Algae Modified Alginate Beads for Improved Cd(II) Removal from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
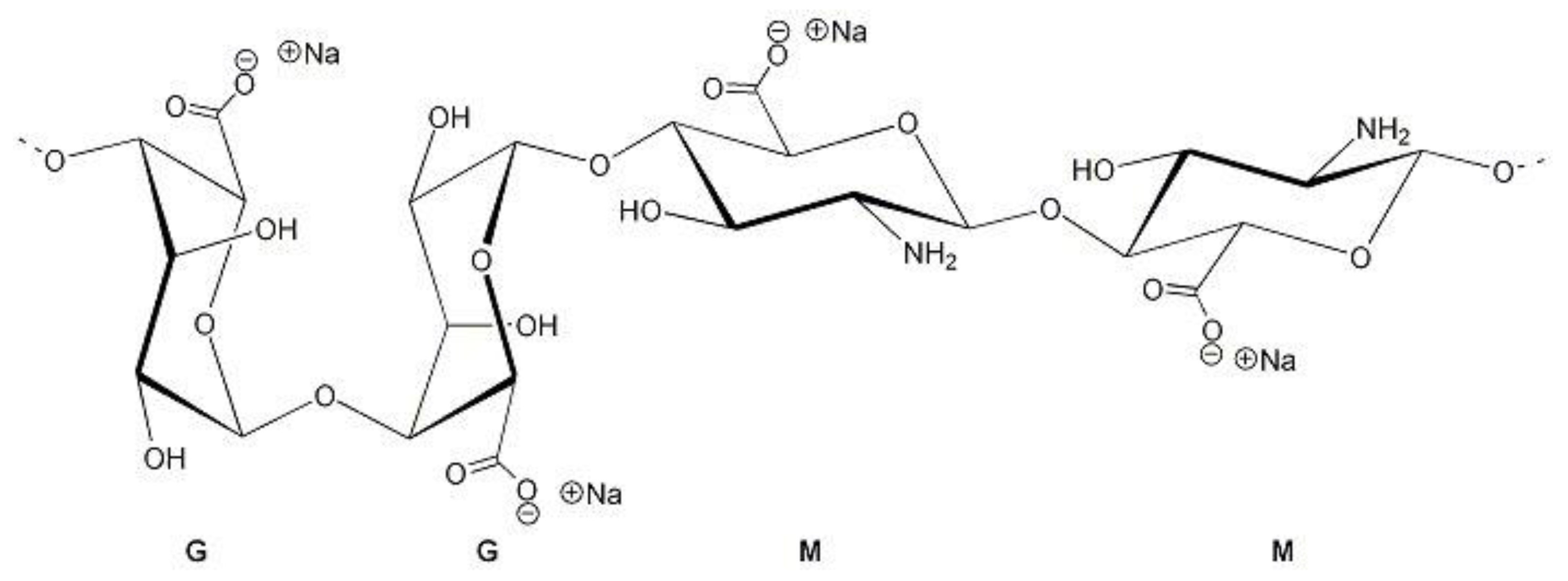
2.1. Beads Synthesis
2.2. Characterization
2.3. Adsorption Experiments
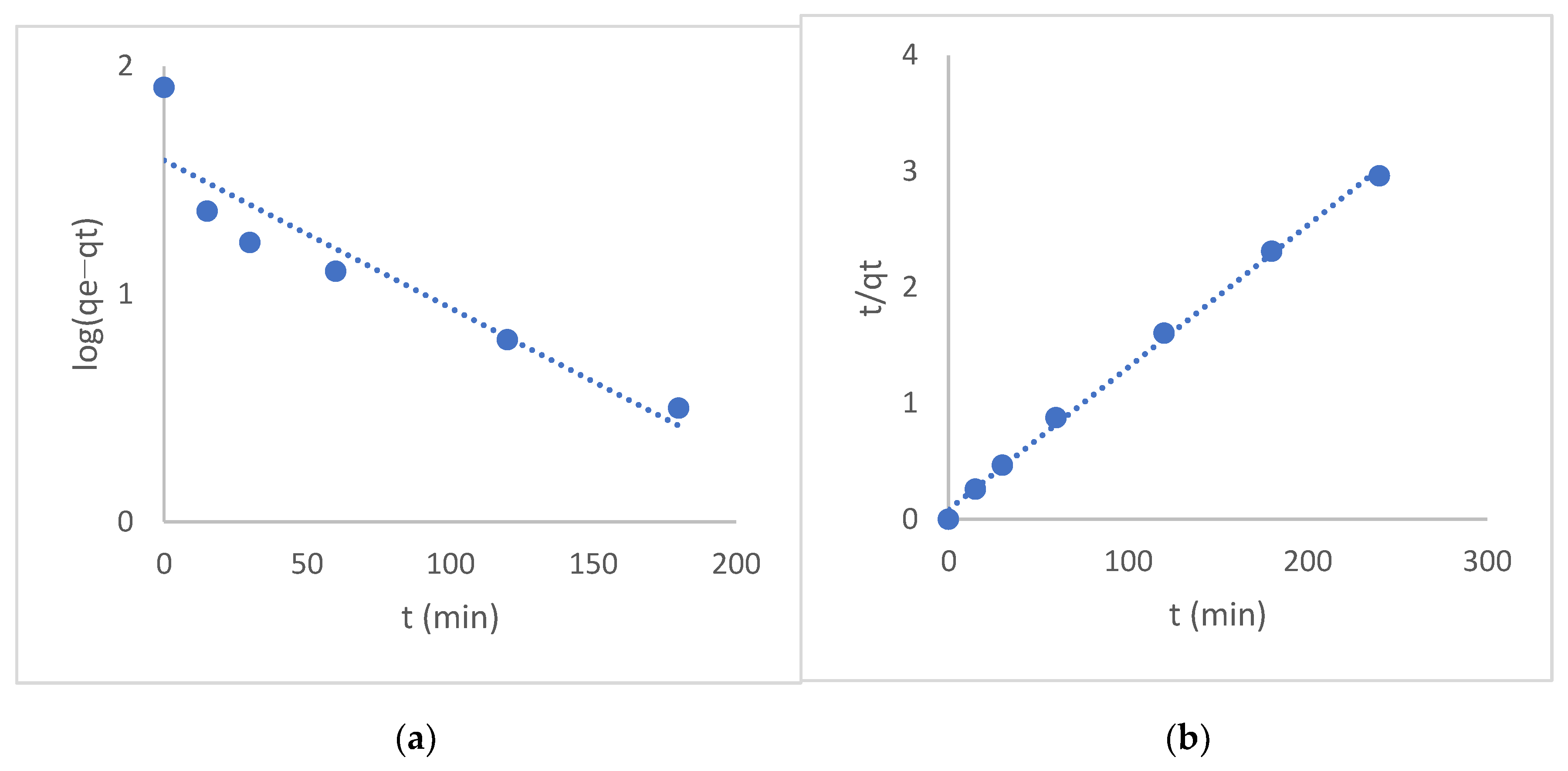
2.4. Kinetic Studies
- qt sorption capacity at time t (mg/g);
- qe sorption capacity at equilibrium (mg/g);
- k1 pseudo-first-order reaction rate constant.
- k2 pseudo-second-order reaction rate constant [14].
- ce the equilibrium concentration of adsorbate (mg/L);
- Q0 the Langmuir constant regarding maximum specific uptake (mg/g);
- b the Langmuir constant related to the affinity of binding sites to the metal ion (L/mg) [14].
- The Freundlich isotherm describes non-ideal and reversible adsorption (Equation (6)):
- qe the amount of Cd(II) adsorbed at equilibrium;
- KF Freundlich constant ((mg/g)/(mg/L)n);
- 1/n the adsorption intensity of surface heterogeneity that ranges between 0 and 1, becoming more heterogeneous as its value gets closer to zero [14].
- The Temkin isotherm contains a factor that explicitly takes into account adsorbent–adsorbate interactions (Equation (7)):
- qe the amount of Cd(II) adsorbed at equilibrium;
- bT the Temkin constant related to the heat of sorption (kJ/mol);
- T the absolute temperature (K);
- R the universal gas constant (8.314 J/mol K);
- AT the Temkin isotherm constant (L/g) [15].
2.5. Thermodynamics of Sorption
3. Results
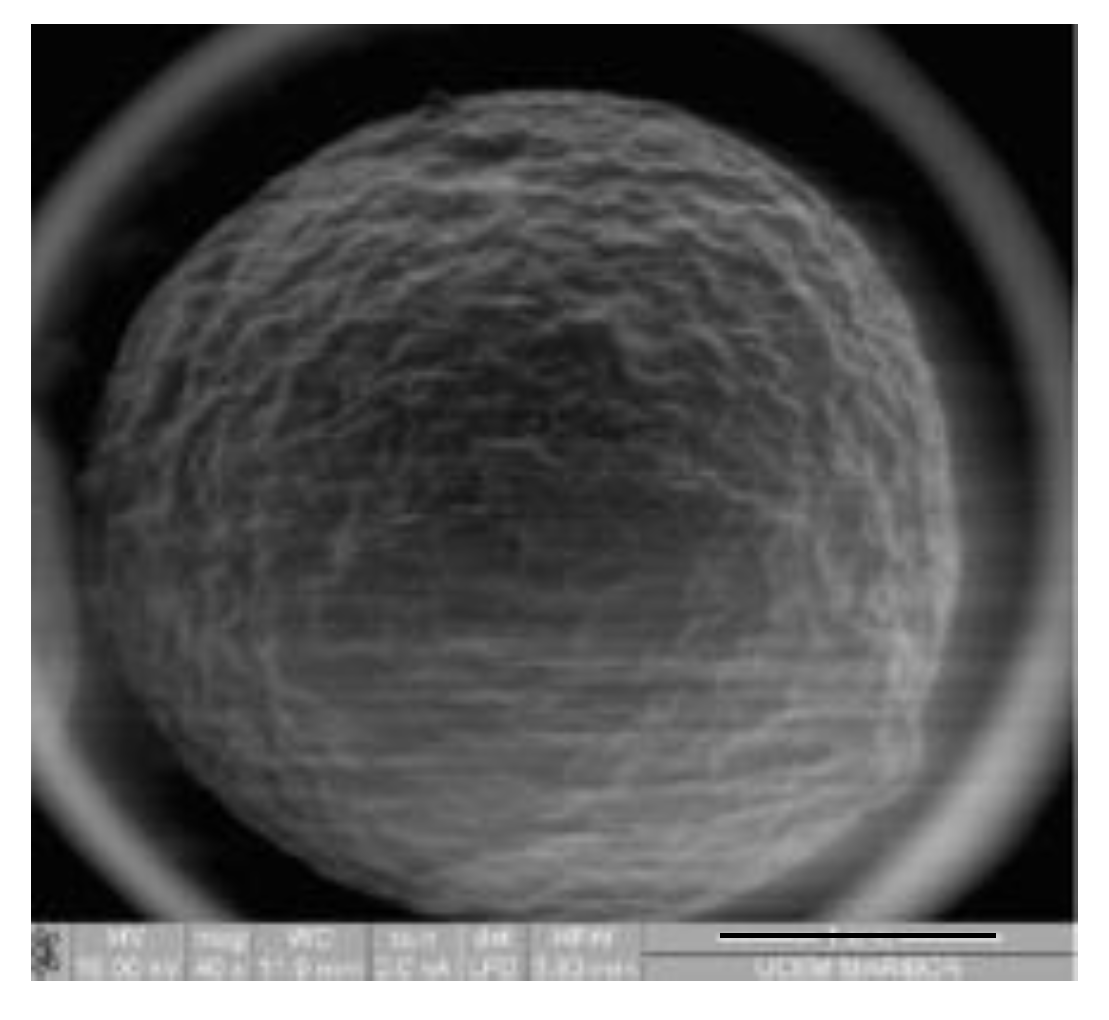
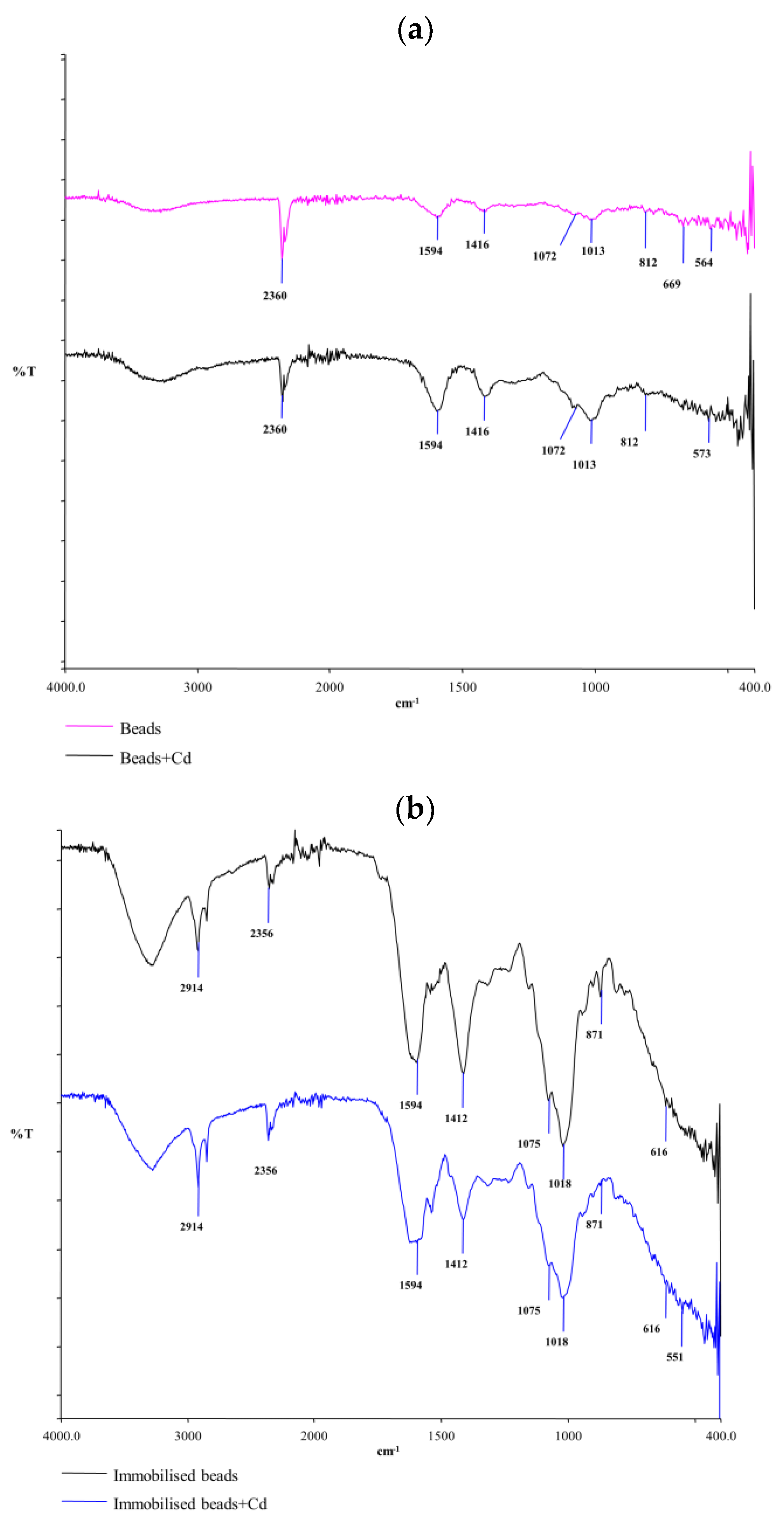
3.1. Characterization Results Study
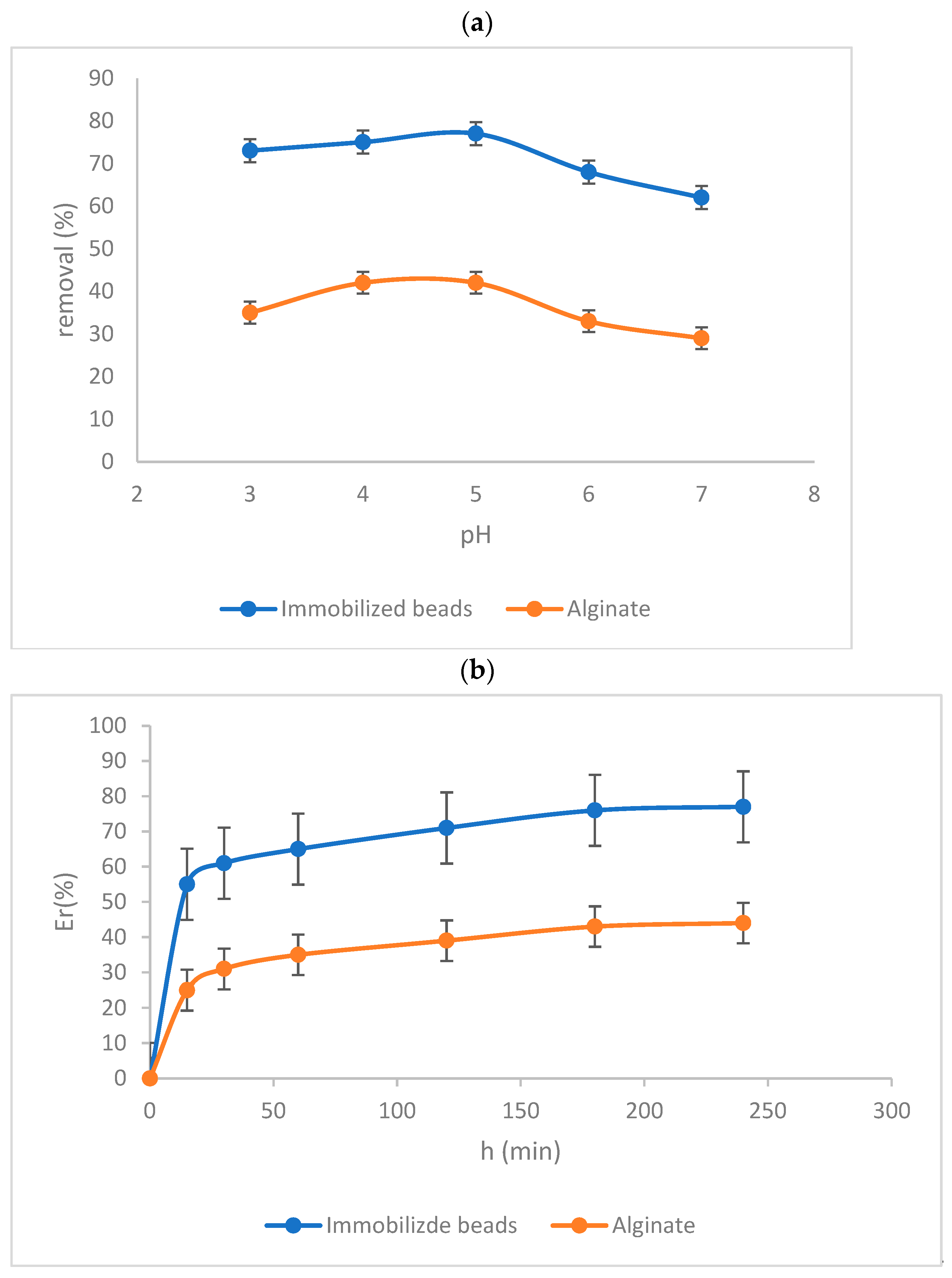
3.2. Study of Influential Factors
3.3. Adsorption Isotherms
3.4. Adsorption Kinetic Studies
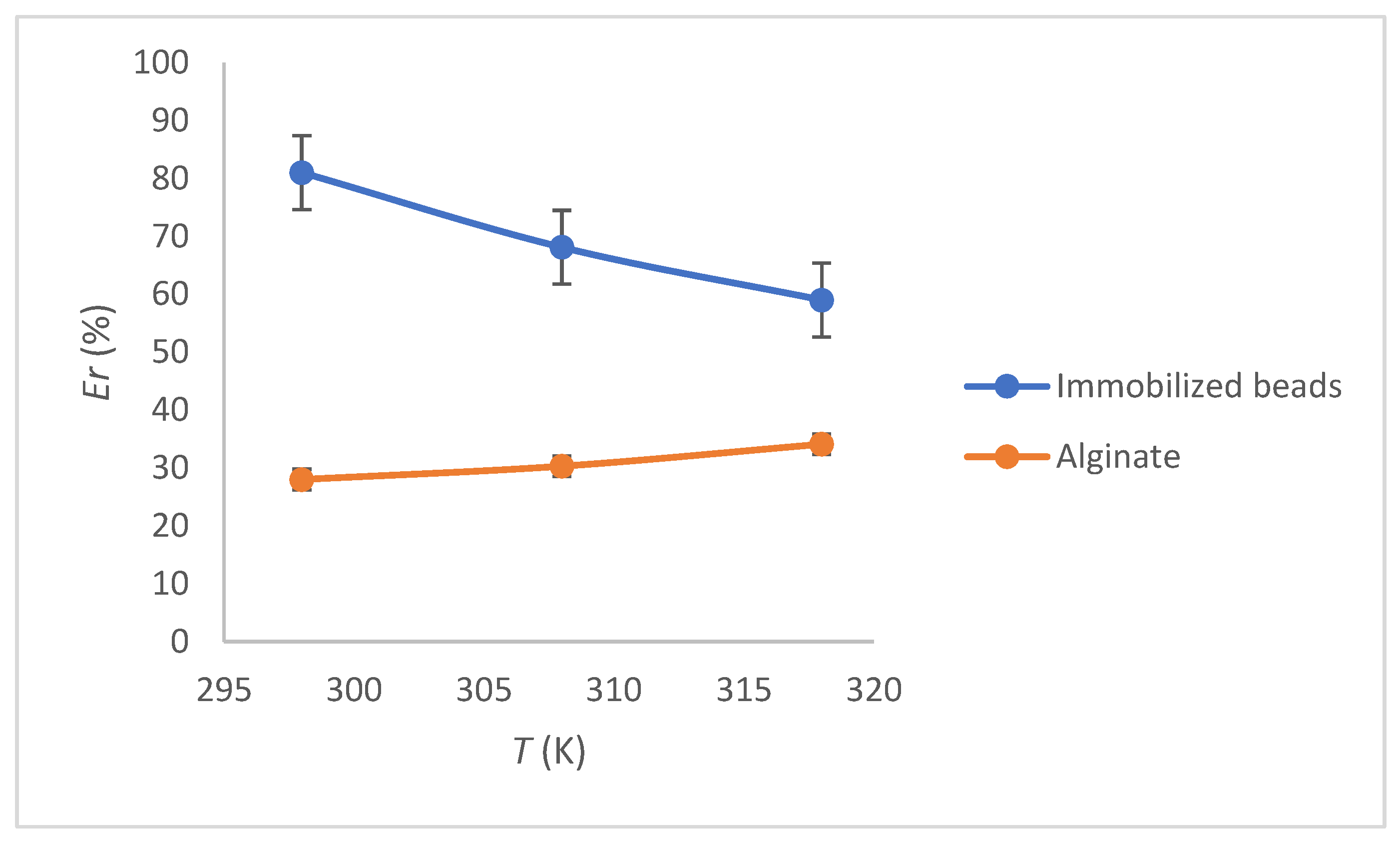
3.5. Thermodynamic Study
3.6. Desorption Study
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrovič, A.; Simonič, M. Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int. J. Environ. Sci. Technol. 2016, 13, 1761–1780. [Google Scholar] [CrossRef]
- Yogeshwaran, V.; Priya, A.K. Removal of Hexavalent Chromium (Cr6+) Using Different Natural Adsorbents—A review. J. Chromatogr. Sep. Technol. 2017, 8, 1000392. [Google Scholar] [CrossRef]
- Gupta, V.; Rastogi, A. Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J. Hazard. Mater. 2009, 163, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Poo, K.-M.; Son, E.-B.; Chang, J.-S.; Ren, X.; Choi, Y.-J.; Chae, K.-J. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-B.; Bhandari, B.R.; Howes, T. Gelation of an alginate film via spraying of calcium chloride droplets. Chem. Eng. Sci. 2018, 183, 1–12. [Google Scholar] [CrossRef]
- Huang, J.; Su, B.; Fei, X.; Che, J.; Yao, T.; Zhang, R.; Yi, S. Enhanced microalgal biomass and lipid production with simultaneous effective removal of Cd using algae-bacteria-activated carbon consortium added with organic carbon source. Chemosphere 2024, 350, 141088. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2008, 101, 1611–1627. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Wan, Y. Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd(II). J. Ind. Eng. Chem. 2018, 61, 161–168. [Google Scholar] [CrossRef]
- Qi, X.; Fu, K.; Yue, M.; Shou, N.; Yuan, X.; Chen, X.; He, C.; Yang, Y.; Shi, Z. Kynurenic acid mediates bacteria-algae consortium in resisting environmental cadmium toxicity. J. Hazard. Mater. 2023, 444 Pt A, 130397. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- ISO 5961:1994; Water Quality—Determination of Cadmium by Atomic Absorption Spectrometry. International Organization of Standardization: Geneve, Switzland, 1994.
- Zheng, H.; Guo, W.; Li, S.; Wu, Q.; Yin, R.; Feng, X.; Du, J.; Ren, N.; Chang, J.-S. Biosorption of cadmium by a lipid extraction residue of lipid-rich microalgae. RSC Adv. 2016, 6, 20051–20057. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Popuri, S.R.; Kalyani, S.; Kachireddy, S.R.; Krishnaiah, A. Biosorption of hexavalant chromium from aqueous solution by using prawn pond algae. Indian J. Chem. 2010, 46, 284–289. [Google Scholar]
- Gu, S.; Lan, C.Q. Lipid-extraction algal biomass for biosorption of bivalent lead and cadmium ions: Kinetics and isotherm. Chem. Eng. Sci. 2023, 276, 118778. [Google Scholar] [CrossRef]
- Janik, W.; Nowotarski, M.; Ledniowska, K.; Shyntum, D.Y.; Krukiewicz, K.; Turczyn, R.; Sabura, E.; Furgoł, S.; Kudła, S.; Dudek, G. Modulation of physicochemical properties and antimicrobial activity of sodium alginate films through the use of chestnut extract and plasticizers. Sci. Rep. 2023, 13, 11530. [Google Scholar] [CrossRef]
- Moreira, J.B.; Santos, T.D.; Cruz, C.G.; da Silveira, J.T.; de Carvalho, L.F.; de Morais, M.G.; Costa, J.A.V. Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields. Polysaccharides 2023, 4, 371–389. [Google Scholar] [CrossRef]
- Loosli, F.; Vitorazi, L.; Berret, J.-F.; Stoll, S. Towards a better understanding on agglomeration mechanisms and thermodynamic properties of TiO2 nanoparticles interacting with natural organic matter. Water Res. 2015, 80, 139–148. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Long, X.; Chen, H.; Huang, T.; Zhang, Y.; Lu, Y.; Tan, J.; Chen, R. Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue. Molecules 2021, 26, 2497. [Google Scholar] [CrossRef] [PubMed]
- Freitas, O.M.; Martins, R.J.; Delerue-Matos, C.M.; Boaventura, R.A. Removal of Cd(II), Zn(II) and Pb(II) from aqueous solutions by brown marine macro algae: Kinetic modelling. J. Hazard. Mater. 2008, 153, 493–501. [Google Scholar] [CrossRef]
- Khalfaoui, M.; Knani, S.; Hachicha, M.; Lamine, A. New theoretical expressions for the five adsorption type isotherms classified by BET based on statistical physics treatment. J. Colloid Interface Sci. 2003, 263, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rao, D.; Zou, L.; Teng, Y.; Yu, H. Capacity and potential mechanisms of Cd(II) adsorption from aqueous solution by blue algae-derived biochars. Sci. Total. Environ. 2021, 767, 145447. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, M.; Yu, X.; Niu, N.; Chen, L. Application of lignin adsorbent in wastewater Treatment: A review. Sep. Purif. Technol. 2022, 302, 122116. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Nonspontaneous and multilayer adsorption of malachite green dye by Acacia nilotica waste with dominance of physisorption. Water Sci. Technol. 2017, 76, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Thajeel, A.S. Isotherm, kinetic and thermodynamic of adsorption of heavy metal ions onto local activated carbon. Aquat. Sci. Technol. 2013, 1, 2168–9148. [Google Scholar] [CrossRef]
- Chegrouche, S.; Mellah, A.; Barkat, M. Removal of strontium from aqueous solutions by adsorption onto activated carbon: Kinetic and thermodynamic studies. Desalination 2009, 235, 306–318. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Mechanism of heavy metal ion biosorption by microalgal cells: A mathematic approach. J. Hazard. Mater. 2024, 463, 132875. [Google Scholar] [CrossRef]
- Kumar, R.; Bhatia, D.; Singh, R.; Rani, S.; Bishnoi, N.R. Sorption of heavy metals from electroplating effluent using immobilized biomass Trichoderma viride in a continuous packed-bed column. Int. Biodeterior. Biodegrad. 2011, 65, 1133–1139. [Google Scholar] [CrossRef]

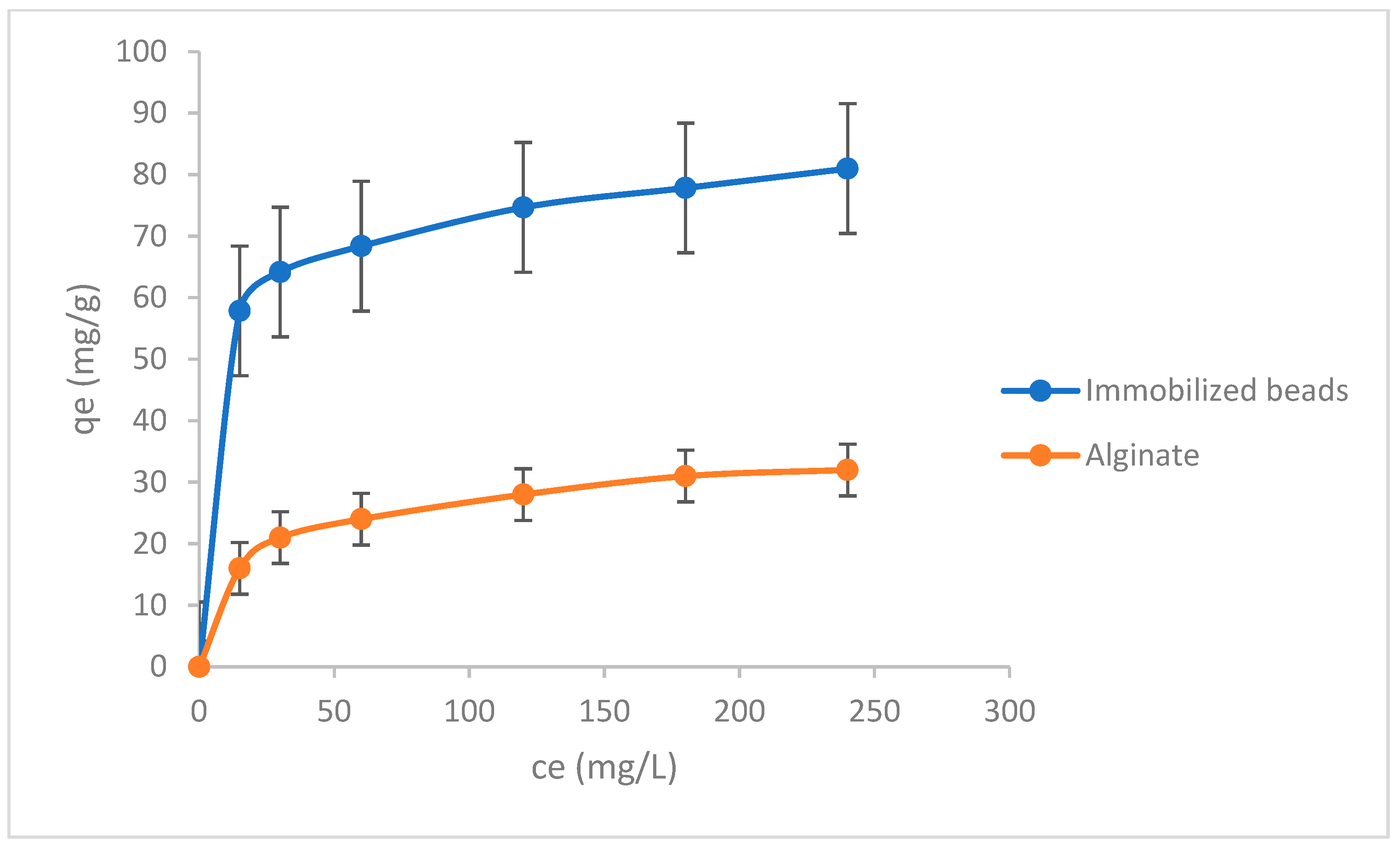

| Model | Konstants | Value |
|---|---|---|
| Freundlich | KF[(mg/g)(mg/L)n] | 4.498 |
| n | 1.595 | |
| R2 | 0.975 | |
| Langmuir | qm (mg/g) | 181.0 |
| b (L/mg) | 0.0108 | |
| R2 | 0.998 | |
| Temkin | bT | 13.9 |
| A (mol/g) | 99.8 | |
| R2 | 0.866 |
| T (K) | ΔGo (kJ/mol) | ΔSo (J/molK) | ΔHo (kJ/mol) |
|---|---|---|---|
| 298 | −10.9 | 6.1 | −12.7 |
| 308 | −10.4 | ||
| 318 | −10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonič, M. Algae Modified Alginate Beads for Improved Cd(II) Removal from Aqueous Solutions. Sustainability 2024, 16, 8174. https://doi.org/10.3390/su16188174
Simonič M. Algae Modified Alginate Beads for Improved Cd(II) Removal from Aqueous Solutions. Sustainability. 2024; 16(18):8174. https://doi.org/10.3390/su16188174
Chicago/Turabian StyleSimonič, Marjana. 2024. "Algae Modified Alginate Beads for Improved Cd(II) Removal from Aqueous Solutions" Sustainability 16, no. 18: 8174. https://doi.org/10.3390/su16188174
APA StyleSimonič, M. (2024). Algae Modified Alginate Beads for Improved Cd(II) Removal from Aqueous Solutions. Sustainability, 16(18), 8174. https://doi.org/10.3390/su16188174






