (N) fertilization | Farmers are traditionally happy with quick response of N fertilizers | Improved extension and wider use of leaf color chart for N application efficiency |
| Higher availability of essential nutrients | Use inorganic fertilizers based on local specific or test-kit-assisted recommendations supplemented by organic and natural fertilizers | Unaffordability and/or unavailability of fertilizers at the right amount and the right place |
|
| Improved nutrient balance, especially by higher potassium (K) application | Increase application of K from inorganic and organic fertilizers |
|
|
| Increased soil carbon stock | Application of biochar |
|
|
| Application of organic fertilizer | Scarcity of feedstock because of off-farm utilization | Optimize the use of other organic fertilizers, such as manure | |
| Improved soil health and nutrient availability by microbes | Inoculation of microbes (N fixing, solubilizing, and antagonistic microbes) | Quick expiration of biofertilizers, causing their ineffectiveness | Intensive technical guidance of handling and use of inoculants |
7. Conclusions and Recommendation
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization); International Fund for Agricultural Development; United Nations Children’s Fund; World Food Programme; World Health Organization. The State of Food Security and Nutrition in the World 2019: Safeguarding Against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/ca5162en/ca5162en.pdf (accessed on 10 January 2023).
- Lestari, P.; Mulya, K.; Utami, D.W.; Satyawan, D.; Supriadi, M. Strategies and Technologies for the Utilization and Improvement of Rice; Indonesian Agency for Agricultural Research and Development: Jakarta, Indonesia, 2020. [Google Scholar]
- BPS (Badan Pusat Statistik). Statistical Yearbook of Indonesia 2023; BPS: Jakarta, Indonesia, 2023. [Google Scholar]
- Grassini, P.; van Bussel, L.G.J.; Van Wart, J.; Wolf, J.; Claessens, L.; Yang, H.; Boogaard, H.; de Groot, H.; van Ittersum, M.K.; Cassman, K.G. How good is good enough? Data requirements for reliable crop yield simulations and yield-gap analysis. Field Crops Res. 2015, 177, 49–63. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.F.; Ferrer, A.; Peigne, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- de Molina, M.G.; Casado, G.I.G. Agroecology and ecological intensification. A discussion from a metabolic point of view. Sustainability 2017, 9, 86. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Fraga-Corral, M.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Chapter 2—Approaches for sustainable food production and consumption systems. In Future Foods; Rajeev Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 23–38. [Google Scholar] [CrossRef]
- Martínez-Camacho, Y.D.; Negrete-Yankelevich, S.; Maldonado-Mendoza, I.E.; Núñez-de la Mora, A.; Amescua-Villela, G. Agroecological management with intra-and interspecific diversification as an alternative to conventional soil nutrient management in family maize farming. Agroecol. Sustain. Food Syst. 2022, 46, 364–391. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Snapp, S.S. Advancing the science and practice of ecological nutrient management for smallholder farmers. Front. Sustain. Food Syst. 2022, 6, 921216. [Google Scholar] [CrossRef]
- Soane, B.D.; Ball, B.C.; Arvidsson, J.; Basch, G.; Moreno, F.; Roger-Estrade, J. No-till in northern, western and south-western Europe: A review of problems and opportunities for crop production and the environment. Soil Till. Res. 2012, 118, 66–87. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. B 2008, 363, 447–465. [Google Scholar] [CrossRef]
- Balmford, A.; Amano, T.; Bartlett, H. The environmental costs and benefits of high-yield farming. Nat. Sustain. 2018, 1, 477–485. [Google Scholar] [CrossRef]
- Dawe, D. Can Indonesia trust the world rice market? Bull. Indones. Econ. Stud. 2008, 44, 115–132. [Google Scholar] [CrossRef]
- Yuan, S.; Stuart, A.M.; Laborte, A.G.; Rattalino Edreira, J.I.; Dobermann, A.; Kien, L.V.N.; Thúy, L.T.; Paothong, K.; Traesang, P.; Tint, K.M.; et al. Southeast Asia must narrow down the yield gap to continue to be a major rice bowl. Nat. Food 2022, 3, 217–226. [Google Scholar] [CrossRef]
- Liu, Y.; Yamauchi, F. Population density, migration, and the returns to human capital and land: Insights from Indonesia. Food Policy 2014, 48, 182–193. [Google Scholar] [CrossRef]
- Mulyani, A.; Kuncoro, D.; Nursyamsi, D.; Agus, F. Analisis konversi lahan sawah: Penggunaan data spasial resolusi tinggi memperlihatkan laju konversi yang mengkhawatirkan. J. Tan. Iklim. 2016, 40, 121–133. [Google Scholar]
- Andrade, J.F.; Cassman, K.G.; Rattalino Edreira, J.I.; Agus, F.; Bala, A.; Deng, N.; Grassini, P. Impact of urbanization trends on production of key staple crops. Ambio 2022, 51, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Agus, F.; Susilawati, H.L.; Surmaini, E. Strategies for Indonesia’s agricultural climate change adaptation and mitigation. J. Sumb. Daya. Lahan. Indo. 2022, 16, 67–81. [Google Scholar] [CrossRef]
- Mulyani, A.; Agus, F. Kebutuhan dan ketersediaan lahan cadangan untuk mewujudkan cita-cita Indonesia sebagai lumbung pangan dunia tahun 2045. Anal. Keb. Pert. 2018, 15, 1–17. [Google Scholar] [CrossRef]
- Mulyani, A. Analisis kapasitas produksi lahan sawah untuk ketahanan pangan nasional menjelang tahun 2045. J. Sumb. Lah. 2022, 16, 33–50. Available online: https://garuda.kemdikbud.go.id/documents/detail/2690966 (accessed on 6 July 2023).
- Agus, F.; Andrade, J.F.; Edreira, J.I.R.; Deng, N.; Purwantomo, D.; Agustiani, N.; Aristya, V.E.; Batubara, S.F.; Herniwati; Hosang, E.Y.; et al. Yield gaps in intensive rice-maize cropping sequences in the humid tropics of Indonesia. Field Crops Res. 2019, 237, 12–22. [Google Scholar] [CrossRef]
- Setyorini, D.; Widowati, L.R.; Rochayati, S. Teknologi Pengelolaan Hara Lahan Sawah Intensifikasi. In Lahan Sawah dan Teknologi Pengelolaannya; Pusat Penelitian Tanah dan Agroklimat: Bogor, Indonesia, 2005. [Google Scholar]
- Husnain, H.; Masunaga, T.; Wakatsuki, T. Field assessment of nutrient balance under intensive rice-farming systems, and its effects on the sustainability of rice production in Java Island, Indonesia. J. Agric. Food Environ. Sci. 2010, 4, 1–11. [Google Scholar]
- Hardjowigeno, S.; Subagyo, H.; Rayes, M. Morfologi dan Klasifikasi Tanah Sawah. In Tanah Sawah dan Teknologi Pengelolaannya; Pusat Penelitian Tanah dan Agroklimat Departemen Pertanian: Bogor, Indonesia, 2005. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.; Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2010. Available online: https://www.nrcs.usda.gov/resources/guides-and-instructions/keys-to-soil-taxonomy (accessed on 9 November 2022).
- Widowati, L.R.; Husnain; Kasno, A.L.; Las, I.; Sarwani, M.; Rochayati, S.; Suryani, E.; Setyorini, D.; Hartatik, W.; Subiksa, M.; et al. Dosis Pupuk N, P, K Untuk Tanaman Padi Pada Lahan Sawah (per Kecamatan); Indonesian Agency for Agricultural Research and Development, Ministry of Agriculture, Republic of Indonesia: Jakarta, Indonesia, 2021; Available online: http://repository.pertanian.go.id/bitstreams/7cf03ed5-9ed4-400d-95c3-37d3d252b8b9/download (accessed on 9 November 2022).
- Prasetyo, B.H. Genesis Tanah Sawah Bukaan Baru. In Tanah Sawah Bukaan Baru; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2007; pp. 25–52. [Google Scholar]
- Calvo, B.E.; Guendehou, S.; Limmeechokchai, B. Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change: Geneva, Swiss, 2019. [Google Scholar]
- Kyuma, K. Paddy Soil Science; Kyoto University Press: Kyoto, Japan, 2004. [Google Scholar]
- Kagawa, A. Foliar water uptake as a source of hydrogen and oxygen in plant biomass. Tree. Physiol. 2022, 42, 2153–2173. [Google Scholar] [CrossRef]
- Somani, L. Micronutrient for Soil and Plant Health; Agrotech Publishing Academy: Udaipur, India, 2008. [Google Scholar]
- Kumar, T.; Jashimuddin, N.M.; Shahjahan, K.H. The sustainable intensification of agroforestry in shifting cultivation areas of Bangladesh. Agrofor. Syst. 2016, 90, 405–416. [Google Scholar] [CrossRef]
- Brady, N.; Weil, R.R. The Nature and Properties of Soil; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Deb, D.L.; Sakal, R.; Datta, S. Fundamentals of Soil Science; Indian Society of Soil Science, Cambridge Printing Works: New Delhi, India, 2009. [Google Scholar]
- Janmohammadi, M.; Abdoli, H.; Sabaghnia, N. The effect of iron, zinc and organic fertilizer on yield of chickpea (Cicerartietinum L.) in mediterranean climate. Acta. Univ. Agric. Silvic. Mendelianae. Brun. 2018, 66, 49–60. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Yang, X. Effects of long-term inorganic and organic fertilization on soil micronutrient status. Comm. Soil. Sci. Plant. Anal. 2015, 46, 1778–1790. [Google Scholar] [CrossRef]
- Central Bureau of Statistics. Agricultural Statistics; Central Bureau of Statistics: Jakarta, Indonesia, 2020. [Google Scholar]
- Kasno, A.; Setyorini, D.; Widowati, L.R.; Rostaman, T. Evaluasi karakteristik, sumbangan hara K air irigasi dan jerami serta respon pemupukan hara kalium pada lahan sawah. J. Agric. 2021, 33, 189–198. Available online: https://ejournal.uksw.edu/agric/article/download/5180/1968/ (accessed on 10 January 2023).
- Sparks, D.L.; Huang, P.M. Physical Chemistry of Soil Potassium. In Potassium in Agriculture; Munson, R.D., Ed.; Soil Science Society of America: Madison, WI, USA, 1985. [Google Scholar]
- Kasno, A.; Setyorini, D.; Suastika, I.W. Pengelolaan hara terpadu pada lahan sawah tadah hujan sebagai upaya peningkatan produksi beras nasional. J. Sumb. Lah. 2020, 14, 15–20. [Google Scholar] [CrossRef]
- Rizzo, G.; Agus, F.; Batubara, S.F.; Andrade, J.F.; Edreira, J.I.R.; Purwantomo, D.K.G.; Anasiru, R.H.; Maintang; Marbun, O.; Ningsih, R.D.; et al. A farmer data-driven approach for prioritization of agricultural research and development: A case study for intensive crop systems in the humid tropics. Field Crop. Res. 2023, 297, 108942. [Google Scholar] [CrossRef]
- Setyorini, D. Uji Tanah Sebagai Dasar Penyusunan Rekomenasi Pemupukan; Balai Penelitian Tanah: Bogor, Indonesia, 2003. [Google Scholar]
- Irawan; Kasno, A.; Nurjaya. Financial benefits of using soil test kit of PUTS for determining dosage of lowland rice fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2020, 648, 012039. [Google Scholar] [CrossRef]
- Setyorini, D.; Adiningsih, J.S.; Rochayati, S. Uji Tanah Sebagai Dasar Rekomendasi Pemupukan. Seri Monograf Sumber Daya Tanah Indonesia; Balai Penelitian Tanah, Badan Litbang Pertanian: Bogor, Indonesia, 2003. [Google Scholar]
- Sukarman; Setyorini, D.; Ritung, S. Metode Percepatan Pemetaan Status Hara Lahan Sawah. In Prosiding Seminar Nasional Teknologi Pemupukan dan Pemulihan Lahan Terdegradasi; Indonesian Agency for Agricultural Research and Development, Ministry of Agriculture: Jakarta, Indonesia, 2012; pp. 141–150. [Google Scholar]
- Pangaribuan, S.M.; Supriadi, S.; Sarifuddi, S. Pemetaan status hara K, Ca, Mg tanah pada kebun kelapa sawit (Elaeis guineensis Jacq) di perkebunan rakyat Kecamatan Hutabayu Raja Kabupaten Simalungun. J. Agroekoteknologi Univ. Sumat. Utara 2013, 1, 987–995. [Google Scholar] [CrossRef]
- Virgawati, S.; Padmini, O.S.; Eko, P.M. Pemetaan NPK Tanah untuk Prediksi Rekomendasi Pemupukan Presisi Pada Tanaman Padi. In Prosiding Seminar Nasional Perhimpunan Teknik Pertanian Indonesia; International Workshop on Biomass Energy: Bogor, Indonesia, 2014; pp. 161–164. [Google Scholar]
- Reuter, D.J.; Robinson, J.B. Plant Analysis: An Interpretation Manual, 2nd ed.; CSIRO Publishing: Victoria, Australia, 1997. [Google Scholar]
- Dobermann, A.; Fairhurst, T.H. Nutrient Disorders and Nutrient Management; Potash and Phosphate Institute of Canada and International Rice Research Institute: Singapore, 2000; Available online: http://books.irri.org/9810427425_content.pdf (accessed on 11 May 2023).
- Grassini, P. (University of Nebraska-Lincoln, Lincoln, NE, USA). Personal communication, 2023.
- Bennet, W.F. Plant Nutrient Utilization And Diagnostic Plant Symptoms. In Nutrient Deficiencies And Toxicities in Crop Plant; Bennett, W.F., Ed.; American Phytopathological Society: St. Paul, MN, USA, 1993; pp. 1–7. [Google Scholar]
- Liew, Y.A.; Omar, S.S.; Husni, M.H.A. Effects of micronutrient fertilizers on the production of MR 219 rice (Oryza sativa L.). Malay. J. Soil. Sci. 2010, 14, 71–82. [Google Scholar]
- Al-Jabri, M.; Soepartini, D.; Ardi, S. Status hara Zn dan pemupukannya di lahan sawah. In Prosiding Lokakarya Nasional Efisiensi Penggunaan Pupuk; Pusat Penelitian Tanah dan Agroklimat: Bogor, Indonesia, 1990; pp. 427–464. [Google Scholar]
- Shukla, R.; Sharma, Y.K.; Shukla, A.K. Molecular mechanism of nutrient uptake in plants. Inter. J. Curr. Res. Aca. Rev. 2014, 2, 142–145. Available online: http://www.ijcrar.com/vol-2-12/Rajni%20Shukla,%20et%20al.pdf (accessed on 11 May 2023).
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Plant. Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil. Sci. Plant. Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1–2, 100007. [Google Scholar] [CrossRef]
- Khan, Z.I.; Hussain, M.I.; Zafar, A. Ecological risk assessment and bioaccumulation of trace element, copper, in wheat varieties irrigated with non-conventional water resources in a semi-arid tropics. Agric. Water Manag. 2022, 269, 107711. [Google Scholar] [CrossRef]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Saha, B.; Ray, M.; Mukhopadhyay, S.K.; Halder, P.; Das, A. Integrated nutrient management (INM) on yield trends and sustainability, nutrient balance and soil fertility in a long-term (30 years) rice-wheat system in the Indo-Gangetic plains of India. J. Plant Nut. 2018, 41, 2365–2375. [Google Scholar] [CrossRef]
- Agus, F.; Setyorini, D.; Hartatik, W.; Lee, S.M.; Sung, J.K.; Shin, J.H. Nutrient balance and vegetable crop production as affected by different sources of organic fertilizers. Korean J. Soil Sci. Fertil. 2009, 42, 1–13. [Google Scholar]
- Hartatik, W.; Setyorini, D. Validasi rekomendasi pemupukan NPK dan pupuk organik pada padi sawah. In Prosiding seminar Sumberdaya Lahan Pertanian; Balai Besar Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2008; pp. 275–283. [Google Scholar]
- Doberman, A.; Fairhurst, T.H. Rice straw management. Better Crops Int. 2002, 16, 7–11. [Google Scholar]
- Cao, Y.; He, Z.; Zhu, T. Organic-C quality as a key driver of microbial nitrogen immobilization in soil: A meta-analysis. Geoderma 2021, 383, 114784. [Google Scholar] [CrossRef]
- Tadesse, T.; Dechassa, N.; Bayu, W.; Gebeyehu, S. Effects of farmyard manure and inorganic fertilizer application on soil physico-chemical properties and nutrient balance in rain-fed lowland rice ecosystem. Sci. Res. 2013, 4, 309–316. [Google Scholar] [CrossRef]
- Ando, K.; Yamaguchi, N.; Kasuya, M.; Oga, T.; Ohashi, Y.; Taki, K. Long-term (nearly a century) effects of fertilizer, lime and rice straw compost application on active aluminum and iron and available phosphorus in paddy fields. Geoderma 2022, 424, 115992. [Google Scholar] [CrossRef]
- Liao, P.; Huang, S.; Van Gestel, N.C.; Zeng, Y.; Wu, Z.; van Groenigen, K.J. Liming and straw retention interact to increase nitrogen uptake and grain yield in a double rice-cropping system. Field Crops Res. 2018, 216, 217–224. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G.; Valori, F. Microbial diversity and microbial activity in the rhizosphere. Cienc. Suelo 2007, 25, 89–97. Available online: http://www.scielo.org.ar/scielo.php?pid=S1850-20672007000100011&script=sci_arttext (accessed on 11 May 2023).
- Sarkar, D.; Sankar, A.; Devika, O.S. Optimizing nutrient use efficiency, productivity, energetics, and economics of red cabbage following mineral fertilization and biopriming with compatible rhizosphere microbes. Sci. Rep. 2021, 11, 15680. [Google Scholar] [CrossRef] [PubMed]
- Burris, R.H.; Roberts, G.P. Biological nitrogen fixation. Annu. Rev. Nutr. 1993, 13, 317–335. [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Roger, P.A. Nitrogen Fixation in Wetland Rice Field. In Current Developments in Biological Nitrogen Fixation; Subba-Rao, N.S., Ed.; Oxford and IBH: New Delhi, India, 1984; pp. 237–276. [Google Scholar]
- Leakey, R.R.B. The role of trees in agroecology and sustainable agriculture in the tropics. Ann. Rev. Phyto 2014, 52, 113–133. [Google Scholar] [CrossRef]
- Razie, F.; Anas, I. Effect of Azotobacter and Azospirillum on growth and yield of rice grown on tidal swamp rice field in South Kalimantan. J. Ilmu Tanah Lingkung. 2008, 10, 41–45. [Google Scholar] [CrossRef]
- Iswandi, A.; Hazra, F.; Rakhmadina, V.D. Potensi oligochitosan, vitazyme dan biofertilizer dalam meningkatkan pertumbuhan dan produksi padi (Oryza sativa L.). J. Ilmu Tanah Lingkung. 2013, 15, 5–11. [Google Scholar] [CrossRef]
- Chatterjee, A.; Singh, S.; Agrawal, C. Role of algae as a biofertilizer. In Algal Green Chemistry Recent Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 189–200. [Google Scholar] [CrossRef]
- Pimratch, S.; Butsat, S.; Kesmala, T. Application of blue-green algae and mineral fertilizers to direct seeding lowland rice. Sci. Asia. 2015, 41, 305–314. [Google Scholar] [CrossRef]
- Roger, P.A.; Kulasooriya, S. Blue Green Algae and Rice; International Rice Research Institute: Los Banos, Philippines, 1980; Available online: http://books.irri.org/971104028X_content.pdf (accessed on 11 May 2023).
- Setiawati, M.R.; Prayoga, M.K.; Stöber, S.; Adinata, K.; Simarmata, T. Performance of rice paddy varieties under various organic soil fertility strategies. Open Agric. 2020, 5, 509–515. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Fitriatin, B.N.; Suryatmana, P.; Simarmata, T. Aplikasi pupuk hayati dan azolla untuk mengurangi dosis pupuk anorganik dan meningkatkan N, P, C organik tanah, dan N, P tanaman, serta hasil padi sawah. J. Agroekotek 2020, 12, 63–76. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Damayani, M.; Herdiyantoro, D.; Suryatmana, P.; Anggraini, D.; Khumairah, F.H. The application dosage of Azolla pinnata in fresh and powder form as organic fertilizer on soil chemical properties, growth and yield of rice plant. AIP Conf. Proc. 2018, 1927, 030017. [Google Scholar]
- Bello, S.K.; Shobayo, A.B.; Ibrahim, M.M.; Alasinrin, S.Y.; Aliyu, I.A.; Yusuf, A.A. Biological nitrogen fixation contributes to soil productivity in tropical agroecologies. Niger. J. Soil Science 2021, 31, 1. [Google Scholar]
- Prasad, R.C.; Prasad, B. Blue green algal inoculation for rice productivity and soil fertility in Nepal. J. Nep. Biotech. Assoc. 2003, 1, 17–20. [Google Scholar]
- Mishra, U.; Pabbi, S. Cyanobacteria: A potential biofertilizer for rice. Resonance 2004, 9, 6–10. Available online: https://link.springer.com/article/10.1007/BF02839213 (accessed on 10 January 2023). [CrossRef]
- Prasad, R.C. Studies on Screening and Biology of Some of the Potential Blue Green Algae (Cyanobacteria) as a Source of Biofertilizers from the Rice Fields of Bagmati and Narayani Zones of Nepal. Ph.D. Thesis, Tribhuvan University, Kathmandu, Nepal, 2005. [Google Scholar]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.S.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta. Immune. Hung 2009, 56, 263–284. [Google Scholar] [CrossRef]
- Puspitawati, M.D.; Anas, I. Pemanfaatan mikrob pelarut fosfat untuk mengurangi dosis pupuk P anorganik pada padi sawah. J. Agron. Indo. 2013, 41, 188–195. Available online: https://journal.ipb.ac.id/index.php/jurnalagronomi/article/view/8095 (accessed on 3 January 2023).
- Sindhu, S.S.; Parmar, P.; Phour, M.; Sehrawat, A. Potassium Solubilizing Microorganisms (KSMs) and its Effect on Plant Growth Improvement. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–185. [Google Scholar]
- Prajapati, K.B.; Modi, H.A. Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIBTech. J. Microbiol. 2012, 1, 8–14. [Google Scholar]
- Megarani, M.M. Subtitusi Sebagian Pupuk Kimia Dengan Pupuk Organik Hayati Pada Budidaya Padi SRI (System of Rice Intensification); Dissertation, Institut Pertanian Bogor: Bogor, Indonesia, 2016; Available online: https://repository.ipb.ac.id/handle/123456789/85147 (accessed on 10 January 2023).
- Shaaban, M.; Wu, L.; Peng, Q.A.; van Zwieten, L.; Chhajro, M.A.; Wu, Y.; Lin, S.; Ahmed, M.M.; Khalid, M.S.; Abid, M.; et al. Influence of ameliorating soil acidity with dolomite on the priming of soil C content and CO2 emission. Environ. Sci. Pollut. Res. 2017, 24, 9241–9250. [Google Scholar] [CrossRef]
- Zhou, Z.-M. Rice yields and soil nutrients response to liming method and dosages in field cultivation. Sains Malays. 2022, 51, 83–94. [Google Scholar] [CrossRef]
- Azman, E.A.; Jusop, S.; Ishak, C.F. Increasing rice production using different lime sources on an acid sulphate soil in Merbok, Malaysia. J. Trop. Agri. Sci. 2014, 37, 223–247. [Google Scholar]
- Duart, V.M.; Garbuio, F.J.; Caires, E.F. Does direct-seeded rice performance improve upon lime and phosphogypsum use? Soil Tillage Res. 2021, 212, 105055. [Google Scholar] [CrossRef]
- Soratto, R.P.; Crusciol, C.A. Dolomite and phosphogypsum surface application effects on annual crops nutrition and yield. J. Agro. 2008, 100, 261–270. [Google Scholar] [CrossRef]
- He, Y.-B.; Huang, D.-Y.; Zhu, Q.-H.; Wang, S.; Liu, S.-L.; He, H.-B.; Zhu, H.-H.; Xu, C. A three-season field study on the in-situ remediation of Cd-contaminated paddy soil using lime, two industrial by-products, and a low-Cd-accumulation rice cultivar. Ecotoxicol. Environ. Saf. 2017, 136, 135–141. [Google Scholar] [CrossRef]
- Duan, M.M.; Wang, S.; Huang, D.Y. Effectiveness of simultaneous applications of lime and zinc/iron foliar sprays to minimize cadmium accumulation in rice. Ecotoxicol. Environ. Saf. 2018, 165, 510–515. [Google Scholar] [CrossRef]
- Xie, T.; Li, Y.; Dong, H.; Liu, W.; Wang, M.; Wang, G. Effects and mechanisms on the reduction of lead accumulation in rice grains through lime amendment. Ecotoxicol. Environ. Saf. 2019, 173, 266–272. [Google Scholar] [CrossRef]
- Kong, L.; Guo, Z.; Peng, C.; Xiao, X.; He, Y. Factors influencing the effectiveness of liming on cadmium reduction in rice: A meta-analysis and decision tree analysis. Sci. Total. Environ. 2021, 779, 146477. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, H.; Li, S.; Zhang, Z.; Liao, Y.; Lu, Y.; Zhou, G.; Gao, S.; Nie, J.; Cao, W. Co-utilizing milk vetch, rice straw, and lime reduces the Cd accumulation of rice grain in two paddy soils in South China. Sci. Total. Environ. 2022, 806, 150622. [Google Scholar] [CrossRef]
- Murtaza, G.; Ditta, A.; Ullah, N.; Usman, M.; Amer, Z. Biochar for the management of nutrient impoverished and metal contaminated soils: Preparation, applications, and prospects. J. Soil. Sci. Plant. Nutr. 2021, 21, 2191–2213. [Google Scholar] [CrossRef]
- Sukartono; Utomo, W.H.; Kusuma, Z.; Nugroho, W.H. Soil fertility status, nutrient uptake, and maize (Zea mays L.) yield following biochar and cattle manure application on sandy soils of Lombok, Indonesia. J. Trop. Agric. 2011, 49, 47–52. Available online: https://jtropag.kau.in/index.php/ojs2/article/view/236/236 (accessed on 8 March 2023).
- Masulili, A.; Utomo, W.H.; Syechfani, M.S. Rice husk biochar for rice based cropping system in acid soil 1: The characteristics of rice husk biochar and sulfate soils and rice growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39. Available online: https://www.ccsenet.org/journal/index.php/jas/article/view/4369 (accessed on 8 March 2023). [CrossRef]
- Lakitan, B.; Alberto, A.; Lindiana, L.; Kartika, K.; Herlinda, S.; Kurnianingsih, A. The benefits of biochar on rice growth and yield in tropical riparian wetland, South Sumatra, Indonesia. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 111–126. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S. Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Eco. Letter. 2005, 8, 1075–1087. [Google Scholar] [CrossRef]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Ben Fekih, I.; Ditta, A.; Xing, S. Role of biochar and plant growth promoting rhizobacteria to enhance soil carbon sequestration: A review. Environ. Mon. Assess. 2019, 191, 251. [Google Scholar] [CrossRef]
- Leonardos, O.H.; Theodoro, S.H.; Assad, M.L. Remineralization for sustainable agriculture: A tropical perspective from a Brazilian viewpoint. Nutr. Cycl. Agroecosyst. 2000, 56, 3–9. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.; Gupta, V.K.; Singh, J.S. The effect of rice husk biochar on soil nutrient status, microbial biomass and paddy productivity of nutrient poor agriculture soils. Catena 2018, 171, 485–493. [Google Scholar]
- Dong, D.; Feng, Q.; Mcgrouther, K.; Yang, M.; Wang, H.; Wu, W. Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. J. Soils Sediments 2015, 15, 153–162. [Google Scholar] [CrossRef]
- Theodoro, S.H.; Leonardos, O.H. The use of rocks to improve family agriculture in Brazil. Acad. Bras. Ciências 2006, 78, 721–730. [Google Scholar] [CrossRef]
- Samobor, V.; Horvat, D.; Kesteli, B.; Jost, M. Effect of stone meal on control of seed-borne diseases in wheat. Agron. Glas. Glas. Hrvat. Agron. Društva 2008, 70, 563–572. Available online: https://hrcak.srce.hr/33136 (accessed on 10 January 2023).
- Wahyudi, A.; Wahyudi, T. A literature study of benefiting K-bearing silicate rocks. Indo. Min. J. 2013, 6, 101–110. Available online: https://jurnal.tekmira.esdm.go.id/index.php/imj/article/download/428/293 (accessed on 10 January 2023).
- Eddy, H.R.; Muksin, I. Karakteristik batuan pembawa kalium di Kecamatan Cluwak, Kabupaten Pati, Provinsi Jawa Tengah. Bul. Sumb. Geol. 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Muksin, I.; Karangan, C. Prospeksi batuan pembawa kalium Kabupaten Pati, Provinsi Jawa Tengah. In Prosiding No. 11 Hasil Kerja Lapangan Tahun 2016, Buku 2. Bidang Mineral; Pusat Sumber Daya Mineral Batubara dan Panas Bumi: Bandung, Indonesia, 2017; pp. 245–252. [Google Scholar]
- Valentin, C.; Agus, F.; Alamban, R. Runoff and sediment losses from 27 upland catchments in Southeast Asia: Impact of rapid land use changes and conservation practices. Agric. Ecosyst. Environ. 2008, 128, 225–238. [Google Scholar] [CrossRef]
- Tran, D.N.L.; Nguyen, T.D.; Pham, T.; Ranula, J.R.F.; Nguyen, T.A. Improving irrigation water use efficiency of robusta coffee (Coffea canephora) production in Lam Dong Province, Vietnam. Sustainability 2021, 13, 6603. [Google Scholar] [CrossRef]
- Fuglie, K.O. Sources of growth in Indonesian agriculture. J. Prod. Anal. 2010, 33, 225–240. [Google Scholar] [CrossRef]
- Devkota, K.P.; Pasuquin, E.; Elmido-Mabilangan, A. Economic and environmental indicators of sustainable rice cultivation: A comparison across intensive irrigated rice cropping systems in six Asian countries. Ecol. Indic. 2019, 105, 199–214. [Google Scholar] [CrossRef]
- Yustika, D.R.; Somura, H.; Budi, Y. Impact of human activities and natural processes on the seasonal variability of river water quality in two watersheds in Lampung, Indonesia. Water 2019, 11, 2363. [Google Scholar] [CrossRef]
- Prastica, R.M.S.; Soeryantono, H.; Marthanty, D.R. Mathematical modelling of hydraulics and water quality characteristics for small dam maintenance. Mag. Civ. Eng. 2022, 109, 10903. [Google Scholar] [CrossRef]
- Tyagi, S.K.; Singh, P.S.D. Need for proper water management for security. Curr. Sci. 2012, 102, 690–695. Available online: https://www.currentscience.ac.in/Volumes/102/05/0690.pdf (accessed on 7 May 2023).
- Setiawan, Y.; Rustiadi, E.; Yoshino, K.; Effendi, H. Assessing the seasonal dynamics of the Java’s paddy field using MODIS satellite images. ISPRS Inter. J. Geo-Info. 2014, 3, 110–129. [Google Scholar] [CrossRef]
- Doni, F.; Zain, C.R.C.M.; Isahak, A. Relationships observed between Trichoderma inoculation and characteristics of rice grown under System of Rice Intensification (SRI) vs. conventional methods of cultivation. Symbiosis 2017, 72, 45–59. [Google Scholar] [CrossRef]
- Siahaan, Y.; Rohmat, D. The implementation of modern irrigation to improve the farmer welfare. IOP Conf. Ser. Earth Environ. Sci. 2019, 286, 012018. [Google Scholar] [CrossRef]
- Anas, I.; Rupela, O.P.; Thiyagarajan, T.M.; Uphoff, N. A review of studies on SRI effects on beneficial organisms in rice soil rhizospheres. Paddy Water Environ. 2011, 9, 53–64. [Google Scholar] [CrossRef]
- Lakitan, B. Research and technology development in Southeast Asian economies are drifting away from agriculture and farmers’ needs. J. Sci. Tech. Pol. Manag. 2019, 10, 251–272. [Google Scholar] [CrossRef]
- Hidayati, N.; Triadiati, T.; Anas, I. Rooting system of rice cultivated under System of Rice Intensification (SRI) method which improving rice yield. HAYATI J. Biosci. 2018, 25, 63–69. [Google Scholar] [CrossRef]
- Bakrie, M.M.; Anas, I.; Idris, K. Application of inorganic and bio-organic fertilizer on System of Rice Intensification. J. Tan. Lingk. 2010, 12, 25–32. [Google Scholar] [CrossRef]
- Razie, F.; Anas, I.; Sutandi, A.; Gunarto, L. Efisiensi serapan hara dan hasil padi pada budidaya SRI di persawahan pasang surut dengan menggunakan kompos diperkaya. J. Agron. Indones. 2013, 41, 89–97. Available online: https://journal.ipb.ac.id/index.php/jurnalagronomi/article/download/7509/5840 (accessed on 7 May 2023).
- Hidayati, N.; Anas, I. Photosynthesis and transpiration rates of rice cultivated under the system of rice intensification and the effects on growth and yield. HAYATI J. Biosci. 2016, 23, 67–72. [Google Scholar] [CrossRef]
- Subardja, V.O.; Anas, I.; Widyastuti, R. Utilization of organic fertilizer to increase paddy growth and productivity using System of Rice Intensification (SRI) method in saline soil. J. Degr. Min. Land. Manag. 2016, 3, 543–549. [Google Scholar] [CrossRef][Green Version]
- Moser, C.M.; Barrett, C.B. The disappointing adoption dynamics of a yield-increasing, low external-input technology: The case of SRI in Madagascar. Agric. Syst. 2003, 76, 1085–1100. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Horie, T.; Randriamihary, H. The key factors for higher productivity in the fields utilizing the system of rice intensification (SRI) in the central highland of Madagascar. Agric. Syst. 2009, 100, 61–71. [Google Scholar] [CrossRef]
- Styger, E.; Aboubacrine, G.; Attaher, M.A.; Uphoff, N. The system of rice intensification as a sustainable agricultural innovation: Introducing, adapting and scaling up a system of rice intensification practices in the Timbuktu region of Mali. Inter. J. Agric. Sustain. 2011, 9, 67–75. [Google Scholar] [CrossRef]
- Thakur, A.K.; Uphoff, N.; Antony, E. An assessment of physiological effects of system of rice intensification (SRI) practices compared with recommended rice cultivation practices in India. Exp. Agric. 2010, 46, 77–98. [Google Scholar] [CrossRef]
- Palanisami, K.; Karunakaran, K.R.; Amarasinghe, U.; Ranganathan, C.R. 2013. Doing different things or doing it differently? Rice intensification practices in 13 states of India. Econ. Political Wkly. 2013, 48, 51–58. Available online: https://cgspace.cgiar.org/handle/10568/40276 (accessed on 10 January 2023).
- Arsil, P.; Sahirman, S.; Hidayat, H.H. The reasons for farmers not to adopt System of Rice Intensification (SRI) as a sustainable agricultural practice: An explorative study. IOP Conf. Ser. Earth Environ. Sci. 2018, 250, 012063. [Google Scholar] [CrossRef]
- Uphoff, N.; Kassam, A.; Stoop, W. A critical assessment of a desk study comparing crop production systems: The example of the “system of rice intensification” versus “best management practice”. Field Crops Res. 2008, 108, 109–114. [Google Scholar] [CrossRef]
- Kassam, A.; Stoop, W.; Uphoff, N. Review of SRI modifications in rice crop and water management and research issues for making further improvements in agricultural and water productivity. Paddy Water Environ. 2011, 9, 163–180. [Google Scholar] [CrossRef]
- Breidenbach, B.; Brenzinger, K.; Brandt, F.B.; Blaser, M.B.; Conrad, R. The effect of crop rotation between wetland rice and upland maize on the microbial communities associated with roots. Plant Soil 2017, 419, 435–445. [Google Scholar] [CrossRef]
- Adhikari, K.; Bhandari, S.; Acharya, S. An overview of azolla in rice production: A review. Rev. Food Agric. 2020, 2, 04–08. [Google Scholar] [CrossRef]
- Yassi, A.; Farid, M.; Anshori, M.F.; Muchtar, H.; Syamsuddin, R.; Adnan, A. The Integrated Minapadi (Rice-Fish) Farming System: Compost and Local Liquid Organic Fertilizer Based on Multiple Evaluation Criteria. Agronomy 2023, 13, 978. [Google Scholar] [CrossRef]
- Gao, H.; Dai, W.; Fang, K.; Yi, X.; Chen, N.; Penttinen, P.; Sha, Z.; Cao, L. Rice-duck co-culture integrated different fertilizers reduce P losses and Pb accumulation in subtropical China. Chemosphere 2020, 245, 125571. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Huang, H.; Liao, X.; Fu, Z.; Zheng, H.; Chen, A.; Chen, C. Mechanism and capacities of reducing ecological cost through rice–duck cultivation. J. Sci. Food. Agric. 2013, 93, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, C.; Li, Z.; Luo, J.; Ren, B.; Chen, C.; Xu, Y.; Ding, J.; Huang, H. Review of Rice–Fish–Duck Symbiosis System in China—One of the Globally Important Ingenious Agricultural Heritage Systems (GIAHS). Sustainability 2023, 15, 1910. [Google Scholar] [CrossRef]
- Simarmata, T.; Proyoga, M.K.; Herdiyantoro, D.; Setiawati, M.R.; Adinata, K.; Stober, S. Climate resilient sustainable agriculture for restoring the soil health and increasing rice productivity as adaptation strategy to climate change in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 748, 012039. [Google Scholar] [CrossRef]
- Yan, X.; Shi, S.; Du, L.; Xing, G. Pathways of N2O emission from rice paddy soil. Soil Biol. Biochem. 2000, 32, 437–440. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, M.; Wang, Y.; Shen, R.; Gou, Z.; Li, J.; Jin, J.; Li, L. Impacts of soil moisture on nitrous oxide emission from croplands: A case study on the rice-based agro-ecosystem in Southeast China. Chem. Chang. Sci. 2000, 2, 207–224. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, S.; Kiese, R.; Castro, S. Soil Greenhouse Gas Pulses from a Tropical Dry Forest; General Assembly Conference; European Geoscience Union: Vienna, Austria, 2019. [Google Scholar]
- Gathorne-Hardy, A. A life cycle assessment (LCA) of greenhouse gas emissions from SRI and flooded rice production in SE India. Taiwan Water Conser. 2013, 61, 111–125. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Singh, Y.V.; Prasanna, R. Pattern of methane emission and water productivity under different methods of rice crop establishment. Paddy Water Environ. 2013, 11, 321–329. [Google Scholar] [CrossRef]
- Ferichani, M.; Prasetya, D.A. System of rice intensification increases rice productivity on saline soil. Paddy Water Environ. 2017, 15, 649–657. [Google Scholar] [CrossRef]
- Setyanto, P.; Pramono, A.; Adriany, T.A.; Susilawati, H.L.; Tokida, T.; Padre, A.T.; Minamikawa, K. Alternate wetting and drying reduces methane emission from a rice paddy in Central Java, Indonesia without yield loss. Soil Sci. Plant Nutr. 2018, 64, 23–30. [Google Scholar] [CrossRef]
- Tirol-Padre, A.; Minamikawa, K.; Tokida, T.; Wassmann, R.; Yagi, K. Site-specific feasibility of alternate wetting and drying as a greenhouse gas mitigation option in irrigated rice fields in Southeast Asia: A synthesis. Soil Sci. Plant Nutr. 2018, 64, 2–13. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil. Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Mekuria, W.; Noble, A. The role of biochar in ameliorating disturbed soils and sequestering soil carbon in tropical agricultural production systems. Appl. Environ. Soil. Sci. 2013, 2013, 354965. [Google Scholar] [CrossRef]
- Shen, J.; Tang, H.; Liu, J.; Wang, C.; Li, Y.; Ge, T.; Jones, D.L.; Wu, J. Contrasting effects of straw and straw-derived biochar amendments on greenhouse gas emissions within double rice cropping systems. Agric. Ecosyst. Environ. 2014, 188, 264–274. [Google Scholar] [CrossRef]
- Shaukat, M.; Samoy-Pascual, K.; Maas, E.D.V.L. Simultaneous effects of biochar and nitrogen fertilization on nitrous oxide and methane emissions from paddy rice. J. Environ. Manag. 2019, 248, 109242. [Google Scholar] [CrossRef] [PubMed]
- Criscuoli, I.; Ventura, M.; Wiedner, K.; Glaser, B.; Panzacchi, P. Woodchips biochar and impact on soil carbon stocks: Results from a two-year field experiment. Forests 2021, 12, 1350. [Google Scholar] [CrossRef]
- Sui, Y.; Gao, J.; Liu, C.; Zhang, W.; Lan, Y.; Li, S.; Meng, J.; Xu, Z.; Tang, L. Interactive effects of straw-derived biochar and N fertilization on soil C storage and rice productivity in rice paddies of Northeast China. Sci. Total. Environ. 2016, 544, 203–210. [Google Scholar] [CrossRef]
- Paudel, S.; Baral, H.; Rojario, A.; Bhatta, K.P. Agroforestry: Opportunities and challenges in Timor-Leste. Forests 2022, 13, 41. [Google Scholar] [CrossRef]
- Arianti, M.; Setyanto, P. The effect of rice straw and manure application on N2O emission and rice yield in crop-livestock integration systems. Food Crop. Agric. Res. 2010, 29, 47–52. [Google Scholar]
- Win, E.P.; Win, K.K.; Bellingrath-Kimura, S.D.; Oo, A.Z. Influence of rice varieties, organic manure and water management on greenhouse gas emissions from paddy rice soils. PLoS ONE 2021, 16, e0253755. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Baruah, K.K. Organic manures and crop residues as fertilizer substitutes: Impact on nitrous oxide emission, plant growth and grain yield in pre-monsoon rice cropping system. J. Environ. Protect. 2015, 6, 755. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Shen, J.; Chen, D.; Zheng, L.; Ge, T.; Li, Y.; Wu, J. Reduction in net greenhouse gas emissions through a combination of pig manure and reduced inorganic fertilizer application in a double-rice cropping system: Three-year results. Agric. Ecosyst. Environ. 2022, 326, 107799. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, Y.; Liu, Y.; Huang, X.; Yang, Y.; Xiong, H.; Yehua, Y.; Xiong, H.; Zhu, H.; Li, Y. Characteristics of Greenhouse Gas Emissions from Yellow Paddy Soils under Long-Term Organic Fertilizer Application. Sustainability 2022, 14, 12574. [Google Scholar] [CrossRef]
- Sembiring, H.A.; Subekti, N.; Nugraha, D.; Priatmojo, B.; Stuart, A.M. Yield gap management under seawater intrusion areas of Indonesia to improve rice productivity and resilience to climate change. Agriculture 2020, 10, 1. [Google Scholar] [CrossRef]
- Wihardjaka, A.; Harsanti, E.S. Potensi produksi gas metana dari tanah sawah tadah hujan di daerah pantai utara bagian Timur Jawa Tengah. Ecolab 2011, 5, 68–88. [Google Scholar] [CrossRef]
- MoEF (Ministry of Environment and Forestry). Indonesia First Biennial Update Report (BUR); MoEF: Jakarta, Indonesia, 2015; Available online: https://unfccc.int/resource/docs/natc/idnbur1.pdf (accessed on 7 May 2023).
- Gaihre, Y.K.; Wassmann, R.; Tirol-Padre, A.; Villegas-Pangga, G.; Aquino, E.; Kimball, B.A. Seasonal assessment of greenhouse gas emissions from irrigated lowland rice fields under infrared warming. Agric. Ecosyst. Environ. 2014, 184, 88–100. [Google Scholar] [CrossRef]
- Locatelli, B.; Pavageau, C.; Pramova, E.; Di Gregorio, M. Integrating climate change mitigation and adaptation in agriculture and forestry: Opportunities and trade-offs. Wiley Interdiscip. Rev. Clim. Chang. 2015, 6, 585–598. [Google Scholar] [CrossRef]
- Thomas, V.G.; Kevan, P.G. Basic principles of agroecology and sustainable agriculture. J. Agric. Environ. Ethics 1993, 6, 1–19. [Google Scholar] [CrossRef]
- Prasad, S.; Malav, L.C.; Choudhary, J. Soil microbiomes for healthy nutrient recycling. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2021; pp. 1–21. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.A.; Othman, Y. Soil health and sustainable agriculture. Sustainability 2020, 1, 4859. [Google Scholar] [CrossRef]
- Song, J.; Cai, D.; Deng, J.; Wang, K.; Shen, Z. Dynamics of paddy field patterns in response to urbanization: A case study of the Hang-Jia-Hu Plain. Sustainability 2015, 7, 13813–13835. [Google Scholar] [CrossRef]
- Sukarjo; Zulaehah, I.; Handayani, C.O.; Zu’Amah, H. Heavy metal pollution assessment in paddy fields and dryland in Bandung District, West Java. IOP Conf. Ser.: Earth Environ. Sci. 2021, 648, 012114. [Google Scholar] [CrossRef]
- Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep. 2020, 10, 13768. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Farooq, M.; Zulfiqar, U.; Hussain, S.; Akbar, N.; Nawaz, A.; Anjum, S. Alternate wetting and drying: A water-saving and ecofriendly rice production system. Agric. Water Manag. 2020, 241, 106363. [Google Scholar] [CrossRef]
- Bambaradeniya, C.N.B.; Amarasinghe, F.P. Biodiversity Associated with Rice Field Agroecosystem in Asian Countries: A Brief Review; Working Paper 63; International Water Management Institute: Colombo, Sri Lanka, 2003. [Google Scholar]
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- ATRBPN (Ministry of Agrarian Affairs and Spatial Planning/National Land Agency). Decree No. 686-SK_PG-03_03-XII-2019; ATRBPN: Jakarta, Indonesia, 2019. [Google Scholar]
- Agustiani, N.; Deng, N.; Edreira, J.I.R. Simulating rice and maize yield potential in the humid tropical environment of Indonesia. Eur. J. Agron. 2019, 101, 10–19. [Google Scholar] [CrossRef]
- Mohammadi, A.; Khoshnevisan, B.; Venkatesh, G.; Eskandari, S. A critical review on advancement and challenges of biochar application in paddy fields: Environmental and life cycle cost analysis. Processes 2020, 8, 1275. [Google Scholar] [CrossRef]
- Mutiara, V.I.; Hariance, R.; Utami, A.S. Incentive program towards sustainability of organic rice certification in Limapuluh Kota Regency, West Sumatra, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 741, 012077. [Google Scholar] [CrossRef]
- Sukayat, Y.; Setiawan, I.; Suharfaputra, U.; Kurnia, G. Determining factors for farmers to engage in sustainable agricultural practices: A case from Indonesia. Sustainability 2023, 15, 10548. [Google Scholar] [CrossRef]
- Leimona, B.; Amaruzaman, S.; Arifin, B.; Yasmin, F.; Hasan, F.; Agusta, H.; Frias, J. Indonesia’s ‘Green Agriculture’ Strategies and Policies: Closing the Gap between Aspirations and Application; Occasional Paper 23; World Agroforestry: Nairobi, Kenya, 2015; Available online: https://apps.worldagroforestry.org/sea/Publications/files/occasionalpaper/OP0003-15.pdf (accessed on 7 May 2023).
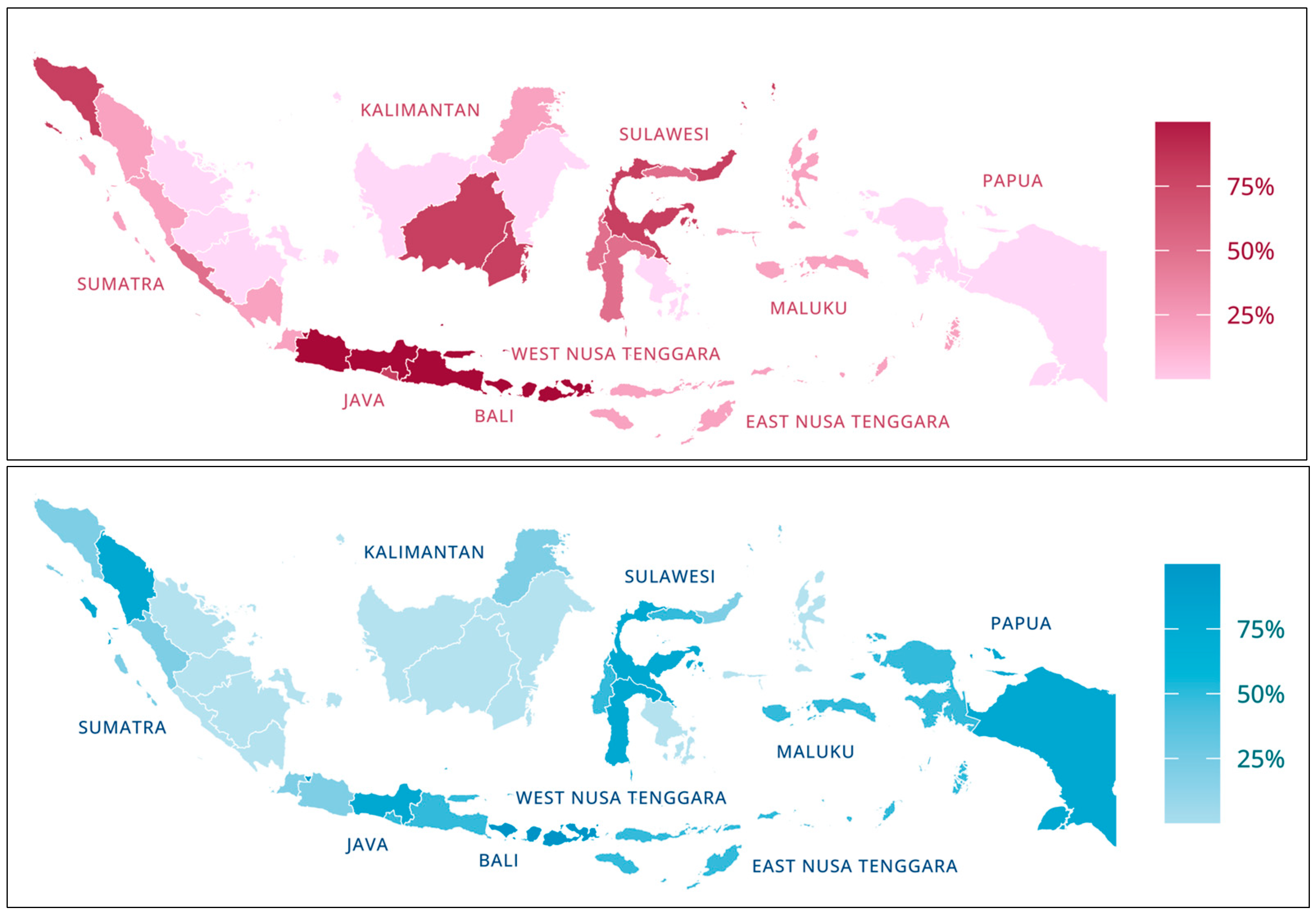
| Soil Nutrient Status | Fertilizer Recommendation |
|---|---|
| P status (mg P kg−1) | Fertilizer rate (kg P ha−1) |
| Low (<87) | 17 |
| Medium (87–174) | 13 |
| High (>174) | 11 |
| K status (mg K kg−1) | Fertilizer rate (kg K ha−1) |
| Low (<83) | 38 |
| Medium (83–166) | 30 |
| High (>166) | 25 |
| Yield Targets (t ha−1) | N Rates (kg ha−1) |
|---|---|
| <6 | 112.5 |
| 6–8 | 135.0 |
| >8 | 157.5 |
| Method | Increase | Reference | |
|---|---|---|---|
| Conventional | SRI | ||
| t ha−1 | t ha−1 | % | |
| 6.0 | 7.9 | 32 | Bakrie et al., 2010 [130] |
| 3.6 | 4.3 | 22 | Razie et al., 2013 [131] |
| 7.0 | 9.0 | 29 | Hidayati and Anas 2016 [132] |
| 5.4 | 7.2 | 33 | Subardja et al., 2016 [133] |
| 4.5 | 6.4 | 42 | Moser and Barrett 2003 [134] |
| 3.8 | 4.6 | 22 | Tsujimoto et al., 2009 [135] |
| 4.8 | 7.6 | 58 | Styger et al., 2011 [136] |
| Treatments | Potential Nutrient Contribution | Yield Increase (t ha−1) | References | |||
|---|---|---|---|---|---|---|
| N | P | K | Micronutrients | |||
| Manure, 2 t ha−1 (dry weight) | 16 kg ha−1 | 14 kg ha−1 | 31 kg ha−1 | NA | NA | [61] |
| Rice straw (5 t ha−1) | 25–40 kg ha−1 | 3.5–5.9 kg ha−1 | 58–83 kg ha−1 | NA | From 2.4 to 4.1 t ha−1 | [62,63] |
| Azotobacter + 50% of NPK normal dose | 50% † | NA | NA | NA | No yield decline | [75] |
| 50% NPK (69 kg N, 4 kg P, 21 kg K ha−1) + 7 t ha−1 of Azolla + 25 kg ha−1 powder of Azotobacter, Azospirillum, N2-fixing bacteria, and P-solubilizing bacteria) | 69 kg ha−1 Ł | 4 kg ha−1 Ł | 21 kg ha−1 Ł | NA | No yield decline | [111] |
| Blue-green algae | 30 to 40 kg ha−1 | NA | NA | NA | From 2.0 to 2.3 t ha−1 | [78] |
| Blue-green algae + 50 kg N ha−1 | 30 to 40 kg ha−1 | NA | NA | NA | From 3.0 to 3.2 t ha−1 | [78] |
| Blue-green algae | NA | NA | NA | NA | 7–22% | [78,82,83,84,85] |
| P-solubilizing microbes | NA | 50% † | NA | NA | NA | [89] |
| K-solubilizing microbes | NA | NA | [90,91] | |||
| Biochar | ||||||
| Stonemeal of K-bearing rocks | NA | NA | 1.6 to 7.3% ‡ | NA | NA | [115,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susanti, W.I.; Cholidah, S.N.; Agus, F. Agroecological Nutrient Management Strategy for Attaining Sustainable Rice Self-Sufficiency in Indonesia. Sustainability 2024, 16, 845. https://doi.org/10.3390/su16020845
Susanti WI, Cholidah SN, Agus F. Agroecological Nutrient Management Strategy for Attaining Sustainable Rice Self-Sufficiency in Indonesia. Sustainability. 2024; 16(2):845. https://doi.org/10.3390/su16020845
Chicago/Turabian StyleSusanti, Winda Ika, Sri Noor Cholidah, and Fahmuddin Agus. 2024. "Agroecological Nutrient Management Strategy for Attaining Sustainable Rice Self-Sufficiency in Indonesia" Sustainability 16, no. 2: 845. https://doi.org/10.3390/su16020845
APA StyleSusanti, W. I., Cholidah, S. N., & Agus, F. (2024). Agroecological Nutrient Management Strategy for Attaining Sustainable Rice Self-Sufficiency in Indonesia. Sustainability, 16(2), 845. https://doi.org/10.3390/su16020845






