Biodiesel from Higher Alcohols for Removal of Crude Oil Spills from Coastal Sediments
Abstract
1. Introduction
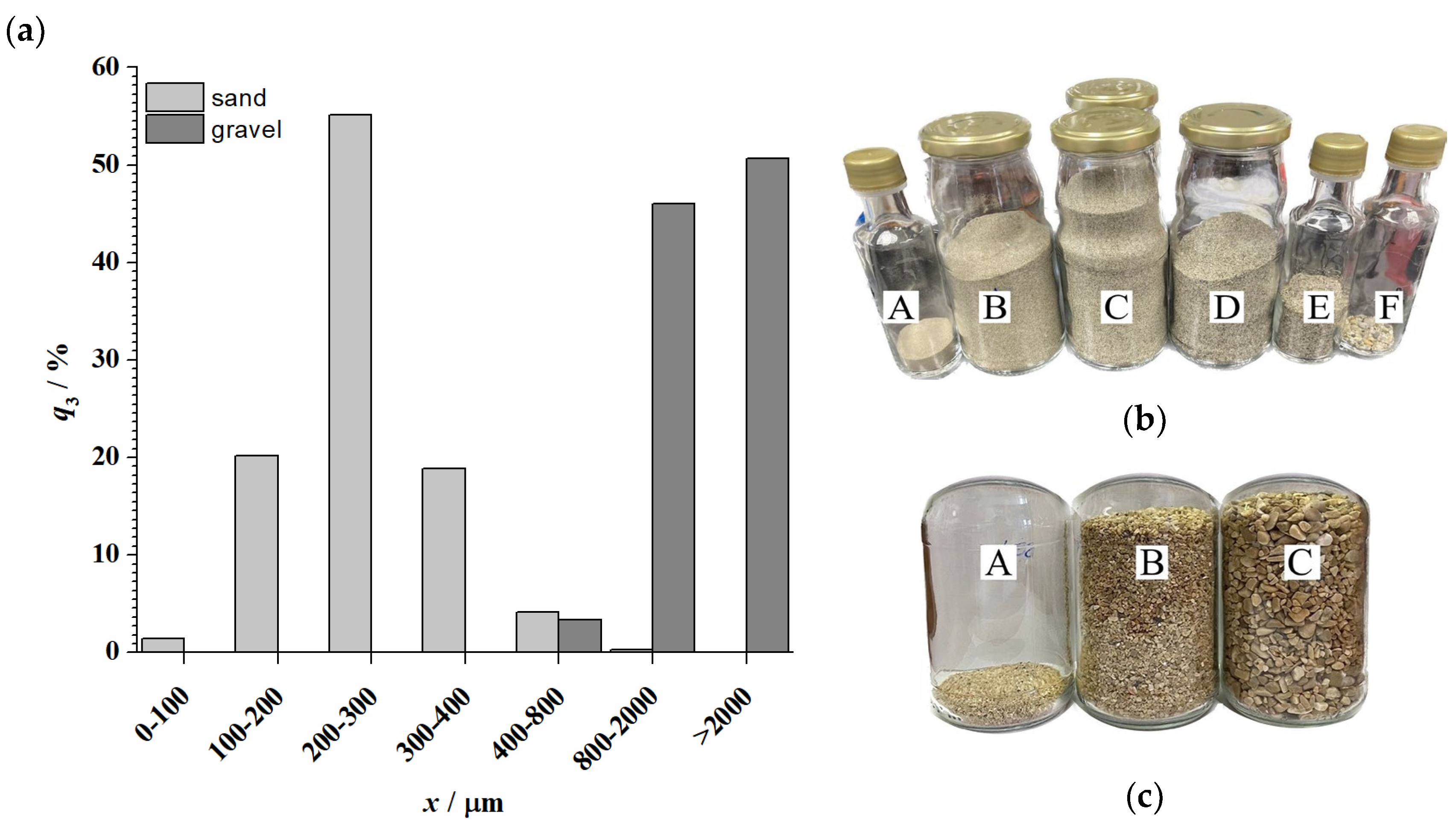
2. Materials
3. Methods
3.1. Synthesis and Characterization of Crude Oil Removing Agent—Biodiesel
3.2. Testing the Crude Oil Removal Efficiency of Biodiesel from Coastal Sediments
4. Results and Discussion
4.1. Synthesis of Biodiesel and Characterization of the Used Materials
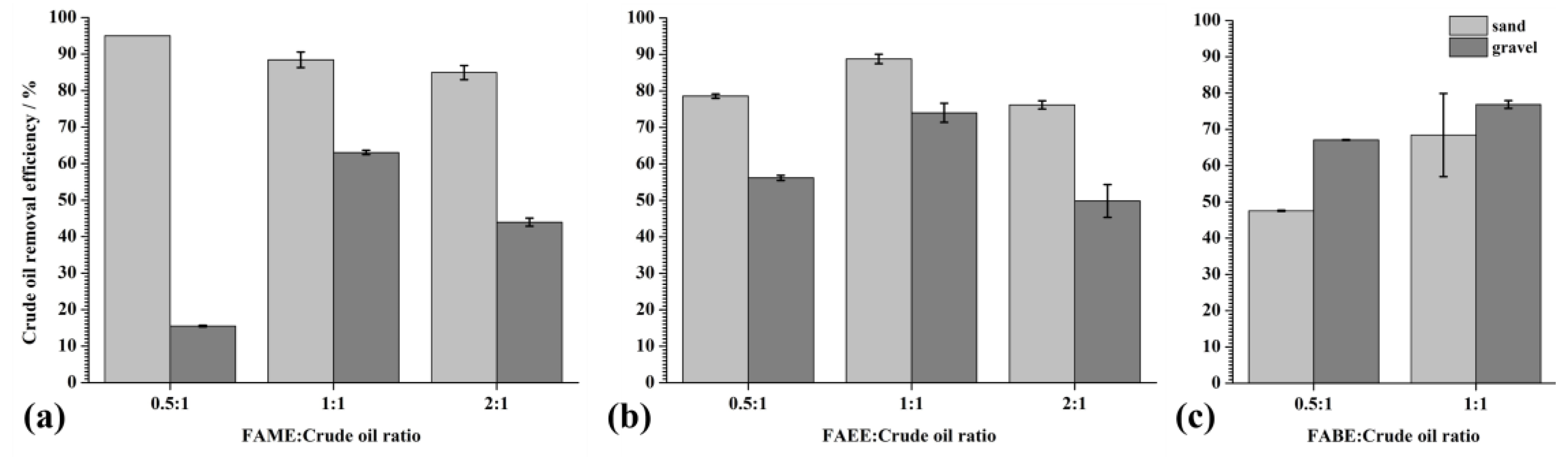
4.2. Testing the CrudeOoil Removal Efficiency of Biodiesel from Coastal Sediments
4.3. The Possibility of Application in a Real System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michael-Igolima, U.; Abbey, S.J. A systematic review on the effectiveness of remediation methods for oil contaminated soils. Environ. Adv. 2022, 9, 100319. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, N.; Arya, S.K.; Khatri, M. Environmental impacts of oil spills and their remediation by magnetic nanomaterials. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100305. [Google Scholar] [CrossRef]
- Zhu, Z.; Merlin, F.; Yang, M.; Lee, K.; Chen, B.; Liu, B.; Cao, Y.; Song, X.; Ye, X.; Li, Q.K.; et al. Recent advances in chemical and biological degradation of spilled oil: A review of dispersants application in the marine environment. J. Hazard. Mater. 2022, 436, 129260. [Google Scholar] [CrossRef]
- Kim, T.; Lee, C.; Lee, J.; Bae, H.; Noh, J.; Hong, S.; Kwon, B.O.; Kim, J.J.; Yim, U.H.; Chang, G.S.; et al. Best available technique for the recovery of marine benthic communities in a gravel shore after the oil spill: A mesocosm-based sediment triad assessment. J. Hazard. Mater. 2022, 435, 128945. [Google Scholar] [CrossRef]
- Desplat, Y.; Warner, J.F.; Blake, E.J.; Vijayan, N.; Cuvelier, M.; Blackwelder, P.; Lopez, J.V. Morphological and transcriptional effects of crude oil and dispersant exposure on the marine sponge Cinachyrella alloclada. Sci. Total Environ. 2023, 878, 162832. [Google Scholar] [CrossRef]
- Jeffery, S.; Hannah, L.C.; Herborg, L.; St Germain, C. Oil spill response planning in Pacific Canada: A tool for identifying vulnerable marine biota. Mar. Policy 2023, 148, 105466. [Google Scholar] [CrossRef]
- Mohammadshirazi, A.; Akram, A.; Rafiee, S.; Bagheri Kalhor, E. Energy and cost analyses of biodiesel production from waste cooking oil. Renew. Sustain. Energy Rev. 2014, 33, 44–49. [Google Scholar] [CrossRef]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. A comparison of used cooking oils: A very heterogeneous feedstock for biodiesel. Bioresour. Technol. 2009, 100, 5796–5801. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from Waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Viornery-Portillo, E.A.; Bravo-Díaz, B.; Mena-Cervantes, V.Y. Life cycle assessment and emission analysis of waste cooking oil biodiesel blend and fossil diesel used in a power generator. Fuel 2020, 281, 118739. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Bouaid, A.; El boulifi, N.; Hahati, K.; Martinez, M.; Aracil, J. Biodiesel production from biobutanol. Improvement of cold flow properties. Chem. Eng. J. 2014, 238, 234–241. [Google Scholar] [CrossRef]
- Yusoff, M.F.M.; Xu, X.; Guo, Z. Comparison of Fatty Acid Methyl and Ethyl Esters as Biodiesel Base Stock: A Review on Processing and Production Requirements. J. Am. Oil Chem. Soc. 2014, 91, 525–531. [Google Scholar] [CrossRef]
- Melikoglu, M.; Singh, V.; Leu, S.Y.; Webb, C.; Lin, C.S.K. Handbook of Biofuels Production, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2016; pp. 237–258. [Google Scholar]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in biobutanol production: How to improve the efficiency? Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Pereira, M.G.; Mudge, S.M. Cleaning oiled shores: Laboratory experiments testing the potential use of vegetable oil biodiesels. Chemosphere 2004, 54, 297–304. [Google Scholar] [CrossRef]
- Fernández-Álvarez, P.; Vila, J.; Garrido-Fernández, J.M.; Grifoll, M.; Lema, J.M. Trials of bioremediation on a beach affected by the heavy oil spill of the Prestige. J. Hazard. Mater. 2006, 137, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.R. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J. Food Sci. Technol. 2018, 55, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 3104; Petroleum products–Transparent and opaque liquids—Determination of kinematic viscosity and calculation of dynamic viscosity. ISO: Geneva, Switzerland, 2020.
- ASTM D664-18e2; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2019.
- EN ISO 12937:2000; Petroleum products—Determination of water—Coulometric Karl Fischer titration method. ISO: London, UK, 2000.
- Cvengroš, J.; Cvengrošová, Z. Used frying oils and fats and their utilization in the production of methyl esters of higher fatty acids. Biomass Bioenergy 2004, 27, 173–181. [Google Scholar] [CrossRef]
- Parlov Vuković, J. Primjena spektroskopije NMR u analizi biodizela. Kem. Ind. 2016, 65, 17–24. [Google Scholar] [CrossRef][Green Version]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Vargas-Ibáñez, L.T.; Cano-Gómez, J.J.; Santos-López, I.A.; Iglesias-Silva, G.A.; López-Lázaro, J.d.L.S.; Alcalá-Rodríguez, M.M.; Villarreal-Mendoza, C.; Armendáriz-Ovalle, C. Surface tensions of biodiesel blends with pentanol and octanol isomers at different conditions: Measurement and new correlation. Fluid Phase Equilibria 2021, 540, 113046. [Google Scholar] [CrossRef]
- Miller, N.J.; Mudge, S.M. The effect of biodiesel on the rate of removal and weathering characteristics of crude oil within artificial sand columns. Spill Sci. Technol. Bull. 1997, 4, 17–33. [Google Scholar] [CrossRef]
- Fernández-Álvarez, P.; Vila, J.; Garrido, J.M.; Grifoll, M.; Feijoo, G.; Lema, J.M. Evaluation of biodiesel as bioremediation agent for the treatment of the shore affected by the heavy oil spill of the Prestige. J. Hazard. Mater. 2007, 147, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Mudge, S.M.; Pereira, G. Stimulating the Biodegradation of Crude Oil with Biodiesel Preliminary Results. Spill Sci. Technol. Bull. 1999, 5, 353–355. [Google Scholar] [CrossRef]
- Xia, W.; Xia, Y.; Li, J.; Zhang, D.; Zhou, Q.; Wang, X. Studies on crude oil removal from pebbles by the application of biodiesel. Mar. Pollut. Bull. 2015, 91, 288–294. [Google Scholar] [CrossRef]
- Wanga, M.; Zhangb, B.; Lic, G.; Wu, T.; Sun, D. Efficient remediation of crude oil-contaminated soil using a solvent/surfactant system. RSC Adv. 2019, 9, 2402–2411. Available online: https://pubs.rsc.org/en/content/articlehtml/2019/ra/c8ra09964b (accessed on 25 August 2024). [CrossRef] [PubMed]
- Urum, K.; Grigson, S.; Pekdemir, T.; McMenamy, S. A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 2006, 62, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y. Feasibility analysis of the remediation of fuel oil-contaminated soil and groundwater around the railroad station of Y city, Korea with surfactant-aided soil flushing. Asia-Pac. J. Chem. Eng. 2013, 8, 461–472. [Google Scholar] [CrossRef]
- Fingas, M. Oil Spill Science and Technology–Prevention, Response and Cleanup; Gulf Professional Publ.: Houston, TX, USA, 2010; pp. 335–336. [Google Scholar]
- Ng, Y.F.; Ge, L.; Chan, W.K.; Tan, S.N.; Hong Yong, J.W.; Yang Tan, T.T. An environmentally friendly approach to treat oil spill: Investigating the biodegradation of petrodiesel in the presence of different biodiesels. Fuel 2015, 139, 523–528. [Google Scholar] [CrossRef]


| Biodiesel | FAME | FAEE | FABE |
|---|---|---|---|
| Temperature (°C) | 60 | 40 | 60 |
| Time (min) | 80 | 60 | 120 |
| Molar ratio of alcohol to oil (mol mol−1) | 6.21:1 | 12:1 | 10:1 |
| Mass fraction of KOH (%) | 2 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotovuša, M.; Huzjak, P.; Zadravec, I.; Zadravec, M.; Parlov Vuković, J.; Faraguna, F. Biodiesel from Higher Alcohols for Removal of Crude Oil Spills from Coastal Sediments. Sustainability 2024, 16, 8574. https://doi.org/10.3390/su16198574
Gotovuša M, Huzjak P, Zadravec I, Zadravec M, Parlov Vuković J, Faraguna F. Biodiesel from Higher Alcohols for Removal of Crude Oil Spills from Coastal Sediments. Sustainability. 2024; 16(19):8574. https://doi.org/10.3390/su16198574
Chicago/Turabian StyleGotovuša, Mia, Paula Huzjak, Ivana Zadravec, Martina Zadravec, Jelena Parlov Vuković, and Fabio Faraguna. 2024. "Biodiesel from Higher Alcohols for Removal of Crude Oil Spills from Coastal Sediments" Sustainability 16, no. 19: 8574. https://doi.org/10.3390/su16198574
APA StyleGotovuša, M., Huzjak, P., Zadravec, I., Zadravec, M., Parlov Vuković, J., & Faraguna, F. (2024). Biodiesel from Higher Alcohols for Removal of Crude Oil Spills from Coastal Sediments. Sustainability, 16(19), 8574. https://doi.org/10.3390/su16198574






